Abstract
The architectonics of the mammalian brain arise from a remarkable range of directed cell migrations, which orchestrate the emergence of cortical neuronal layers and pattern brain circuitry. At different stages of cortical histogenesis, specific modes of cell motility are essential to the stepwise formation of cortical architecture. These movements range from interkinetic nuclear movements at the ventricular zone (VZ), to migrations of early-born, postmitotic polymorphic cells into the preplate, to the radial migration of precursors of cortical output neurons across the thickening cortical wall, and the vast, tangential migrations of interneurons from the basal forebrain into the emerging cortical layers. In all cases, acto-myosin motors act in concert with cell adhesion receptor systems to provide the force and traction needed for forward movement. As key regulators of actin and microtubule cytoskeletons, cell polarity, and adhesion, the Rho GTPases play a critical role in CNS neuronal migration. This review will focus on the different types of migration in the developing neocortex and cerebellar cortex, and the role of the Rho GTPases, their regulators and effectors in these CNS migrations, with particular emphasis on their involvement in radial migration.
Introduction
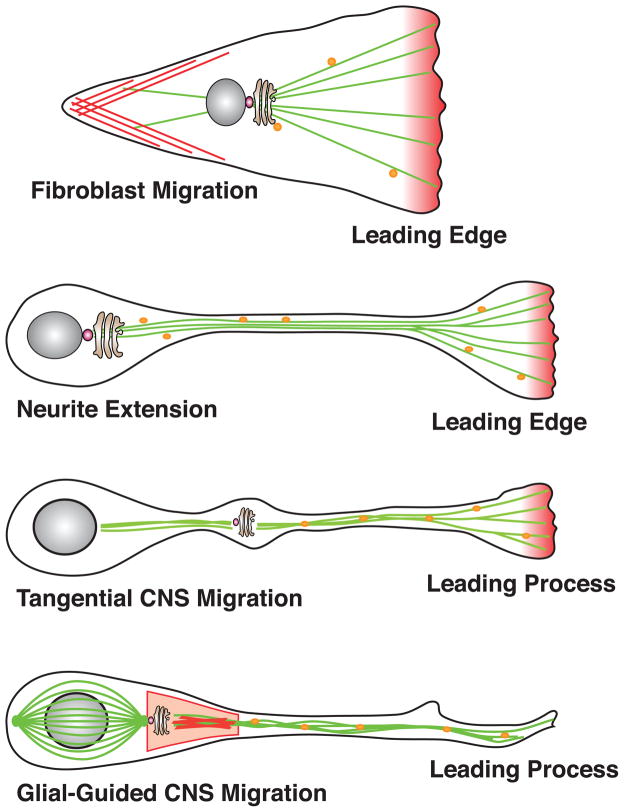
Over the past decade, remarkable progress has been made in our understanding of the mechanisms of cell migration. In general, cell migration involves polarization of the cell in the direction of movement, the formation of a broad lamellipodium and filopodia, and directed vesicle trafficking toward the leading edge (Ridley et al., 2003; Heasman and Ridley, 2008). Just behind the leading edge, Myosin II motors act in concert with integrin-based adhesions to provide traction for cell locomotion (Gupton et al., 2002; Ridley et al., 2003; Giannone et al., 2004; Hu et al., 2007; Vicente-Manzanares et al., 2009), and adhesions are disassembled at the rear of the cell to allow detachment. Spike-like, actin-driven filopodia serve as sensors to explore the local environment (Ridley et al., 2003; Heasman and Ridley, 2008). Although many neuronal migrations in the developing brain can be described by this general model, several highly specialized modes of movement establish the neuronal layers during corticogenesis. These include tangential migrations along emerging axon tracts, chain migrations of neurons ensheathed in a glial network and neuronal migrations along the radial glial fiber network (Fig. 1).
Figure 1.
Modes of Cell Migration in the Developing CNS. Fibroblast (top cell) locomotion and and growth cone extension during neurite outgrowth (second cell) occurs by a general model of motility that involves the formation of an actin-rich lamellipodial protrusion at the leading edge in the front of the cell. Tangentially migrating CNS neurons (third cell) extend a leading process and form a swelling that contains the centrosome and Golgi, which moves into the leading process, followed by nuclear translocation. Glial-guided neuronal migration (fourth cell) involves the extension of a leading process, acto-myosin contractility in the proximal portion of the leading process, and forward movement of the centrosome prior to the nucleus. Red zones, actin; green, microtubules; orange circles, vesicles; purple circles, centrosome.
The Rho family of GTPases are critical regulators of key steps in cell migration. They are low molecular weight guanine nucleotide binding proteins, which in general function as binary molecular switches that cycle between an active GTP-bound and inactive GDP-bound state. Their activity can be positively influenced by guanine nucleotide exchange factors (GEFs) that promote the exchange of GDP for GTP (Schmidt and Hall, 2002; Rossman et al., 2005), and negatively influenced by GTPase activating proteins (GAPs) that increase the intrinsic rate of GTP hydrolysis of the GTPase (Bernards, 2003; Bernards and Settleman, 2004) and by guanine nucleotide dissociation inhibitors (GDIs) that sequester and solubilize the GDP-bound GTPase and prevent the exchange of GDP for GTP (Zalcman et al., 1999; Hoffman et al., 2000; DerMardirossian and Bokoch, 2005). Active, GTP-bound Rho GTPases transduce upstream signals by interacting with effector molecules (Van Aelst and D’Souza-Schorey, 1997; Bishop and Hall, 2000; Gundersen, 2002; Bokoch, 2003; Riento and Ridley, 2003; Burridge and Wennerberg, 2004; Govek et al., 2005; Bustelo et al., 2007; Kurisu and Takenawa, 2009). Spatial and temporal specificity of Rho GTPase signaling are achieved in part via spatio-temporal regulation of regulatory molecules (Etienne-Manneville and Hall, 2002), GEFs that link Rho GTPases to specific effectors through scaffolding molecules (Buchsbaum et al., 2002; 2003; Jaffe et al., 2004), post-translational modifications such as lipid modification and phosphorylation (Ward et al., 2004; Roberts et al., 2008), and by ubiquitin-mediated proteosome degradation (Senadheera et al., 2001; Doye et al., 2002; Wang et al., 2003; Ward et al., 2004; Riento et al., 2005; Visvikis et al., 2008). In general during migration, Cdc42 plays an integral role in polarizing the centrosome and defining the direction of movement, Rac and Cdc42 are classic regulators of actin-based lamellipodia and filopodia formation, respectively, and Rho regulates cell contractility. All three of these GTPases also play a role in cell adhesion (Ridley, 2001; Fukata et al., 2003; Raftopoulou and Hall, 2004). While the effects of the Rho GTPases on non-neuronal cell migration have been studied extensively, the part that they play in CNS migrations is only beginning to be elucidated with the advent of advanced techniques that allow us to examine the contribution of individual molecules in migrating neurons in vivo.
This review will provide a brief description of migrations critical for cortical and cerebellar histogenesis, and highlight the role of the Rho GTPases, their regulators and effectors in these different modes of CNS migrations.
Cell Migrations in Cortical Development
Interkinetic Nuclear Movements in the Cortical Ventricular Zone
In the mouse, neural precursors in the neuroepithelium lining the third ventricle of the emerging telencephalic vesicle proliferate just after neural tube closure (E9.25). Like all epithelia, the neuroepithelium of the telencephalic VZ has an apical-basal polarity. In contrast to most epithelia in the mouse embryo, however, the apical surface of progenitor cells in the telencephalic neuroepithelium is oriented toward the ventricular surface, where they form adherens junctions that are dependent upon the conserved Par6 polarity complex. During neurogenesis, interphase progenitor cells span the epithelium, moving toward the ventricular surface just prior to mitosis. During G1, the nucleus of proliferating progenitor cells moves away from the ventricular surface as the progenitor cell extends toward the pial surface. During the S phase of the cycle, the nucleus is located close to the basal, pial surface, and moves back toward the apical, ventricular surface just prior to entering mitosis (Sauer and Walker, 1959) (Fig. 2). The translocation of the nucleus throughout the cell cycle is termed interkinetic nuclear migration. These cyclic interkinetic nuclear migrations involve dynamic changes in the microtubule (MT) and actin cytoskeletons, as well as cytoskeletal and adhesion regulatory molecules (Tsai et al., 2005; Xie et al., 2007; Del Bene et al., 2008; Latasa et al., 2009; Norden et al., 2009; Schenk et al., 2009; Zhang et al., 2009; Buchman et al., 2010; Ge et al., 2010; Sottocornola et al., 2010). Among the proteins that regulate neurogenesis and interkinetic nuclear movements are the Rho GTPase family members, Rac1 and Cdc42.
Figure 2.
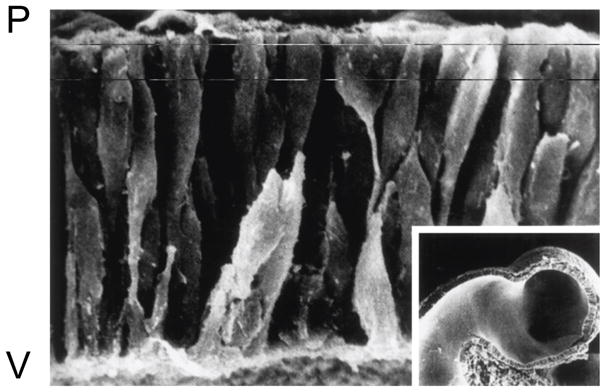
Scanning Electron Microscopy of Neural Progenitors in the Cerebral Vesicle. After neural tube closure (E9.25), the neuroepithelium along the third ventricle contains interphase progenitor cells that extend from the outer pial surface (P) to the inner ventricular surface (V), and mitotic cells are located closer to the ventricular surface. The movement of the nucleus from the ventricular surface to the pial surface and back again, during the cell cycle, is called interkinetic migration. (Inset) A low-power view of the hamster cerebral vesicle, corresponding roughly to that of a human embryo at the end of the first month of gestation. From Sidman and Rakic (1973).
Recent studies have revealed a role for Rac1 in the earliest stages of neural progenitor production and fate specification. Rac1 is present at the ventricular surface during cortical development (Minobe et al., 2009), and Rac1 deficiency in the telencephalic VZ of mouse embryos causes a reduction in neural progenitors, which is due in part to increased apoptosis, resulting in microcephaly (Chen et al., 2009). Rac1 inhibition, using a dominant negative mutant, also retards nuclear migration and causes failures in cytokinesis (Minobe et al., 2009). These findings suggest that Rac1 may regulate self-renewal, survival, and interkinetic nuclear migration of neocortical progenitor cells.
While less is known about Rac1 in cortical neurogenesis, the role of Cdc42 in this process has been studied more extensively. Cdc42 deficiency in telencephalic progenitor cells also causes defects in forebrain development, which are characterized by an increase in neuron number, defective radial glia/progenitor fibers, and holoprosencephaly. Cdc42 protein is enriched at the apical/ventricular side of the neuroepithelium, and is present in basally located postmitotic neurons. Loss of Cdc42 abolishes the localization of Par complex proteins, E-cadherin, β-catenin, and Numb to adherens junctions at the apical surface of neural progenitors, thereby causing a loss of adherens junctions, failure of interkinetic nuclear migration, and an increase in basal mitoses and neuron number (Cappello et al., 2006; Chen et al., 2006). Apical localization of Cdc42, as well as other polarity proteins, is dependent upon the myristoylated alanine-rich C-kinase substrate protein (MARCKS), which is required for radial progenitor localization at the VZ and formation of the radial glial scaffold (Weimer et al., 2009). The formation of adherens junctions and the ability of Cdc42 to interact with polarity proteins also appears to be dependent on ceramide in the apical membrane of the neuroepithelium (Wang et al., 2008), and Connexin 43 (Cx43) may modulate Cdc42 phosphorylation during interkinetic nuclear migration (Liu et al., 2010). Taken together, these findings suggest that Cdc42 regulates adherens junction formation, interkinetic nuclear migration, and the self-renewal of VZ progenitors, as well as radial glial scaffold formation.
Insight into how Cdc42 affects the fate of VZ progenitors comes from experiments on the Par polarity complex members, Par6, Par3, and atypical Protein Kinase C (aPKC), in neocortical development. The binding of active, GTP-bound Cdc42 (or Rac1) to Par6 (Joberty et al., 2000; Lin et al., 2000; Qiu et al., 2000; Noda et al., 2001; Garrard et al., 2003) activates aPKC to phosphorylate proteins (Qiu et al., 2000; Etienne-Manneville and Hall, 2001). Similar to Cdc42 deficiency, loss of aPKCλ affects adherens junction formation, causes retraction of apical processes, and impairs interkinetic nuclear migration, resulting in disordered neuroepithelial tissue architecture (Imai et al., 2006). Loss of Cdc42 and Par3 also both cause a decrease in self-renewing progenitors and an increase in neurons, while overexpression of Par3 and Par6 promote the generation of self-renewing progenitors. The finding that the effects of Par3 and Par6 on progenitor self-renewal were similar in vitro and in vivo suggests that the observed defects in progenitor self-renewal likely resulted from a reduction in Par protein levels, as opposed to a loss of tissue polarity or coordinated apico-basal fate determinants (Costa et al., 2008). Thus, Cdc42 likely promotes self-renewing progenitor cell divisions at the expense of neurogenic differentiation in the developing cerebral cortex by regulating the levels and/or activity of apically localized Par-complex proteins.
Development of Secondary Germinal Zones
Beginning on about day E13 in the mouse, a secondary germinal zone, called the subventricular zone (SVZ), forms above the VZ and expands significantly as cortical development proceeds (Molyneaux et al., 2007). Neurogenesis in this zone produces a variety of neurons, including precursors of olfactory bulb GABAergic neurons, which migrate from the cortical SVZ into the olfactory bulb along a pathway called the rostral migratory stream (RMS) (Luskin, 1993). Proliferating precursors in the RMS migrate at speeds up to 122 μm/h (Wichterle et al., 1997; Murase and Horwitz, 2002) as a chain of neurons ensheathed bya protective layer of glial cells in a posterior toanterior direction to populate the olfactory bulb (Lois et al., 1996). This migration continues in the adult brain, where recent studies show that newly generated neurons produce Slit1, a diffusible protein whose receptor, Robo, is produced by the astrocytic meshwork through which these neurons migrate (Doetsch et al., 1997; Kaneko et al., 2010). This finding suggests that neuron-glial interactions control glial morphological differentiation needed to maintain this glial network. Repulsion of migratory cells from the anterior SVZ by Slit involves downregulation of Cdc42 activity via the Slit-Robo GAP (srGAP) 1 (Wong et al., 2001). In addition to providing a thoroughfare for RMS migration, astrocytes in this region also concentrate BDNF released by vascular endothelial cells (Snapyan et al., 2009), take up GABA released by migrating neurons (Bolteus and Bordey, 2004), and secrete other factors that promote migration. Several different adhesion receptor systems are necessary for RMS migration, including polysialiated NCAM (Battista and Rutishauser, 2010) and α1, αv, and β1 integrins (Murase and Horwitz, 2002). Of note, while deletion of Rac1 in the VZ of the telencephalon using Fox1-Cre severely impairs tangential migration of telencephalon-derived interneurons, deletion of Rac1 in the SVZ of the ventral telencephalon using Dlx5/6-Cre-IRES-EGFP does not impair migration (Chen et al., 2007). Furthermore, repression of a Rac/Cdc42 effector, the p21-activated serine/threonine kinase PAK3, by Dlx transcription factors is thought to be required to restrain neuritic growth and promote tangential migration (Cobos et al., 2007). Thus Rac1 appears to be dispensable for the motility of SVZ-derived interneurons, and limiting a potential Rac1 signaling pathway seems to facilitate tangential migration.
Neuronal Migrations During Preplate and Cortical Plate Stages of Cortical Development
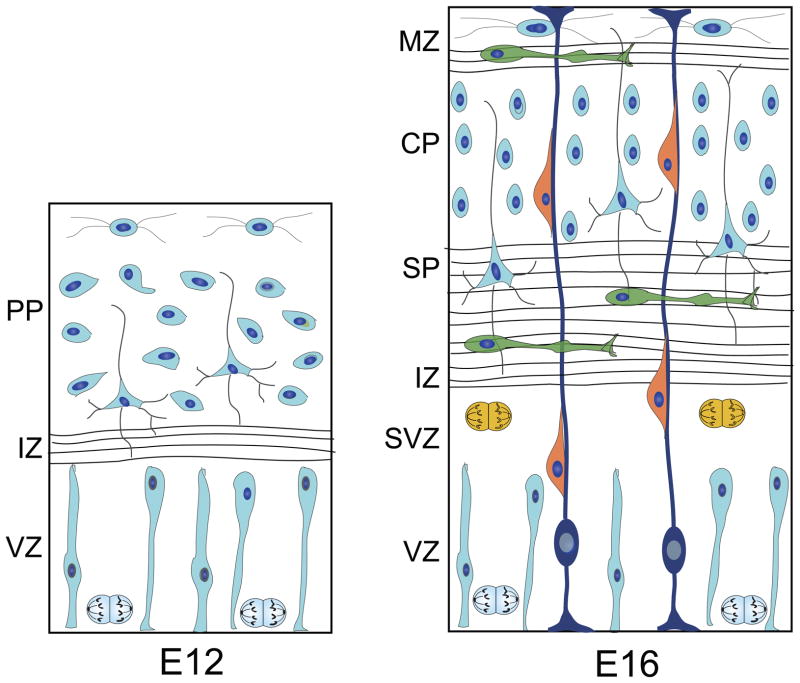
In the mouse, the earliest neurons generated in the telencephalic VZ become postmitotic on, or before, the eleventh day of gestation (E11). These early-born, polymorphic neurons settle in a narrow laminar zone above the neuroepithelium, termed the preplate (Caviness, 1982; Wood, 1992; McConnell et al., 1994; Del Rio et al., 2000; Zecevic and Rakic, 2001) (Fig. 3). They include Cajal-Retzius cells, GABAergic interneurons, and future subplate projection neurons (Caviness, 1982; Marin-Padilla, 1998; Del Rio et al., 2000). Preplate neurons migrate within the thin preplate zone in a dorso-ventral direction at an average speed of 18 μm/h. During periods of movement, these neurons rapidly extend and retract a broad lamellipodium (Schneider et al., 2010).
Figure 3.
Embryonic Development of the Cerebral Cortex. The ventricular zone (VZ) contains proliferating progenitors of neurons and glia (blue mitotic cells). The first postmitotic, neuronal precursors move above the VZ where they settle in a narrow zone, the preplate (PP). Preplate neurons pioneer cortical axons to both cortical and sub-cortical targets. In-growing axons establish the intermediate zone (IZ). After E13, the preplate splits into the marginal zone (MZ), or future layer I, which contains Cajal-Retzius cells, and the subplate (SP), a transient population of neurons. By E16, the cortical plate thickens as precursors of large, output neurons (orange cells), as well as of some interneurons, migrate along radial glia (navy cells) to establish the neuronal layers. A large population of interneurons (green cells), nearly 80% in the developing murine neocortex, migrate into the developing cortex from the basal forebrain in a trajectory that is tangential to the radial plane. These neurons migrate into the emerging cortical laminae along axons in the IZ and the MZ. Neurogenesis (blue and yellow mitotic cells) continues in both the VZ and the subventricular zone (SVZ), a zone of dividing cells above the VZ which generates neuronal precursors that migrate rostrally, forming the rostral migratory stream.
Between E11 and E12, preplate neurons pioneer the first axon projections of the neocortex, with some preplate neurons extending descending axons to subcortical targets and others extending corticocortical axons to targets in the ipsilateral or contralateral hemisphere via the anterior commissure. Like tangentially migrating preplate neurons, the growth cones of extending axons also exhibit a general mode of motility that includes the formation of a broad lamellipodium and leading edge. In addition, axon guidance receptor systems localized to the tips of actin-based filopodia control the directionality of movement. The Rho GTPases are key mediators of signaling by guidance cues in neurons, and RhoA signaling increases Myosin II activity to promote axon retraction and growth cone collapse, while Rac1 and Cdc42 regulate lamellipodial and filopodial formation in growth cones and promote axon extension and guidance (Gallo and Letourneau, 2004). A recent report shows that Rac1-induced lamellipodia formation in growth cones requires the Wiskott-Aldrich syndrome protein (WASP) family verprolin-homologous protein (WAVE) complex and Arp2/3, which regulates actin nucleation and polymerization to induce cytoskeletal remodeling (Tahirovic et al., 2010). Of note, the finding that pharmacological inhibition of Rho/Rho kinase signaling in precerebellar neurons (PCN) enhanced axon extension and impaired migration toward a netrin-1 source, while pharmacological inhibition of Rac and Cdc42 impaired neurite outgrowth without affecting migration, suggests that axon outgrowth and nuclear translocation can be uncoupled and rely on distinct signaling pathways (Causeret et al., 2004). The role of Rho GTPases in axon formation and guidance has been reviewed by (Govek et al., 2005; Koh, 2006; Yoshimura et al., 2006; Witte and Bradke, 2008; Yi et al., 2010).
Tangential migrations, first described by McConnell and Colleagues, occur in the IZ along these pioneering axons that provide a thoroughfare across the radial plane of the thickening cortical wall (O’Rourke et al., 1992; Anderson et al., 1997). These tangentially migrating cells include massive populations of interneurons that emigrate into the telencephalon from the ganglonic eminence in the basal forebrain. Interneurons migrate into the cortex through the marginal zone and the intermediate zone beginning at E14–16 in the mouse, with huge numbers moving into the cortex as the neuronal laminae form. Tangentially migrating interneurons undergo a specialized form of migration that involves cyclical steps of leading process extension or branching followed by nuclear translocation. Interestingly, leading process extension and branching ceases during nuclear translocation. A swelling also forms that contains the centrosome and the Golgi apparatus, accompanied by centriole splitting, and moves into the leading process prior to nuclear movement. Nuclear movement is dependent upon the activity of Myosin II, which accumulates in the rear of the cell (Bellion et al., 2005). A particularly unique and interesting aspect of interneuron migration is the extension or branching of a new leading process along the pre-existing leading process or on the cell body when the neuron changes direction (Polleux et al., 2002; Martini et al., 2009). Rac1 is required for the tangential migration of olfactory and cortical interneurons derived from the telencephalic VZ, since migration of these interneurons is severely impaired by deletion of Rac1 in the VZ using Fox1-Cre. These defects in migration may be due to a failure in acquisition of migratory competency during differentiation of progenitors for ventral telencephalon-derived interneurons, rather than a general defect in motility, and may be dependent, at least in part, on limited extension of corticofugal axons (Chen et al., 2007).
Formation of the Cortical Plate and Neuronal Layers
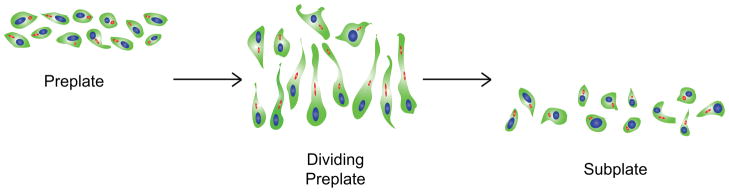
In the emerging neocortex, neurons generated after E13 pattern a second transient layer in the middle of the preplate, the cortical plate. During cortical plate formation, preplate neurons segregate into a superficial marginal zone containing Cajal-Retzius cells and a deeper layer, the subplate (Caviness, 1982; Wood, 1992; McConnell et al., 1994; Del Rio et al., 2000; Molnar et al., 2006) (Fig. 3). Just before the cortical plate forms, a series of changes occur in the morphology and orientation of preplate neurons, leading to a pseudo-columnar patterning of preplate neurons. At E12.5, a stage prior to preplate splitting, preplate neurons have a polymorphic shape that is randomly aligned relative to the radial axis. Over the next 12 hours, preplate neurons undergo changes in polarity, changing from a polymorphic shape to a bipolar morphology, and assemble into a transient, columnar pattern as the preplate neurons segregate into the superficial marginal zone and underlying subplate (Schneider et al., 2010) (Fig. 4). Our recent observations of active changes in preplate/subplate neuron morphology and orientation during preplate splitting suggest that polarity signaling pathways, as well as Rho GTPase pathways, might be required for the radially migrating neurons to migrate past subplate neurons during formation of the cortical plate.
Figure 4.
Preplate Neurons Undergo Changes in Cell Polarity Prior to Preplate Separation and Cortical Plate Formation. Beginning on E12.5, preplate neurons undergo transient changes in polarity, as randomly oriented neurons align with the radial axis by E13.5, forming a transiently columnar pseudolayer just prior to the separation of the preplate into the marginal zone and subplate. Red, Golgi. (Adapted from (Schneider et al., 2010).)
Glial-Guided Neuronal Migration and the Formation of Neuronal Laminae
After the segregation of preplate cells into the marginal zone (Kostovic I, 1974; Luskin and Shatz, 1985), cortical plate and subplate neuronal precursors undergo a mode of migration unique in cortical regions of the developing mammalian brain. Precursors of cortical large output neurons generated in the VZ of the developing neocortex adhere to and migrate on the fibers of radial glial cells that span the entire cortical wall, with an apical end-foot at the ventricular surface and a basal process at the pial surface. This gliophilic mode of migration was first proposed by Rakic from Golgi and EM studies (Rakic, 1972). As development proceeds, later born cohorts of neuronal precursors migrate past existing cortical layers to form the inside-out sequence of six cortical layers (Rakic, 1974; Luskin and Shatz, 1985). In the developing human neocortex, directed migrations establish the principal neuronal layers by the end of the second trimester (Sidman, 1973). In addition to providing a substrate for neuronal migrations, radial glia serve as neural progenitors (Noctor et al., 2001; Noctor et al., 2002). Genetic analyses by Anthony and Heintz show that (1) radial glia are neuronal progenitors in all regions of the developing CNS, (2) the vast majority of neurons in all brain regions derive from radial glia, and (3) radial glia within different regions of the CNS pass through their neurogenic stage of development at distinct time points (Anthony et al., 2004). Thus radial glia serve as a scaffold for the neurons they generate to migrate and populate the cortical plate. The role of the Rho GTPases in glial-guided neuronal migration in the neocortex will be discussed in the Rho GTPase section below.
The Developing Cerebellum
The cerebellum, like the neocortex, consists of an outer cortical structure and set of subcortical nuclei, the cerebellar nuclei, which project to cerebellar targets. Progenitors in the neuropeithelial ventricular zone (VZ) along the dorsomedial surface of the IVth ventricle give rise to the principal output neuron of the cerebellar cortex, the Purkinje cell, neurons of the cerebellar nuclei and more than half a dozen types of cerebellar interneurons, including Golgi, basket and stellate cells. A secondary germinal zone forms along the anterior portion of the rhombic lip, which generates the cerebellar granule neuron, as well as a subpopulation of neurons of the cerebellar nuclei (Wang et al., 2005; Fink et al., 2006) and neurons of several pre-cerebellar nuclei (Dymecki and Tomasiewicz, 1998; Wingate and Hatten, 1999; Machold and Fishell, 2005). Direct evidence for precursor cell migration along radial glia in the embryonic cerebellum comes from confocal imaging studies on transgenic mice expressing markers for transcription factors in neurons of the cerebellar nuclei and the Purkinje neurons. Between E10 and E12, postmitotic precursors of neurons of the cerebellar nuclei, identified by their expression of the transcription factors Lhx2/9, Meis 1/2, and Irx3, migrate radially along the emerging glial fiber system to form a superficial zone across the dorsal surface of the cerebellar anlagen. Between E11 and E14, postmitotic precursors of the Purkinje neuron, identified by expression of the LIM transcription factors Lhx1 and Lhx5, migrate away from the VZ along radial glial fibers and assemble into a broad zone in the core of the anlagen (Morales and Hatten, 2006).
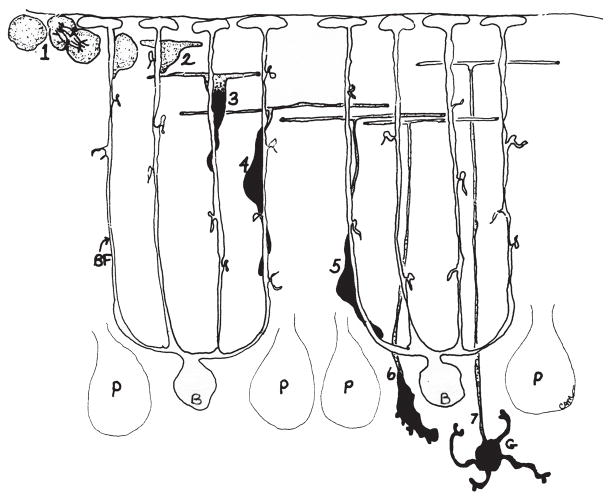
As discussed above, progenitors in the secondary germinal zone of the cerebellar anlagen, the EGL, give rise to cerebellar granule neurons. The remarkable abundance of GCPs, up to several million in a neonatal mouse, has facilitated correlated in vitro and in vivo studies on glial-guided neuronal migration. As GCPs exit the cell cycle, they move to deeper aspects of the EGL where they assume a bipolar shape and extend two axons, called parallel fibers. Localization of the centrosome plays a key role in determining where a neurite or process will form. During parallel fiber formation, the centrosome localizes to one pole of the cell where a neurite extends parallel to the axis of the EGL. Subsequently, the centrosome moves to the opposite pole of the cell and extends a second neurite parallel to the axis of the EGL (Zmuda and Rivas, 1998). GCPs then extend a descending, leading process perpendicular to the axis of the EGL, attach to the radial fiber of a Bergmann glial cell, and migrate through the molecular layer, past the Purkinje cell layer, to the inner granular layer (IGL), trailing a T-shaped axon behind (Rakic, 1971; Edmondson and Hatten, 1987; Komuro and Rakic, 1995; 1998). After migration into the emerging IGL, multiple dendrites form, which establish synaptic connections with in-growing afferent mossy fibers (Fig. 5). The peak period of GCP proliferation and granule neuron migration occurs around postnatal day (P)8, and both processes are complete by P15, at which time the EGL disappears. Contribution of the Rho GTPases to glial-guided neuronal migration in the cerebellum will be discussed in the Rho GTPase section below.
Figure 5.
Granule Cell Development and Radial, Glial Guided Migration in the Postnatal Cerebellum. During postnatal cerebellar development, granule cell progenitors (GCPs) proliferate in the outer region of the external granule layer (EGL) (1). After exiting the cell cycle, GCPs move into the lower aspect of the EGL and commence differentiation, extending bipolar axons, the parallel fibers (2). Thereafter, the cell soma extends a leading process in the direction of migration (3), trailing a “T-shaped” axon behind, and migrates along Bergmann glial fibers (BF) through the molecular layer and Purkinje cell layer (4–6) into the internal granular layer, where they form dendrites and synaptic connections (7). (P, Purkinje cell; B, Bergmann glial cell; G, mature granule neuron.) (Drawing provided by Dr. Carol A. Mason).
Cellular and Molecular Mechanisms of Radial Glial-Guided Migration
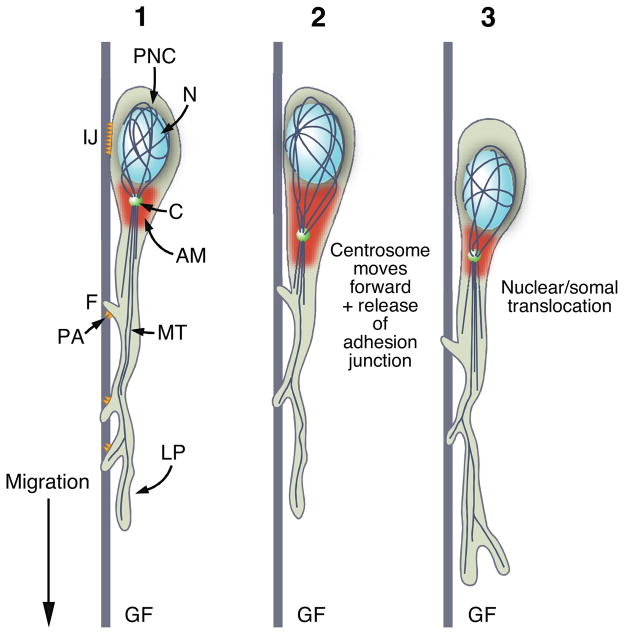
As first described by Rakic, glial-guided neuronal migration involves alignment of postmitotic neurons with a system of radial glial fibers during periods of cortical formation in the neocortex, hippocampal formation and cerebellum (Rakic, 1971; 1972; 1974). Interestingly, live imaging studies on migrating neurons from these three brain regions (Edmondson and Hatten, 1987; Gasser and Hatten, 1990), as well as on migrating neurons from heterotypic brain regions (Gasser and Hatten, 1990), revealed that neuronal migration is not dependent on region-specific features of radial glia. Indeed, a similar mode of movement occurs when CNS neurons migrate on glass fibers with the same geometric parameters as glial fibers (Fishman and Hatten, 1993). Live imaging studies of cerebellar granule neuron migration in vitro revealed features of glial-guided migration that were distinct from the general crawling movement of metazoan cells. These unique features include the extension of a highly polarized, leading process in the direction of migration, the assembly of an interstitial adhesion junction beneath the cell soma (Gregory et al., 1988), localization of acto-myosin contractile motors in the proximal portion of the leading process (Solecki et al., 2009), and the formation of a perinuclear cage of tubulin to maintain the posterior positioning of the nucleus. The migration cycle involves forward movement of the centrosome into the proximal portion of the leading process preceding translocation of the neuronal nucleus (Edmondson and Hatten, 1987; Solecki et al., 2004), the activation of acto-myosin motors located in the proximal aspect of the leading process (Solecki et al., 2009), and the release of the adhesion junction, allowing the cell soma to glide forward along the glial fiber until a new adhesion forms (Fig. 6).
Figure 6.
Cellular Mechanisms of Radial, Glial-Guided Migration in the CNS. (1) During radial, glial-guided migration of CNS neurons, the neuron polarizes and forms a leading process (LP) in the direction of migration. The centrosome (C) and golgi apparatus (not shown) are localized forward of the nucleus (N), which is enwrapped in a perinuclear tubulin cage (PNC). Microtubules (MT) extend from the centrosome into the leading process, and F-actin and acto-myosin motors (AM) are enriched in the proximal portion of the leading process. A specialized interstitial adhesion junction (IJ) forms beneath the neuronal soma and punctae adherentia (PA) form beneath short filopodia (F) that eminate from the leading process and enwrap the glial fiber (GF). (2) The migration cycle involves forward movement of the centrosome (C) prior to forward movement of the nucleus (N) and soma. Swelling of the proximal area of the leading process occurs, and dissolution of the interstitial junction is required to release adhesion from the glial fiber and allow the neuron to glide forward. After the nucleus and soma move forward as the neuron takes a step along the glial fiber (3), a new interstitial adhesion junction forms and the migration cycle starts again so that migration continues in a cyclical, saltatory manner. (Adapted from (Solecki et al., 2006).)
Recent, live imaging studies have revealed the contribution of actin and microtubule regulatory molecules in this process. Dynamic rearrangement of the actin cytoskeleton occurs in the proximal region of the leading process, where Myosin II motors localize. Myosin II activity, which is regulated by the conserved mPar6α polarity complex, is required for both centrosomal and somal motility (Solecki et al., 2004; Solecki et al., 2009). The microtubule cytoskeleton is further regulated by cytoplasmic dynein and its cofactors LIS1 and Doublecortin (DCX) to couple centrosomal and nuclear movement (Faulkner et al., 2000; Smith et al., 2000; Coquelle et al., 2002; Tai et al., 2002; Tanaka et al., 2004; Tsai et al., 2005; Mesngon et al., 2006; Tsai et al., 2007; Umeshima et al., 2007). Given the key role of the Rho GTPases in regulating the actin and microtubule cytoskeletons, the role of the Rho GTPases in radial migration will be discussed in detail below.
Insight into the critical role of adhesion and receptor trafficking during neuronal migration comes from studies on receptor systems that function in glial-guided neuronal migration, including Astrotactin (ASTN1), Neuregulin/ErbB4, and BDNF. The neuronal protein ASTN1 is a well-studied receptor for neuronal migration along glial fibers (Edmondson et al., 1988; Fishell and Hatten, 1991) that is expressed by neurons in both the cerebellum and cerebral cortex (Zheng et al., 1996). Mice lacking ASTN1 have slowed cerebellar granule neuron migration, a decrease in neuron-glial binding and abnormal Purkinje cell development compared with wild-type littermates (Adams et al., 2002). Recent studies have identified a second member of the Astrotactin receptor family, ASTN2, which regulates the cell surface expression and trafficking of ASTN1 critical for granule cell migration (Wilson et al., 2010). The importance of trafficking to neuronal migration is further highlighted by the finding that inhibition of clathrin-mediated endocytosis brings migration to a halt (Zhou et al., 2007; Wilson et al., 2010). Neuregulin binds ErbB4 on the glial surface and provides signals that maintain glial process formation (Anton et al., 1997; Rio et al., 1997), and BDNF stimulates granule neuron migration (Borghesani et al., 2002). Surprisingly integrin-based adhesions, which are essential for most types of cell migration, are not required for glial-guided neuronal migration in the developing brain (Edmondson et al., 1988; Fishell and Hatten, 1991; Belvindrah et al., 2007).
Insight into the importance of receptor trafficking to neuronal migration also comes from experiments that provide a new model for the defects observed in the reeler mutant mouse. In the reeler mouse, preplate segregation into a superficial marginal zone and deeper subplate zone fails and the arrival of cortical plate stage neurons generates a broad “superplate” rather than a cortical plate (Caviness, 1982; Goffinet, 1984; Tissir and Goffinet, 2003). Although the failure of preplate splitting has been attributed to defects in the cessation of the migration of later-generated neurons destined for the cortical plate (Caviness, 1982; Goffinet, 1984), recent studies on changes in polarity and orientation of preplate neurons revealed that these neurons in rl/rl neocortex fail to adopt a bipolar shape and align with the radial axis like their counterparts in wild type neocortex. These studies identify the earliest known defect in reeler cortical patterning, and suggest that defects in preplate neuron polarity precede defects in neuronal migration during early stages of reeler corticogenesis. The failure of preplate neurons to transiently polarize in reeler mice further suggests that the protein and pathway responsible, Reelin (RELN), might converge on conserved polarity signaling pathways to control neuronal alignment and polarity during preplate splitting. The most widely accepted model for RELN signaling postulates that RELN, an extracellular matrix glycoprotein, binds to Low density lipoprotein (LDL) receptors, including apoliprotein E receptor 2 (apoER2) and very low density lipoprotein (VLDL) receptors (D’Arcangelo et al., 1999; Hiesberger et al., 1999; Trommsdorff et al., 1999; Hack et al., 2007), and activates Src family tyrosine kinases (STKs), which subsequently phosphorylate vertebrate homologues of the Drosophila protein Disabled 1 (DAB1) (Howell et al., 1997; D’Arcangelo et al., 1999; Hiesberger et al., 1999; Howell et al., 1999; Jossin et al., 2003; Kuo et al., 2005). Experiments by Mikoshiba and colleagues have suggested a novel role for DAB1 in RELN signaling, namely that DAB1 regulates the expression of cell surface RELN and trafficking of RELN receptors (Morimura et al., 2005). This model is based on the observation that RELN is internalized by clathrin-dependent endocytosis, and the fact that adaptor proteins with a PTB domain, including DAB1, regulate intracellular trafficking of transmembrane receptors that contain the cytoplasmic NPXY motif (Mishra et al., 2002). In this model, expression of DAB1 increases the expression of RELN receptors on the cell surface and tyrosine phosphorylation of DAB1 initiates intracellular trafficking of both RELN and its receptors (Morimura et al., 2005), which affects cortical plate formation and cortical lamination.
The ability of the Rho GTPases to regulate cell adhesion and motility (Ridley, 2001; Fukata et al., 2003; Raftopoulou and Hall, 2004), as well as clathrin-dependent and independent endocytosis (Symons and Rusk, 2003), opens up the possibility that they may regulate the formation and dissolution of adhesion junctions through receptor trafficking during neuronal migration.
The Rho GTPases in Radial Glial-Guided Neuronal Migration
Rac in Radial Migration
Conditional knockout of Rac1 in the forebrain, as well as in utero electroporation studies in embryonic mice, revealed a requirement for Rac, its regulators, and effectors, in radial migration. Deletion of Rac1 in the telencephalic VZ, using Foxg1-Cre mice, showed surprisingly that Rac1 is not essential for neuritogenesis, but that it has important functions in axonal guidance and migration. Rac1 deletion results in a larger percentage of neurons in deeper regions of the developing neocortex, and a smaller percentage in upper, superficial layers compared to controls. However, Rac1 deficiency appears to delay the onset or reduce the speed of cortical neuron migration rather than inhibit it entirely (Chen et al., 2007). Neural precursors electroporated with either constitutively active (CA) RacV12 or dominant negative (DN) RacN17 exhibit retarded migration and a failure to form a leading process, suggesting that fine regulation of Rac activity levels and cycling of the GTPase are required for morphological polarization and migration (Konno et al., 2005; Kawauchi et al., 2006). Notably, the effects of DN RacN17 on radial migration are more severe than those of the conditional knockout, and may reflect the ability of the DN mutant to bind/sequestor regulators utilized by other Rho GTPases and signaling pathways. A very recent report provides evidence that migration defects caused by loss of Rac1 in Foxg1-Cre mice may be due, at least in part, to defects in radial glial organization resulting from an inability to anchor their pial endfeet in the basement membrane (Leone et al., 2010).
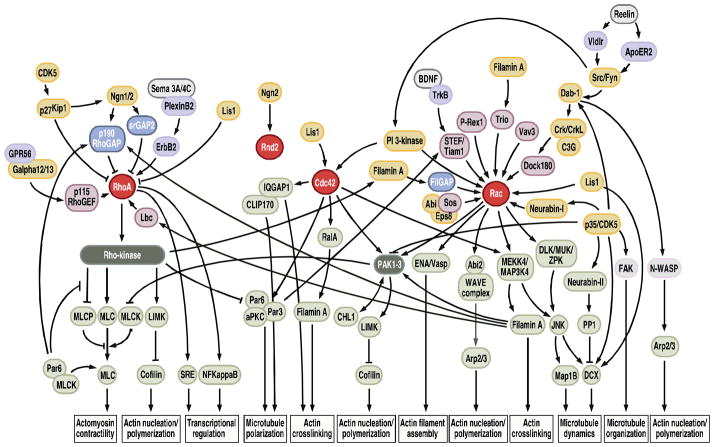
A role for Rac in radial migration is further supported by a number of studies showing involvement of Rac regulators in this process (Fig. 7). These regulators include guanine nucleotide exchange factor (GEFs), such as STEF, Tiam1, P-Rex1, and Vav3. STEF and Tiam1 are Rac specific GEFs detectable in regions of the brain where neuronal migration and neurite outgrowth occur (Ehler et al., 1997; Yoshizawa et al., 2002), and they are both required for neurite formation in neuronal cell lines and primary neurons (Leeuwen et al., 1997; Matsuo et al., 2002; Matsuo et al., 2003). In the developing neocortex, STEF and Tiam1 are present in cells in the IZ and CP (Yoshizawa et al., 2002; Kawauchi et al., 2003), and in utero electroporation of neural progenitors with DN STEF/Tiam causes cells to stall in the IZ without affecting their differentiation (Kawauchi et al., 2003). A study in cerebellar granule cell progenitors (GCPs) shows that Tiam1 and Rac act downstream of BDNF/TrkB signaling to mediate BDNF-induced directional migration, which involves polarized endocytosis of BDNF and TrkB in vitro and in cerebellar cortical slices (Zhou et al., 2007).
Figure 7.
Rho GTPase Signaling Involved in Radial Migration. Some of the key molecules implicated in Rho GTPase signaling that play a role in radial migration, and potential interactors, are shown here (see text for details).
P-Rex1 is another Rac-specific GEF that is also expressed in the IZ and lower CP of the developing neocortex, although its expression is slightly more restricted than that of STEF and Tiam1, and it is expressed in Purkinje cells and possibly Bergmann glia of the developing and adult cerebellum. In migrating neurons in the cortical IZ, P-Rex1 protein localizes in the leading process and adjacent cytoplasmic region, and electroporation of DN P-Rex1 that lacks the Dbl-homology (DH) domain into embryonic cortex causes neuronal precursors to stall in the IZ (Yoshizawa et al., 2005).
Vav family members are Rac/Rho GEFs that have been shown to play a role in axon guidance in retina neurons and in Purkinje cell spinogenesis (Luo et al., 1996; Cowan et al., 2005). Vav3 is expressed at high levels in Purkinje cells and GCPs of the cerebellum, and regulates Purkinje cell dendritogenesis, the timely migration of GCPs from the EGL to the IGL, and the survival of mature granule neurons (Quevedo et al., 2010)
Other molecules known to act upstream of Rac that play a role in radial migration include two kinases, Phosphatidylinositol (PI) 3-kinase and Cdk5. PI 3-kinase produces phosphatidylinositol 3,4,5-triphosphate (PI[3,4,5]P3), and regulates cell polarization and migration via Rac, Cdc42, and/or Akt (protein kinase B) (Fukata et al., 2003). Expression of CA and DN PI 3-kinase mutants by in utero electroporation in neural progenitors markedly inhibited radial migration, and likely did so by acting upon both Rac1 and Cdc42, since a PI 3-kinase inhibitor suppressed the formation of GTP-bound Rac1 and Cdc42 and radial migration in neocortical slices (Konno et al., 2005).
Cyclin-dependent kinase 5 (Cdk5) is a neuron specific cyclin-dependent kinase regulated by subunits p35 and p39, which can complex with activated, GTP-bound Rac through p35, resulting in downregulation of the kinase activity of the Rac/Cdc42 effector PAK (Nikolic et al., 1998). Mutations in Cdk5 and p35 both cause an inversion of cortical layers (Ohshima et al., 1996; Chae et al., 1997; Gilmore et al., 1998; Ko et al., 2001; Ohshima et al., 2007). Introduction of DN Cdk5 via in utero electroporation arrests cells in the IZ (Kawauchi et al., 2003; 2006; Nguyen et al., 2006), and Cdk5 deficient neurons fail to transition from the multipolar to the bipolar stage, form a leading process and translocate (Ohshima et al., 2007). p35 deficiency causes improper neuronal-glial interaction and aberrant branched migration instead of somal translocation (Gupta et al., 2003). Cdk5 may regulate Rac with regards to radial migration through the phosphorylation of Neurabin-1, a neuronal-specific F-actin-binding protein. Neurabin-1 negatively regulates Rac1 activity in the presence of Cdk5/p35, and both overexpression and knockdown of Neurabin-1 by in utero electroporation perturbs radial migration, with prominent effects on the leading process (Causeret et al., 2007). Neurabin-II may also negatively regulate Rac signaling by antagonizing the effects of JNK (c-jun N-terminal kinase), a member of the mitogen-activated protein kinase (MAPK) family known to act downstream of Rac (Davis, 2000). Neurabin II promotes the dephosphorylation of JNK-mediated phosphorylation sites on Doublecortin (DCX) through protein phosphatase 1 (PP1) (see JNK paragraph below for more information on JNK and DCX) (Shmueli et al., 2006; Tsukada et al., 2006). In addition to Neurabin, Cdk5 also targets both Reelin (RELN) and Lis1-related signaling proteins, and actin and microtubule regulatory molecules, including DCX, Ndel1, DAB1, and focal adhesion kinase (FAK) (Niethammer et al., 2000; Sasaki et al., 2000; Keshvara et al., 2002; Xie et al., 2003; Tanaka et al., 2004). Thus, Cdk5 may coordinately regulate different signaling pathways, and the actin and microtubule cytoskeletons, to effect radial migration.
Potential regulation of Rac downstream of the RELN/DAB1 signaling pathway may also occur during radial migration. When DAB1 is tyrosine phosphorylated, it provides a scaffold for signaling complexes, including Crk and CrkL adaptors, and CrkII links phosphorylated DAB1 to the Rac GEF DOCK180/DOCK1 (Ballif et al., 2004; Chen et al., 2004; Huang et al., 2004). Expression of phosphorylated DAB1 interferes with CrkII-dependent cell migration of Nara Bladder Tumor II (NBT-II) cells, and a loss-of-function mutation in myoblast city, the DOCK180 homologue in Drosophila, rescues the rough-eye phenotype in Drosophila caused by exogenous expression of phosphorylated mouse Dab1 (Chen et al., 2004), suggesting that DAB1 tyrosine phoshorylation may downregulate Rac activity. The absence of DAB1 Crk/CrkL binding sites, or Crk and CrkL deficiency, causes defects in cortical layering and loss of activity of the Crk-regulated Ras family guanine nucleotide exchange factor C3G (RapGEF1, Grf2) (Sanada et al., 2004; Park and Curran, 2008; Feng and Cooper, 2009). C3G-deficient mouse embryos also fail to split the preplate, form a cortical plate, and migrate, resulting in an arrest of neurons in the multipolar state and defects in migration (Voss et al., 2008). Thus, a likely scenario is that DAB1, Crk-DOCK180, and Rac1 as a complex contribute to radial migration.
Molecules that generally transduce Rac signaling also play a part in radial migration and include regulators of both the actin and microtubule cytoskeletons (Fig. 7). Actin regulators known to act downstream of Rac that play a role in radial migration include, PAK, Ena/VASP, Abi2, and Filamin A. The Pak (p21-activated kinase) family of serine/threonine kinases, which includes Pak1–6, can be activated by, and serves as an effector for, both Rac and Cdc42 (Jaffer and Chernoff, 2002; Bokoch, 2003). Recent studies reveal a role for Pak in cortical radial migration. Pak1 expression is high in migrating neurons, and both loss and gain of Pak1 function using in utero electroporation to express CA and DN Pak1 mutants perturbs the neuronal morphology and radial migration of projection neurons. Overexpression of hyperactivated Pak1 causes neurons to arrest in the intermediate zone (IZ) with short processes that curve or branch and bear small protrusions. Those that make it to the CP have broader leading processes with extensive lamellipodia. Loss of Pak1 disrupts the morphology of migrating neurons, which accumulate in the IZ and deep cortical layers. Neurons in the IZ elaborate disorganized extensions and broad lamellipodial protrusions that surround the soma, and those migrating in the cortical plate have a shorter leading process. Unexpectedly, neurons with reduced Pak1 expression that make it to the upper cortical plate aberrantly enter into the marginal zone, suggesting an inability to cease migration, perhaps due to an inability to dissociate from radial glia (Causeret et al., 2009). Pak 1–3 may also cooperate with the cell adhesion molecule close homologue of L1 (CHL1) to regulate morphological development of the leading process/apical dendrite of embryonic cortical neurons (Demyanenko et al., 2010). Thus Pak appears to play a major role in proper neurite and leading process formation during radial migration.
Members of the Ena/Vasp family (MENA (mammalian enabled), VASP, and EVL) control the elongation of unbranched actin filaments. They are localized in regions of dynamic actin reorganization, such as in the lamellipodium at the leading edge of motile cells (Reinhard et al., 1992; Gertler et al., 1996). In primary neurons, Mena is concentrated in the distal tips of growth cone filopodia and regulates axon guidance (Lanier et al., 1999). In developing cortical neurons, Ena/VASP proteins are highly expressed in IZ neurons and CP neurons bordering Reelin-expressing Cajal-Retzius cells. Loss of Ena/VASP function causes neuronal ectopias, alters intralayer positioning in the CP, and blocks axon fiber tract formation. The cortical fiber tract defects result from a failure in neurite initiation, which are preceded by a failure to form bundled actin filaments and filopodia (Goh et al., 2002; Kwiatkowski et al., 2007).
The Abl-interactor (Abi) family of adaptor proteins bind Abl tyrosine kinases (Dai and Pendergast, 1995; Shi et al., 1995) and modulate Rac activity by acting both downstream and upstream of this GTPase. Abi1 and Abi2 bind a WAVE-interacting complex downstream of Rac to regulate Arp2/3-dependent actin polymerization (Eden et al., 2002; Soderling et al., 2002; Kunda et al., 2003; Rogers et al., 2003; Echarri et al., 2004; Innocenti et al., 2004; Steffen et al., 2004), and Abi1 complexes with Eps8 and Sos GEF to directly increase Rac activity and regulate actin dynamics (Scita et al., 1999; Scita et al., 2001; Innocenti et al., 2002; Innocenti et al., 2004). A role for Abi2 in neuronal migration has been reported. Abi2 is highly expressed in the brain and developing cortex, and is prominent in the MZ and along the border of the lateral ventricles, where it colocalizes with β-catenin. Although cortical layers appear to form normally, pyramidal cells are not radially aligned in adult mice. Downregulation of Abi expression by RNA interference in epithelial cells suggests that these defects could arise from impaired adherens junction formation and downregulation of the WAVE actin-nucleation promoting factor (Grove et al., 2004).
Filamin-A is an actin binding protein that can bind Rac1 (Marti et al., 1997; Ohta et al., 1999; Stossel et al., 2001; Zhou et al., 2010), the Rac GEF Trio (Bellanger et al., 2000), and the Rac GAP FilGAP (Ohta et al., 2006), as well as positively regulate the Rac effector PAK (Vadlamudi et al., 2002; Maceyka et al., 2008). Mutations in the Filamin A gene, FLNa, cause the cortical malformation periventricular heterotopia (PH) that results from a failure in neuronal migration (Fox et al., 1998; Feng and Walsh, 2004; Sarkisian et al., 2008). Filamin-A expression is high in the IZ above the SVZ and in the CP of the developing cortex (Fox et al., 1998; Nagano et al., 2002; Sato and Nagano, 2005). Expression of a Filamin A mutant lacking the actin-binding domain arrests the migration of post-mitotic neurons in the IZ of the mouse neocortex, and impairs acquisition of a bipolar morphology in the SVZ/IZ, whereas Filamin A overexpression, achieved by downregulating the Filamin A-interacting protein that induces Filamin A degradation (FILIP), increases the number of bipolar cells (Nagano et al., 2004; Sato and Nagano, 2005). These findings suggest that Filamin A is enriched in post-mitotic, migratory neurons and that its ability to cross-link actin is required for its function in migration. In agreement with these data, FLNa mutations that cause PH are often truncations or disruptions of the actin-binding domain (Fox et al., 1998; Feng and Walsh, 2004; Sarkisian et al., 2008). Filamin-A is also regulated by MEKK4/MAP3K4 (Sarkisian et al., 2006), a Rac1/Cdc42 binding protein and upstream MAPKKK for JNK (Davis, 2000). Similar to mutations in Filamin A, loss of MEKK4 causes periventricular heterotopias (Sarkisian et al., 2006). This association suggests that Filamin A might enhance signaling from Rac1 to JNK, and in doing so may cross-talk with the microtubule cytoskeleton (see JNK paragraph below).
While JNK is known to act downstream of Rac to regulate stress response (Johnson and Nakamura, 2007), a novel role for JNK in microtubule regulation critical to radial migration has been reported. JNK expression is strong in the IZ (Hirai et al., 2002; Kawauchi et al., 2003), and its activity is negatively affected by expression of DN RacN17 (Kawauchi et al., 2003). Administration of a JNK inhibitor, or in utero electroporation of DN JNK, retards migration (Kawauchi et al., 2003; Hirai et al., 2006), with migrating cells possessing a leading process that is twisted and irregular. Examination of cultured neurons revealed that activated JNK is detected along microtubules in processes, and a JNK inhibitor causes twisted, irregular, thick microtubules and shorter neurites, as well as decreased phosphorylation of microtubule associated protein 1B (Map1B), which is required for MT stability (Kawauchi et al., 2003). Map1B is also present in migrating IZ cells (Cheng et al., 1999), its overexpression overstabilizes microtubules and perturbs neuronal migration (Kawauchi et al., 2005), and Map1B knockout mice exhibit abnormal cortical lamination (Gonzalez-Billault et al., 2005). JNK also phosphorylates DCX, which is localized to neurite tips by JNK interacting protein (JIP) bound to kinesin, to regulate neurite outgrowth and the velocity of migrating neurons (Gdalyahu et al., 2004). The non-phosphorylated forms of Map1B and DCX interact with and stabilize microtubules, and phosphorylation of these proteins decreases their affinity for microtubules, leading to increased microtubule dynamics. Thus JNK, through the phosphorylation of these substrates, increases microtubule dynamics in migrating neurons. Activity of JNK in migrating cortical neurons is regulated by upstream kinases, including the dual leucine zipper kinase (DLK)/MAPK upstream protein kinase (MUK)/leucine zipper protein kinase (ZPK) and MEKK4/MAP3K4. DLK, like JNK, is predominantly expressed in the IZ (Hirai et al., 2002; Kawauchi et al., 2003). Overexpression of DLK perturbs radial migration (Hirai et al., 2002), and genetic disruption of DLK decreases JNK activity and the phosphorylation of known JNK substrates, including c-Jun and DCX, and impairs axon growth and radial migration of neocortical pyramidal neurons (Hirai et al., 2006). As mentioned above, MEKK4/MAP3K4 is also associated with migration defects and links JNK with Filamin A (Sarkisian et al., 2006).
All together, these findings provide strong support for a role for Rac in radial migration. However, perturbation of Rac function, as well as of a number of the Rac-interacting proteins discussed above generally does not inhibit migration entirely, but rather delays migration. In addition, Rac signaling does not appear to affect cell polarization, but causes defects in the morphology of the leading process. Interestingly, a study on rhombic lip cell migration in the developing chicken cerebellum comes to this same conclusion. In this system, expression of DN RacN17 suppresses the generation of protrusions at the tip of the leading process and impairs leading process extension, but does not alter overall polarity. PI 3-kinase and Pak also regulate leading process formation and morphology, respectively, in these cells (Sakakibara and Horwitz, 2006).
Cdc42 in Radial Migration
Although the role of Cdc42 in radial migration has not been as extensively examined compared to Rac, studies suggest that Cdc42 is important for this process. In the developing neocortex, perturbation of Cdc42 activity retards radial migration (Konno et al., 2005), and some insight into the role of Cdc42 in CNS migration comes from experiments implicating Cdc42 in Lis1 signaling (Fig. 7). Lis1 (Pafah1b1) is a subunit of the platelet activating factor acetylhydrolase 1b (Pafah1b) (Hattori et al., 1994), and mutations in LIS1/PAFAH1B1 and associated signaling molecules are associated with lissencephaly. Lissencephaly is a cortical malformation characterized by lack of (agyria), or reduction in (pachygyria), normal cerebral surface convolutions, resulting in a “smooth brain”, thickening of the cortex, abnormal cortical lamination, mental retardation and epilepsy (Reiner et al., 1993; Pang et al., 2008; Spalice et al., 2009). Lis1 is required not only for radial migration, but also for other steps in neuronal development leading up to migration, including maintenance of neuroepithelial stem cells, interkinetic nuclear movement and progression of neural progenitors through the cell cycle in the VZ, as well as the formation of process outgrowth (Tsai et al., 2005; Yingling et al., 2008).
Regulation of cell motility and migration by Lis1 involves activation of Cdc42, and the formation of a complex that contains Lis1, active GTP-bound Cdc42, IQGAP1, and CLIP-170 (Kholmanskikh et al., 2006). IQGAP1 was identified as an effector of Cdc42 and Rac1 (Hart et al., 1996; Kuroda et al., 1996), and is an actin-binding protein that stabilizes actin filaments at the leading edge (Fukata et al., 1997). It interacts with the microtubule stabilizing protein CLIP170 to connect microtubule plus ends to peripheral actin filaments (Fukata et al., 2002; Watanabe et al., 2004). Activation of Cdc42 and Rac1 appears to target IQGAPs and CLIP-170 to specific cortical areas to polarize microtubules and regulate the front-rear polarization of migrating cells (Fukata et al., 2002; Watanabe et al., 2004) and promote axon outgrowth (Wang et al., 2007). In addition to acting as an effector for Cdc42 and Rac1, IQGAP1 inhibits the GTPase activity of Cdc42 and stabilizes the active, GTP-bound form of this GTPase (Ho et al., 1999). In migrating cerebellar granule neurons, calcium influx enhances neuronal motility through Lis1 activation of Cdc42, leading to the perimembrane localization of IQGAP1 and CLIP-170, which presumably tethers microtubule ends to the actin cytoskeleton to regulate migration. In turn, IQGAP1 may stabilize and thus enhance Cdc42 activity in migrating neurons (Kholmanskikh et al., 2006). Cdc42 and Lis1 also appear to be partners of IQGAP1 in the non-radial migration of neuronal precursor cells of the subventricular zone (SVZ) and the rostral migratory stream (RMS) (Balenci et al., 2007).
While these findings implicate Cdc42 in radial migration, the contribution of Cdc42 to different stages of the migration cycle, and the various signaling pathways involved, remain to be elucidated. Cdc42 regulates cell polarization and centrosomal reorientation during directed non-neuronal cell migration (Etienne-Manneville and Hall, 2001; Palazzo et al., 2001; Tzima et al., 2003; Cau and Hall, 2005; Gomes et al., 2005; Gundersen et al., 2005; Lee et al., 2005) and is the central regulator of overall polarity in migrating rhombic lip cells in the developing chicken cerebellum (Sakakibara and Horwitz, 2006); however, it remains to be determined whether this is the case in radially migrating CNS neurons. Interestingly, wild type (WT) Cdc42 localizes to the perinuclear region on the side of the leading process in migrating cortical neurons, hinting at a potential role in polarization of the neuron during glial-guided neuronal migration (Konno et al., 2005). It will be critical to determine where active Cdc42 localizes throughout the migration cycle in CNS neurons, and whether known Cdc42 interactors, such as the conserved Par6 polarity complex, mediate its effects on migration, or whether Cdc42 regulates glial-guided radial migration independent of these proteins.
Rho in Radial Migration
Strict regulation of RhoA levels and activity appear to be required for radial migration. In the developing rodent neocortex, RhoA mRNA expression is high in the premigratory cortical VZ and SVZ, and low in cells migrating in the IZ, while RhoB mRNA expression is high only in the CP (Olenik et al., 1999; Ge et al., 2006; Nguyen et al., 2006). In the developing cerebellum, RhoA expression is high in cells in the EGL, IGL, and Purkinje cell layer (Richard et al., 2008). Electroporation of the VZ of embryonic mouse cortex with RhoA or DN-RhoA, followed by slice culture, shows that ectopic expression of RhoA blocks radial migration, whereas interfering with RhoA function promotes migration. In addition, loss of a negative regulator of Rho, p190 RhoGAP, causes defects in forebrain development, including impaired layering of the cerebral cortex (Brouns et al., 2000). Taken together, these findings suggest that downregulation of RhoA activity is required for the radial migration of neurons. However, despite these findings, active RhoA has been reported in the leading process of migrating cerebellar GCPs (Guan et al., 2007), suggesting that lower levels of RhoA may still play a role in radial migration (see further below).
The proneural basic helix–loop–helix (bHLH) transcription factors Ngn1 and 2 initiate differentiation and migration in the neocortex by upregulating factors required for migration, such as DCX and p35, and downregulating opposing factors, such as RhoA. Ectopic expression of Ngn1 downregulates both RhoA levels and activity, and Ngn2 deficiency increases RhoA expression. Loss of Ngn1, Ngn2, or both, also expands the zone of RhoA expression in the developing neocortex, with higher RhoA expression in the IZ compared to controls (Ge et al., 2006). Importantly, Ngn2 is required for radial migration (Mattar et al., 2004; Schuurmans et al., 2004; Hand et al., 2005; Ge et al., 2006; Nguyen et al., 2006; Heng et al., 2008), and specifies the polarity of the leading process during the initiation of migration by a mechanism that is independent of its transactivation properties and proneural function. The migration defect observed in the Ngn2 knockout mice can be rescued by expressing DN RhoAN19 in cortical progenitors (Hand et al., 2005). Consistent with a negative role for Ngn2 in regulating RhoA, Ngn2 knockout also causes a decrease in expression of two negative Rho GTPase regulators, p190RhoGAP-B/RhoGAP5/ARHGAP5 and srGAP2/formin binding protein 2 (FNBP2) (Mattar et al., 2004; Hand et al., 2005).
RhoA signaling during radial migration is also downregulated by a signaling pathway that involves Cdk5 and the cyclin-dependent kinase (CDK) inhibitor p27(Kip1) (Fig. 7). Cdk5 phosphorylates and stabilizes p27 to maintain the amount of p27 in post-mitotic neurons (Kawauchi et al., 2006). Similar to loss of Cdk5 function, p27 deficiency prevents cortical precursors from migrating to the CP (Kawauchi et al., 2003; 2006; Nguyen et al., 2006). Both DN Cdk5 and p27 shRNA expressing cells in the lower IZ are round with thin processes instead of multipolar, but only DN Cdk5 expressing cells in the upper IZ lack a normal leading process (Kawauchi et al., 2006). Interestingly, p27 regulates neuronal differentiation and radial migration by two distinct mechanisms, which are independent of its role in cell cycle regulation. p27 promotes neuronal differentiation by stabilizing Ngn2 protein, an activity carried out by the N-terminal half of the protein, and it promotes neuronal migration by blocking RhoA signaling, an activity that resides in its C-terminal half. Expression of Ngn2 rescues differentiation defects, and expression of DN RhoAN19 rescues the migration defects caused by knock-down of p27 in vivo. Inhibition of RhoA signaling using an inhibitor for the Rho effector Rho-kinase also rescues p27 siRNA migration defects in cortical slices (Nguyen et al., 2006). By suppressing the RhoA/Rho-kinase pathway, Cdk5-p27 signaling can modulate F-actin in migrating cells. Rho-kinase phosphorylates and activates the LIM (Lin-11, Isl-1 and Mec-3) domain-containing kinases, which in turn phosphorylate and inactivate cofilin, an actin filament depolymerizing/severing factor (Maekawa et al., 1999; Sumi et al., 1999; Ohashi et al., 2000; Ohashi et al., 2000; Amano et al., 2001; Sumi et al., 2001). In fact, p27 decreases cofilin phosphorylation by suppressing the RhoA/Rho-kinase pathway in fibroblasts (Besson et al., 2004), and p27 deficient cortical neurons contain less F-actin than control cells (Kawauchi et al., 2006). A CDK inhibitor, expression of DN Cdk5 and expression of p27 shRNA all cause an increase in cofilin phosphorylation in cultured cortical neurons, and phosphorylation of cofilin induced by the CDK inhibitor can be suppressed by expression of DN Rho-kinase or DN RhoAN19. The importance of cofilin phosporylation to radial migration is further highlighted by the arrest of neuronal precursors in the IZ of embryonic cortices electroporated with a phosphorylation deficient cofilin mutant (Kawauchi et al., 2006).
While considerable downregulation of RhoA levels and activity appear to be required for cortical neurons to migrate successfully, lower levels of RhoA may still be necessary to regulate actomyosin contractility and drive motility. The actin-based motor Myosin II contracts actin filaments to generate the force needed to power cell motility and turn over actin-based adhesions (Gupton et al., 2002; Webb et al., 2004; Gupton and Waterman-Storer, 2006; Vicente-Manzanares et al., 2009; Vicente-Manzanares et al., 2009), and Myosin IIB is the predominant Myosin II motor expressed in the nervous system (Kawamoto and Adelstein, 1991; Rochlin et al., 1995). Rho-kinase regulates myosin activity downstream of RhoA by directly phosphorylating and activating myosin light chain (MLC), and by phosphorylating and inactivating MLC phosphatase (MLCP), thereby indirectly increasing MLC phosphorylation and activation (Amano et al., 1996; Kimura et al., 1996). Myosin IIB mutant mice display an abnormal pattern of cerebellar foliation, and defects in GCP migration along Bergmann glial fibers (Ma et al., 2004). Myosin II motors and F-actin are enriched in the leading process of migrating neurons, and Myosin II activity is required for high actin dynamics in this region, and for centrosomal and somal motility (Schaar and McConnell, 2005; Solecki et al., 2009). In cortical neurons, inhibition of Myosin IIB appears to regulate nuclear, but not centrosomal movement (Tsai et al., 2007). The mechanism by which Myosin II regulates centrosomal and somal movement in migrating neurons may involve the conserved polarity protein mPar6α, which localizes to the centrosome and also regulates centrosomal and somal movement (Solecki et al., 2004). Ectopic expression of Par6α inhibits Rho-kinase phosphorylation of MLCP, leading to enhanced MLCP dephosphorylation of MLC, and ultimately a reduction in the acto-myosin contractility that drives neuronal migration. Par6 also interacts with MLC and myosin light chain kinase (MLCK), a positive regulator of myosin activity. Disruption of Par6-MLC binding via overexpression of the IQ-like domain of Par6α inhibits MLC phosphorylation and increases the turnover time of F-actin in the leading process of migrating neurons (Solecki et al., 2009) (Fig. 7). In the future, it will be of interest to determine whether low levels of RhoA activity regulate centrosome positioning and nuclear movement during radial glial-guided migration.
Rho/Rho-kinase signaling may also be affected by Filamin A, an actin binding protein associated with the cortical malformation periventricular heterotopia (PH) (Fox et al., 1998; Feng and Walsh, 2004; Sarkisian et al., 2008; Zhou et al., 2010) discussed in detail in the Rac in Radial Migration section above. In addition to binding Rac and Cdc42 signaling components (Ohta et al., 1999; Bellanger et al., 2000; Vadlamudi et al., 2002; Ohta et al., 2006), Filamin A can associate with the Rho GEF Lbc (Pi et al., 2002) and form a complex with Rho-kinase (Ueda et al., 2003), which phosphorylates and stimulates the Rac GAP FilGAP to inactivate Rac (Tseng et al., 2004). Filamin A also promotes the accumulation of p190RhoGAP in lipid rafts (Mammoto et al., 2007). Thus Filamin A appears to be capable of both activating and inactivating the Rho GTPases Rac and Rho (Fig. 7). Filamin A may increase Trio/Rac/Pak signaling during cell protrusion, while decreasing Rho activity through p190RhoGAP. Conversely, during cell retraction, Filamin A may stimulate Lbc to increase Rho/Rho-kinase activity, while decreasing Rac activity through FilGAP (Zhou et al., 2010). However, despite all of these possibilities, how Filamin A interacts with Rho GTPase signaling during radial migration remains to be defined.
A recent study suggests that positive regulation of RhoA by Semaphorin-Plexin signaling may facilitate radial migration. Semaphorin 3A (Sema 3A) expression is highest in superficial cortical layers (Polleux et al., 2000), and Sema 3A and 3F have been shown to set the directionality of cortical neuron migration. Knockdown of Sema3A receptors, including Neuropilin and Plexin subunits, as well as the application of exogenous Sema3A, delays radial migration (Chen et al., 2008). Mice lacking Plexin-B2 exhibit neural tube closure defects and a variety of other developmental brain defects, including exencephaly, enlargement of the ventricles, hypotrophy of the VZ, disruption of the ventricular wall, and ventricular ectopias. Plexin-B2 is required for proliferation of VZ neuroblasts, and proliferation and migration of GCPs in the cerebellum, dentate gyrus, and olfactory bulb. Signaling analysis in a heterologous system shows that Sema 4C binding to Plexin-B2 activates the receptor tyrosine kinase ErbB-2 and RhoA (Fig. 7), suggesting that these molecules may play a role in proliferation and the ability of neural precursors to migrate directionally (Deng et al., 2007).
There is also a possibility that Rho signaling may regulate termination of radial neuronal migration through a link with the heterotrimeric G proteins Gα12 and Gα13 and the orphan G protein-coupled receptor GPR56 (Fig. 7). The heterotrimeric G proteins Gα12 and Gα13 link G-protein-coupled receptors to actomyosin-based cellular contractility, and are required for the proper termination of radial migration by cortical neurons. Simultaneous ablation of both Gα12 and Gα13 genes results in neuronal ectopia of the cerebral and cerebellar cortices due to overmigration of cortical plate neurons and cerebellar Purkinje cells, respectively (Moers et al., 2008). Gα12 and Gα13 couple with the orphan G protein-coupled receptor GPR56, which is highly expressed in neural progenitor cells and inhibits their migration. Coupling of GPR56 with Gα12 and Gα13 induces Rho-dependent activation of serum-responsive element (SRE) and NFkappaB transcription and actin reorganization, which can be inhibited by expression of the RGS domain of the p115 Rho-specific GEF (p115 RhoGEF RGS) and DN RhoN19. In addition, the effects of an anti-GPR56 antibody that acts as an agonist and inhibits migration can be attenuated by p115 RhoGEF RGS, C3 exoenzyme that inhibits Rho, and GPR56 knockdown. While the GPR56-related migration experiments were performed using neurospheres, and therefore were not in vivo, these findings provide a potential mechanism whereby Rho might participate in the termination of migration during cortical development (Iguchi et al., 2008). Indeed, while RhoA is mainly expressed in the VZ at early embryonic stages of neocortical development, it appears also to be present in the CP at later stages after cells have finished migrating (Olenik et al., 1999). RhoA might therefore transduce stop signals that prevent neurons from migrating too far.
Taken together, these studies provide a picture whereby a reduction in RhoA levels and activity are required to commence migration, and persistently low levels may regulate actin reorganization, acto-myosin contractility, and transcription required for the mechanics of motility and the cessation of movement. Precisely how Rho might regulate the cellular mechanisms of glial-guided migration, however, remains to be defined.
Rnd2 in Radial Migration
The Rnd proteins constitute a unique branch of the Rho GTPases that have low intrinsic GTPase activity and are thought to be constitutively active. As a result, they are also insensitive to the effects of classical Rho GTPase regulators, including GEFs and GAPs. There are three Rnd family members, called Rnd1, Rnd2 and Rnd3/RhoE. The Rnd proteins have been shown to regulate neurite formation, axon extension, and dendritic development, as well as cell-cycle control (Riento et al., 2005; Chardin, 2006). Rnd1 and Rnd3/RhoE proteins regulate reorganization of the actin cytoskeleton by reducing cellular levels of GTP-bound RhoA through a p190RhoGAP mechanism, and RhoE sequesters Rho-kinase to prevent it from phosphorylating MLCP (Riento et al., 2003; Wennerberg et al., 2003; Riento et al., 2005; Chardin, 2006). In contrast, Rnd2 interacts with the effector Pragmin to stimulate RhoA/Rho-kinase activity and induce cell contraction (Tanaka et al., 2006). Rnd2 also interacts with the protein Rapostlin in a GTP-dependent manner, which binds directly to microtubules and regulates reorganization of both actin filaments and microtubules to induce neurite branching (Fujita et al., 2002). Most recently, Rnd2 has been shown to play a critical role in cortical radial migration (Nakamura et al., 2006; Heng et al., 2008).
During cortical development, Rnd2 expression is intense in basal/intermediate progenitors and radially migrating neurons in the SVZ and IZ, respectively, weak in the VZ progenitors, and sharply downregulated in cells in the CP (Nakamura et al., 2006; Heng et al., 2008). In utero electroporation of Rnd2 or CA Rnd2V16 resulted in a failure of neurons to migrate to upper cortical layers. However, cells electroporated with DN Rnd2N21 migrated similar to control cells, a result which likely reflects an inability of this mutant to utilize and titrate out GEFs the way a traditional DN Rho GTPase mutant does (Nakamura et al., 2006). Knockdown of Rnd2 by ex vivo electroporation of cortices, followed by organotypic slice cultures, markedly inhibited radial migration without affecting the proliferation of cortical progenitors, their specification to a cortical neuron identity, or the organization of the radial glia scaffold. Neurons with compromised Rnd2 expression also exhibited morphological defects, including an increased fraction of IZ neurons with a multipolar morphology and increased length of the longest neurite, and those with a unipolar or bipolar shape had branched leading processes compared to control neurons. Neuronal processes in Rnd2 knockdown cells were also more unstable. Not only did Ngn2 directly induce Rnd2 expression, and Rnd2 knockdown defects in radial migration were similar to those observed upon deletion of the Ngn2 gene, but Rnd2 expression in Ngn2 deficient neurons resulted in a remarkable rescue of their ability to migrate (Heng et al., 2008). Thus Rnd2 is a major effector of Ngn2 function in the promotion of migration.
Conclusion
Over the last decade, remarkable progress has been made in understanding the mechanisms of neuronal migration in vivo. The advent of in utero electroporation, advanced live imaging techniques, and the increasing availability of conditional mutant mouse strains, have allowed us to examine the contribution of individual molecules to specific stages of neuronal development. Using these experimental methods, we have learned that the Rho GTPases play critical and unique roles in neurogenesis and radial migration. During neurogenesis in the cortex, Rac and Cdc42 regulate the self-renewal of neural progenitors, and, Cdc42, in particular, regulates interkinetic nuclear migration. During radial migration, Cdc42 likely polarizes migrating neurons, while Rac regulates leading process formation and protrusion. Although down-regulation of RhoA is required for migration, low levels likely persist to regulate acto-myosin contractility required to generate the force needed to drive motility. In addition, Rnd2 has emerged as a critical and strong regulator of radial migration. These Rho GTPases act within multiple signaling pathways, including those associated with cortical malformations, which ultimately converge on the actin and microtubule cytoskeletons. Thus the Rho GTPases integrate and propagate signals from various upstream regulators to produce the morphological changes needed to progress through development and undergo directed movement. Since the function of the Rho GTPases during neurogenesis and migration is dependent on strict regulation of not only their levels, but their also activity, it will be interesting to see how Rho GTPase levels and activity are localized and regulated in different subcellular compartments of live, developing and migrating neurons in vivo. It will also be critical to define how all of these signaling pathways required for radial migration come together to produce the complex architectonics of the human brain associated with higher cognitive functions.
Acknowledgments
Given the large scope of this review, we apologize for not being able to cite all of our colleagues who have contributed to this field. Supported by National Institutes of Health–National Institute of Neurological Disorders and Stroke Grant RO1 NS 051778-05 (M.E.H.) and National Institute of Mental Health Grant RO1 MH082808 (L.V.A.). We are grateful to Mr. James Duffy for help with the illustrations in Figures 3, 6 & 7, to Dr. Carol A. Mason for providing the drawing in Figure 5, and to Mr. William Carey for proofreading the manuscript.
References
- Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129:965–972. doi: 10.1242/dev.129.4.965. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano T, Tanabe K, Eto T, Narumiya S, Mizuno K. LIM-kinase 2 induces formation of stress fibres, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem J. 2001;354:149–159. doi: 10.1042/0264-6021:3540149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- Balenci L, Saoudi Y, Grunwald D, Deloulme JC, Bouron A, Bernards A, Baudier J. IQGAP1 regulates adult neural progenitors in vivo and vascular endothelial growth factor-triggered neural progenitor migration in vitro. J Neurosci. 2007;27:4716–4724. doi: 10.1523/JNEUROSCI.0830-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol. 2004;14:606–610. doi: 10.1016/j.cub.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Battista D, Rutishauser U. Removal of polysialic acid triggers dispersion of subventricularly derived neuroblasts into surrounding CNS tissues. J Neurosci. 2010;30:3995–4003. doi: 10.1523/JNEUROSCI.4382-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol. 2000;2:888–892. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- Bellion A, Baudoin JP, Alvarez C, Bornens M, Metin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci. 2005;25:5691–5699. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R, Hankel S, Walker J, Patton BL, Muller U. Beta1 integrins control the formation of cell chains in the adult rostral migratory stream. J Neurosci. 2007;27:2704–2717. doi: 10.1523/JNEUROSCI.2991-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003;1603:47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- Bernards A, Settleman J. GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 2004;14:377–385. doi: 10.1016/j.tcb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. BDNF stimulates migration of cerebellar granule cells. Development. 2002;129:1435–1442. doi: 10.1242/dev.129.6.1435. [DOI] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, Bronson RT, Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–4903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- Buchman JJ, Tseng HC, Zhou Y, Frank CL, Xie Z, Tsai LH. Cdk5rap2 interacts with pericentrin to maintain the neural progenitor pool in the developing neocortex. Neuron. 2010;66:386–402. doi: 10.1016/j.neuron.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Buchsbaum RJ, Connolly BA, Feig LA. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol. 2002;22:4073–4085. doi: 10.1128/MCB.22.12.4073-4085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum RJ, Connolly BA, Feig LA. Regulation of p70 S6 kinase by complex formation between the Rac guanine nucleotide exchange factor (Rac-GEF) Tiam1 and the scaffold spinophilin. J Biol Chem. 2003;278:18833–18841. doi: 10.1074/jbc.M207876200. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, Brakebusch C, Gotz M. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- Causeret F, Hidalgo-Sanchez M, Fort P, Backer S, Popoff MR, Gauthier-Rouviere C, Bloch-Gallego E. Distinct roles of Rac1/Cdc42 and Rho/Rock for axon outgrowth and nucleokinesis of precerebellar neurons toward netrin 1. Development. 2004;131:2841–2852. doi: 10.1242/dev.01162. [DOI] [PubMed] [Google Scholar]
- Causeret F, Jacobs T, Terao M, Heath O, Hoshino M, Nikolic M. Neurabin-I is phosphorylated by Cdk5: implications for neuronal morphogenesis and cortical migration. Mol Biol Cell. 2007;18:4327–4342. doi: 10.1091/mbc.E07-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causeret F, Terao M, Jacobs T, Nishimura YV, Yanagawa Y, Obata K, Hoshino M, Nikolic M. The p21-activated kinase is required for neuronal migration in the cerebral cortex. Cereb Cortex. 2009;19:861–875. doi: 10.1093/cercor/bhn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7:54–62. doi: 10.1038/nrm1788. [DOI] [PubMed] [Google Scholar]
- Chen G, Sima J, Jin M, Wang KY, Xue XJ, Zheng W, Ding YQ, Yuan XB. Semaphorin-3A guides radial migration of cortical neurons during development. Nat Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- Chen K, Ochalski PG, Tran TS, Sahir N, Schubert M, Pramatarova A, Howell BW. Interaction between Dab1 and CrkII is promoted by Reelin signaling. J Cell Sci. 2004;117:4527–4536. doi: 10.1242/jcs.01320. [DOI] [PubMed] [Google Scholar]
- Chen L, Liao G, Waclaw RR, Burns KA, Linquist D, Campbell K, Zheng Y, Kuan CY. Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons. J Neurosci. 2007;27:3884–3893. doi: 10.1523/JNEUROSCI.3509-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liao G, Yang L, Campbell K, Nakafuku M, Kuan CY, Zheng Y. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci U S A. 2006;103:16520–16525. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Melendez J, Campbell K, Kuan CY, Zheng Y. Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly. Dev Biol. 2009;325:162–170. doi: 10.1016/j.ydbio.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Krueger BK, Bambrick LL. MAP5 expression in proliferating neuroblasts. Brain Res Dev Brain Res. 1999;113:107–113. doi: 10.1016/s0165-3806(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle FM, Caspi M, Cordelieres FP, Dompierre JP, Dujardin DL, Koifman C, Martin P, Hoogenraad CC, Akhmanova A, Galjart N, De Mey JR, Reiner O. LIS1, CLIP-170’s key to the dynein/dynactin pathway. Mol Cell Biol. 2002;22:3089–3102. doi: 10.1128/MCB.22.9.3089-3102.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MR, Wen G, Lepier A, Schroeder T, Gotz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Dai Z, Pendergast AM. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995;9:2569–2582. doi: 10.1101/gad.9.21.2569. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–1065. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio JA, Martinez A, Auladell C, Soriano E. Developmental history of the subplate and developing white matter in the murine neocortex. Neuronal organization and relationship with the main afferent systems at embryonic and perinatal stages. Cereb Cortex. 2000;10:784–801. doi: 10.1093/cercor/10.8.784. [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Halberstadt AI, Rao RS, Maness PF. CHL1 cooperates with PAK1–3 to regulate morphological differentiation of embryonic cortical neurons. Neuroscience. 2010;165:107–115. doi: 10.1016/j.neuroscience.2009.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Hirschberg A, Worzfeld T, Penachioni JY, Korostylev A, Swiercz JM, Vodrazka P, Mauti O, Stoeckli ET, Tamagnone L, Offermanns S, Kuner R. Plexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo. J Neurosci. 2007;27:6333–6347. doi: 10.1523/JNEUROSCI.5381-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doye A, Mettouchi A, Bossis G, Clement R, Buisson-Touati C, Flatau G, Gagnoux L, Piechaczyk M, Boquet P, Lemichez E. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell. 2002;111:553–564. doi: 10.1016/s0092-8674(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Dymecki SM, Tomasiewicz H. Using Flp-recombinase to characterize expansion of Wnt1-expressing neural progenitors in the mouse. Dev Biol. 1998;201:57–65. doi: 10.1006/dbio.1998.8971. [DOI] [PubMed] [Google Scholar]
- Echarri A, Lai MJ, Robinson MR, Pendergast AM. Abl interactor 1 (Abi-1) wave-binding and SNARE domains regulate its nucleocytoplasmic shuttling, lamellipodium localization, and wave-1 levels. Mol Cell Biol. 2004;24:4979–4993. doi: 10.1128/MCB.24.11.4979-4993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- Edmondson JC, Hatten ME. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J Neurosci. 1987;7:1928–1934. doi: 10.1523/JNEUROSCI.07-06-01928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson JC, Liem RK, Kuster JE, Hatten ME. Astrotactin: a novel neuronal cell surface antigen that mediates neuron-astroglial interactions in cerebellar microcultures. J Cell Biol. 1988;106:505–517. doi: 10.1083/jcb.106.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler E, van Leeuwen F, Collard JG, Salinas PC. Expression of Tiam-1 in the developing brain suggests a role for the Tiam-1-Rac signaling pathway in cell migration and neurite outgrowth. Mol Cell Neurosci. 1997;9:1–12. doi: 10.1006/mcne.1997.0602. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Faulkner NE, Dujardin DL, Tai CY, Vaughan KT, O’Connell CB, Wang Y, Vallee RB. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Feng L, Cooper JA. Dual functions of Dab1 during brain development. Mol Cell Biol. 2009;29:324–332. doi: 10.1128/MCB.00663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- Fink AJ, Englund C, Daza RA, Pham D, Lau C, Nivison M, Kowalczyk T, Hevner RF. Development of the deep cerebellar nuclei: transcription factors and cell migration from the rhombic lip. J Neurosci. 2006;26:3066–3076. doi: 10.1523/JNEUROSCI.5203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Hatten ME. Astrotactin provides a receptor system for CNS neuronal migration. Development. 1991;113:755–765. doi: 10.1242/dev.113.3.755. [DOI] [PubMed] [Google Scholar]
- Fishman RB, Hatten ME. Multiple receptor systems promote CNS neural migration. J Neurosci. 1993;13:3485–3495. doi: 10.1523/JNEUROSCI.13-08-03485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng Y, Graham DA, Scheffer IE, Dobyns WB, Hirsch BA, Radtke RA, Berkovic SF, Huttenlocher PR, Walsh CA. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21:1315–1325. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- Fujita H, Katoh H, Ishikawa Y, Mori K, Negishi M. Rapostlin is a novel effector of Rnd2 GTPase inducing neurite branching. J Biol Chem. 2002;277:45428–45434. doi: 10.1074/jbc.M208090200. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58:92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- Garrard SM, Capaldo CT, Gao L, Rosen MK, Macara IG, Tomchick DR. Structure of Cdc42 in a complex with the GTPase-binding domain of the cell polarity protein, Par6. Embo J. 2003;22:1125–1133. doi: 10.1093/emboj/cdg110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser UE, Hatten ME. Central nervous system neurons migrate on astroglial fibers from heterotypic brain regions in vitro. Proc Natl Acad Sci U S A. 1990;87:4543–4547. doi: 10.1073/pnas.87.12.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser UE, Hatten ME. Neuron-glia interactions of rat hippocampal cells in vitro: glial-guided neuronal migration and neuronal regulation of glial differentiation. J Neurosci. 1990;10:1276–1285. doi: 10.1523/JNEUROSCI.10-04-01276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdalyahu A, Ghosh I, Levy T, Sapir T, Sapoznik S, Fishler Y, Azoulai D, Reiner O. DCX, a new mediator of the JNK pathway. Embo J. 2004;23:823–832. doi: 10.1038/sj.emboj.7600079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, He F, Kim KJ, Blanchi B, Coskun V, Nguyen L, Wu X, Zhao J, Heng JI, Martinowich K, Tao J, Wu H, Castro D, Sobeih MM, Corfas G, Gleeson JG, Greenberg ME, Guillemot F, Sun YE. Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc Natl Acad Sci U S A. 2006;103:1319–1324. doi: 10.1073/pnas.0510419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Frank CL, Calderon de Anda F, Tsai LH. Hook3 interacts with PCM1 to regulate pericentriolar material assembly and the timing of neurogenesis. Neuron. 2010;65:191–203. doi: 10.1016/j.neuron.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffinet AM. Events governing organization of postmigratory neurons: studies on brain development in normal and reeler mice. Brain Res. 1984;319:261–296. doi: 10.1016/0165-0173(84)90013-4. [DOI] [PubMed] [Google Scholar]
- Goh KL, Cai L, Cepko CL, Gertler FB. Ena/VASP proteins regulate cortical neuronal positioning. Curr Biol. 2002;12:565–569. doi: 10.1016/s0960-9822(02)00725-x. [DOI] [PubMed] [Google Scholar]
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Billault C, Del Rio JA, Urena JM, Jimenez-Mateos EM, Barallobre MJ, Pascual M, Pujadas L, Simo S, Torre AL, Gavin R, Wandosell F, Soriano E, Avila J. A role of MAP1B in Reelin-dependent neuronal migration. Cereb Cortex. 2005;15:1134–1145. doi: 10.1093/cercor/bhh213. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Gregory WA, Edmondson JC, Hatten ME, Mason CA. Cytology and neuron-glial apposition of migrating cerebellar granule cells in vitro. J Neurosci. 1988;8:1728–1738. doi: 10.1523/JNEUROSCI.08-05-01728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan CB, Xu HT, Jin M, Yuan XB, Poo MM. Long-range Ca2+ signaling from growth cone to soma mediates reversal of neuronal migration induced by slit-2. Cell. 2007;129:385–395. doi: 10.1016/j.cell.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Gundersen GG. Evolutionary conservation of microtubule-capture mechanisms. Nat Rev Mol Cell Biol. 2002;3:296–304. doi: 10.1038/nrm777. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Gomes ER. Regulation of microtubules by Rho GTPases in migrating cells. Novartis Found Symp. 2005;269:106–116. discussion 116–126, 223–130. [PubMed] [Google Scholar]
- Gupta A, Sanada K, Miyamoto DT, Rovelstad S, Nadarajah B, Pearlman AL, Brunstrom J, Tsai LH. Layering defect in p35 deficiency is linked to improper neuronal-glial interaction in radial migration. Nat Neurosci. 2003;6:1284–1291. doi: 10.1038/nn1151. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Salmon WC, Waterman-Storer CM. Converging populations of f-actin promote breakage of associated microtubules to spatially regulate microtubule turnover in migrating cells. Curr Biol. 2002;12:1891–1899. doi: 10.1016/s0960-9822(02)01276-9. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, Frotscher M. Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development. 2007;134:3883–3891. doi: 10.1242/dev.005447. [DOI] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, Schuurmans C, Guillemot F, Polleux F. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase [corrected] Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Heng JI, Nguyen L, Castro DS, Zimmer C, Wildner H, Armant O, Skowronska-Krawczyk D, Bedogni F, Matter JM, Hevner R, Guillemot F. Neurogenin 2 controls cortical neuron migration through regulation of Rnd2. Nature. 2008;455:114–118. doi: 10.1038/nature07198. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Hirai S, Cui de F, Miyata T, Ogawa M, Kiyonari H, Suda Y, Aizawa S, Banba Y, Ohno S. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci. 2006;26:11992–12002. doi: 10.1523/JNEUROSCI.2272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S, Kawaguchi A, Hirasawa R, Baba M, Ohnishi T, Ohno S. MAPK-upstream protein kinase (MUK) regulates the radial migration of immature neurons in telencephalon of mouse embryo. Development. 2002;129:4483–4495. doi: 10.1242/dev.129.19.4483. [DOI] [PubMed] [Google Scholar]
- Ho YD, Joyal JL, Li Z, Sacks DB. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J Biol Chem. 1999;274:464–470. doi: 10.1074/jbc.274.1.464. [DOI] [PubMed] [Google Scholar]
- Hoffman GR, Nassar N, Cerione RA. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000;100:345–356. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine [corrected] phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- Huang Y, Magdaleno S, Hopkins R, Slaughter C, Curran T, Keshvara L. Tyrosine phosphorylated Disabled 1 recruits Crk family adapter proteins. Biochem Biophys Res Commun. 2004;318:204–212. doi: 10.1016/j.bbrc.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem. 2008;283:14469–14478. doi: 10.1074/jbc.M708919200. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Tenca P, Frittoli E, Faretta M, Tocchetti A, Di Fiore PP, Scita G. Mechanisms through which Sos-1 coordinates the activation of Ras and Rac. J Cell Biol. 2002;156:125–136. doi: 10.1083/jcb.200108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti M, Zucconi A, Disanza A, Frittoli E, Areces LB, Steffen A, Stradal TE, Di Fiore PP, Carlier MF, Scita G. Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat Cell Biol. 2004;6:319–327. doi: 10.1038/ncb1105. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Aspenstrom P, Hall A. Human CNK1 acts as a scaffold protein, linking Rho and Ras signal transduction pathways. Mol Cell Biol. 2004;24:1736–1746. doi: 10.1128/MCB.24.4.1736-1746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol. 2002;34:713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Ogawa M, Metin C, Tissir F, Goffinet AM. Inhibition of SRC family kinases and non-classical protein kinases C induce a reeler-like malformation of cortical plate development. J Neurosci. 2003;23:9953–9959. doi: 10.1523/JNEUROSCI.23-30-09953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Marin O, Koike M, Hirota Y, Uchiyama Y, Wu JY, Lu Q, Tessier-Lavigne M, Alvarez-Buylla A, Okano H, Rubenstein JL, Sawamoto K. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron. 2010;67:213–223. doi: 10.1016/j.neuron.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Adelstein RS. Chicken nonmuscle myosin heavy chains: differential expression of two mRNAs and evidence for two different polypeptides. J Cell Biol. 1991;112:915–924. doi: 10.1083/jcb.112.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. Embo J. 2003;22:4190–4201. doi: 10.1093/emboj/cdg413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nishimura YV, Nabeshima Y, Hoshino M. MAP1B phosphorylation is differentially regulated by Cdk5/p35, Cdk5/p25, and JNK. Biochem Biophys Res Commun. 2005;331:50–55. doi: 10.1016/j.bbrc.2005.03.132. [DOI] [PubMed] [Google Scholar]
- Keshvara L, Magdaleno S, Benhayon D, Curran T. Cyclin-dependent kinase 5 phosphorylates disabled 1 independently of Reelin signaling. J Neurosci. 2002;22:4869–4877. doi: 10.1523/JNEUROSCI.22-12-04869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholmanskikh SS, Koeller HB, Wynshaw-Boris A, Gomez T, Letourneau PC, Ross ME. Calcium-dependent interaction of Lis1 with IQGAP1 and Cdc42 promotes neuronal motility. Nat Neurosci. 2006;9:50–57. doi: 10.1038/nn1619. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh CG. Rho GTPases and their regulators in neuronal functions and development. Neurosignals. 2006;15:228–237. doi: 10.1159/000101527. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Dynamics of granule cell migration: a confocal microscopic study in acute cerebellar slice preparations. J Neurosci. 1995;15:1110–1120. doi: 10.1523/JNEUROSCI.15-02-01110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci. 1998;18:1478–1490. doi: 10.1523/JNEUROSCI.18-04-01478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno D, Yoshimura S, Hori K, Maruoka H, Sobue K. Involvement of the phosphatidylinositol 3-kinase/rac1 and cdc42 pathways in radial migration of cortical neurons. J Biol Chem. 2005;280:5082–5088. doi: 10.1074/jbc.M408251200. [DOI] [PubMed] [Google Scholar]
- Kostovic IMM. Anat Rec. Vol. 178. 1974. A new interpretation of the laminar development of the cerebral cortex: synaptogenesis in different layers of neopallium in the human fetus. American association of anatomists. Eighty seventh annual session; p. 395. [Google Scholar]
- Kunda P, Craig G, Dominguez V, Baum B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol. 2003;13:1867–1875. doi: 10.1016/j.cub.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisu S, Takenawa T. The WASP and WAVE family proteins. Genome Biol. 2009;10:226. doi: 10.1186/gb-2009-10-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, Bronson RT, Mori S, Fassler R, Gertler FB. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron. 2007;56:441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Lanier LM, Gates MA, Witke W, Menzies AS, Wehman AM, Macklis JD, Kwiatkowski D, Soriano P, Gertler FB. Mena is required for neurulation and commissure formation. Neuron. 1999;22:313–325. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Latasa MJ, Cisneros E, Frade JM. Cell cycle control of Notch signaling and the functional regionalization of the neuroepithelium during vertebrate neurogenesis. Int J Dev Biol. 2009;53:895–908. doi: 10.1387/ijdb.082721ml. [DOI] [PubMed] [Google Scholar]
- Lee JS, Chang MI, Tseng Y, Wirtz D. Cdc42 mediates nucleus movement and MTOC polarization in Swiss 3T3 fibroblasts under mechanical shear stress. Mol Biol Cell. 2005;16:871–880. doi: 10.1091/mbc.E03-12-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwen FN, Kain HE, Kammen RA, Michiels F, Kranenburg OW, Collard JG. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Srinivasan K, Brakebusch C, McConnell SK. The rho GTPase Rac1 is required for proliferation and survival of progenitors in the developing forebrain. Dev Neurobiol. 2010;70:659–678. doi: 10.1002/dneu.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- Liu X, Hashimoto-Torii K, Torii M, Ding C, Rakic P. Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J Neurosci. 2010;30:4197–4209. doi: 10.1523/JNEUROSCI.4187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Neurogenesis of the cat’s primary visual cortex. J Comp Neurol. 1985;242:611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Ma X, Kawamoto S, Hara Y, Adelstein RS. A point mutation in the motor domain of nonmuscle myosin II-B impairs migration of distinct groups of neurons. Mol Biol Cell. 2004;15:2568–2579. doi: 10.1091/mbc.E03-11-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol. 2008;28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J Cell Sci. 2007;120:456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- Marti A, Luo Z, Cunningham C, Ohta Y, Hartwig J, Stossel TP, Kyriakis JM, Avruch J. Actin-binding protein-280 binds the stress-activated protein kinase (SAPK) activator SEK-1 and is required for tumor necrosis factor-alpha activation of SAPK in melanoma cells. J Biol Chem. 1997;272:2620–2628. doi: 10.1074/jbc.272.5.2620. [DOI] [PubMed] [Google Scholar]
- Martini FJ, Valiente M, Lopez Bendito G, Szabo G, Moya F, Valdeolmillos M, Marin O. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development. 2009;136:41–50. doi: 10.1242/dev.025502. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Hoshino M, Yoshizawa M, Nabeshima Y. Characterization of STEF, a guanine nucleotide exchange factor for Rac1, required for neurite growth. J Biol Chem. 2002;277:2860–2868. doi: 10.1074/jbc.M106186200. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Terao M, Nabeshima Y, Hoshino M. Roles of STEF/Tiam1, guanine nucleotide exchange factors for Rac1, in regulation of growth cone morphology. Mol Cell Neurosci. 2003;24:69–81. doi: 10.1016/s1044-7431(03)00122-2. [DOI] [PubMed] [Google Scholar]
- Mattar P, Britz O, Johannes C, Nieto M, Ma L, Rebeyka A, Klenin N, Polleux F, Guillemot F, Schuurmans C. A screen for downstream effectors of Neurogenin2 in the embryonic neocortex. Dev Biol. 2004;273:373–389. doi: 10.1016/j.ydbio.2004.06.013. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate pioneers and the formation of descending connections from cerebral cortex. J Neurosci. 1994;14:1892–1907. doi: 10.1523/JNEUROSCI.14-04-01892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesngon MT, Tarricone C, Hebbar S, Guillotte AM, Schmitt EW, Lanier L, Musacchio A, King SJ, Smith DS. Regulation of cytoplasmic dynein ATPase by Lis1. J Neurosci. 2006;26:2132–2139. doi: 10.1523/JNEUROSCI.5095-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minobe S, Sakakibara A, Ohdachi T, Kanda R, Kimura M, Nakatani S, Tadokoro R, Ochiai W, Nishizawa Y, Mizoguchi A, Kawauchi T, Miyata T. Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci Res. 2009;63:294–301. doi: 10.1016/j.neures.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moers A, Nurnberg A, Goebbels S, Wettschureck N, Offermanns S. Galpha12/Galpha13 deficiency causes localized overmigration of neurons in the developing cerebral and cerebellar cortices. Mol Cell Biol. 2008;28:1480–1488. doi: 10.1128/MCB.00651-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Metin C, Stoykova A, Tarabykin V, Price DJ, Francis F, Meyer G, Dehay C, Kennedy H. Comparative aspects of cerebral cortical development. The European journal of neuroscience. 2006;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Morales D, Hatten ME. Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J Neurosci. 2006;26:12226–12236. doi: 10.1523/JNEUROSCI.3493-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura T, Hattori M, Ogawa M, Mikoshiba K. Disabled1 regulates the intracellular trafficking of reelin receptors. J Biol Chem. 2005;280:16901–16908. doi: 10.1074/jbc.M409048200. [DOI] [PubMed] [Google Scholar]
- Murase S, Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci. 2002;22:3568–3579. doi: 10.1523/JNEUROSCI.22-09-03568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Morikubo S, Sato M. Filamin A and FILIP (Filamin A-Interacting Protein) regulate cell polarity and motility in neocortical subventricular and intermediate zones during radial migration. J Neurosci. 2004;24:9648–9657. doi: 10.1523/JNEUROSCI.2363-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Yoneda T, Hatanaka Y, Kubota C, Murakami F, Sato M. Filamin A-interacting protein (FILIP) regulates cortical cell migration out of the ventricular zone. Nat Cell Biol. 2002;4:495–501. doi: 10.1038/ncb808. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yamashita Y, Tamamaki N, Katoh H, Kaneko T, Negishi M. In vivo function of Rnd2 in the development of neocortical pyramidal neurons. Neurosci Res. 2006;54:149–153. doi: 10.1016/j.neures.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee MS, Morabito M, Tsai LH. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Noctor S, Flint A, Weissman T, Dammerman R, Kriegstein A. Neurons derived from radial glial cells establish radial units in neocortexre. Nature. 2001;8:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Takeya R, Ohno S, Naito S, Ito T, Sumimoto H. Human homologues of the Caenorhabditis elegans cell polarity protein PAR6 as an adaptor that links the small GTPases Rac and Cdc42 to atypical protein kinase C. Genes Cells. 2001;6:107–119. doi: 10.1046/j.1365-2443.2001.00404.x. [DOI] [PubMed] [Google Scholar]
- Norden C, Young S, Link BA, Harris WA. Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell. 2009;138:1195–1208. doi: 10.1016/j.cell.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke NA, Dailey ME, Smith SJ, McConnell SK. Diverse migratory pathways in the developing cerebral cortex. Science. 1992;258:299–302. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Hosoya T, Takahashi K, Hing H, Mizuno K. A Drosophila homolog of LIM-kinase phosphorylates cofilin and induces actin cytoskeletal reorganization. Biochem Biophys Res Commun. 2000;276:1178–1185. doi: 10.1006/bbrc.2000.3599. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Hirasawa M, Tabata H, Mutoh T, Adachi T, Suzuki H, Saruta K, Iwasato T, Itohara S, Hashimoto M, Nakajima K, Ogawa M, Kulkarni AB, Mikoshiba K. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development. 2007;134:2273–2282. doi: 10.1242/dev.02854. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci U S A. 1999;96:2122–2128. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenik C, Aktories K, Meyer DK. Differential expression of the small GTP-binding proteins RhoA, RhoB, Cdc42u and Cdc42b in developing rat neocortex. Brain Res Mol Brain Res. 1999;70:9–17. doi: 10.1016/s0169-328x(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol. 2001;11:1536–1541. doi: 10.1016/s0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Pang T, Atefy R, Sheen V. Malformations of cortical development. Neurologist. 2008;14:181–191. doi: 10.1097/NRL.0b013e31816606b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Curran T. Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway. J Neurosci. 2008;28:13551–13562. doi: 10.1523/JNEUROSCI.4323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Spurney RF, Tu Q, Hinson T, Quarles LD. Calcium-sensing receptor activation of rho involves filamin and rho-guanine nucleotide exchange factor. Endocrinology. 2002;143:3830–3838. doi: 10.1210/en.2002-220240. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Abo A, Steven Martin G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr Biol. 2000;10:697–707. doi: 10.1016/s0960-9822(00)00535-2. [DOI] [PubMed] [Google Scholar]
- Quevedo C, Sauzeau V, Menacho-Marquez M, Castro-Castro A, Bustelo XR. Vav3-deficient mice exhibit a transient delay in cerebellar development. Mol Biol Cell. 2010;21:1125–1139. doi: 10.1091/mbc.E09-04-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Halbrugge M, Scheer U, Wiegand C, Jockusch BM, Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. Embo J. 1992;11:2063–2070. doi: 10.1002/j.1460-2075.1992.tb05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard V, Dulon D, Hafidi A. Expression of Rho GTPases Rho-A and Rac1 in the adult and developing gerbil cerebellum. Int J Dev Neurosci. 2008;26:723–732. doi: 10.1016/j.ijdevneu.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Riento K, Totty N, Villalonga P, Garg R, Guasch R, Ridley AJ. RhoE function is regulated by ROCK I-mediated phosphorylation. Embo J. 2005;24:1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K, Villalonga P, Garg R, Ridley A. Function and regulation of RhoE. Biochem Soc Trans. 2005;33:649–651. doi: 10.1042/BST0330649. [DOI] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, Cox AD, Wilson O, Kirschmeier P, Der CJ. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J Biol Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108 ( Pt 12):3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Wiedemann U, Stuurman N, Vale RD. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol. 2003;162:1079–1088. doi: 10.1083/jcb.200303023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Sakakibara A, Horwitz AF. Mechanism of polarized protrusion formation on neuronal precursors migrating in the developing chicken cerebellum. J Cell Sci. 2006;119:3583–3592. doi: 10.1242/jcs.03080. [DOI] [PubMed] [Google Scholar]
- Sanada K, Gupta A, Tsai LH. Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron. 2004;42:197–211. doi: 10.1016/s0896-6273(04)00222-3. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, Bartley CM, Chi H, Nakamura F, Hashimoto-Torii K, Torii M, Flavell RA, Rakic P. MEKK4 signaling regulates filamin expression and neuronal migration. Neuron. 2006;52:789–801. doi: 10.1016/j.neuron.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisian MR, Bartley CM, Rakic P. Trouble making the first move: interpreting arrested neuronal migration in the cerebral cortex. Trends Neurosci. 2008;31:54–61. doi: 10.1016/j.tins.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, Hirotsune S. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Sato M, Nagano T. Involvement of filamin A and filamin A-interacting protein (FILIP) in controlling the start and cell shape of radially migrating cortical neurons. Anat Sci Int. 2005;80:19–29. doi: 10.1111/j.1447-073x.2005.00101.x. [DOI] [PubMed] [Google Scholar]
- Sauer ME, Walker BE. Radioautographic study of interkinetic nuclear migration in the neural tube. Proc Soc Exp Biol Med. 1959;101:557–560. doi: 10.3181/00379727-101-25014. [DOI] [PubMed] [Google Scholar]
- Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci U S A. 2005;102:13652–13657. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk J, Wilsch-Brauninger M, Calegari F, Huttner WB. Myosin II is required for interkinetic nuclear migration of neural progenitors. Proc Natl Acad Sci U S A. 2009;106:16487–16492. doi: 10.1073/pnas.0908928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Schneider S, Gulacsi A, Hatten ME. Lrp12/Mig13a Reveals Changing Patterns of Preplate Neuronal Polarity during Corticogenesis that Are Absent in Reeler Mutant Mice. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans C, Armant O, Nieto M, Stenman JM, Britz O, Klenin N, Brown C, Langevin LM, Seibt J, Tang H, Cunningham JM, Dyck R, Walsh C, Campbell K, Polleux F, Guillemot F. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. Embo J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore PP. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- Scita G, Tenca P, Areces LB, Tocchetti A, Frittoli E, Giardina G, Ponzanelli I, Sini P, Innocenti M, Di Fiore PP. An effector region in Eps8 is responsible for the activation of the Rac-specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J Cell Biol. 2001;154:1031–1044. doi: 10.1083/jcb.200103146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senadheera D, Haataja L, Groffen J, Heisterkamp N. The small GTPase Rac interacts with ubiquitination complex proteins Cullin-1 and CDC23. Int J Mol Med. 2001;8:127–133. doi: 10.3892/ijmm.8.2.127. [DOI] [PubMed] [Google Scholar]
- Shi Y, Alin K, Goff SP. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 1995;9:2583–2597. doi: 10.1101/gad.9.21.2583. [DOI] [PubMed] [Google Scholar]
- Shmueli A, Gdalyahu A, Sapoznik S, Sapir T, Tsukada M, Reiner O. Site-specific dephosphorylation of doublecortin (DCX) by protein phosphatase 1 (PP1) Mol Cell Neurosci. 2006;32:15–26. doi: 10.1016/j.mcn.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000;2:767–775. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Binns KL, Wayman GA, Davee SM, Ong SH, Pawson T, Scott JD. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nat Cell Biol. 2002;4:970–975. doi: 10.1038/ncb886. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Govek EE, Tomoda T, Hatten ME. Neuronal polarity in CNS development. Genes Dev. 2006;20:2639–2647. doi: 10.1101/gad.1462506. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron. 2009;63:63–80. doi: 10.1016/j.neuron.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocornola R, Royer C, Vives V, Tordella L, Zhong S, Wang Y, Ratnayaka I, Shipman M, Cheung A, Gaston-Massuet C, Ferretti P, Molnar Z, Lu X. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell. 2010;19:126–137. doi: 10.1016/j.devcel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Spalice A, Parisi P, Nicita F, Pizzardi G, Del Balzo F, Iannetti P. Neuronal migration disorders: clinical, neuroradiologic and genetics aspects. Acta Paediatr. 2009;98:421–433. doi: 10.1111/j.1651-2227.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- Steffen A, Rottner K, Ehinger J, Innocenti M, Scita G, Wehland J, Stradal TE. Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. Embo J. 2004;23:749–759. doi: 10.1038/sj.emboj.7600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem. 2001;276:670–676. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- Sumi T, Matsumoto K, Takai Y, Nakamura T. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J Cell Biol. 1999;147:1519–1532. doi: 10.1083/jcb.147.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M, Rusk N. Control of vesicular trafficking by Rho GTPases. Curr Biol. 2003;13:R409–418. doi: 10.1016/s0960-9822(03)00324-5. [DOI] [PubMed] [Google Scholar]
- Tahirovic S, Hellal F, Neukirchen D, Hindges R, Garvalov BK, Flynn KC, Stradal TE, Chrostek-Grashoff A, Brakebusch C, Bradke F. Rac1 regulates neuronal polarization through the WAVE complex. J Neurosci. 2010;30:6930–6943. doi: 10.1523/JNEUROSCI.5395-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CY, Dujardin DL, Faulkner NE, Vallee RB. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol. 2002;156:959–968. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Katoh H, Negishi M. Pragmin, a novel effector of Rnd2 GTPase, stimulates RhoA activity. J Biol Chem. 2006;281:10355–10364. doi: 10.1074/jbc.M511314200. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004;165:709–721. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, Tsai LH, Gleeson JG. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron. 2004;41:215–227. doi: 10.1016/s0896-6273(03)00852-3. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y, An KM, Esue O, Wirtz D. The bimodal role of filamin in controlling the architecture and mechanics of F-actin networks. J Biol Chem. 2004;279:1819–1826. doi: 10.1074/jbc.M306090200. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Prokscha A, Eichele G. Neurabin II mediates doublecortin-dephosphorylation on actin filaments. Biochem Biophys Res Commun. 2006;343:839–847. doi: 10.1016/j.bbrc.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem. 2003;278:31020–31023. doi: 10.1074/jbc.M301179200. [DOI] [PubMed] [Google Scholar]
- Ueda K, Ohta Y, Hosoya H. The carboxy-terminal pleckstrin homology domain of ROCK interacts with filamin-A. Biochem Biophys Res Commun. 2003;301:886–890. doi: 10.1016/s0006-291x(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Umeshima H, Hirano T, Kengaku M. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc Natl Acad Sci U S A. 2007;104:16182–16187. doi: 10.1073/pnas.0708047104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvikis O, Lores P, Boyer L, Chardin P, Lemichez E, Gacon G. Activated Rac1, but not the tumorigenic variant Rac1b, is ubiquitinated on Lys 147 through a JNK-regulated process. Febs J. 2008;275:386–396. doi: 10.1111/j.1742-4658.2007.06209.x. [DOI] [PubMed] [Google Scholar]
- Voss AK, Britto JM, Dixon MP, Sheikh BN, Collin C, Tan SS, Thomas T. C3G regulates cortical neuron migration, preplate splitting and radial glial cell attachment. Development. 2008;135:2139–2149. doi: 10.1242/dev.016725. [DOI] [PubMed] [Google Scholar]
- Wang G, Krishnamurthy K, Chiang YW, Dasgupta S, Bieberich E. Regulation of neural progenitor cell motility by ceramide and potential implications for mouse brain development. J Neurochem. 2008;106:718–733. doi: 10.1111/j.1471-4159.2008.05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- Wang S, Watanabe T, Noritake J, Fukata M, Yoshimura T, Itoh N, Harada T, Nakagawa M, Matsuura Y, Arimura N, Kaibuchi K. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 2007;120:567–577. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Ward Y, Spinelli B, Quon MJ, Chen H, Ikeda SR, Kelly K. Phosphorylation of critical serine residues in Gem separates cytoskeletal reorganization from down-regulation of calcium channel activity. Mol Cell Biol. 2004;24:651–661. doi: 10.1128/MCB.24.2.651-661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Weimer JM, Yokota Y, Stanco A, Stumpo DJ, Blackshear PJ, Anton ES. MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development. 2009;136:2965–2975. doi: 10.1242/dev.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg K, Forget MA, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, Der CJ, Hansen SH. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr Biol. 2003;13:1106–1115. doi: 10.1016/s0960-9822(03)00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997;18:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- Wilson PM, Fryer RH, Fang Y, Hatten ME. Astn2, a novel member of the astrotactin gene family, regulates the trafficking of ASTN1 during glial-guided neuronal migration. J Neurosci. 2010;30:8529–8540. doi: 10.1523/JNEUROSCI.0032-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate RJ, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol. 2008;18:479–487. doi: 10.1016/j.conb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, Wu JY, Xiong WC, Rao Y. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- Wood J, Martin S, Price DJ. Evidence that the earliest generated cells of the murine cerebral cortex form a transient population in the subplate and marginal zone. Developmental Brain Research. 1992;66:137–140. doi: 10.1016/0165-3806(92)90150-u. [DOI] [PubMed] [Google Scholar]
- Xie Z, Moy LY, Sanada K, Zhou Y, Buchman JJ, Tsai LH. Cep120 and TACCs control interkinetic nuclear migration and the neural progenitor pool. Neuron. 2007;56:79–93. doi: 10.1016/j.neuron.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD. TGF-beta signaling specifies axons during brain development. Cell. 2010;142:144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling J, Youn YH, Darling D, Toyo-Oka K, Pramparo T, Hirotsune S, Wynshaw-Boris A. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–486. doi: 10.1016/j.cell.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Arimura N, Kaibuchi K. Signaling networks in neuronal polarization. J Neurosci. 2006;26:10626–10630. doi: 10.1523/JNEUROSCI.3824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Hoshino M, Sone M, Nabeshima Y. Expression of stef, an activator of Rac1, correlates with the stages of neuronal morphological development in the mouse brain. Mech Dev. 2002;113:65–68. doi: 10.1016/s0925-4773(01)00650-5. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Kawauchi T, Sone M, Nishimura YV, Terao M, Chihama K, Nabeshima Y, Hoshino M. Involvement of a Rac activator, P-Rex1, in neurotrophin-derived signaling and neuronal migration. J Neurosci. 2005;25:4406–4419. doi: 10.1523/JNEUROSCI.4955-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalcman G, Dorseuil O, Garcia-Ranea JA, Gacon G, Camonis J. RhoGAPs and RhoGDIs, (His)stories of two families. Prog Mol Subcell Biol. 1999;22:85–113. doi: 10.1007/978-3-642-58591-3_5. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Development of layer I neurons in the primate cerebral cortex. J Neurosci. 2001;21:5607–5619. doi: 10.1523/JNEUROSCI.21-15-05607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Heintz N, Hatten ME. CNS gene encoding astrotactin, which supports neuronal migration along glial fibers. Science. 1996;272:417–419. doi: 10.1126/science.272.5260.417. [DOI] [PubMed] [Google Scholar]
- Zhou AX, Hartwig JH, Akyurek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20:113–123. doi: 10.1016/j.tcb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal RA. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007;55:53–68. doi: 10.1016/j.neuron.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda JF, Rivas RJ. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskeleton. 1998;41:18–38. doi: 10.1002/(SICI)1097-0169(1998)41:1<18::AID-CM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]