Summary
Objective
This study investigated a novel approach to induce chondrogenic differentiation of human mesenchymal stem cells (hMSC). We hypothesized that a structured three-dimensional co-culture using hMSC and chondrocytes would provide chondroinductive cues to hMSC without inducing hypertrophy.
Method
In an effort to promote optimal chondrogenic differentiation of hMSC, we created bilaminar cell pellets (BCPs), which consist of a spherical population of hMSC encased within a layer of juvenile chondrocytes (JC). In addition to histologic analyses, we examined proteoglycan content and expression of chondrogenic and hypertrophic genes in BCPs, JC pellets, and hMSC pellets grown in the presence or absence of TGFβ following 21 days of culture in either growth or chondrogenic media.
Results
In either growth or chondrogenic media, we observed that BCPs and JC pellets produced more proteoglycan than hMSC pellets treated with TGFβ. BCPs and JC pellets also exhibited higher expression of the chondrogenic genes Sox9, aggrecan, and collagen 2A1, and lower expression of the hypertrophic genes matrix metalloproteinase-13, Runx2, collagen 1A1, and collagen 10A1 than hMSC pellets. Histologic analyses suggest that juvenile chondrocytes promote chondrogenic differentiation of cells in BCPs without hypertrophy. Furthermore, when cultured in hypoxic and inflammatory conditions intended to mimic the injured joint microenvironment, BCPs produced significantly more proteoglycan than either JC pellets or hMSC pellets.
Conclusion
The BCP co-culture promotes a chondrogenic phenotype without hypertrophy and, relative to pellet cultures of hMSCs or JCs alone, is more resistant to the adverse conditions anticipated at the site of articular cartilage repair.
Keywords: mesenchymal stem cell, chondrocyte, coculture, cartilage, osteoarthritis, tissue engineering
Introduction
Damaged hyaline articular cartilage has a poor capacity for self-repair. Injured articular cartilage is often replaced by fibrocartilaginous tissue which does not exhibit the same functional and mechanical properties as hyaline cartilage (1) and can lead to osteoarthritis. A cell-based approach to cartilage repair has the potential to overcome limitations of current osteoarthritis therapies to restore the unique biological and mechanical properties of this tissue. This approach requires an abundant source of cells that can proliferate, synthesize proteoglycan, and express collagen II and aggrecan. Autologous chondrocytes are not optimal because the harvesting procedure damages healthy cartilage. Donor chondrocytes can be obtained only in small numbers. Furthermore, both chondrocyte populations often dedifferentiate during in vitro expansion (2). In contrast, autologous human mesenchymal stem cells (hMSC) can be harvested and expanded readily. However, hMSC require exogenous growth factors to induce chondrogenic differentiation that can present unique challenges (3, 4).
Chondrogenic induction of hMSC is commonly performed using three-dimensional pellet cultures in media containing transforming growth factor-β (TGFβ) (5-9). TGFβ is a potent chondroinductive agent, but in vitro it drives cells to adopt a hypertrophic phenotype in which cells terminally differentiate, undergo apoptosis, and produce matrix with inferior mechanical properties. Though chondrocyte hypertrophy is essential in endochondral ossification, it is problematic for the regeneration of articular cartilage in which chondrocytes retain a stable phenotype. As a result, a successful tissue engineering approach should promote robust chondrogenic differentiation of hMSC and cartilage matrix synthesis without hypertrophy, particularly when challenged by the inflammatory and hypoxic microenvironment of the injured or osteoarthritic joint.
Co-culture of stem cells with a differentiated cell population has been explored as an alternative to growth factors to direct stem cell differentiation (10). Rather than supplying a single growth factor, these cellular interactions have the potential to generate a multitude of biochemical and physical cues in a more physiologic dose, duration, and sequence. Chondrocyte co-culture with hMSC can overcome the hMSC requirement for exogenous growth factors to enhance chondrogenic differentiation (11-19). While the mechanisms remain unclear, both chondrocyte and hMSC-derived soluble factors have been implicated in the chondroinductive effects of co-culture. However, conditioned media is insufficient to confer this advantage (11, 16), suggesting the need for bidirectional exchange of soluble factors between the two cell types and/or a gradient of soluble factors, neither of which are replicated by application of conditioned media.
In vivo and in vitro data clearly indicate the importance of physical and spatial cues in cell differentiation (20, 21). For example, physical interactions among mesenchymal precursors and surrounding cell types induce instructive signaling events that self-promote chondrogenesis in development (21, 22). Therefore, we hypothesized that a structured bilaminar co-culture of hMSC with chondrocytes would provide a combination of physical, spatial, and biochemical cues to promote optimal chondrogenic differentiation and performance in the adverse conditions anticipated in an injured joint. Previous studies showed that structured co-culture of intervertebral disc cells with hMSC outperformed mixed co-culture (23). To determine if structured co-culture is also an advantage for chondrogenesis, we created a three-dimensional co-culture in which a spherical population of hMSC is surrounded by a layer of commercially prepared juvenile chondrocytes (JC) derived from cadaveric tissue from young donors (20, 23, 24). The chondroinductive capacity of these bilaminar cell pellets (BCPs) was evaluated in vitro in conditions that are optimal for chondrogenesis as well as in conditions intended to mimic the injured articular cartilage microenvironment.
Materials and Methods
Cell culture
Commercially available bone marrow-derived human mesenchymal stem cells (hMSC) from three female donors (Lonza, MD) were expanded to the seventh passage in growth media (DMEM low glucose, 1% penicillin/streptomycin and 10% fetal bovine serum) at 37° C with 5% CO2. Culture media was changed three times a week. Juvenile human chondrocytes were used as the instructive cell population (JC) and were derived from cadaveric male donor tissue less than 13 years old (ISTO Technologies, Inc.) (24, 25, 26). JC were incorporated into pellets immediately after thawing.
Pellet formation
Three types of pellets were formed, each consisting of 5×105 cells: pellets of 100% single cell type (hMSC or JC) and pellets of hMSC and JC organized into a bilaminar cell pellet (BCP). To form 100% single cell type pellets, 5×105 cells were centrifuged in a 15 mL polypropylene tube at 300g for 5 min. To create BCPs, 3.75×105 hMSC were centrifuged in a 15 mL polypropylene tube at 300g for 5 min. Subsequently, 1.25×105 JC were added to the same tube and centrifuged again at 300g for 5 min. Following centrifugation, pellets were cultured for three days in polypropylene tubes with 2 mL growth media with caps loosened to allow for gas exchange. During this time, cells rearrange to create a layer of JC that surrounds the hMSC pellet, resulting in a bilaminar spherical configuration (20; unpublished observations, M. Cooke and T Alliston). Pellets were then transferred to ultra-low attachment 24 well plates (Corning, NY) and cultured for 21 days before harvesting. Growth media or chondrogenic media (DMEM high glucose, 1% pen/strep, 1% non-essential amino acids, 1% HEPES, 160 μM L-proline, 200 μM ascorbic acid, 0.1 μM dexamethasone, 3×10-9 M sodium selenite, 10 μg/mL transferrin, 10 μg/mL insulin) was changed three times a week. Pellets were cultured in several conditions: normoxia; hypoxia generated by an incubator maintaining 2% oxygen; inflammatory conditions (10 ng/mL TNF-α and 10 ng/mL IL-1β); or hypoxia with inflammatory conditions.
Sulfated glycosaminoglycan (GAG) and DNA content
Pellets were digested in papain (20 U/mL in PBS) at 60° C overnight. Each digested lysate was independently assayed for GAG content using the dimethylmethylene blue assay (DMMB) and for DNA content using the Quant-iT PicoGreen kit (Invitrogen, CA). For each spectrophotometric DMMB assay, GAG content was quantified using a standard curve generated with bovine chondroitin sulfate (Sigma, MO). Absorption was measured at 525 nm using a spectrophotometer. In growth media experiments, lysates were further digested with DNAse Solution (50 mM Tris-HCL ph7, 10 mM MgCl2, 50 μg/mL BSA, 1 uL DNAse I per sample) (Roche, Germany) for 30 minutes at 37° C prior to performing the DMMB assay, which decreased the variability between technical replicates without compromising the GAG measurements. The DMMB assay was preformed in triplicate and the PicoGreen assay in duplicate for each sample. For each condition using growth media, 10 pellets were assayed (10 biological replicates of 1 pellet each, N=10). For each condition using chondrogenic media, 24 pellets were assayed (8 biological replicates of 3 pellets each, N=8). Each experiment was powered to compensate for the high variability in this assay.
Histological analysis
Pellets were fixed in 4% paraformaldehyde at 4° C overnight, dehydrated with sequential ethanol washes, embedded in paraffin and sectioned at 7 micron thickness. Immunohistochemistry was performed as described in the manufacturer's instructions (Mach 4 Universal HRP-Polymer Kit with DAB, Biocare Medical, CA) with a 1:50 dilution of the primary mouse anti-aggrecan antibody (SC-73693, Santa Cruz Biotechnology, CA). 3,3′-Diaminobenzidine (DAB) and a hematoxylin counterstain were used to visualize bound antibodies. For all histological analyses, figures show representative images of N≥3 biological replicates.
In situ hybridization was performed as described (27). Briefly, sections were hybridized with 35S-labeled riboprobes that recognize human aggrecan mRNA and human collagen-2α1 mRNA. Sections were counterstained with Hoechst dye (Sigma, MO) to visualize nuclei. Hybridization signals were detected using darkfield illumination and the nuclear stain was detected with epifluorescence.
Lipophilic tracers, DiI and DiO (Invitrogen, OR) were used to label different cell populations. Briefly, prior to creating pellets some of the cells were incubated with 12.5 μg DiI or DiO per 1 mL media for three hours. Cells were washed and pelleted as described above. BCP structure was observed in these pellets using a fluorescence microscope, Axiovert 40 CFL (Zeiss, NY).
Fluorescence in situ hybridization (FISH): To localize cells of hMSC or JC origin within the BCPs, we used FISH. Female hMSC and male JC were distinguished using dual-color human X and Y chromosome DNA probes, CEP X Spectrum orange / CEP Y Spectrum green (Vysis, Inc., IL). Sections were deparaffinized, rehydrated, and pre-treated using the Histology FISH Accessory Kit (DAKO, Denmark) prior to digestion for 40 minutes at 37° C with 500 μg pepsin (Mallinckrodt, MO) per 1 mL 0.9% sodium chloride. Sections were hybridized with FISH probes overnight at 45° C, washed with the provided buffers, counterstained with DAPI II (Vysis, Inc), mounted, and visualized with single-band excitation filters for rhodamine (excitation-525 nm, emission-552 nm), FITC (excitation-490 nm, emission-525 nm), and DAPI (excitation-350 nm, emission-470 nm) to visualize the X chromosome, Y chromosome and nucleus respectively.
Analysis of gene expression
For pellets cultured in growth media, the total RNA was isolated and purified as described in product protocols for the QIAshredder and RNeasy Mini Kits (Qiagen, Germany). RNA was reverse transcribed using an iScript kit (Bio-Rad Laboratories, CA). Five pellets of the same type and culture condition were pooled for each biological replicate. A total of 5 biological replicates were analyzed for each condition. Quantitative RT-PCR (Bio-Rad CFX96) assessed gene expression using Taqman primer sets for each cDNA (Applied Biosystems, CA; Table 1). For pellets cultured in chondrogenic media, the total RNA was isolated and purified as described in product protocols for the Cells to CT Kit (Invitrogen). Quantitative RT-PCR assessed gene expression using SYBR Green primer sets for each cDNA (Integrated DNA Technologies; Table 2). For all conditions, analysis was preformed using primers and probes for human Sox9, aggrecan, collagen 1A1, collagen 2A1, collagen 10A1, matrix metalloproteinase 13 (MMP13), Runx2 and either L-19 or β-2-microglobulin (B2M). Fold changes were calculated according to the comparative Ct method (ΔΔCt Method) and normalized to either L19 or B2M. Statistics show the average and 95% confidence interval of 5 biological replicates for each condition (N=5).
Table 1. Taqman Probe/Primer Sets (Applied Biosystems).
| Human Collagen I | Hs01028956_m1 |
| Human Collagen II | Hs00156568_m1 |
| Human Aggrecan | Hs00153936_m1 |
| Human Sox9 | Hs00165814_m1 |
| Human β-2-Microglobulin | Hs99999907_m1 |
Table 2. SYBR Green Primers.
| SYBR Green Primer | Forward | Reverse |
|---|---|---|
| Human Sox9 | 5-GAC TTC CGC GAC GTG GAC-3 | 5-GTT GGG CGG CAG GTA CTG-3 |
| Human Aggrecan | 5-TCG AGG ACA GCG AGG CC-3 | 5-TCG AGG GTG TAG CGT GTA GAG A-3 |
| Human Collagen I | 5-GTG GAA ACC CGA GCC CTG CC-3 | 5-TCC CTT GGG TCC CTC GAC GC-3 |
| Human Collagen II | 5-AGG GCC AGG ATG TCC GGC AA-3 | 5-ACG AGG TCC AGG GGC ACC TTT T-3 |
| Human Collagen X | 5-CAA GGC ACC ATC TCC AGG AA-3 | 5-AAA GGG TAT TTG TGG CAG CAT ATT-3 |
| Human Matrix Metalloproteinase 13 | 5-TCT GAA CTG GGT CTT CCA AAA-3 | 5-GCA TCT ACT TTA TCA CCA ATT CCT-3 |
| Human L-19 | 5-GGG ATT TGC ATT CAG AGA TCA G-3 | 5-GGA AGG GCA TCT CGT AAG-3 |
Analysis of protein expression
Pellets were lysed in RIPA lysis buffer at 4° C (28). Lysates were sonicated 3 × 15 seconds on ice, clarified by centrifugation, and assessed by SDS-PAGE and Western analysis using rabbit anti-Sox9 (SC-20095, Santa Cruz Biotechnology, CA) and mouse anti-β Actin (ab8226, Abcam, MA) as previously described (29). Western blot images are representative of N≥3 images. MMP13 protein expression was evaluated using a commercially available Enzyme Activity Assay for Human Active MMP13 (R&D Systems, MN), in conditioned media that was collected after 72 hours from the indicated conditions and concentrated with Amicon®Ultra-4 centrifugal filter devices (Millipore, Ireland). Statistics show the average and 95% confidence interval of 3 biological replicates for each condition (N=3).
Statistical analysis
Data analysis was preformed using standard analysis of variance procedures (ANOVA, Minitab Software, PA) to calculate group means and to test for the effects of pellet type (hMSC, hMSC+TGFβ, JC, BCP; entered as categorical variables) and culture conditions (normal media, hypoxia with inflammatory cytokines, hypoxia alone, and inflammatory cytokines alone) on the measured parameters of interest (L19, B2M, aggrecan, collagen 2A1, Sox9, Runx 2, MMP13, collagen 1A1 and collagen 10A1, entered as continuous predictors). When indicated, Tukey's post-hoc tests were used to judge pair-wise differences. Graphs represent the average of biological replicates; error bars describe 95% confidence intervals, and p-values less than 0.001 are noted with an asterisk (*) unless otherwise noted in the figure or figure legend. A group of pellets of any given culture condition and cell type is derived from the same cell sources and therefore individual pellets of a group do not represent true biologic replicates. However, as the pellets were grown separately for 21 days in culture we attribute differences between the pellets to both tehnical variability and biologic variability.
Results
Chondroinduction and hypertrophy of hMSCs
Consistent with previous studies (5-7, 30), hMSC pellet culture in chondrogenic media containing TGFβ induced robust expression of aggrecan mRNA (Figure 1A) and protein (Figure 1B) and other chondrogenic genes (data not shown), particularly relative to hMSC pellets grown in the same conditions without TGFβ. However, hMSC pellets cultured with TGFβ also expressed high levels of the hypertrophic chondrocyte marker genes, MMP13, Runx2, Collagen I, and Collagen X (Figure 1C and data not shown). Consistent with these results, TGFβ treatment of hMSC also induced MMP13 protein expression (Figure 1D).
Figure 1. hMSC pellets cultured with TGF-β express aggrecan, but also the hypertrophic gene MMP13.
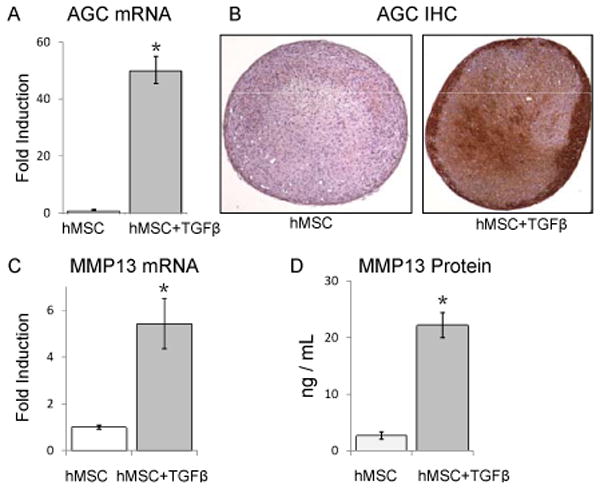
(*, p<0.001). hMSC pellets cultured in chondrogenic media for 21 days with TGFβ (5ng/mL) express more aggrecan and MMP13 mRNA than comparable pellets grown in the absence of TGFβ, N=5 (A, C). The gene expression data is confirmed by protein analyses: aggrecan immunohistochemistry reveals greater staining in hMSC pellets cultured with TGFβ compared to those without TGFβ, N≥3 (B), and more MMP13 was detected in media of hMSC pellets cultured with TGFβ than those cultured without TGFβ, N=3 (D). Error bars represent 95% confidence intervals.
BCP coculture promotes proteoglycan production
We hypothesized that structured BCP co-culture is sufficient to induce chondrogenic differentiation without hypertrophy. The BCP is a structured pellet co-culture comprised of a spherical population of hMSC surrounded by a layer of juvenile chondrocytes (JC) (Figure 2A, B). To test this hypothesis, we first assessed proteoglycan content produced by the pellets cultured in growth media that contains no added chondroinductive growth factors (Figure 2C). When BCPs were cultured for 21 days in growth media (without chondroinductive growth factors such as TGFβ), the BCPs had significantly greater presence of glycosaminoglycan (GAG) per cell than the hMSC pellets (2.03 fold increase p = 0.0015, Figure 3). No significant differences in GAG production were apparent between the BCP and JC pellet group (p = 0.6033). These results suggest that BCP co-culture enhances proteoglycan production by one or both cell types. Furthermore, the BCP co-culture was able to produce an equivalent amount of proteoglycan as the 100% JC pellets despite the fact that BCPs only contain 25% JC.
Figure 2. BCPs produce significantly more GAG than hMSC pellets.
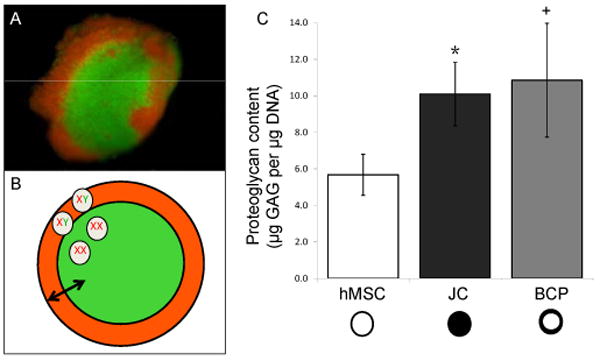
(+, p = 0.0015 compared to hMSC; *, p = 0.0121 compared to hMSC). BCPs constructed with cell populations labeled with DiI (red) or DiO (green), N≥3 (A), show bilaminar structure as portrayed in a schematic (B). BCPs are intended to facilitate inductive interactions (arrow) between an outer layer of male juvenile chondrocytes (XY, orange), and a core of female hMSC (XX, green). Following 21 days of culture in growth media, BCPs containing 75% hMSC and 25% JC produce as much proteoglycan as pellets containing 100% JC as determined by DMMB analysis N=10 (C). Error bars represent 95% confidence intervals.
Figure 3. BCP co-culture is sufficient to induce chondrogenic differentiation of hMSC.
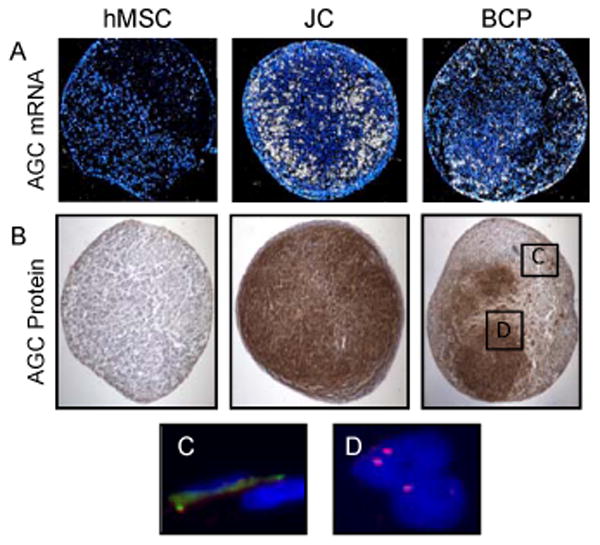
In situ hybridization (A) and immunohistochemistry (B) reveal that JC and BCP pellets express qualitatively more aggrecan mRNA and protein than hMSC pellets. FISH detection of male JC (C) and female hMSC (D), respectively, in boxed regions of (B). N≥3 for all.
As a first step in determining which cells in the pellets were responsible for the production of proteoglycan, we next assessed aggrecan mRNA and protein expression histologically (Figure 3A, B). In situ hybridization and immunohistochemistry (IHC) revealed that after 21 days of culture with growth media, the BCPs qualitatively demonstrated more aggrecan mRNA and protein expression compared to hMSC pellets (Figure 3A, B). Furthermore, the aggrecan mRNA and protein was detected centrally in the BCP, not only in the juvenile chondrocyte shell. The JC pellets showed qualitatively more aggrecan than either the BCPs or hMSC pellets.
Bilaminar structure was retained in BCPs
To determine if the BCP co-culture retained its bilaminar structured form throughout the 21 day differentiation period, we evaluated the location of male chondrocytes and female hMSC within the BCP co-culture by fluorescence in situ hybridization. Even after 21 days of culture, the male JCs remained at the periphery of the BCP surrounding the female hMSC (Figure 3C, D). Adjacent section FISH and aggrecan IHC confirmed that cells in the aggrecan-rich center of the BCP are derived from a female donor, suggesting that hMSC in BCPs can undergo chondrogenic differentiation.
BCPs induce chondrogenic differentiation without hypertrophy
We further quantified the chondrogenic performances of BCPs relative to hMSC alone, which require TGFβ for chondrogenic differentiation. After 21 days of culture in growth media, the BCP co-culture had greater expression of the chondrogenic genes Sox9 (3.81 fold, p<0.0001), Col II (948 fold, p<0.0001) and Aggrecan (353 fold, p<0.0001) than hMSC pellets cultured with TGFβ (Figure 4A). Not only did BCPs express more mRNA for anabolic chondrocyte genes relative to hMSC pellets cultured with TGFβ, but they also expressed less mRNA for hypertrophic marker genes, Runx2 (59.5% decrease p<0.0001), Collagen X (99.6% decrease p<0.0001) and MMP13 (89.5% decrease p<0.0001) (Figure 4C). These results are consistent with protein analyses, which show increased endogenous Sox9 protein expression and reduced secreted MMP13 protein in BCPs compared to hMSC pellets cultured with TGFβ (Figure 4B, 4D).
Figure 4. Unlike TGF-β, BCP co-culture induces chondrogenic differentiation of hMSC without hypertrophy.
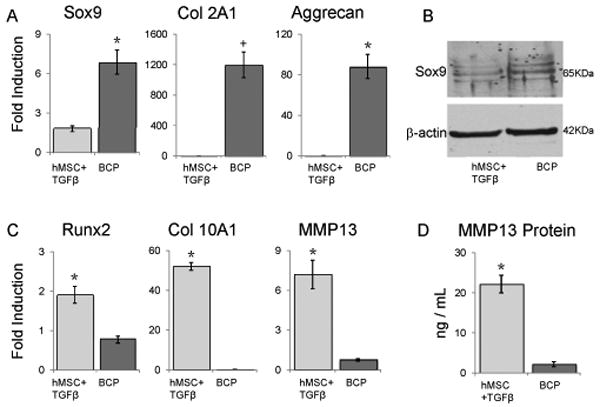
(*, p<0.001; +, p = 0.0021). Following 21 days of culture in growth media, BCPs exhibit enhanced chondrogenic differentiation relative to TGF-β treated hMSC pellets, as shown by increased mRNA expression for anabolic chondrocyte marker genes Sox9, collagen 2A1, and aggrecan (A) and reduced mRNA expression of hypertrophic genes Runx2, Col 10A1 and MMP13 (C) as assessed by quantitative RT-PCR. Data are normalized to β-2-macroglobulin expression and show averages of 5 biological replicates. The gene expression data is confirmed by protein analyses: Western blotting confirms the upregulation of Sox9 in the BCPs compared to the TGFβ-treated hMSCs, N≥3 (B), and an enzyme activity assay confirms the upregulation of MMP13 in the TGFβ treated hMSCs compared to the BCPs N=3 (D). Error bars represent 95% confidence intervals.
Similar data were observed when the pellets were cultured in chondrogenic media
Chondrogenic media supplements growth media with additional factors that encourage chondrogenic differentiation. Accordingly, the studies were repeated with chondrogenic media to compare the BCP model to a chondrocyte pellet culture in optimal chondrogenic conditions. Unlike growth media, in which BCP and JC pellets produce equivalent levels of proteoglycan, in chondrogenic media, the JC pellets produced the greatest amount of proteoglycan (18% more than BCPs p = 0.0038, 123% more than hMSC p < 0.0001). This was confirmed with aggrecan immunohistochemistry staining (Figure 5A,B). JC pellets showed maximal expression of the chondrogenic genes Sox9 (p < 0.0001), collagen 2A1 (p < 0.0001), and aggrecan (p < 0.0001) (Figure 5C). After 21 days of culture in chondrogenic media, compared to hMSC pellets treated with TGFβ, the BCPs produced more proteoglycan (p < 0.0001) and had equivalent expression of the chondrogenic gene collagen 2A1. In addition, hMSCs treated with TGFβ had greater expression of the hypertrophic genes MMP13, collagen 10A1 and collagen 1A1 compared to all other pellet types (p < 0.0001) (Figure 5C). These results are consistent with those in growth media, demonstrating that BCP coculture promotes chondrogenic gene expression without hypertrophy.
Figure 5. BCP co-culture performs similarly in chondrogenic media compared to culture in growth.
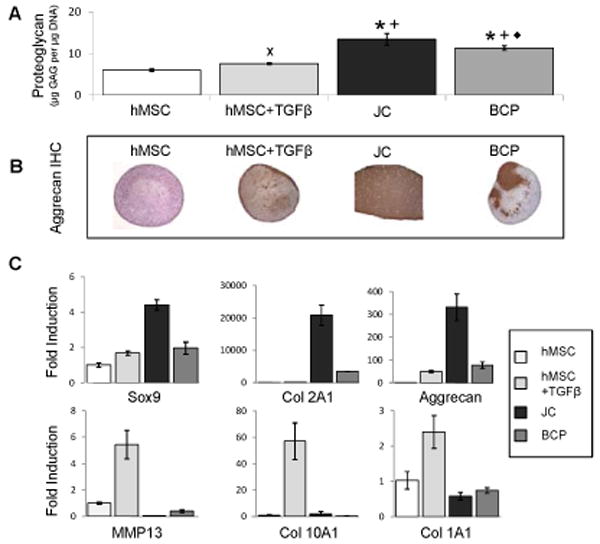
media (*, p<0.001 compared to hMSC; +, p <0.001 compared to TGFβ treated hMSC; ◆, p = 0.0038 compared to JC; x, p = 0.045 compared to hMSC). In chondrogenic media, BCP and JC pellets produce more proteoglycan compared to hMSC in the presence or absence of TGFβ as assessed by DMMB, N=8 (A), a result that is consistent with Aggrecan IHC N≥3 (B). JC pellets showed increased expression of chondrogenic genes relative to BCPs and hMSC+TGFβ pellets. hMSC+TGFβ pellets had greater expression of hypertrophic genes than BCP pellets N=5 (C). Error bars represent 95% confidence intervals.
BCPs continue to produce proteoglycan in hypoxic and inflammatory conditions
The injured articular cartilage microenvironment is hypoxic and inflammatory. As expected, the proteoglycan synthesis of the JC pellets was impaired in the presence of inflammatory cytokines. In contrast, BCPs cultured in hypoxic, inflammatory, or combined conditions produced more proteoglycan than either hMSC and JC pellets (all p < 0.02, specific p values listed in Figure 6A). Elevated proteoglycan levels in BCPs occur even without significant differences in cell number (Figure 6B), suggesting that BCP co-culture protects proteoglycan synthesis rather than cell proliferation or viability.
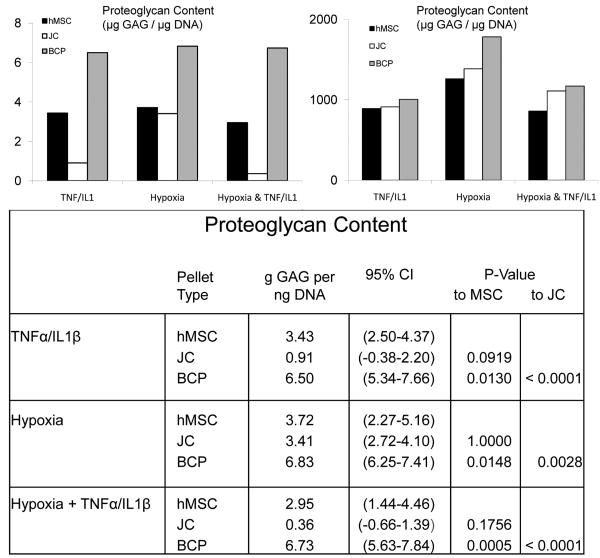
Figure 6. BCPs produce more GAG than hMSC or JC pellets when subjected to conditions that mimic the hypoxic, proinflammatory environment found in cartilage.
defects (*, p <.001). A DMMB assay revealed a significant advantage for BCP proteoglycan production relative to JC or hMSC pellets that were cultured with TNF-α (10 ng/mL) and IL1-β cytokines (10 ng/mL), and/or at 2% O2 for 21 days, N=10. Results were normalized to DNA content, N=10 (B), which showed no statistical differences between the cell types (hMSC, JC and BCP) within the conditions: TNFα/IL1β, Hypoxia or Hypoxia + TNFα/IL1β).
Discussion
Our results demonstrates that a structured three-dimensional co-culture, in which a spherical population of human mesenchymal stem cells (hMSC) is surrounded by a layer of juvenile chondrocytes (JC), promotes a chondrogenic phenotype without the induction of chondrocyte hypertrophy. The amount of proteoglycan produced by BCPs was nearly equivalent to JC pellets even though the BCPs contain only 25% JC cells. Furthermore, the BCPs produce more proteoglycan than hMSC pellets or hMSC pellets treated with TGFβ. Taken together, these data indicate that the structured co-culture of juvenile chondrocytes and hMSC in the BCP are inducing chondrogenic differentiation. Most notably, in the setting of inflammatory cytokines and/or hypoxia, BCPs outperform JC as well as hMSC pellets in the production of proteoglycan. Therefore, the unique combination of physical, spatial, and biochemical cues provided by BCP co-culture overcomes several obstacles that currently limit the utility of hMSC for articular cartilage repair.
Unlike TGFβ-induced chondroinduction of hMSC, chondrogenic differentiation in BCPs occurred with reduced hypertrophy. The observation that BCP co-culture directs a more stable chondrogenic differentiation than does TGFβ is consistent with those of Aung, et al., who found that the co-culture of hydrogel-encapsulated hMSC with chondrocytes enhanced differentiation without hypertrophy. Chondrocyte conditioned media was unable to replicate this effect or that of BCP co-culture (16, data not shown). Therefore, chondroinduction requires an exchange of soluble factors between the two cell populations, and/or a spatial gradient of these factors. Although mass spectrometry studies by Aung, et al. identified many soluble factors present only in co-cultured media, which of the co-culture-specific cues provided by BCP co-culture are responsible for this stable chondrogenic differentiation remains unclear. Nonetheless, several studies suggest factors that may be involved. Specifically, TGFβ is known to play an important role in the induction of chondrocyte lineage selection and in the regulation of chondrogenic hypertrophy (5). BCPs that were cultured with SB431542, a specific pharmacologic inhibitor of the type I TGFβ receptor, showed a reduced expression of both pro-chondrogenic (collagen 2A1 and aggrecan) and hypertrophic (MMP13 and collagen 10A1) genes (data not shown). Likely, the combination of factors produced by BCP co-culture modulate the hMSC response to TGFβ, such that the anabolic effects are enhanced, while hypertrophy-inducing effects are suppressed. Other factors may include agonists and antagonists of the fibroblast growth factor (FGF), hedgehog, bone morphogenetic protein (BMP) and Wnt pathways (22, 31).
While several studies demonstrate the important role of soluble factors in the chondroinductive effects of co-culture (10, 12-17, 19), physical and spatial cues may also participate (18, 23). In development, the spatial juxtaposition of the perichondrium with the undifferentiated mesenchymal cells provides a critical signaling feedback loop that self-promotes chondrogenesis (22). A computational model predicts that a graded diffusion of soluble factors may impact chondrogenic differentiation (16). Our observations in BCPs may provide experimental support for this model, suggesting that cellular crosstalk between two cell populations across a boundary or gradient may enhance the inductive effect. The report by Allon, et al., provides further support for this conclusion by demonstrating that the structured BCP configuration is superior to mixed co-cultures of MSCs and chondrocyte-related nucleus pulposus cells (23). Since the BCP configuration used herein is based on Allon, et al., we anticipate but have not yet verified similar advantages of BCP culture over random mixtures of hMSC and chondrocytes. Finally, physical cues ranging from extracellular matrix stiffness to topographical cues have been shown to direct cell fate and may contribute to the chondroinductive effects of BCPs (32-35); important points to consider as cells and engineered biomaterials are combined to develop stem cell-based therapies.
Another key advantage of BCP co-culture is the ability of BCPs to resist the adverse hypoxic and inflammatory conditions anticipated in injured articular cartilage, which are recognized challenges to the success of cartilage tissue engineering for the treatment of osteoarthritis. This study found that BCPs maintain their chondrogenic potential under hypoxic and inflammatory conditions, producing more proteoglycan than pellets composed of either cell type alone. While hMSC are susceptible to hypoxia and JC are susceptible to inflammatory conditions, the combined cell populations in the BCP conferred resistance to these adverse environments, possibly due to the well-documented anti-inflammatory capability of hMSC (36, 37) and the adaptation of chondrocytes to hypoxic conditions. Though these results are provocative, a limitation of our study is that we used two cytokines and a hypoxia to mimic the complex microenvironment of the injured joint. Additional studies are necessary to evaluate the performance of BCP under in vivo conditions that more effectively model the healthy and injured articular cartilage microenvironment.
Further study is also needed to conclusively demonstrate which of the two cell populations in BCPs are responsible for the increased proteoglycan production and chondrogenic gene expression. Several prior studies have explored whether the MSC, chondrocytes, or both are responsible for enhanced chondrogenesis in co-cultures (12-14, 17, 19). The possibility that BCP co-culture induces chondrogenic differentiation of hMSC is supported by several lines of evidence. First, the magnitude of proteoglycan production by BCPs and JC pellets is comparable, even though BCPs contain 75% hMSC. Second, histological analyses of BCPs show aggrecan mRNA and proteoglycan deposition throughout the BCP, not only in the outer shell composed of juvenile chondrocytes. Third, FISH analysis confirmed that aggrecan and safranin-O-positive regions of BCPs contained only hMSC, and that the JC were confined to the pellet surface even after 21 days of culture. Fourth, similar analyses of bilaminar cell pellets composed of 25% bovine nucleus pulposus cells and 75% human MSC showed an induction of human (not bovine) collagen 2A1 and aggrecan expression after 21 days of culture (38). These results are consistent with those in which co-culture of hMSC with chondrocytes or cartilage matrix, for example, in a transwell, can increase expression of chondrocyte marker genes by hMSC (12, 13, 39). Alternatively, hMSC may promote increased levels of proteoglycan production by the juvenile chondrocytes (40). Human MSC are known to generate instructive signals for other cell types, and several other studies demonstrate that MSC increase chondrogenic capacity of chondrocytes in pellet co-cultures of these two cell types (14, 17, 19). While our data suggest that BCP co-culture promotes chondrogenic differentiation of hMSC, it is does not exclude the possibility that hMSC enhance the chondrogenic activity of the human chondrocytes. Indeed, both mechanisms likely contribute.
In summary, our data indicate that the structured cellular interactions within BCPs promote a robust chondrogenic phenotype, as demonstrated by proteoglycan production and chondrogenic gene expression. Importantly, BCP co-culture addresses two major limitations of cartilage tissue engineering: a limited chondrocyte supply and the generation of chondrocytes without hypertrophy. hMSC are plentiful, therefore BCP formation may be used to expand the limited supply of chondrocytes for cartilage bioengineering. Furthermore, BCP co-culture does not require exogenous growth factors nor does it exhibit a hypertrophic phenotype. Although under chondrogenic conditions JC pellets express genes indicative of a chondrogenic phenotype, chondrocytes are limited in supply and also have impaired production of proteoglycan under inflammatory conditions that are seen in osteoarthritic joints. By contrast, BCPs do not appear to be negatively affected when exposed to inflammatory and hypoxic conditions. Consequently, BCPs may provide significant advantages as a therapeutic approach for cartilage regeneration.
Acknowledgments
The authors gratefully acknowledge the support of ISTO Technologies, who supplied the instructive chondrocytes, as well as Kristin Butcher, Ellen Liebenberg and Eunice Fu for their invaluable technical support.
Role of the Funding Source: This project was funded by UCSF Quarterly PACCTR Fellowship, the UCSF Quarterly Dean's Research Fellowship Program, and HHMI Medical Research Fellowship to MEC; NIH R01DE018234 to RSM; NIH R01DE016402 to RAS; NIH R01AR052712, NIH R01AR 049786, and a CIRM Disease Team Planning Grant, DT1-00656-1 to JCL, and NIH RO1DE019284 to TA.
Footnotes
Author Contributions: All authors contributed intellectually in study design and participated in the development of this manuscript. Specifically, MC planned, performed or participated in all experiments and wrote the manuscript with mentorship and supervision by TA. AA, mentored by JL, planned and performed the experiments using growth media and mentored MC. TC performed analysis of gene expression with mentorship from AK. RM provided support for histological analyses. RS provided support for in situ hybridization studies. All authors participated in monthly meetings to design the study, analyze data, and interpret results.
Conflict of Interest: The authors have no conflicts of interest.
References
- 1.Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. doi: 10.22203/ecm.v009a04. [DOI] [PubMed] [Google Scholar]
- 2.Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39 1:S58–65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 4.Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor regulation of growth factors in articular chondrocytes. J Biol Chem. 2009;284:6697–704. doi: 10.1074/jbc.M807859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–88. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo JU, Barthel TS, Nishimura K, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg. 1998;80:1745–57. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 8.Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–61. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derynck R, Schneider RA, Piek E, Alliston T. TGF-β family signaling in mesenchymal differentiation. In: Derynck R, Miyazono K, editors. The TGF-β Family. Cold Spring Harbor Press; Woodbury, NY: 2008. pp. 613–55. [Google Scholar]
- 10.Hendriks J, Riesle J, van Blitterswijk CA. Co-culture in cartilage tissue engineering. J Tiss Engineer Regen Med. 2007;1:170–8. doi: 10.1002/term.19. [DOI] [PubMed] [Google Scholar]
- 11.Jikko A, Kato Y, Hiranuma H, Fuchihata H. Inhibition of chondrocyte terminal differentiation and matrix calcification by soluble factors released by articular chondrocytes. Calc Tiss Intl. 1999;65:276–9. doi: 10.1007/s002239900698. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed N, Dreier R, Gopferich A, Grifka J, Grassel S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem. 2007;20:665–78. doi: 10.1159/000107728. [DOI] [PubMed] [Google Scholar]
- 13.Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–4. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- 14.Mo XT, Guo SC, Xie HQ, Deng L, Zhi W, Ziang Z, et al. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45:42–51. doi: 10.1016/j.bone.2008.07.240. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, et al. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 31:9406–14. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 16.Aung A, Gupta G, Majid G, Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 63:148–58. doi: 10.1002/art.30086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tiss Engineer. 17:1425–36. doi: 10.1089/ten.TEA.2010.0517. [DOI] [PubMed] [Google Scholar]
- 18.Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tiss Engineer. 17:1137–45. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuchiya K, Chen G, Ushida T, Matsuno T, Tateishi T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mat Sci Engineer C. 2004;24:391–6. [Google Scholar]
- 20.Allon A, Schneider R, Lotz J. Co-culture of adult mesenchymal stem cells and nucleus pulposus cells in bilaminar pellets for intervetebral disc regeneration. SAS Journal. 2009;3:41–6. doi: 10.1016/SASJ-2009-0005-NT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarth Cartil. 2000;8:309–34. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 22.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 23.Allon AA, Aurouer N, Yoo BB, Liebenberg EC, Buser Z, Lotz JC. Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model. Spine J. 2010;10:1089–97. doi: 10.1016/j.spinee.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. ClinOrthop Rel Res. 2001:S280–94. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 25.Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: Implications for biologic repair of joint articular cartilage. Stem Cell Res. 2009;4:57–68. doi: 10.1016/j.scr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Adkisson HD, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colnot CI, Helms JA. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev. 2001;100:245–250. doi: 10.1016/s0925-4773(00)00532-3. [DOI] [PubMed] [Google Scholar]
- 28.Balooch G, Balooch M, Nalla RK, Schilling S, Filvaroff EH, Marshall GW, et al. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci U S A. 2005;102:18813–18. doi: 10.1073/pnas.0507417102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24:2543–55. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mwale F, Stachura D, Roughley P, Antoniou J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006;24:1791–1798. doi: 10.1002/jor.20200. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Kronenberg H. Minireview: transcriptional regulation in development of bone. Endocrinoly. 2005;146:1012–7. doi: 10.1210/en.2004-1343. [DOI] [PubMed] [Google Scholar]
- 32.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–50. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 36.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–25. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 37.Gotherstrom C. Immunomodulation by multipotent mesenchymal stromal cells. Transplant. 2007;84:S35–7. doi: 10.1097/01.tp.0000269200.67707.c8. [DOI] [PubMed] [Google Scholar]
- 38.Allon A, Butcher K, Schneider R, Lotz J. Structured coculture of mesenchymal stem cells and disc cells enhances differentiation and proliferation. 2011 doi: 10.1159/000332985. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi KH, Choi BH, Park SR, Kim BJ, Min BH. The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials. 31:5355–65. doi: 10.1016/j.biomaterials.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 40.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. [Google Scholar]