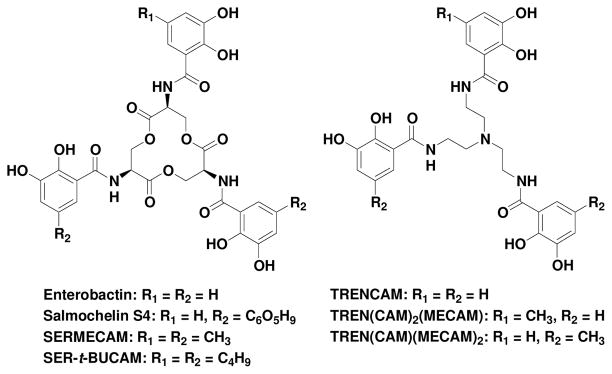
Abstract
The mammalian protein siderocalin binds and inactivates the ferric complex of the bacterial siderophore enterobactin with a Kd value similar to that of the bacterial receptor FepA. However microorganisms can evade this immune response by structural modifications of the siderophore. The binding of siderophores by siderocalin relies in part on electrostatic interactions and does not depend greatly on what metal is in the complex. It is also sterically limited by the rigid conformation of the protein calyx; methylation of the three catecholate rings of enterobactin hinders siderocalin recognition. The siderocalin binding has been probed for a series of enterobactin analogues in order to investigate in detail the specificity of siderocalin recognition.
The mammalian protein siderocalin (also called lipocalin 2, NGAL, 24p3 and uterocalin)2–4 complements the general antibacterial iron-depletion defense of the innate immune system by specifically binding to bacterial ferric and apo siderophores, preventing significant, early bacteraemia.3 Since bacterial growth and infection rely on adequate iron supply,5 it is important to understand the specificity and limitations of this process. A number of pathogenic strains of enteric bacteria produce the siderophore enterobactin (Ent), a powerful iron scavenger (pFeIII = 34.3), to mediate their iron acquisition (Fig. 1).6 However, the sequestration of the ferric complex of enterobactin [FeIII(Ent)]3− by siderocalin, as an immune response against pathogenic invasion, may explain the use of alternate or modified siderophores by successful pathogens.3,7,8 Strains such as S. typhimurium LT2 and E. coli CFT073 harbor the five-gene iroA locus that enables the production of C-glycosylated Ent analogues, salmochelins.7 The diglucosyl-Ent, Salmochelin S4 (Fig. 1), first isolated from S. enterica, incorporates glucose moieties at the 5′ position of two catecholamide rings.9,10 Such modifications introduce steric hindrance significant enough to preclude binding to siderocalin.11 Hence, while siderocalin binds [FeIII(Ent)]3− with a Kd similar to that of the native bacterial receptor FepA,12 siderocalin sequestration of ferric-siderophore complexes can still be evaded by the microorganism.
Figure 1.
Enterobactin, salmochelin S4 and synthetic analogues.
The crystal structure of the siderocalin-[FeIII(Ent)]3− adduct has previously revealed that the siderophore/siderocalin recognition mechanism is dependent upon hybrid electrostatic/cation-π interactions in the highly positively charged protein calyx.4,13 The work presented herein probes this electrostatic interaction and shows that siderocalin-recognition is not significantly affected by most metal substitutions or scaffold alteration of [FeIII(Ent)]3−. In contrast, the steric constraints imposed by the conformation of the protein calyx play a major role in siderocalin-binding.
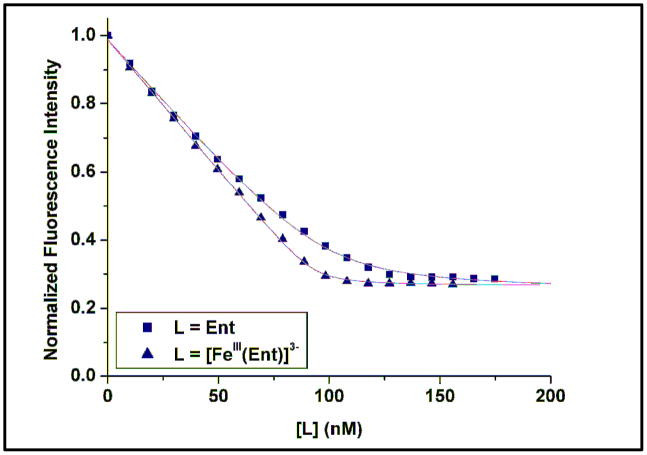
The affinity of siderocalin for a siderophore can be quantified by monitoring the fluorescence of the protein upon siderophore-binding.13 The equilibrium dissociation constants of siderocalin for the various metal complexes [AlIII(Ent)]3−, [GaIII(Ent)]3−, [InIII(Ent)]3− and [VIV(Ent)]2−, as well as for the apo-siderophore Ent, were determined (Table 1). In each case, aliquots of freshly prepared solutions of the ligand (6 μM, pH 7.4) were added to a siderocalin solution (100 nM, pH 7.4). The fluorescence intensity of the protein solution (λexc = 281 nm, λem = 340 nm) was monitored after each addition and a 5 min equilibration time (Fig. 2). Analysis of the data afforded similar Kd values to [FeIII(Ent)]3−, on the order of 0.4 nM, for the AlIII, GaIII and InIII complexes. While the bacterial receptor FepA distinguishes between Ent complexes of iron and other common metals,14 siderocalin does not show the same ability to discriminate between different trianionic metal-Ent chelates. However, the affinity of the protein for the VIV complex of Ent is somewhat weaker. This effect can be attributed to the attenuation of the electrostatic interaction between the positively charged protein residues (Arg81, Lys125, and Lys134) and the dianionic complex. Siderocalin was also found to bind the apo-siderophore Ent tightly, although with a lower affinity than that measured for the ferric complex. Ent is not only less negatively charged than [FeIII(Ent)]3− (only one catecholate oxygen atom is fully deprotonated at pH 7.4),14 its conformation in solution has more degrees of freedom, since the catecholate rings can rotate around the amide bonds.15
Table 1.
Siderocalin Affinity for Ent and Its Complexes at pH 7.4
| Siderocalin Ligand | Kd, nM (esd’s) |
|---|---|
| [FeIII(Ent)]3− | 0.41(11)a |
| [AlIII(Ent)]3− | 0.34(1) |
| [GaIII(Ent)]3− | 0.37(15) |
| [InIII(Ent)]3− | 0.30(2) |
| [VIV(Ent)]2− | 2.26(9) |
| Ent− | 3.57(2) |
The Kd value for [FeIII(Ent)]3− was determined in a previous study.13
Figure 2.
Fluorescence quenching titrations measuring the affinity of siderocalin for Ent and [FeIII(Ent)]3− at pH 7.4. Symbols give the fluorescence measurements at 340 nm and lines give the calculated fits.
In order to further probe the steric constraints imposed by the rigid calyx of the protein, two analogues of enterobactin and salmochelin S4, were synthesized: SERMECAM and SER-t-BUCAM. These incorporate the same serine trilactone backbone of the natural siderophores, but also contain bulky alkyl (methyl and t-butyl respectively) substituents on the 5′ position of each catecholamide unit (Fig. 1). Coupling of the trilactone tri-hydrochloride salt to the methyl or t-butyl substituted benzyl-protected catechol units, followed by catalytic hydrogenation, afforded the new ligands in good yields (Scheme S1). The affinity of siderocalin for the ferric complexes of these two synthetic analogues was subsequently investigated. The intrinsic fluorescence of the system was not significantly quenched upon addition of [FeIII(SERMECAM)]3− or [FeIII(SER-t-BUCAM)]3− (Fig. 3 and S2). Co-crystallization of siderocalin in the presence of each ferric complex also confirmed no binding of these compounds by the protein. Colorless crystals were obtained in both cases (Fig. 3 and S2), indicative of the absence of the ferric-catecholate complexes in the calyx of the protein, since such ferric complexes exhibit a characteristic deep red color resulting from the intense ligand-to-metal charge transfer transitions.13,14 Addition of bulky alkyl groups on all catecholate units of Ent therefore introduces sufficient steric hindrance to preclude binding to siderocalin.
Figure 3.
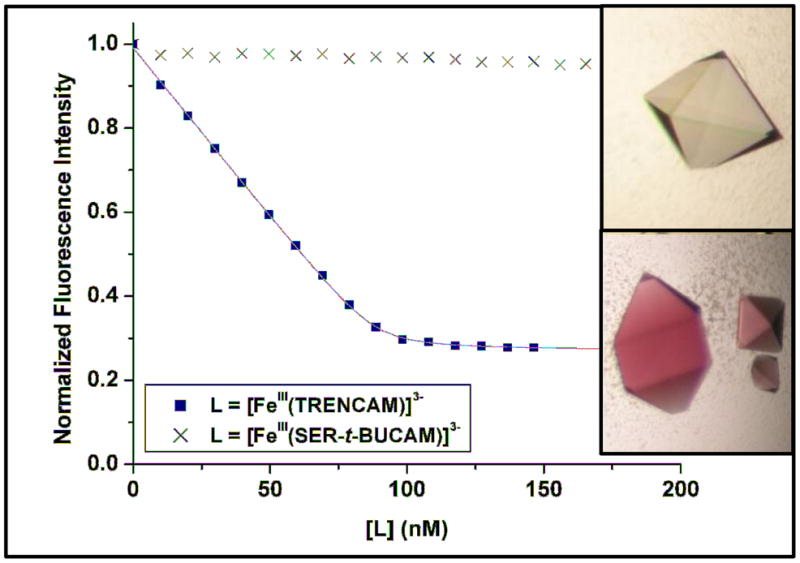
Fluorescence quenching titrations to measure the affinity of siderocalin (100 nM) for [FeIII(TRENCAM)]3− and [FeIII(SER-t-BUCAM)]3−. Symbols give the fluorescence measurements at 340 nm, and lines give the calculated fits. Insert: siderocalin co-crystallized in the presence of [FeIII(SER-t-BUCAM)]3− (top) and [FeIII(TRENCAM)]3− (bottom).
In an attempt to determine the maximal size and shape of the siderophore that can be bound by the protein, another series of analogues was designed: TRENCAM,16 TREN(CAM)2(MECAM) and TREN(CAM)(MECAM)2. These form a sequence of tripodal ligands, with a systematic addition of methyl groups on the 5′ position of the aromatic rings (Fig. 1). The TREN scaffold was used (instead of the triserine cyclic frame) to permit slow addition of thiazolide activated catecholamide units, and thereby control the stoichiometry of the substituted moieties (Scheme S2). The affinity of siderocalin for [FeIII(TRENCAM)]3− was measured by fluorescence titrations (Fig. 3). The similar equilibrium dissociation constants obtained for the ferric complexes of TRENCAM and Ent (Table 2) demonstrate that replacing the triserine backbone by the tripodal TREN scaffold does not affect siderocalin recognition. Therefore TREN(CAM)2(MECAM) and TREN(CAM)(MECAM)2 are relevant intermediates between Ent and the bulky SERMECAM. Each insertion of a methyl group on the catecholate rings of the tripodal ligands resulted in a slight decrease of the affinity of siderocalin, as indicated by the increase of the measured Kd values (Table 2). Although the structure of TREN(CAM)(MECAM)2 does not interfere with the conformation of the protein calyx, insertion of a third methyl group, as on SERMECAM, hinders siderocalin-binding.
Table 2.
Siderocalin Affinity for Ferric Ent and Salmochelin S4 Analogues at pH 7.4
| Siderocalin Ligand | Kd, nM (esd’s) |
|---|---|
| [FeIII(SERMECAM)]3− | No Binding |
| [FeIII(SER-t-BUCAM)]3− | No Binding |
| [FeIII(TRENCAM)]3− | 0.32(1) |
| [FeIII(TREN(CAM)2(MECAM)]3− | 0.49(6) |
| [FeIII(TREN(CAM)(MECAM)2]3− | 1.93(70) |
While our results demonstrate that electrostatics contribute extensively to the overall affinity of siderocalin for [FeIII(Ent)]3−, cation-π interactions likely dominate. Gaps in the siderocalin-[FeIII(Ent)]3− adduct structure suggest positions on the siderophore where modifications could be accommodated.13 The siderocalin calyx tolerates considerable variation of the binding siderophores, such as replacement of the Ent trilactone with the TREN scaffold, while simultaneously imposing significant steric constraints, such as not tolerating methylation of the three catecholate rings. Our data support the idea that Ent glucosylation may, in part, be a bacterial response to evade siderocalin-mediated iron depletion.
Acknowledgments
This work was supported by the National Institutes of Health (K.N.R.: AI11744, R.K.S.: AI59432). We thank Anh Phan for help with the synthesis, Wendy Paulsene for help with the protein, and Trisha Hoette for useful discussions.
Footnotes
Supporting Information Available: Detailed experimental procedures for ligand synthesis, fluorescence titrations and co-crystallization assay. Synthetic Schemes and additional titration data are also available. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Paper #76 in the series “Coordination Chemistry of Microbial Iron Transport”. For the previous paper, see: Abergel RJ, Warner JA, Shuh DK, Raymond KN. J Am Chem Soc. 2006;128:0000–0000. doi: 10.1021/ja062046j.
- 2.Devireddy LR, Teodoro JG, Richard FA, Green MR. Science. 2001;293:829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- 3.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 4.Goetz DH, Sirkku TW, Armen RS, Bratt T, Borregaard N, Strong RK. Biochemistry. 2000;39:1935–1941. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- 5.Dertz EA, Raymond KN. In: Comprehensive Coordination Chemistry - II. McCleverty J, Meyer T, editors. Vol. 8. Pergamon; Oxford: 2003. pp. 141–168. [Google Scholar]
- 6.Raymond KN, Dertz EA, Kim SS. Proc Natl Acad Sci USA. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischbach MA, Lin H, Liu DR, Walsh CT. Nature Chem Biol. 2006;2:132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 8.Holmes MA, Paulsene W, Xu J, Ratledge C, Strong RK. Structure. 2005;13:29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Hantke K, Nicholson G, Rabsch W, Winkelmann G. Proc Natl Acad Sci USA. 2003;100:3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H, Fischbach MA, Liu DR, Walsh CT. J Am Chem Soc. 2005;127:11075–11084. doi: 10.1021/ja0522027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, Aderem A, Smith KD. Submitted for publication. 2006 doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton SM, Igo JD, Scott DC, Klebba PE. Mol Microbiol. 1999;32:1153–1165. doi: 10.1046/j.1365-2958.1999.01424.x. [DOI] [PubMed] [Google Scholar]
- 13.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 14.Loomis LD, Raymond KN. Inorg Chem. 1991;30:906–911. [Google Scholar]
- 15.Studies are in progress to investigate the pH dependence of siderocalin binding to apo-Ent.
- 16.Stack TDP, Karpishin TB, Raymond KN. J Am Chem Soc. 1992;114:1512–1514. [Google Scholar]