INTRODUCTION
Autism spectrum disorder (ASD) is a complex developmental disorder that generally presents in early childhood and is defined by marked delays and impairments in social reciprocity, expressive and receptive communication, imaginative play, as well as restricted range and repertoire of interests and activities. It is often, but not always, associated with intellectual disabilities (American Psychiatric Association, 2000). Children with ASD may have a diagnosis of autism, Asperger syndrome, Pervasive Developmental Disorder (PDD) or Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS); all of which are defined by varying degrees of behaviours in the above mentioned diagnostic criteria. One developmental area that is often overlooked in the clinical evaluation and in the planning of early intervention is the motor development of young children with ASD (Rosenbaum, 2005). Both Asperger (translated in Frith) (1991) and Kanner (1943), credited with early descriptions of what is now called ASD, attached considerable weight on motor clumsiness in their early clinical descriptions of the disorder. Discussion of the motor behaviour of children with ASD has historically focused on stereotyped and repetitive movements such as hand flapping or body rocking (Leary & Hill, 1996; Richler, Bishop, Kleinke, & Lord, 2007). Although stereotyped and repetitive motor behaviours are a criterion for ASD, parents and individuals who work with children with ASD also describe their gross motor and fine motor skills to be atypical and/or delayed (Chawarska et al., 2007; Landa & Garrett-Mayer, 2006).
Recent empirical research has investigated the motor development and motor skill proficiency of children with ASD. The research has consistently found that infants and children with ASD experience both gross and fine motor delays, and/or atypical motor patterns (Berkeley, Zittel, Pitney, & Nichols, 2001; Ghaziuddin & Butler, 1998; Green et al., 2002; Leary & Hill, 1996; Ozonoff et al., 2008; Vernazza-Martin et al., 2005). Many of these studies have examined the motor skills of older children with ASD and the research questions vary widely; variables studied include fundamental motor skills, motor planning, and fine motor skills. Despite the limitations of previous studies they have consistently found motor deficits or delays in children with ASD.
Ghaziuddin and Butler (1998) compared the motor skills of 12 children with autism, 12 with Asperger syndrome, and 12 with PDD- NOS, using The Bruininks-Oseretsky Test of Motor Proficiency (Bruininks, 1978). The most significant finding of this study was that all children with ASD showed problems with motor coordination. Miyahara and colleagues (1997) tested children 8–12 years of age with Asperger syndrome (n=26) and non-specific learning disabilities with no autism (n=16) on the Movement Assessment Battery for Children (Movement ABC) (Henderson & Sugden, 1992). They found that both the groups displayed significant delays or difficulties with motor coordination. They also found no significant correlation between IQ scores and the Movement ABC test scores. Green and colleagues (2002) compared children between 6–10 years of age with Asperger syndrome (n=11) and developmental coordination disorder (n= 9) also using the Movement ABC. Results indicated that all children with Asperger syndrome met criteria for a diagnosis of motor impairment (Green, et al., 2002). In sum, the research has demonstrated that older children with ASD have difficulties in the motor domain.
The motor development of toddlers and preschool age children with ASD has emerged as an area of interest due, in part, to the increased need for early diagnosis and the increasing evidence that children with ASD exhibit atypical motor characteristics. In a sample of children 21–41 months of age Provost and colleagues (2007) investigated the gross motor skills of 19 children with ASD, a group with a developmental delay (n=19), and a group with a developmental delay but no motor problems (n=18) using the Bayley Scales of Infant Development −2 (Bayley, 1993), and The Peabody Developmental Motor Scales −2 (Folio & Fewell, 2000). Results indicated that all the toddlers were delayed in at least one area of motor development. Jasmin and colleagues (2009) also used the Peabody Developmental Motor Scales-2 and in addition to The Sensory Profile (Dunn, 1999)and Vineland Adaptive Behaviour Scales-2 (Sparrow, Cicchetti, & Balla, 2005); in their sample of 3–4 year old children with ASD (n=35) they found atypical sensory responses, very poor motor skills and daily living skills. Landa and Garrett-Mayer (2006), in their prospective study of 87 infants at risk for ASD used the Mullen Scales of Early Learning and evaluated children with (n= 60) and without ASD (n= 27) at 6, 14, and 24 months. They found that the children in the ASD group performed significantly worse than the other groups on their gross and fine motor skills as early as 14 months and nearly half of the ASD group showed “developmental worsening” between 14 and 24 months. These studies indicate that delays and/or atypical patterns in gross motor development appear to emerge early in young children with ASD.
Despite the recent interest in the motor skills of young children with ASD, there are several limitations of the previously published studies; for example, small sample sizes (n <30), and the inclusion of only high functioning children with relatively high IQ's. High IQ is a particularly relevant limitation because a large proportion of children with ASD have intellectual disabilities that are in the moderate to severe range. Finally, in the literature on the motor skills of children with ASD, diagnosis is often not confirmed by objective diagnostic measures, but by parent report. What this study adds to the literature is a well controlled analysis (e.g. diagnosis and IQ), of the gross and fine motor skills of a large cross-sectional sample of very young children with ASD in addition to a longitudinal analysis of a relatively large sample.
The purpose of this paper is to first describe and compare the objectively measured gross motor and fine motor skills, using the Mullen Scale of Early Learning (MSEL), of a cross-sectional group of 162 children with ASD (12–36 months). Secondly, we will describe the gross motor and fine motor skills of 58 children with ASD longitudinally over two time points (approximately 12 months apart). The longitudinal analysis allows for the observation of individual patterns of change and provides some contrast for possible effects of recruitment differences by age in the cross-sectional group. It is hypothesized that the gross and fine motor skills of children with ASD will be delayed for their age and the delay will persist upon follow up evaluation.
METHOD
Data was collected at three types of sites: 1) Four North Carolina state-funded autism centres, 2) a Chicago autism clinic within a private university hospital and 3) an autism centre in Michigan affiliated with a public university. The participants were part of two large longitudinal investigations in which the developmental trajectories of children with, or at risk for, ASD in the toddler years or early preschool years were studied using multiple measures over several years. This sample of convenience, which was drawn from the larger datasets met the following criteria in the dataset: a) a best estimate (final) diagnosis of ASD, b) reported ages of independent sitting and walking that were less than 48 months c) a score on the MSEL gross motor sub-scale, d) a score on the VABS gross motor sub-scale, and e) ratio verbal and non-verbal IQs.
Participants in the larger studies were recruited locally at each site through physician referral, parent referral (e.g. a younger sibling of a child with ASD), advertisements, and local preschool centres. Not all children recruited into the large longitudinal studies received an ASD diagnosis; however, only children who met the diagnostic criteria for ASD are included in this study. This study does not differentiate sub-types of ASD, participants were included if they met the diagnostic criteria for ASD (including Autism, Asperger Syndrome, PDD and PDD-NOS) based on the diagnostic tests and clinician judgment and the other inclusion criteria for this study. Written informed consent was obtained from all parents and/or legal guardians and institutional review boards approved all procedures. Due to the longitudinal nature of these investigations most of the participants had multiple assessments over many years; therefore the most recent, or best, diagnosis was used for each participant in this paper. Best diagnosis is the final, stable diagnosis that the children received based on all available information and corroborated by an assessment by a clinician who was blind to all earlier diagnoses and assessments. As part of the two longitudinal studies, multiple developmental and diagnostic measures were administered but only the measures relevant to this paper on the motor skills of preschool age children with ASD are described.
Diagnostic Instruments
Caregivers for all children were administered the Autism Diagnostic Instrument-Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994), a standardized parent interview designed to distinguish children with ASD from other populations with developmental delays. All the children were administered the Autism Diagnostic Observation Schedule (ADOS) (Lord, Rutter, DiLavore, & Risi, 1999) or its precursor, the Pre-Linguistic Autism Diagnostic Observation Schedule (PL-ADOS) (DiLavore, Lord, & Rutter, 1995), in order to acquire diagnostic information through direct observation of the children by a trained clinician. The participants at the Michigan center were administered a “toddler research” version of the ADOS (Luyster et al., 2009). Best estimate diagnoses were determined using the standardized algorithms of the ADOS and ADI-R (Lord et al., 2006) in conjunction with the clinical judgment of experienced psychologists (Lord, et al., 1999; Luyster et al., 2005). If children met the criteria for ASD based on the results of the combined diagnostic tests (e.g. ADOS, ADI-R) in conjunction with clinician judgement they were deemed eligible for this study.
Each member of the research clinical teams, at all centres, established inter-rater reliability exceeding 90% exact agreement (kappa>0.70) for all items on the ADI-R and 80% exact agreement (kappa>0.60) on codes for the PL-ADOS, ADOS, and `toddler ADOS' for three consecutive administrations before the studies began. Reliability was maintained over time through consensus coding of approximately every sixth administration with a second rater who was blind to referral status.
Average age of “best” or “final” diagnosis was 95.25 (± 37.28) months (7.9 years ± 3.1 years) for the cross-sectional group and 112.27 (± 21.29) months (9.4 years ±1.7 years) for the longitudinal group. Due to the fact that this paper is a secondary analysis of data collected in multiple longitudinal studies, most of the participants were followed into late childhood explaining how the “final” or “stable” diagnosis reported is in late childhood. The purpose of this paper is to describe the motor skills of children with ASD when they were toddlers. A limitation of previous research is that often children with ASD are not identified early enough to study their early development, or motor skills are not a research question in early diagnosis studies, and therefore retrospective reports or videos are used to study early motor skills. This study uses direct measures of motor skills at an early age with a confirmed diagnosis that may have emerged later; however, all children in this study had a confirmed ASD diagnosis.
Psychometric Instruments
All children were administered the MSEL, a developmental test intended for children aged from birth to 68 months of age (Mullen, 1989, 1995). Scores on the MSEL are organized into 5 domains including: gross motor, fine motor, visual reception (nonverbal problem solving), receptive language, and expressive language. IQ was derived for all participants from the fine motor, visual reception, receptive language and expressive language subtests. When it was not possible to extrapolate deviation IQ scores or composite scores (due to very low IQ for age), the early learning composite score was not used, ratio IQs were used. Ratio verbal IQ was calculated by taking the mean age equivalent of the expressive and receptive language subtests, dividing by chronological age, and multiplying by 100. Ratio non-verbal IQ was calculated in the same manner using the age equivalents from the fine motor and visual reception subtests; this method has been used in other studies (Richler, et al., 2007).
The gross and fine motor subtests of the MSEL were used to assess the gross and fine motor skills of the children with ASD. The gross motor sub-test is not included in the early learning composite which is why it is sometimes not administered by clinicians in the interest of time (and why an even larger sample was not available from the database using our inclusion criteria). Additionally, the MSEL is a developmental test used almost exclusively by psychologists, it is not commonly used by people in the kinesiology or physical therapy domains – and is therefore not as detailed as some other early motor development assessments. However the MSEL is standardized and available for use in the clinical setting. For the purpose of this paper early gross motor skills are the fundamental skills that children learn and use to explore and navigate their environment. All children in the current study had learned how to walk and were developing more complex gross motor skills such as walking up stairs, running, kicking a ball, standing on one foot and jumping.
The Vineland Adaptive Behavior Scales (VABS) (Sparrow, et al., 2005) were also administered immediately following the ADI-R. The VABS is a standardized parent-report measure of everyday adaptive functioning and yields domain scores in the areas of communication, daily living skills, social skills, and motor development (fine motor and gross motor).
Participant Sample
162 children between the ages of 12 and 36 months were identified from a large research database. All children were tested for Fragile X and tuberous sclerosis at the time of entry into the study, and had no known genetic disorders, although this does not preclude the possibility that genetic disorders could be identified today. In addition, 58 children in the cross-sectional group had a second assessment (an average of 12 months later) that also fit the criteria of this study, allowing for longitudinal analyses.
Statistical Analysis
For Study 1, the cross-sectional group of 162 children was separated into three age groups (12–24 months, 25–30 months, and 31–36 months) for analysis. A gross motor difference variable was calculated by taking the absolute difference of each child's chronological age and his or her respective gross motor age equivalent obtained from the MSEL. This variable quantifies the amount of motor delay in months regardless of chronological age. The same formula was used to compute a fine motor difference score for the children's fine motor skills. This computation was employed because it was not possible to compute standard scores (T-scores) for these subtests because many children fell below the basal norms (T ≤ 20). Analysis of covariance (ANCOVA) was performed to compare the three age groups' gross motor difference variable using the MSEL age equivalent on the visual reception subtest (non-verbal problem solving) as the covariate. Post-hoc comparisons with Bonferonni corrections were used when a significant main effect was found. This analysis was repeated for the fine motor difference variable.
A correlation analysis (Pearson Product Moment Correlation) was also conducted for the cross-sectional group of children to evaluate the relationship between parent report measures of motor skills on the VABS and direct testing using the MSEL. The purpose of this analysis was to lend a second layer of confidence that the MSEL gross motor and fine motor subtests. In accordance with our specific focus on motor skills, only the gross motor and fine motor scales on the VABS were analyzed with the gross motor and fine motor scales on the MSEL.
For Study 2, a repeated measures ANCOVA was also performed on the gross motor difference variable to compare the gross motor skills of 58 children with ASD at two different time points while co-varying the visual reception age equivalent on the MSEL (non-verbal problem solving). This same analysis was also conducted using the fine motor difference variable for fine motor skills.
RESULTS
Study 1
Preliminary analyses revealed no differences in motor skills between the 3 data collection sites; therefore all sites were combined for further analysis. Also, there were no group differences found for gender (p = 0.39). The participants were placed by age into three groups (12–24 months, 25–30 months, and 31–36 months) for analysis (Table 1). Due to the small number of children (n=8) who met our inclusion criteria in the 12–18 month range and due to the paucity of data on children with ASD at this age group they were included with the 19–24 month group. There were also no differences in the motor skills between children 12–18 months and 19–24 months (p=.078) therefore these two age groups were collapsed. Mean ratio verbal IQs across age groups ranged from 4.76– 109.26 (mean= 37.36, SD, 23.39). Analysis of variance (ANOVA) on ratio verbal IQ revealed a significant main effect for group (F(2, 159)= 6.42; p < 0.01); post-hoc analysis showed significant differences in ratio verbal IQ between the first age group (12–24 months) and all older groups (p < 0.01) (Table 1). Mean ratio non-verbal IQ for the three groups ranged from 24.24–121.43 (mean= 70.77, SD= 19.57) and consistently decreased with age. The ANOVA on ratio non-verbal IQ revealed group differences (F(2, 159)= 17.72; p < 0.001), with all post-hoc group comparisons significantly different from one another (p < 0.05) on ratio non-verbal IQ. Thus, although all the children in each age group had cognitive delays, the older children had more significant delays than the younger children. Characteristics of the 162 children in the cross-sectional analysis are presented in Table 1.
Table 1.
Descriptive variables for cross-sectional participants
| 12–24 months | 25– 30 months | 31– 36 months | |
|---|---|---|---|
| N | 34 | 55 | 73 |
| Gender | M= 28, F= 6 | M= 51, F= 4 | M= 61, F= 12 |
| Race/ Ethnicity | C= 27, AA= 5, B= 2 | C= 42, B= 13 | C= 42, AA= 27, A= 2, B= 1 |
| Chronological Age | |||
| Mean | 20.42 | 27.71 | 33.32 |
| SD | ± 2.93 | ± 1.81 | ± 1.56 |
| Range | 14– 24 | 25– 30 | 31– 36 |
| Nonverbal Age Equivalent (MSEL Visual Reception Subtest)* | |||
| Mean | 17.32 | 20.38 | 21.51 |
| SD | ± 3.58 | ± 5.31 | ± 7.50 |
| Range | 12– 25 | 8– 32 | 7– 39 |
| Ratio Nonverbal IQ | |||
| Mean | 85.16 | 71.87 | 63.24 |
| SD | ± 16.58 | ± 16.13 | ± 19.48 |
| Range | 52.17– 121.43 | 35.71– 109.26 | 24.24– 113.24 |
| Ratio Verbal IQ | |||
| Mean | 49.68 | 34.87 | 33.52 |
| SD | ± 27.35 | ± 19.42 | ± 22.49 |
| Range | 4.76– 100.00 | 11.54– 109.26 | 6.94– 107.35 |
| Vineland Fine Motor Age Equivalent | |||
| Mean | 16.88 | 18.56 | 20.26 |
| SD | ± 5.06 | ± 3.69 | ± 4.93 |
| Range | 5– 27 | 12– 30 | 6– 37 |
| Vineland Gross Motor Age Equivalent | |||
| Mean | 18.45 | 21.82 | 22.75 |
| SD | ± 3.43 | ± 3.64 | ± 5.33 |
| Range | 12– 26 | 15– 35 | 12– 44 |
Note: M= male, F= female; Mean all ages in months; C= Caucasian, AA= African American, A= Asian, B= Biracial
Visual Reception subtest of the Mullen Scales of Early Learning
Motor Skills
Although the inclusion criteria required the onset of independent sitting before 48 months; on the ADI-R, parents of children in Study 1 reported that their children attained independent sitting at an average age of 7.19 months (SD= 2.24); and independent walking at an average age of 13.73 months (SD= 3.88). These results for sitting and walking fall within typical ranges for children without developmental disabilities (average of 12–18 months for walking and 6–9 months for sitting).
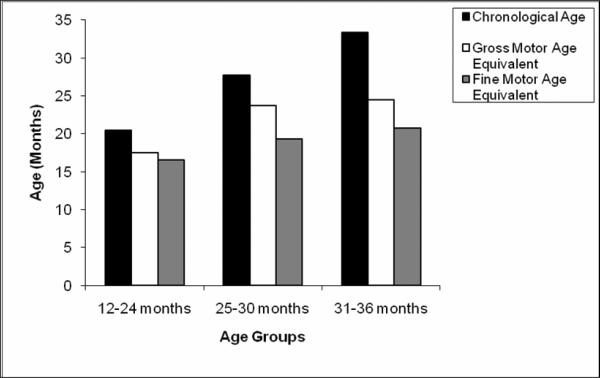
The gross motor age equivalents for all the children in each age group were below what would be expected for chronological age level (Figure 1). The differences between chronological age and gross motor age equivalent increased progressively from 12 to 36 months. The gross motor skills of the children in the 12–24 month group were an average of 3.50 months behind what would be expected (mean age equivalent = 17.47 [SD= 3.18] months), the 25–30 month group were 5.13 months behind (mean age equivalent = 23.75 [SD= 4.40] months) and children in the 31–36 month group were 9.18 months behind (mean age equivalents= 24.44 [SD= 5.98] months). This clearly demonstrates that the gross motor skills of the children with ASD were behind for chronological age at each cross-sectional age point. The amount of delay, or months behind what is expected for chronological age is the `motor difference' variable (e.g. 5.13 for the 25–30 month olds). The question is whether or not this developmental delay increases with age. An ANCOVA revealed a significant main effect for the gross motor difference variable (F(1,2) = 45.06, p < .001). Post-hoc analysis was performed with Bonferroni corrections and revealed significant difference between all three age groups. There was a significant difference between the 12– 24 month age group and the 25– 30 month age group (p < 0.01) on the gross motor difference variable. Likewise, the gross motor difference variable was significantly different between the 25– 30 month age group and the 31–36 month age group (p < 0.001), and finally between the 12– 24 month age group and the 31–36 month group (p < 0.001).
Figure 1.
Motor profile by age for 162 children with ASD (Gross motor and Fine motor scales of the MSEL).
The fine motor skills of the children in the 12– 24 month group were on average 3.94 months behind chronological age (mean age equivalent = 16.50 [SD= 2.86] months), the 25– 30 month group were 8.38 months behind chronological age (mean age equivalents= 19.33 [SD= 4.21] months) and differences for the 31– 36 month age group were on average 12.77 months behind chronological age (mean age equivalents= 20.74 [SD= 6.59] months) (Figure 1). While it is clear that the fine motor skills of the children with ASD were delayed for chronological age, the question was whether or not the delays become more pronounced with age. ANCOVA revealed a significant main effect was found for the fine motor difference variable (F (1,2) = 124.74; p < 0.001). Post-hoc analysis with Bonferroni corrections were performed across all pair-wise comparisons. The fine motor difference variable for the 25– 30 month age group was significantly larger than the 12– 24 month age group (p < 0.001). The fine motor difference variable for the 31– 36 month age group was significantly larger than the 25– 30 month age group (p < 0.001) and the 12– 24 month age group (p < 0.001) (Figure 1).
Correlation Analysis
A correlation analysis was conducted on the MSEL gross motor and fine motor subtests scores and the scores on the VABS where parents reported their perception of their child's motor skills. In addition, average ages of independent sitting and walking were correlated with the motor scores on the MSEL and the VABS. The MSEL scores on the gross motor subtest were significantly related to the scores on the VABS gross motor subtest (r = 0.61, p<.001), and the fine motor subtests were also significantly related (r = 0.43, p<.001). Significant negative correlations were found between the average age of sitting and average age of walking onset with the MSEL gross motor age equivalent scores respectively (sitting r = −0.33, p <.001; and walking r = −0.46, p <.001); significant negative correlations were also found between the gross motor scores on the VABS and sitting (r = −0.32, p<.001), and walking (r = −0.39, p<.001). Indicating earlier sitting and walking were both related to better gross motor proficiency in toddlers with ASD.
Study 2
Characteristics for the 58 children in Study 2 are presented in Table 2. These children were also included in Study 1.
Table 2.
Descriptive variables for longitudinal participants
| Time 1 | Time 2 | |
|---|---|---|
| N | 58 | 58 |
| Gender | M= 51, F= 7 | M= 51, F= 7 |
| Race/ Ethnicity | C= 25, AA= 33 | C= 25, AA= 33 |
| Chronological Age | ||
| Mean | 29.72 | 41.60 |
| SD | ±4.55 | ±4.96 |
| Range | 16– 36 | 24– 48 |
| Nonverbal Age Equivalent (MSEL Visual Reception Subtest)* | ||
| Mean | 18.03 | 25.10 |
| SD | ± 5.42 | ±6.60 |
| Range | 7–30 | 11– 42 |
| Ratio Nonverbal IQ | ||
| Mean | 62.45 | 59.52 |
| SD | ±18.57 | ±16.11 |
| Range | 25.81– 106.25 | 32.22– 104.17 |
| Ratio Verbal IQ | ||
| Mean | 28.72 | 36.55 |
| SD | ±16.39 | ±19.13 |
| Range | 8.06– 87.50 | 8.75– 77.94 |
| Vineland Fine Motor Age Equivalent | ||
| Mean | 19.10 | 22.71 |
| SD | ± 4.67 | ± 5.19 |
| Range | 6– 32 | 10– 37 |
| Vineland Gross Motor Age Equivalent | ||
| Mean | 21.05 | 25.76 |
| SD | ± 5.46 | ± 5.21 |
| Range | 16– 37 | 12– 44 |
Note: M= male, F= female; Mean all ages in months; C= Caucasian, AA= African American, A= Asian, B= Biracial
Visual Reception subtest of the Mullen Scales of Early Learning
Motor Skills
A repeated measures ANCOVA was performed to analyze the gross motor difference variable between the two time points with non-verbal problem solving covaried. Results indicated a significant difference in the gross motor-difference variable at the two time points (F(1,1)= 39.36; p< 0.001). The same analysis on the fine motor difference variable revealed a significant difference for the two time points (F(1,1)= 23.52; p<0.01) (Figure 2). These results demonstrate that the delay in the gross and fine motor skills of these 58 children with ASD became significantly larger; in other words, they fell significantly further behind their chronological age at time point 2 in terms of gross and fine motor skills.
Figure 2.
Longitudinal motor profile of 58 children with ASD (Gross motor and Fine motor scales of the MSEL).
DISCUSSION
The results of the cross-sectional analysis in Study 1 provides evidence, from a large sample, that very young children with ASD have significant motor delays and the delays become more pronounced with age. Landa and Garrett-Mayer (2006) conducted a longitudinal study of preschool aged children with and without ASD, measured using the MSEL, at 6, 14, and 24 months. They too found that between 14 and 24 months the children with ASD (n=13) had significant developmental delays in terms of motor skill development and these delays broadened considerably as the children got older. The developmental trajectory of the motor skills in children with ASD showed the greatest slowing when compared to the other two groups in their study (children with language delays and children with typical development). This same study also found that the children in the ASD group had decreasing scores on the visual reception subtest and the expressive language subtests, results similar to those of our study (Landa & Garrett-Mayer, 2006). However, with a cross-sectional sample of our study, we cannot differentiate between children whose motor skills get worse or the chance that children who happened to be referred to these projects at older ages had worse motor skills as well as more impaired non-verbal problem solving and verbal IQs. The result of the longitudinal analysis in Study 2 supports the findings of Study 1; we found the developmental trajectory of both fine and gross motor skills slowed down when studied longitudinally in these 58 children with ASD. In both Study 1 and Study 2, the developmental gap for gross and fine motor skills widened significantly at the older ages.
Movement is a primary element of “active play” in young children (Pellegrini & Smith, 1998). Active play facilitates the development of motor skills, social skills, an understanding of the world, daily living skills, and adaptive behaviour; it also provides a unique opportunity for young children to be physically active and play games with peers (Pellegrini & Smith, 1998; Ridgers, Stratton, & Fairclough, 2006; Sutera et al., 2007). Children with ASD, by definition, have difficulties in the social domain, thereby limiting their time engaged in play, with or without other children (Dewey, Lord, & Magill, 1988). The children with ASD in this study achieved their motor milestones within typical ranges which is similar to the findings of other studies (Dawson, Osterling, Meltzoff, & Kuhl, 2000; Ozonoff, et al., 2008). However as the toddlers with ASD got older their fundamental motor skills fell significantly behind what would be expected for their chronological age. Fundamental gross motor skills are complex and require coordination, motor planning and control. These skills are often learned through imitation in social contexts. We hypothesize that the slowing of gross motor development found in this study is partly due to the fact that gross motor skills are commonly explored and discovered during self-directed or self-regulated learning both with and without peers during play. This type of play and social engagement is a challenge for children with ASD. It is also possible that tactile sensitivities and/or aversions could contribute to the delays in fundamental motor skills. Therefore we propose that the limitations in motor proficiency demonstrated by the toddlers in this study may create a cycle where poor motor skills constrain social interactions, and poor social interactions constrain motor skill development.
The results of both the cross-sectional analysis and the longitudinal analysis also indicate that fine motor skills are also delayed in children with ASD and the children fall further behind as they get older. It is not clear why children with ASD have poor fine motor skills, but the impact on early learning opportunities and daily living skills is not trivial (Jasmin, et al., 2009). It is possible that tactile sensitivities and an overall lack of social imitation contribute to these fine motor delays; however, the relative contributions of social and imitative deficits to fine motor skills is far from clear. This is an area that requires further study and emphasis in early interventions for children with ASD.
In our results the variations in patterns of non-verbal IQ across the cross-sectional and longitudinal samples suggest that some of the differences could be related to recruitment differences and different ages. The youngest children were from more recent longitudinal early identification studies at the Michigan site and so may reflect increasingly sophisticated identification of milder cases of ASD even at young ages (Lord, et al., 2006; Luyster, et al., 2009). Nevertheless, the finding of increasingly disparate motor functioning both for gross and fine motor skill held true in our longitudinal sample as well, even when non-verbal IQ was going up. Therefore this finding is not likely due to differences in the samples from the different studies. It is well known that children with intellectual disabilities often have delays in their overall early development, including their motor skills (Ozonoff, et al., 2008). Given the fact that the older children in this sample had lower ratio verbal and non-verbal IQs than the younger cross-sectional age groups, the motor delays found in this older group could be related to the corresponding intellectual disabilities. However, all the analyses in both Studies 1 and 2 were covaried by non-verbal problem solving skills to account for this possibility and the results still demonstrated significant delays in motor skills with the delays becoming more pronounced with age. Therefore, low cognitive ability is not entirely responsible for the motor difficulties of young children with ASD. The results of the longitudinal study demonstrate that the developmental gap in the gross and fine motor skills significantly widened; in other words, they fell significantly further behind their chronological age at time point 2 in terms of gross and fine motor skills. This is particularly important because the mean ratio non-verbal IQ of the cross-sectional groups decreased with age, while the ratio non-verbal IQ of the longitudinal group remained relatively stable despite differences in the motor age difference scores. Other factors could contribute to this developmental worsening such as lack of early intervention services focused on the motor domain, lack of emphasis on active play by parents and caregivers, lack of opportunity for active play, or medications that may cause lethargic responses and therefore less practice. Further research is needed to better understand the cause or reason for the developmental worsening in fine and gross motor skills in toddler with ASD.
These results support the findings of Ozonoff and colleagues (2008) who concluded that the motor deficits in children with ASD are not secondary problems, or caused by deficits in cognitive skills. It is hypothesized that there is an underlying brain deficit that is related to traditional ASD characteristics. The scope of this study does not allow us to delineate the etiology of the motor deficits in children with ASD. The results do, however, indicate that the deficits are present very early in development and may become more pronounced with age; therefore, motor deficits should not be a secondary concern in planning and implementing early interventions for children with ASD. All too often motor skills are not considered important enough when verbal, behavioural and social deficits take precedence for parents.
Surprisingly, this study found two results that are not typical - a downward trend over time in ratio verbal IQ between the first two age groups in Study 1 and a plateau in verbal IQ between the second two age groups. However, participant inclusion in this study was obtained through existing projects, and not all children who met the age eligibility criteria had the gross motor scores which would have allowed them entrance into the study. It is possible that the children who were eligible by age for this study, but were not included, had stronger gross motor skills and because of this, the examining clinician decided not to administer the gross motor sub-test. As discussed, cohort differences may also have affected the longitudinal sample, therefore, the fact that there were similar findings for the fine motor sub-test (which is required to complete a full-scale score on the MSEL and for the longitudinal sample) is important.
A limitation of this study includes the use of the gross motor subtest on the MSEL to measure motor proficiency. There are several motor skill tests that are more commonly used in adapted physical education, physical and occupational therapy for differentiating the motor skills of young children with and without disabilities (e.g. Peabody Developmental Motor Scales-2) which would be better able to evaluate motor skills (Ozonoff, et al., 2008). It is also possible that there could have been a ceiling effect for the 30–36 month group; although, Figure 1 demonstrates that the average gross motor age was below the ceiling. The MSEL is a commonly administered developmental test in the field of psychology and has been validated; this allows for information to be gathered on the gross motor skills of children who have not been referred for a motor skill evaluation. Additionally, the correlation of the gross motor MSEL subtests with the VABS gross motor subtest and the sitting/walking developmental milestones strengthens this data indicating that the MSEL scores were an accurate reflection of the gross motor skills of the participants. Finally, this was a secondary data analysis of an extremely large data-set where the participants were drawn from several different early intervention studies. Strict inclusion criteria were applied to the analyses included here. However, different site locations and recruitment methods are a limitation of this study.
Early intervention for children with ASD is of paramount importance. The results of this study add to a growing body of literature that the motor development of children with ASD (both fine motor and gross motor) should not be ignored in early intervention services. Children with ASD should receive appropriate therapy that focuses on their motor development. In the absence of therapeutic avenues available to parents, early “kinder-gym” type of activities should be promoted where gross motor play and activities are facilitated. All too often the focus is on “sitting still” and “paying attention” that the importance of active play is ignored. Motor development is critical to the overall development of the child.
CONCLUSIONS
The gross and fine motor skills of young children with ASD are delayed and become progressively more delayed with age, even when controlling for nonverbal problem solving skills. Unfortunately, fundamental motor skills and physical activity are frequently neglected in fields such as psychology (Rosenbaum, 2005) and the motor skills of young children with ASD are habitually not a priority for early intervention teams who may focus primarily on communication and behavioural concerns. In order to prevent further decline in motor skills, and potential further isolation from social interactions with peers, gross motor and fine motor (which is less often neglected) assessments and programmes should be included in the early intervention plans for children with ASD. It is our belief that the impact of active play and movement on young children is important for more than just language, imitation, and overall cognitive development. Gross motor and fine motor skills are also being learned and practiced and these motor skills contribute to overall developmental outcomes for all children.
Acknowledgements
Support for this project was provided in part from funding from the Simons Foundation, FirstWords and the following grants: NICHD U19 HD355482. The Neurobiology and Genetics of Autism. 06/01/97-5/31/07. NIMH RO1 MH081873-01A1. Longitudinal Studies of Autism Spectrum Disorders: 2 to 23. 09/01/08-05/31/13.
Reference List
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. Second Edition ed. The Psychological Coorporation; San Antonio: 1993. [Google Scholar]
- Berkeley SL, Zittel LL, Pitney LV, Nichols SE. Locomotor and object control skills of children diagnosed with autism. Adapted Physical Activity Quarterly. 2001;18:405–416. [Google Scholar]
- Bruininks RH. Bruininks-Oseretsky Test of Motor Proficiency Examiner's Manual. American Guidance Service; Circle Pines, MN: 1978. [Google Scholar]
- Chawarska K, Paul R, Klin A, Hannigen S, Dichtel LE, Volkmar F. Parental recognition of developmental problems in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):62–72. doi: 10.1007/s10803-006-0330-8. [DOI] [PubMed] [Google Scholar]
- Dawson G, Osterling J, Meltzoff AN, Kuhl P. Case study of the development of an infant with autism from birth to two years of age. Journal of Applied Developmental Psychology. 2000;21(3):299–313. doi: 10.1016/S0193-3973(99)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey D, Lord C, Magill L. Qualitative assessment of the effect of play materials in dyadic peer interactions of children with autism. Canadian Journal of Psychology. 1988;42(2):242–260. doi: 10.1037/h0084183. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, Rutter M. Pre-Linguistic Autism Diagnostic Observation Schedule (PLADOS) Journal of Autism and Developmental Disorders. 1995;25(4):355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Dunn W. Sensory Profile: Pearson. 1999. [Google Scholar]
- Folio MR, Fewell RR. Peabody Developmental Motor Scales. Second Edition ed. Pro-Ed; Austin, TX: 2000. [Google Scholar]
- Frith U. Autism and Asperger's syndrome. Cambridge University Press; Cambridge: 1991. [Google Scholar]
- Ghaziuddin M, Butler E. Clumsiness in autism and Asperger syndrome: a further report. Journal of Intellectual Disability Research. 1998;42(Pt 1):43–48. doi: 10.1046/j.1365-2788.1998.00065.x. [DOI] [PubMed] [Google Scholar]
- Green D, Baird G, Barnett AL, Henderson L, Huber J, Henderson SE. The severity and nature of motor impairment in Asperger's syndrome: a comparison with specific developmental disorder of motor function. Journal of Child Psychology and Psychiatry. 2002;43(5):655–668. doi: 10.1111/1469-7610.00054. [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden DA. Movement Assessment Battery for Children. The Psychological Coorporation; London: 1992. [Google Scholar]
- Jasmin E, Couture M, McKinley P, Reid G, Fombonne E, Gisel E. Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(2):231–241. doi: 10.1007/s10803-008-0617-z. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Leary MR, Hill DA. Moving on: autism and movement disturbance. Mental Retardardation. 1996;34(1):39–53. [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles: 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, Lord C. The Autism Diagnostic Observation Schedule-toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(9):1305–1320. doi: 10.1007/s10803-009-0746-z. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster R, Richler J, Risi S, Hsu WL, Dawson G, Bernier R, Lord C. Early regression in social communication in autism spectrum disorders: a CPEA Study. Developmental Neuropsychology. 2005;27(3):311–336. doi: 10.1207/s15326942dn2703_2. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Tsujii M, Hori M, Nakanishi K, Kageyama H, Sugiyama T. Brief report: motor incoordination in children with Asperger syndrome and learning disabilities. Journal of Autism and Developmental Disorders. 1997;27(5):595–603. doi: 10.1023/a:1025834211548. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. T.O.T.A.L. Child; Cranston, RI: 1989. [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. American Guidance Service Inc; Circle Pines, MN: 1995. [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, Rogers SJ. Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders. 2008;38(4):644–656. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini AD, Smith PK. Physical activity play: the nature and function of a neglected aspect of playing. Child Development. 1998;69(3):577–598. [PubMed] [Google Scholar]
- Provost B, Lopez BR, Heimerl S. A comparison of motor delays in young children: autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders. 2007;37(2):321–328. doi: 10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Richler J, Bishop SL, Kleinke JR, Lord C. Restricted and repetitive behaviors in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):73–85. doi: 10.1007/s10803-006-0332-6. [DOI] [PubMed] [Google Scholar]
- Ridgers ND, Stratton G, Fairclough SJ. Physical activity levels of children during school playtime. Sports Medicine. 2006;36(4):359–371. doi: 10.2165/00007256-200636040-00005. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. The Cinderella of psychology: the neglect of motor control in the science of mental life and behavior. American Psychologist. 2005;60(4):308–317. doi: 10.1037/0003-066X.60.4.308. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, Balla D. Vineland-II Adaptive Behavior Scales. American Guidance Service; Circles Pines, MN: 2005. [Google Scholar]
- Sutera S, Pandey J, Esser EL, Rosenthal MA, Wilson LB, Barton M, Fein D. Predictors of optimal outcome in toddlers diagnosed with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2007;37:98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Vernazza-Martin S, Martin N, Vernazza A, Lepellec-Muller A, Rufo M, Massion J, Assaiante C. Goal directed locomotion and balance control in autistic children. Journal of Autism and Developmental Disorders. 2005;35(1):91–102. doi: 10.1007/s10803-004-1037-3. [DOI] [PubMed] [Google Scholar]