Abstract
We report the first total synthesis of (–)-N-methylwelwitindolinone C isothiocyanate. Our route features a number of key transformations, including an indolyne cyclization to assemble the [4.3.1]-bicyclic scaffold, as well as a late-stage intramolecular nitrene insertion to functionalize the C11 bridgehead carbon en route to the natural product.
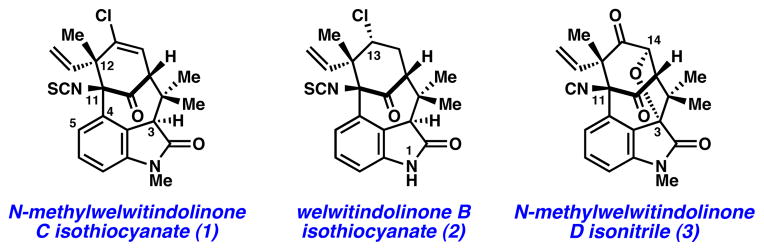
The welwitindolinones are a unique class of natural products isolated from the blue-green algae Hapalosiphon welwitschii and Westiella intricata.1 Ten welwitindolinones have been identified to date, nine of which possess [4.3.1]-bicyclic cores (e.g., 1–3, Figure 1).2 Although compact in size, each of these natural products contains a dense array of functionality that has plagued synthetic efforts for nearly two decades. To date, more than ten laboratories have reported progress toward the bicyclic welwitindolinones.3,4 Whereas these exhaustive efforts have resulted in several elegant methods for bicycle generation, completion of these targets has remained a formidable challenge. In fact, the only total synthesis of a [4.3.1]-bicyclic welwitindolinone was recently achieved by Rawal and co-workers, with their breakthrough synthesis of (±)−3 in 2011.5
Figure 1.
[4.3.1]-Bicyclic welwitindolinones 1–3.
With the aim of synthesizing alkaloids 1–3 and other family members, we selected 1 as our initial synthetic target. Of note, welwitindolinone 1 was uniquely found to reverse P-glycoprotein-mediated multiple drug resistance (MDR) to a variety of anti-cancer drugs in human cancer cell lines, and is therefore a promising lead for the treatment of drug resistant tumors.6 The densely functionalized bicyclic framework of 1 presents numerous synthetic challenges, including a 3,4-disubstituted oxindole, a heavily substituted cyclohexyl ring, and a bridgehead isothiocyanate substituent at C11. In this communication, we report the first total synthesis of (–)-N-methylwelwitindolinone C isothiocyanate (1).
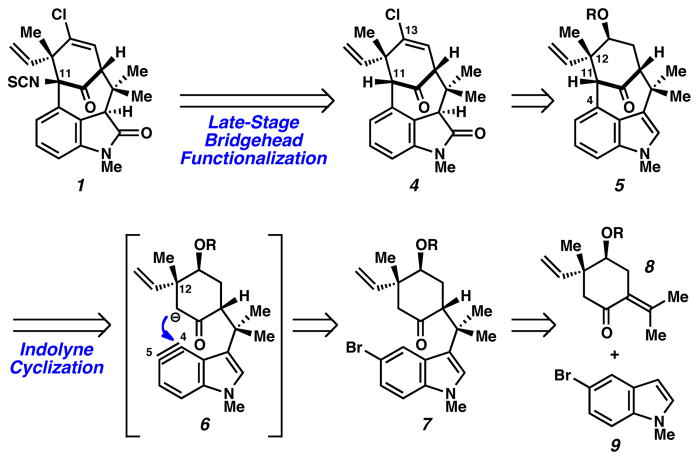
Retrosynthetically, it was envisioned that 1 would be derived from bicycle 4 through late-stage functionalization of the C11 bridgehead position (Scheme 1). In turn, intermediate 4 would arise from indole precursor 5 by introduction of the vinyl chloride and oxindole moieties. In the key complexity generating step, the [4.3.1]-bicycle would be fashioned through intramolecular addition of an enolate onto an in situ-generated “indolyne” species (see transition structure 6).7 The use of an indolyne intermediate8,9 was considered advantageous as the high reactivity of the aryne would permit the assembly of the congested C4–C11 bond linkage, where a tertiary center would be introduced adjacent to the C12 quaternary stereocenter. Of note, the indolyne would be inherently electrophilic, representing an uncommon umpolung of the indole’s typical reactivity. Bromoindole 7 was thought to be a suitable precursor to the desired indolyne via the classic dehydrohalo-genation method for aryne generation. Finally, cyclohexyl derivative 8 and indole 9 were identified as suitable starting fragments.
Scheme 1.
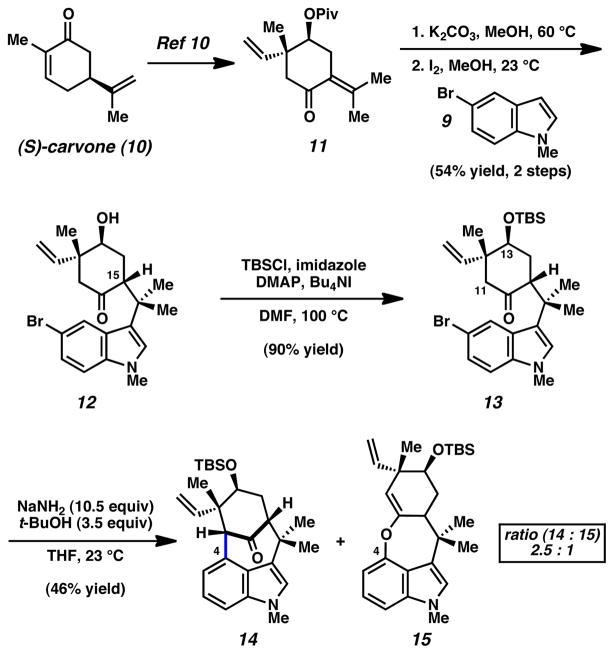
Our synthesis commenced with the concise preparation of the key [4.3.1]-bicycle (Scheme 2). (S)-Carvone (10) was elaborated to enone 11 using the robust five step procedure reported by Natsume in the enantiomeric series.10 Subsequent pivalate cleavage, followed by I2-promoted addition of bromoindole 9,11 furnished adduct 12 in 54% yield over two steps.12 TBS-protection of 12 provided silylether 13, which in turn was employed in the critical indolyne cyclization. To our delight, treatment of 13 with NaNH2 and t-BuOH in THF at ambient temperatures3p,13 led to indolyne adducts 14 and 15 in a combined 46% yield (2.5: 1 ratio).14,15 Although O-arylated product 15 was observed,16 the major product 14 possesses the desired [4.3.1]-bicyclic framework of the natural product and is available in gram quantities.17 Moreover, it was believed that bicycle 14 was suitably functionalized to allow for the ultimate completion of the natural product synthesis.
Scheme 2.
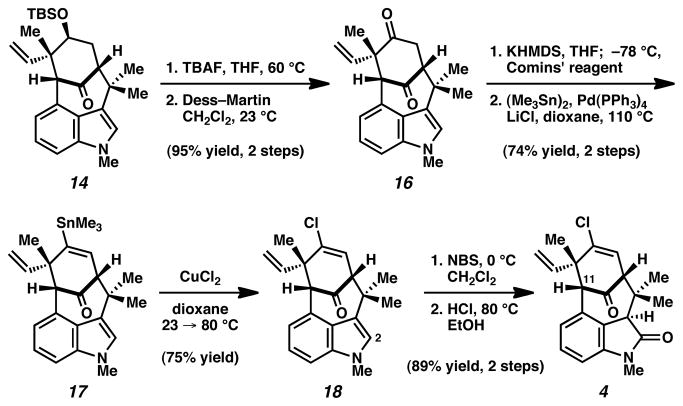
Having assembled the bicyclic framework of the natural product, we focused efforts on introduction of the vinyl chloride and oxindole moieties (Scheme 3). Desilylation of 14, followed by Dess–Martin oxidation, smoothly furnished diketone 16. Subsequently, a sequence involving triflation and Pd-catalyzed stannylation provided vinyl stannane 17.18 Exposure of 17 to CuCl2 in dioxane afforded vinyl chloride 18.19 To arrive at the necessary oxindole, a two-step procedure involving sequential C2 bromination and hydrolysis was employed to deliver late-stage intermediate 4.7
Scheme 3.
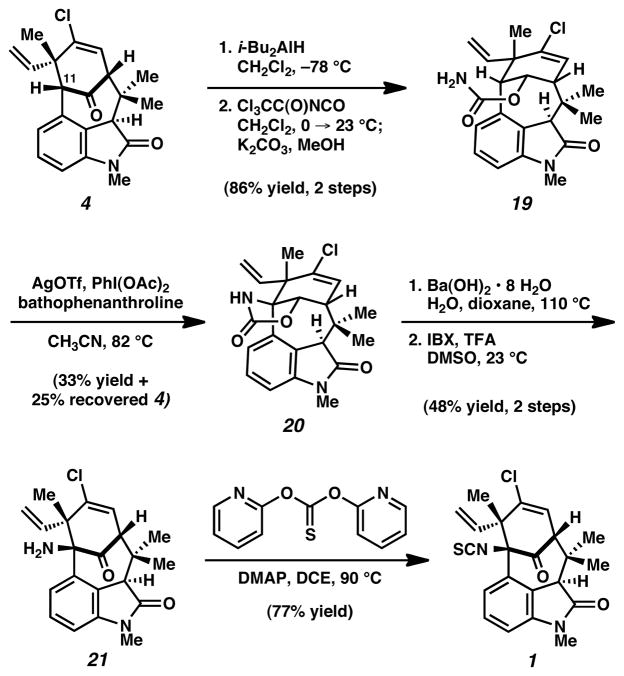
With intermediate 4 lacking only the isothiocyanate substituent, we turned our attention to functionalization of the sterically congested C11 bridgehead position.20 Unfortunately, attempts to substitute C11 through intermolecular processes were unsuccessful.21 As a workaround, we postulated that an intramolecular nitrene C–H insertion might be more fruitful.22,23 Ketone reduction of 4 proceeded efficiently using i-Bu2AlH to furnish a secondary alcohol intermediate as a single diastereomer (Scheme 4). Subsequent carbamoylation furnished 19,23 the key substrate for the critical C–H insertion reaction. The cyclization of carbamate 19 was attempted using a variety of reaction conditions that had previously been used to construct 5-membered oxazolidinones fused to cyclohexyl rings.24 Although use of Rh catalysis furnished ketone 4 rather than the desired product 20,25 Ag catalysis24b,c was found to be more effective. Upon treatment of 19 with AgOTf, bathophenanthroline, and PhI(OAc)2 in CH3CN at elevated temperatures, the desired nitrene insertion took place to deliver oxazolidinone 20 as the major product. Ketone 4 was also recovered, and could be recycled through our synthetic route. Nonetheless, hydrolysis of 20 followed by IBX oxidation generated the penultimate intermediate 21. With aminoketone 21 in hand, final introduction of the isothiocyanate3m,26 furnished 1. Spectral data for synthetic 1 was identical in all respects to that reported for the natural product.1a,27
Scheme 4.
In summary, we have achieved the first total synthesis of N-methylwelwitindolinone C isothiocyanate (1). Our enantio-specific route proceeds in 17 steps from known carvone derivative 11 and features a number of key transformations, including: (a) an indolyne cyclization to assemble the [4.3.1]-bicycle, (b) late-stage introduction of the vinyl chloride and oxindole moieties, and (c) a nitrene insertion reaction to functionalize the sterically congested C11 bridgehead position. Our synthesis of (–)-(1) validates the use of indolynes as intermediates in complex molecule synthesis and provides a promising entryway to the other [4.3.1]-bicyclic welwitindolinones.
Supplementary Material
Acknowledgments
The authors are grateful to the NIH-NIGMS (R01 GM090007), Boehringer Ingelheim, DuPont, Eli Lilly, Amgen, the University of California, Los Angeles, the ACS Organic Division (fellowship to K.W.Q.), Bristol–Myers Squibb (fellowship to K.W.Q.), and the Foote Family (fellowships to A.D.H. and K.W.Q.), for financial support. We thank the Garcia–Garibay laboratory (UCLA) for access to instrumentation, Professor Justin Du Bois for helpful discussions, Dr. John Greaves (UC Irvine) for mass spectra, and Xia Tian, Haoxuan Wang, and Sonam Kumar for experimental assistance.
Footnotes
Supporting Information Available. Detailed experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Stramann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J Am Chem Soc. 1994;116:9935–9942. [Google Scholar]; (b) Jimenez JL, Huber U, Smith CD, Patterson GML. J Nat Prod. 1999;62:569–572. doi: 10.1021/np980485t. [DOI] [PubMed] [Google Scholar]
- 2.Welwitindolinone A isonitrile, a unique welwitindolinone that possesses a C3 spirooxindoline core, has been synthesized independently by the Baran and Wood groups; see: Baran PS, Richter JM. J Am Chem Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r.Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J Am Chem Soc. 2006;128:1448–1449. doi: 10.1021/ja057640s.
- 3.(a) Konopelski JP, Deng H, Schiemann K, Keane JM, Olmstead MM. Synlett. 1998:1105–1107. [Google Scholar]; (b) Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J Am Chem Soc. 1999;121:6326–6327. [Google Scholar]; (c) Kaoudi T, Ouiclet-Sire B, Seguin S, Zard SZ. Angew Chem, Int Ed. 2000;39:731–733. [PubMed] [Google Scholar]; (d) Deng H, Konopelski JP. Org Lett. 2001;3:3001–3004. doi: 10.1021/ol016379r. [DOI] [PubMed] [Google Scholar]; (e) Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835–6838. [Google Scholar]; (f) López-Alvarado P, García-Granda S, Ivarez-Rúa C, Avendaño C. Eur J Org Chem. 2002:1702–1707. [Google Scholar]; (g) MacKay JA, Bishop RL, Rawal VH. Org Lett. 2005;7:3421–3424. doi: 10.1021/ol051043t. [DOI] [PubMed] [Google Scholar]; (h) Baudoux J, Blake AJ, Simpkins NS. Org Lett. 2005;7:4087–4089. doi: 10.1021/ol051239t. [DOI] [PubMed] [Google Scholar]; (i) Greshock TJ, Funk RL. Org Lett. 2006;8:2643–2645. doi: 10.1021/ol0608799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Lauchli R, Shea KJ. Org Lett. 2006;8:5287–5289. doi: 10.1021/ol0620747. [DOI] [PubMed] [Google Scholar]; (k) Guthikonda K, Caliando BJ, Du Bois J. Abstracts of Papers, 232nd ACS National Meeting; September, 2006; abstr ORGN-002. [Google Scholar]; (l) Xia J, Brown LE, Konopelski JP. J Org Chem. 2007;72:6885–6890. doi: 10.1021/jo071156l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. J Am Chem Soc. 2008;130:17938–17945. doi: 10.1021/ja806981k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Boissel V, Simpkins NS, Bhalay G, Blake AJ, Lewis W. Chem Commun. 2009:1398–1400. doi: 10.1039/b820674k. [DOI] [PubMed] [Google Scholar]; (o) Boissel V, Simpkins NS, Bhalay G. Tetrahedron Lett. 2009;50:3283–3286. [Google Scholar]; (p) Tian X, Huters AD, Douglas CJ, Garg NK. Org Lett. 2009;11:2349–2351. doi: 10.1021/ol9007684. [DOI] [PubMed] [Google Scholar]; (q) Trost BM, McDougall PJ. Org Lett. 2009;11:3782–3785. doi: 10.1021/ol901499b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Brailsford JA, Lauchli R, Shea KJ. Org Lett. 2009;11:5330–5333. doi: 10.1021/ol902173g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Freeman DB, et al. Tetrahedron. 2010;66:6647–6655. doi: 10.1016/j.tet.2010.04.131. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Heidebrecht RW, Jr, Gulledge B, Martin SF. Org Lett. 2010;12:2492–2495. doi: 10.1021/ol1006373. [DOI] [PMC free article] [PubMed] [Google Scholar]; (u) Ruiz M, López-Alvarado P, Menéndez JC. Org Biomol Chem. 2010;8:4521–4523. doi: 10.1039/c0ob00382d. [DOI] [PubMed] [Google Scholar]
- 4.For pertinent reviews, see: Brown LE, Konopelski JP. Org Prep Proc Intl. 2008;40:411–445.Avendaño C, Menéndez JC. Curr Org Synth. 2004;1:65–82.
- 5.Bhat V, Allan KM, Rawal VH. J Am Chem Soc. 2011;133:5798–5801. doi: 10.1021/ja201834u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Smith CD, Zilfou JT, Stratmann K, Patterson GML, Moore RE. Mol Pharmacol. 1995;47:241–247. [PubMed] [Google Scholar]; (b) Zhang X, Smith CD. Mol Pharmacol. 1996;49:288–294. [PubMed] [Google Scholar]
- 7.For a model system study of this transformation, see ref 3p.
- 8.For seminal indolyne studies, see: Julia M, Huang Y, Igolen J. C R Acad Sci, Ser C. 1967;265:110–112.Igolen J, Kolb A. C R Acad Sci, Ser C. 1969;269:54–56.Julia M, Le Goffic F, Igolen J, Baillarge M. Tetrahedron Lett. 1969;10:1569–1571.For related studies, see: Julia M, Goffic FL, Igolen J, Baillarge M. C R Acad Sci, Ser C. 1967;264:118–120.Julia M, Igolen J, Kolb M. C R Acad Sci, Ser C. 1971;273:1776–1777.
- 9.For recent indolyne studies, see: Bronner SM, Bahnck KB, Garg NK. Org Lett. 2009;11:1007–1010. doi: 10.1021/ol802958a.Cheong PHY, Paton RS, Bronner SM, Im GY, Garg NK, Houk KN. J Am Chem Soc. 2010;132:1267–1269. doi: 10.1021/ja9098643.Im GY, Bronner SM, Goetz AE, Paton RS, Cheong PHY, Houk KN, Garg NK. J Am Chem Soc. 2010;132:17933–17944. doi: 10.1021/ja1086485.Bronner SM, Goetz AE, Garg NK. J Am Chem Soc. 2011;133:3832–3835. doi: 10.1021/ja200437g.Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org Lett. 2007;9:4135–4137. doi: 10.1021/ol701595n.Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63–65. doi: 10.1016/j.tetlet.2008.10.086.Buszek KR, Brown N, Luo D. Org Lett. 2009;11:201–204. doi: 10.1021/ol802425m.Brown N, Luo D, Decapo JA, Buszek KR. Tetrahedron Lett. 2009;50:7113–7115. doi: 10.1016/j.tetlet.2009.09.083.Garr AN, Luo D, Brown N, Cramer CJ, Buszek KR, VanderVelde D. Org Lett. 2010;12:96–99. doi: 10.1021/ol902415s.Thornton PD, Brown N, Hill D, Neunswander B, Lushington GH, Santini C, Buszek KR. ACS Comb Sci. doi: 10.1021/co2000289.Nguyen TD, Webster R, Lautens M. Org Lett. 2011;13:1370–1373. doi: 10.1021/ol200057j.
- 10.Sakagami M, Muratake H, Natsume M. Chem Pharm Bull. 1994;42:1393–1398. [Google Scholar]
- 11.Wang SY, Ji SJ, Loh TP. Synlett. 2003;15:2377–2379. [Google Scholar]
- 12.The C15 epimer of 12 was also obtained in 22% yield. Upon treatment of this compound with DBU in heated toluene, a separable mixture of 12 and epi-12 is readily obtained.
- 13.Caubere P. Acc Chem Res. 1974;7:301–308. [Google Scholar]
- 14.Variations in reaction conditions (e.g., temperature, stoichiometry, counterion, etc.) did not lead to improvements in the conversion of 13 to 14.
- 15.The remaining balance of mass in the indolyne cyclization is largely attributed to aminoindole products, which presumably form by intermolecular addition of −NH2 to the indolyne intermediate. Attempts to suppress this undesired reaction pathway have been unsuccessful.
- 16.O-arylated product 15 is often isolated with small amounts of the isomeric tetrasubstituted olefin.
- 17.Interestingly, the C13 epimer of substrate 13 does not undergo conversion to the corresponding [4.3.1]-bicycle.
- 18.Wulff WD, Peterson GA, Bauta WE, Chan KS, Faron KL, Gilbertson SR, Kaesler RW, Yang DC, Murray CK. J Org Chem. 1986;51:277–279. [Google Scholar]
- 19.Simpkins SME, Kariuki BM, Aricó CS, Cox LR. Org Lett. 2003;5:3971–3974. doi: 10.1021/ol035536e. [DOI] [PubMed] [Google Scholar]
- 20.Exhaustive efforts to effect indolyne cyclization of substrates bearing N- or C-substituents at C11 were unsuccessful, thus preventing earlier installation of the C11 bridgehead functionality.
- 21.Intermolecular functionalization methods that were tested include bridgehead enolate chemistry, nitrene insertion reactions, and radical halogenations.
- 22.(a) Davies HML, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Collet F, Lescot C, Liang C, Dauban P. Dalton Trans. 2010;39:10401–10413. doi: 10.1039/c0dt00283f. [DOI] [PubMed] [Google Scholar]
- 23.For an elegant late-stage nitrene insertion in natural product total synthesis, see: Hinman A, Du Bois J. J Am Chem Soc. 2003;125:11510–11511. doi: 10.1021/ja0368305.
- 24.For intramolecular nitrene C–H insertion reactions using carbamate substrates, see: Espino CG, Du Bois J. Angew Chem, Int Ed. 2001;40:598–600.Li Z, Capretto DA, Rahaman R, He C. Angew Chem, Int Ed. 2007;46:5184–5186. doi: 10.1002/anie.200700760.Cui Y, He C. Angew Chem, Int Ed. 2004;43:4210–4212. doi: 10.1002/anie.200454243.
- 25.Ketone 4 likely forms by a pathway involving initial insertion into the α C–H bond; for related observations, see: Hinman AW. PhD Dissertation. Stanford University; Stanford, CA: 2004.
- 26.Kim S, Yi KY. J Org Chem. 1986;51:2613–2615. [Google Scholar]
- 27.A sample of natural 1 was not available for direct comparison.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.