Abstract
We show that arylpalladium(II) reagents linked to biotin and indocyanine dye residues can be prepared by decarboxylative palladation of appropriately substituted electron-rich benzoic acid derivatives. When prepared under the conditions described, these organometallic intermediates are tolerant of air and water, can be stored for several months in solution in dimethylsulfoxide, and permit biotin- and indocyanine dye-labeling of functionally complex olefinic substrates in water by Heck-type coupling reactions.
The value of selective bond-forming reactions between reciprocally reactive functional groups in water at ambient temperature has been noted by Sharpless and coworkers and was strikingly illustrated by them with disclosure of the copper-catalyzed [3+2]-dipolar cycloaddition reaction of azides and alkynes.1 Bertozzi and coworkers as well as others have developed strain-promoted cycloaddition reactions that proceed efficiently in water without the use of catalysts.2 In this work we report preliminary steps towards the development of a different type of non-catalyzed (carbon-carbon) bond-forming reaction in water: a stoichiometric Heck-type coupling of discrete, storable arylpalladium(II) reagents with olefinic reactants. We describe the synthesis of three specific arylpalladium(II) reagents bearing biotin and indocyanine dye labels and demonstrate that these can be coupled with functionally complex olefinic reactants in aqueous media at ambient temperature.
Previously we showed that 2,4,5-trimethoxybenzoic acid undergoes rapid decarboxylative palladation upon warming to 80 °C in the presence of palladium(II) trifluoroacetate (1.2 equiv) in dimethylsulfoxide (DMSO), forming a crystallographically characterizable arylpalladium(II) trifluoroacetate intermediate.3 Subsequent addition of an alkene led to an efficient Heck-type coupling reaction with the arylpalladium(II) intermediate (in DMSO).4 Here we show that benzoic acid derivatives bearing biotin and indocyanine dye residues also undergo decarboxylative palladation under somewhat modified conditions, providing spectroscopically characterizable arylpalladium(II) intermediates that display extraordinary stability to storage (in solution in DMSO) and function effectively as reagents for biotin- and indocyanine dye-labeling of olefinic reactants in water.
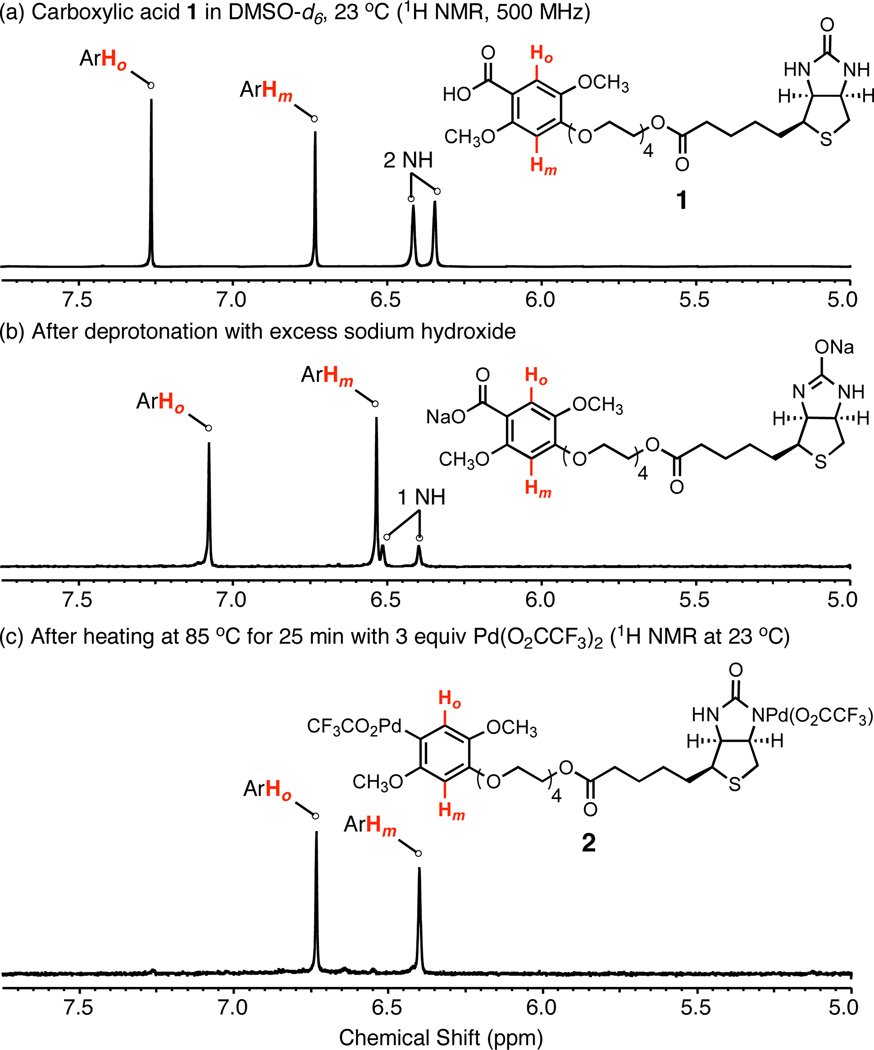
Decarboxylative palladation of the biotin-linked benzoic acid derivative 1 (Figure 1), synthesized in seven steps from 2,5-dimethoxybenzaldehyde and D-biotin (Supporting Information), was studied first. Substrate 1 was chosen because electron-rich benzoic acids (and their sodium salts) with the 2,4,5-substitution pattern had previously been shown to be particularly good substrates for decarboxylative palladation.4 When substrate 1 (monosodium salt) was heated with palladium(II) trifluoroacetate (1–3 equiv) in DMSO-d6 at 85 °C for 15 min we observed that the expected arylpalladium(II) intermediate had formed, but was contaminated with the product of protonolysis of the arylpalladium(II) intermediate. When instead substrate 1 was first deprotonated with an excess of sodium hydroxide in DMSO-d6 (1H NMR analysis suggests that a dianion is formed, see Figure 1b) decarboxylative palladation in the presence of 3.0 equiv of palladium(II) trifluoroacetate was remarkably clean and efficient, forming a stable arylpalladium(II) intermediate (2), as evident from 1H and 13C NMR analysis (Figure 1c and Supporting Information).5 A marked upfield shifting of the biotin carbonyl carbon resonance in the 13C NMR spectrum of the product suggests that the cyclic urea is complexed with palladium. While we depict the complex as N-bound in structure 2, we do not rule out η3- or O-bound complexes, nor the possibility of dinuclear complexes (see Supporting Information for examples of like-shifted amido and imido palladium complexes). Stock solutions of reagent 2 in DMSO-d6 are stable to handling in the air at ambient temperature and, when stored frozen at −20 °C with thawing just prior to use in coupling reactions (vide infra), can be used reliably for several months. While inspection of the 1H and 13C NMR spectra (Figure 1c and Supporting Information) suggests that the decarboxylative palladation reaction was quite efficient, a more quantitative assessment of the yield of the transformation was obtained by protiodepalladation with excess dithiothreitol (DTT) in DMSO at 23 °C (>90% yield, Supporting Information) and, later, by the efficiencies observed in subsequent Heck-type coupling reactions (up to 70%, vide infra).
Figure 1.
Decarboxylative palladation of biotin-linked benzoic acid derivative 1.
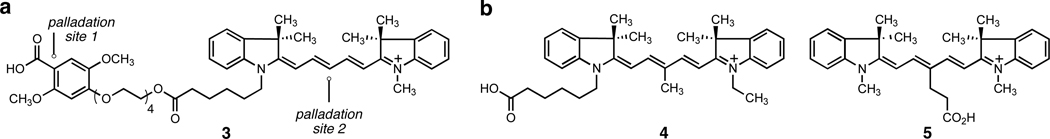
Decarboxylative palladation of the indocyanine dye-linked benzoic acid derivative 3 (Figure 2a), also synthesized in seven steps (Supporting Information), was studied next. The Cy5 indocyanine dye residue of substrate 3 proved not to be compatible with the conditions developed for decarboxylative palladation, for heating of substrate 3 with 1–3 equiv of palladium(II) trifluoroacetate at 80 °C for 25 min in DMSO-d6 led to byproducts arising from palladation of the central methine carbon of the dye (Figure 2), as well as the expected decarboxylative palladation product, the major component of the mixture.
Figure 2.
(a) A Cy5-linked benzoic acid derivative (3) is palladated at two sites. (b) Structures of indocyanine dyes 4 and 5.
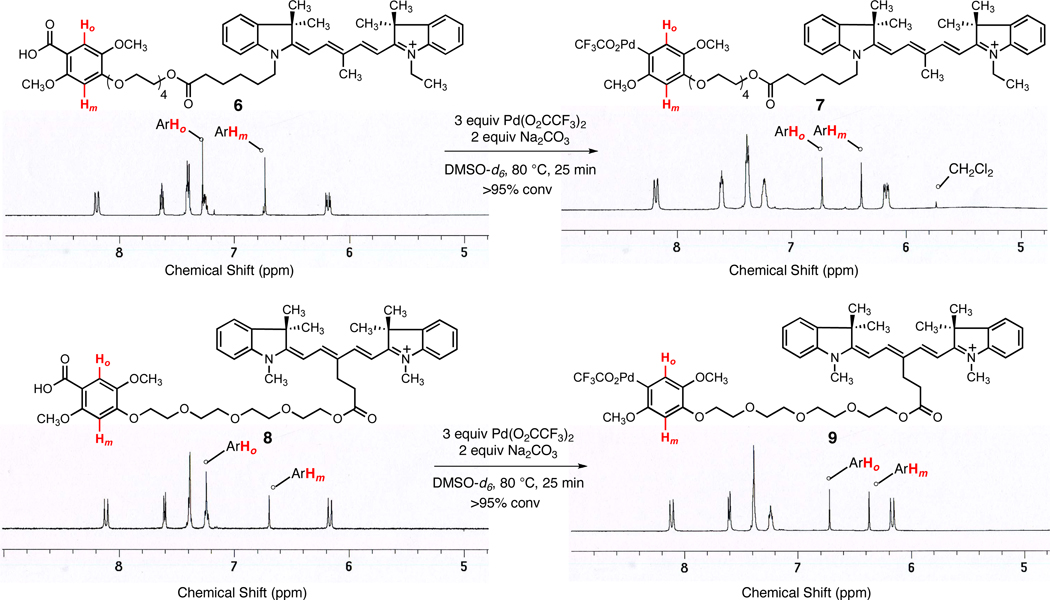
This complication was easily circumvented by substitution of the central carbon of the dye. The unsymmetrically and symmetrically substituted Cy5 dyes 4 and 5,6 respectively (Figure 2b), were synthesized in two to four steps from commercially available materials (see Supporting Information). These were linked to the 2,4,5-substituted benzoic acid derivative as before. When each of the Cy5 derivatives 6 and 8 was heated with 3.0 equiv of palladium(II) trifluoroacetate and 2.0 equiv of sodium carbonate in DMSO-d6 at 80 °C for 25 min, decarboxylative palladation to form a stable arylpalladium(II) intermediate (7 and 9, respectively) was both clean and efficient, as evident from 1H and 13C NMR analysis (Figure 3 and Supporting Information).7 Treatment of each arylpalladium(II) trifluoroacetate intermediate with 3.0 equiv of sodium picolinate at 23 °C led to a rapid ligand exchange reaction (1H NMR analysis) to afford an arylpalladium(II) picolinate intermediate that was characterizable by high-resolution mass spectrometric analysis (Supporting Information).8 As with reagent 2, stock solutions of reagents 7 and 9 in DMSO-d6 were stable to handling in the air at ambient temperature, but were stored frozen at −20 °C with thawing just prior to use in coupling reactions.
Figure 3.
Decarboxylative Palladation of Cy5-Linked Benzoic Acid Derivatives 6 and 8.
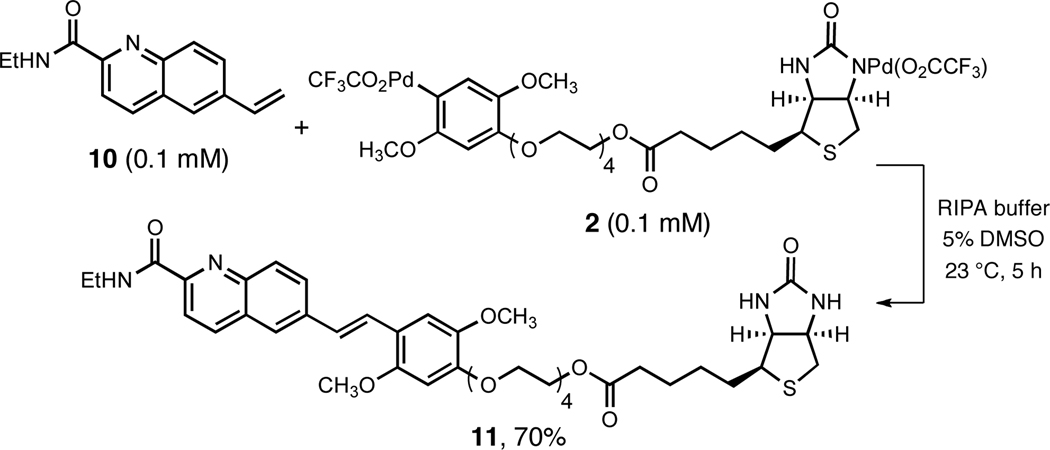
We initiated our studies of Heck-type coupling reactions with the biotin-linked arylpalladium(II) reagent 2 using DMSO as solvent and the 6-vinylquinaldic acid derivative 10 as substrate (Scheme 1), chosen because it served as a model for a projected mechanistic study of saframycins9 and because the coupling product was anticipated to be fluorescent.10 When a stock solution of the biotin-linked arylpalladium(II) reagent 2 in DMSO (18 mM, 1.4 equiv) was combined with substrate 10 (1 equiv) in the air at 23 °C (20 h), the trans coupling product 11 was obtained in 68% yield (isolation by rp-HPLC). Next we evaluated the same coupling reaction in water, limiting the total concentration of DMSO to 5%, in the presence of varying buffer compositions and using equimolar amounts of each substrate, but at much higher dilution (0.1 mM). The coupling product 11 was formed in yields ranging from 35–70% in most common buffer solutions at 23 °C within 4–5 h (rp-HPLC, based on an internal standard, Supporting Information). Optimum results were obtained using RIPA buffer (which contains 1% Triton X-100 and 0.1% SDS, 70% yield, Scheme 1). Lipshutz and coworkers have studied extensively organometallic coupling reactions in water, including conventional Heck reactions of aryl halides using exogenous palladium catalysts, and have noted that surfactants are critical to achieve successful coupling in the systems they examined.11 We too have observed beneficial effects of surfactants in the (non-catalytic) transformations we have examined, including the Heck-type coupling of 2 and 10, but in the latter case surfactants were not absolutely required.12 Lastly, we found that while the efficiency of Heck-type coupling between 2 and 10 was greatly attenuated in the presence of tris(2-carboxyethyl)phosphine, ascorbic acid, or DTT, amines or pyridine-based ligands were well tolerated.
Scheme 1.
Heck-type Coupling Reaction of Reagent 2 in Aqueous Media
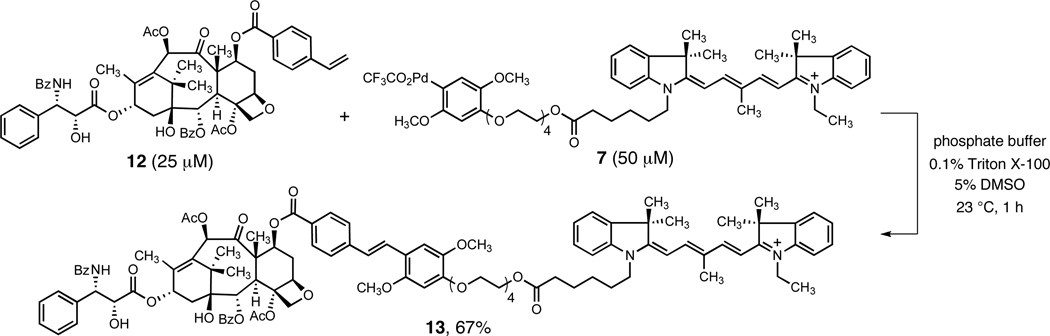
In a different test of the feasibility of Heck-type coupling, the unsymmetrically substituted Cy5 arylpalladium(II) reagent 7 (14 mM, 1 equiv) was mixed with the taxol-derived substrate 12 (1.5 equiv) in DMSO in the air at 23 °C for 2 h. The trans Heck-type coupling product 13 was obtained as a blue solid in 70% yield after isolation by flash-column chromatography. Remarkably, when substrate 12 (25 µM, a >500-fold dilution, 1 equiv) was combined with reagent 7 (50 µM, 2 equiv) in aqueous potassium phosphate buffer solution (pH 8.0, 0.1% Triton X-100) containing 5% DMSO at 23 °C, the coupling product 13 was formed in 67% yield after 1 h (rp-HPLC, based on an internal standard, Scheme 2 and Supporting Information). Variation of pH (5.8–8.0) in the latter system was found not to influence the yield of the coupling product (63–68% yield of 13, 1 h). Coupling was slower but equally efficient when Tris buffer was used in lieu of phosphate buffer (23% yield after 3 h, 70% yield after 17 h, 23 °C). In both cases we found that Triton X-100 was an essential additive (<5% coupling in its absence), consistent with the prior observations of Lipshutz and coworkers, although we found that lower loadings of surfactant were required in this particular system (0.1% versus 5–15%).
Scheme 2.
Heck-type Coupling Reaction of the Styryl-Modified Taxol Derivative 12 and Reagent 7 in Aqueous Media
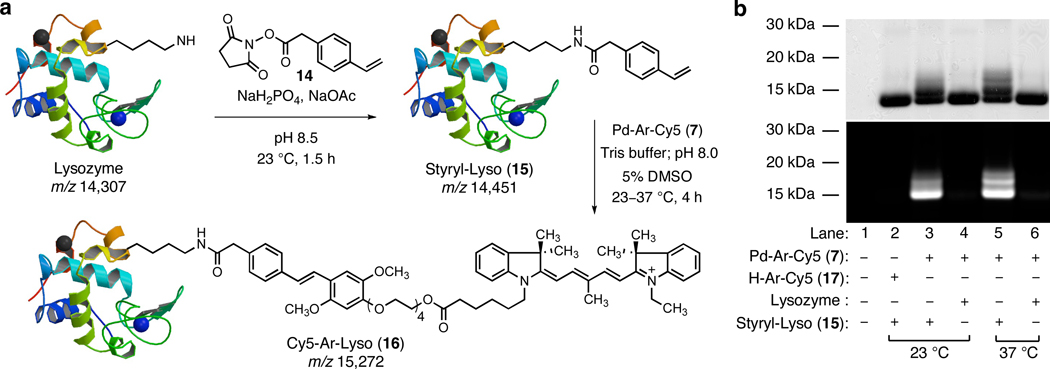
We next evaluated the feasibility of labeling a protein substrate that had been covalently modified to present an alkene function. Acylation of lysozyme (mwt 14,307) with styryl succinimide (14) at pH 8.5 for 1.5 h (Figure 4a) gave rise to a mixture comprising approximately 34% unmodified lysozyme, 40% singly modified lysozyme (15, mwt 14,451), and lesser amounts of doubly and triply modified lysozyme (20% and 6%, respectively), as determined by LC-MS analysis (see Supporting Information).13 When this protein mixture (39 µM) was incubated with the Cy5-linked reagent 7 (115 µM, abbreviated as Pd-Ar-Cy5 in Figure 4) in pH 8.0 Tris buffer containing 5% DMSO at 23 °C or, separately, at 37 °C for 4 h, a major fluorescent band of ~15 kDa mwt was observed by denaturing SDS-PAGE analysis, as well as a minor fluorescent band of higher molecular weight (~16 kDa, believed to be the doubly modified Heck-type coupling product, see Figure 3b, lanes 3 and 5). High-resolution LC-MS analysis of the product mixture after purification by size-exclusion chromatography showed a major product peak of mwt 15,273, corresponding to the singly modified Heck-type coupling product (est. conversion 75%). Control experiments conducted with the styryl-modified lysozyme substrate and a non-palladated Cy5 dye reactant (the protodepalladation product, H-Ar-Cy5, 17), or with unmodified lysozyme and Cy5-linked reagent 7, did not produce any significant fluorescent bands (lanes 2 or 6 and 4, respectively, Figure 4b).
Figure 4.
(a) Styryl residues were introduced by the reaction of lysine residues with NHS-ester 14. Subsequent treatment with Pd-Ar-Cy5 reagent 7 led to labeling of the modified protein by the dye. (b) SDS-PAGE with visualization by fluorescence imaging (bottom) and Coomassie staining (top).
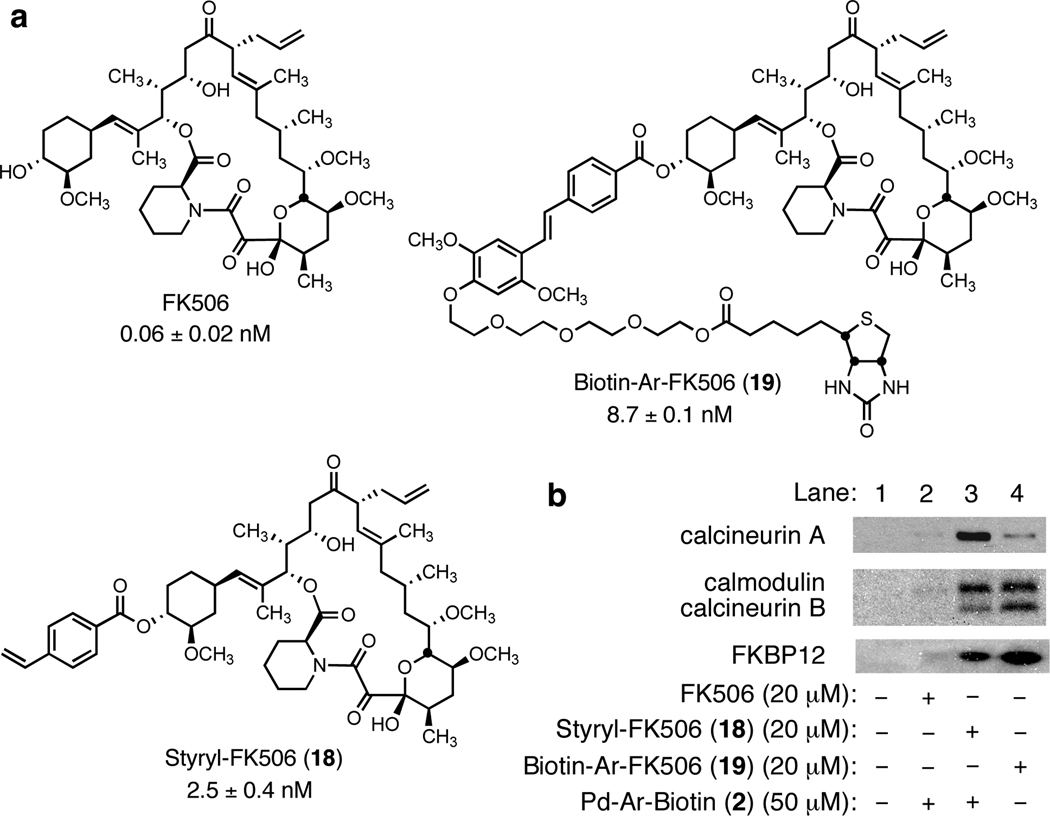
We next conducted an affinity-enrichment experiment using the biotin-conjugated arylpalladium(II) reagent 2 and the styryl-modified FK-506 probe 18 (Figure 5a, see Supporting Information for its synthesis and biological evaluation using an NFAT reporter gene assay).14, 15 Fresh cell lysate from healthy Chinese Hamster Ovary (CHO) cells was diluted to a total protein concentration of 1.0 mg/mL with Tris-buffered saline containing Triton X-100 (pH 8.0) and the diluted protein solution was treated with affinity probe 18 (0.02 mM) for 4 h at 4 °C. This sample was then incubated with the arylpalladium(II) reagent 2 (0.05 M) for 14 h at 23 °C. Dithiothreitol was added, followed by an amine-based palladium scavenger resin. Proteins complexed to the biotin-conjugated probe molecule were then sequestered onto streptavidin-agarose. These were collected by centrifugation, and then washed. Bound proteins were released by heat denaturation, the denatured proteins were separated by SDS-PAGE and the separated bands were analyzed by Western blot (Figure 5b) with quantification by spot densitometry. Under these optimized conditions, we isolated FKBP12 as well as the three known secondary binding proteins, calmodulin and calcineurins A and B (Figure 5b, lane 3). An affinity-isolation experiment using the purified Heck-type coupling product prepared by an independent route (compound 19, lane 4) was conducted simultaneously for comparison, and also led to isolation of the same four proteins, albeit with somewhat different relative efficiencies. These results were confirmed in several replications of the experiment.
Figure 5.
(a) Structures of FK506 and FK506-based affinity probes and their IC50 values in the NFAT reporter gene assay. (b) Western-blot detection of the FK506–immunosuppressive complex.
In summary, we describe protocols for the synthesis of storable arylpalladium(II) reagents bearing biotin and indocyanine-dye residues. We demonstrate that these reagents function effectively in Heck-type coupling reactions with styrene-containing substrates in aqueous media at 23 °C, including a stryl-modified protein substrate and a styryl-modified FK-506 analog in the presence of a cell lysate containing its protein receptors. Because we use stoichiometric organopalladium(II) complexes as reagents, subsequent coupling reactions do not rely upon any catalytic cycle, which we believe to be advantageous. In addition to potential further applications of the reagents in chemistry and chemical biology,16 we envision opportunities for structural refinement and modification of the arylpalladium(II) reagents.
Supplementary Material
ACKNOWLEDGMENT
We express our appreciation to David Liu, Tobias Ritter, Alan Saghatelian, Stuart Schreiber, and Joshua Vaughan for helpful discussions. R.L.S. gratefully acknowledges Eli Lilly for an Organic Chemistry Fellowship. This research was supported by NSF grant CHE-0749566 and NIH grant CA-047148.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Complete experimental procedures and spectral data are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Lewis WG, Green LG, Grynszpan F, Radic Z, Carlier PR, Taylor P, Finn MG, Sharpless KB. Angew. Chem., Int. Ed. 2002;41:1053–1057. doi: 10.1002/1521-3773(20020315)41:6<1053::aid-anie1053>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]; (b) Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J. Am. Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 2.(a) Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]; (b) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ning XH, Guo J, Wolfert MA, Boons G-J. Angew. Chem., Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Blackman ML, Royzen M, Fox JM. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka D, Romeril SP, Myers AG. J. Am. Chem. Soc. 2005;127:10323–10333. doi: 10.1021/ja052099l. [DOI] [PubMed] [Google Scholar]
- 4.(a) Myers AG, Tanaka D, Mannion MR. J. Am. Chem. Soc. 2002;124:11250–11251. doi: 10.1021/ja027523m. [DOI] [PubMed] [Google Scholar]; (b) Tanaka D, Myers AG. Org. Lett. 2004;6:433–436. doi: 10.1021/ol0363467. [DOI] [PubMed] [Google Scholar]
- 5.Decarboxylative palladation did not proceed to completion when fewer than 3 equiv of palladium(II) trifluoroacetate were used.
- 6.Our synthesis of Cy5 dye 5 was modeled after the following report: Shao F, Weissleder R, Hilderbrand SA. Bioconjugate Chem. 2008;19:2487–2491. doi: 10.1021/bc800417b.
- 7.Decarboxylative palladation could also be achieved efficiently by warming substrates 6 or 8 with palladium(II) trifluoroacetate (3 equiv) in the absence of sodium carbonate. We observe that in the absence of sodium carbonate, however, the arylpalladium(II) reagents 7 or 9 were less stable, undergoing slow protiodepalladation over the course of several weeks upon storage at −20 °C.
- 8.We have been unsuccessful in characterizing directly the arylpalladium(II) intermediates 2, 7, or 9 by mass spectrometry in spite of many attempts to do so.
- 9.Xing C, LaPorte JR, Barbay JK, Myers AG. Proc. Natl. Acad. Sci. USA. 2004;101:5862–5866. doi: 10.1073/pnas.0307476101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Gennari G, Galiazzo G, Bortolus P. Chem. Phys. Lett. 1989;157:194–199. [Google Scholar]; (b) Gennari G, Bortolus P, Galiazzo G. J. Mol. Struct. 1991;249:189–202. [Google Scholar]
- 11.(a) Lipshutz BH, Taft BR. Org. Lett. 2008;10:1329–1332. doi: 10.1021/ol702755g. [DOI] [PubMed] [Google Scholar]; (b) Lipshutz BH, Ghorai S, Abela AR, Moser R, Nishikata T, Duplais C, Krasovskiy A, Gaston RD, Gadwood RC. J. Org. Chem. 2011;76:4379–4391. doi: 10.1021/jo101974u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lipshutz BH, Ghorai S, Leong WWY, Taft BR, Krogstad DV. J. Org. Chem. 2011;76:5061–5073. doi: 10.1021/jo200746y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For examples of intermolecular Heck reactions in water employing exogenous palladium catalysts, see: Genet J-P, Blart E, Savignac M. Synlett. 1992:715–717. Jeffery T. Tetrahedron. 1996;52:10113–10130. Uozumi Y, Kimura T. Synlett. 2002:2045–2048. DeVasher RB, Moore LR, Shaughnessy KH. J. Org. Chem. 2004;69:7919–7927. doi: 10.1021/jo048910c. Kodama K, Fukuzawa S, Nakayama H, Kigawa T, Sakamoto K, Yabuki T, Matsuda N, Shirouzu M, Takio K, Tachibana K, Yokoyama S. ChemBioChem. 2006;7:134–139. doi: 10.1002/cbic.200500290.
- 13.For recent examples of selective modification of lysine residues of chicken egg lysozyme, see: Hooker JM, Esser-Kahn AP, Francis MB. J. Am. Chem. Soc. 2006;128:15558–15559. doi: 10.1021/ja064088d. Song W, Wang Y, Qu J, Madden MM, Lin Q. Angew. Chem. Int., Ed. 2008;47:2832–2835. doi: 10.1002/anie.200705805.
- 14.(a) Harding MW, Galat A, Uehling DE, Schreiber SL. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]; (b) Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 15.In recent years, the FK506–immunosuppressive complex has been widely targeted to assess affinity-isolation methods Tamura T, Terada T, Tanaka A. Bioconjugate Chem. 2003;14:1222–1230. doi: 10.1021/bc034099l. Kanoh N, Honda K, Simizu S, Muroi M, Osada H. Angew. Chem. Int., Ed. 2005;44:3559–3562. doi: 10.1002/anie.200462370. Takahashi T, Shiyama T, Mori T, Hosoya K, Tanaka A. Anal. Bioanal. Chem. 2006;385:122–127. doi: 10.1007/s00216-006-0335-3. Peddibhotla S, Dang Y, Liu JO, Romo D. J. Am. Chem. Soc. 2007;129:12222–12231. doi: 10.1021/ja0733686. Wang X, Imber BS, Schreiber SL. Bioconjugate Chem. 2008;19:585–587. doi: 10.1021/bc700297j.
- 16.Preliminary experiments suggest that labeling of ribonucleic acids can also be achieved by the present methodology.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.