Abstract
Background
The latest report by the World Cancer Research Fund/American Institute of Cancer Research concluded that there is convincing evidence that adult height and obesity are risk factors for colorectal cancer. However, studies relating body fatness during early life to the risk of colorectal cancer or adenoma are scarce.
Methods
In the Nurses' Health Study II, participants recalled adult attained height and body shape at ages 5, 10 and 20 years (using a 9-level pictogram: 1= most lean body shape, 9= most overweight body shape) at baseline. Among 32,707 women who had at least one lower bowel endoscopy between 1991 and 2005, 2,327 colorectal adenomas were documented.
Results
Adult height was positively associated with risk of colorectal adenoma (multivariate odds ratio (OR) per 2 inch increment 1.05, 95% confidence interval (CI) 1.01-1.09). Comparing women who were overweight (body shape level 6 or higher) to women who were most lean (body shape level 1), ORs (95% CI, p-trend) of colorectal adenoma for body shapes at ages 5, 10, and 20 years were 1.44 (1.04-1.99, 0.01), 1.21 (0.93-1.56, 0.05) and 1.03 (0.74-1.42, 0.58), respectively. Adjustment for adult body mass index did not change results substantially. The positive associations for body fatness at ages 5 and 10 years as well as adult height were restricted to distal adenoma, while not seen for proximal or rectal adenoma.
Conclusion
Higher height and body fatness during childhood was associated with increased risk of distal adenoma later in life, independent of adult body weight.
Keywords: childhood, adolescence, obesity, height, colorectal adenoma
Introduction
The latest report by the World Cancer Research Fund and the American Institute of Cancer Research concluded that there is convincing evidence that adult obesity and adult attained height are risk factors for colorectal cancer (1). It is known that childhood and adolescence obesity in the US and worldwide pose a major public health problem through immediate and long-term adverse health outcomes such as insulin resistance, early-onset type 2 diabetes mellitus, hypertension, and hyperlipidemia (2, 3). However, the impact of early life obesity on cancer risk later in life is less well studied (4, 5). Early life obesity is associated with alterations in basal insulin levels (6), which might influence early steps in carcinogenesis, as there is evidence that the positive association between obesity and colorectal cancer is at least in part explainable by high circulating insulin concentrations (7). Studies of body fatness during early life in relation to risk of colorectal cancer are scarce, and are often limited with regard to statistical power or the ability to adjust for potential confounders (8-15). To our knowledge, no study has related early life body fatness to the occurrence of colorectal adenoma, a precursor for colorectal cancer (16). Adult attained height may reflect exposure to insulin-like growth factor 1 (IGF-1) during the growth period (17) and has been positively associated with colorectal cancer (18-20).
The aim of this study was to prospectively investigate the association between adult height and body fatness during early life in relation to risk of colorectal adenoma in a large cohort of US women.
Material and Methods
Study population
The Nurses' Health Study II is a prospective cohort including 116,671 female registered nurses aged between 25 and 42 years (96% premenopausal) when they responded to a mailed questionnaire inquiring about life style factors and medical history in 1989. Every two years thereafter, participants were mailed follow-up questionnaires to update risk factors for diseases as well as to ascertain newly diagnosed diseases. In 1991 and every four years thereafter a validated semiquantitative food frequency questionnaire (FFQ) was also mailed to the participants (21). Follow-up rate for each 2-year cycle has been approximately 90%.
For this analysis, only women who had reported at least one lower gastrointestinal endoscopic procedure (colonoscopy or sigmoidoscopy) during the study period (June 1991-December 2005) were included, because colorectal adenomas are usually asymptomatic and diagnosed during routine screening or endoscopic procedures performed for symptoms not related to adenomas. After exclusion of women with a history of cancer or colorectal polyps before baseline and women who did not report on body fatness at ages 5, 10, or 20 years, a total of 32,707 women were eligible for this analysis. This study was approved by the Institutional Review Boards of the Brigham and Women's Hospital and the Harvard School of Public Health.
Assessment of early life body fatness
On the 1989 baseline questionnaire, participants recalled their body fatness at ages 5, 10 and 20 years and at baseline by means of a 9-level figure drawing (figure 1), which was originally developed by Stunkard and colleagues (22). In a validation study within the Third Harvard Growth Study, Pearson correlation coefficients between body fatness during early life recalled by participants at age 71-76 years and measured BMI at approximately the recalled ages were 0.60 for age 5 years, 0.65 for age 10 years, and 0.66 for age 20 years (23). In our study, although two estimates of body fatness close to each other in time were highly correlated, the correlation between body fatness at different ages decreased depending on the time between the two ages (for example, Spearman correlation coefficients between body shape at age 5 with that at ages 10 year, 20 years and at baseline were 0.81, 0.51, and 0.30, respectively).
Figure 1.
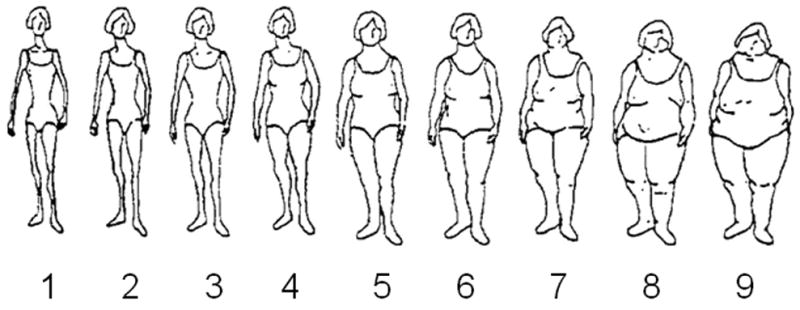
Figure drawing used to assess body fatness at ages 5, 10 and 20 years in the Nurses' Health Study II in 1989. Figure reproduced from Stunkard et al. (22) with permission from Lippincott Williams & Wilkins, Philadelphia, Pennsylvania.
Assessment of adult body weight, and waist and hip circumference
Participants reported their current body weight, height as well as body weight at the age of 18 years on the baseline questionnaire. Current body weight was updated on each of the biennial mailed questionnaires. In a validation study among 140 Nurses' Health Study I participants, Pearson correlation coefficients (after adjustment for age and random within-person variability) between self-reported weight and the average of 2 measurements performed by trained technicians approximately 6 months apart was 0.98 (24). On the 1993 questionnaire, participants also reported self-measured waist and hip circumference.
Ascertainment of cases
When a colorectal polyp was reported on one of the follow-up questionnaires, participants were mailed a consent form requesting permission to obtain medical records. Study investigators blinded to the exposure status of women verified colorectal adenomas by medical records and extracted information on anatomic location, histological type, and size of the reported polyps. We classified confirmed adenoma cases by anatomic site (proximal: cecum, ascending colon, hepatic flexure, transverse colon or splenic flexure; distal: descending or sigmoid colon; rectal: rectosigmoid junction or rectum) and stage (advanced: adenomas ≥1cm in size or any mention of villous features or high-grade dysplasia; non-advanced: <1cm in size and tubular).
Between 1991 and 2005, 2,327 participants were diagnosed with colorectal adenoma (proximal colon: 1,037; distal colon: 1,119; rectum: 403; non-advanced adenomas: 1,137; advanced adenomas: 680).
Sigmoidoscopies versus colonoscopies
Between 1991 and 2001 the NHS II follow-up questionnaires only inquired whether the participants had a lower bowel endoscopy during the past 2 years (i.e. either sigmoidoscopy or colonoscopy). Therefore we do not know whether some of the women who reported a lower bowel endoscopy may have only received a sigmoidoscopy which only covers the rectum and the distal colon versus a colonoscopy which also covers the proximal colon. Starting with the 2003 questionnaire we inquired about sigmoidoscopies and colonoscopies separately. In addition, on the 2005 questionnaire participants were asked to provide information on when they had a sigmoidoscopy versus colonoscopy in the past (1994 or earlier, 1995-96, 1997-98, 1999-2000, 2001-2 and 2003 and up). To assess whether it was appropriate to include proximal adenoma as an outcome, we compared baseline characteristics of women who reported at least one sigmoidoscopy but no colonoscopy (10% of women in this study who also responded to the 2005 question) with those of women who reported at least one colonoscopy but no sigmoidoscopy (57%) and of women who reported both procedures (33%). Most baseline characteristics (age, adult BMI, physical activity and aspirin use) did not differ considerably by type of lower bowel endoscopy. However, women who never had a colonoscopy were less likely to be current smokers and to report a family history of colorectal cancer, and more likely to report routine screening as a reason for endoscopy. The proportion of proximal adenomas observed in our study (45%) exceeds those observed in other colonoscopy-based studies (25, 26), providing further support that most lower bowel endoscopies in our cohort were colonoscopies. Therefore, we also included proximal adenoma as an outcome and all statistical models were adjusted for family history of colorectal cancer, reason for endoscopy and smoking (see below).
Statistical analysis
We examined associations between adult height and body fatness at ages 5, 10 and 20 years and risk of colorectal adenoma using multivariable logistic regression calculating odds ratios (OR) to approximate relative risks. Because of limited numbers in the higher body fatness categories (27), we categorized the 9-level figure drawings into 6 categories (#1, #2, #3, #4, # 5, #6 or higher) for the main analyses and into 5 categories (#1, #2, #3, #4, #5 or higher) for analyses of adenoma subtypes. In addition to the models using categories of height and early life body shape, we computed ORs for a 2-inch increment in adult height and for a 1-unit increment in body fatness at each age. Tests for trend were performed by including height or body fatness at each age (values 1 to 9) as a continuous variable into the models.
We created a basic multivariate model with known risk factors for adenoma which included age at baseline, family history of colorectal cancer (first degree relative, yes/no), time period of endoscopy (2 year study period interval), number of reported endoscopies during the study period (one endoscopy versus ≥2 endoscopies), reason for most recent endoscopy (screening versus symptoms), height, pack-years of smoking, current physical activity, aspirin use, alcohol intake, dietary intake of red meat, processed meat, folate and calcium (for detailed information on definition of covariates see footnotes in tables 2 and 3). Non-dietary covariates that were assessed repeatedly in follow-up questionnaires (physical activity, aspirin use) were updated to best represent the exposure in the 2-year interval before the most recent endoscopy. Cumulative updated dietary intake was calculated by averaging the dietary intakes from all available food frequency questionnaires (1991, 1995, 1997, 1999, and 2003) up to the start of the 2-year interval before the most recent endoscopy. Other potential confounders were also added separately to the basic model (birth weight, age at menarche, oral contraceptive use, menopausal status/postmenopausal hormone use, physical activity at different ages (age 12, 22 years), pack years of smoking before age 20 years, current dietary intake (energy, alcohol, fat, fiber), and dietary intake during high school (energy, fat, fiber, calcium, folate, red and processed meat)) but none of these variables appreciably changed the ORs for height or any of the pictogram variables and were therefore not included in the final model (equivalent to basic model).
Table 2. Association between height, body shape at ages 5, 10 and 20 years and colorectal adenoma.
| Ca/Co | Adj. ORa | 95% CI | ptrend | Adj. ORb | 95% CI | ptrend | |
|---|---|---|---|---|---|---|---|
| Height (inches) | |||||||
| 50-<62 | 175/2546 | 1 | 1 | ||||
| 62-<65 | 826/11171 | 1.07 | (0.89,1.28) | 1.07 | (0.90,1.28) | ||
| 65-<68 | 928/11907 | 1.13 | (0.95,1.35) | 1.13 | (0.95,1.36) | ||
| ≥68 | 398/4756 | 1.26 | (1.03,1.53) | 1.26 | (1.04,1.53) | ||
| Per 2 inch increment | 1.05 | (1.01,1.09) | 0.01 | 1.05 | (1.01,1.09) | 0.01 | |
| Pictogram at age 5 years | |||||||
| 1 | 542/7632 | 1 | 1 | ||||
| 2 | 705/9468 | 1.07 | (0.94,1.21) | 1.06 | (0.94,1.20) | ||
| 3 | 593/7243 | 1.17 | (1.03,1.33) | 1.15 | (1.01,1.31) | ||
| 4 | 289/3925 | 1.04 | (0.88,1.21) | 1.01 | (0.86,1.19) | ||
| 5 | 147/1631 | 1.23 | (1.00,1.51) | 1.20 | (0.97,1.47) | ||
| ≥6 | 51/481 | 1.44 | (1.04,1.99) | 1.40 | (1.01,1.94) | ||
| Per 1-unit increase | 1.05 | (1.01,1.08) | 0.01 | 1.04 | (1.00,1.08) | 0.04 | |
| Pictogram at age 10 years | |||||||
| 1 | 427/5792 | 1 | 1 | ||||
| 2 | 655/9090 | 1.02 | (0.89,1.16) | 1.01 | (0.89,1.16) | ||
| 3 | 541/6831 | 1.08 | (0.94,1.25) | 1.06 | (0.92,1.23) | ||
| 4 | 365/4871 | 1.05 | (0.90,1.23) | 1.02 | (0.87,1.20) | ||
| 5 | 251/2841 | 1.14 | (0.96,1.36) | 1.11 | (0.93,1.33) | ||
| ≥6 | 88/955 | 1.21 | (0.93,1.56) | 1.17 | (0.90,1.52) | ||
| Per 1-unit increase | 1.03 | (1.00,1.07) | 0.05 | 1.02 | (0.99,1.06) | 0.16 | |
| Pictogram at age 20 years | |||||||
| 1 | 113/1551 | 1 | 1 | ||||
| 2 | 610/8028 | 1.04 | (0.83,1.29) | 1.03 | (0.83,1.29) | ||
| 3 | 854/11333 | 1.06 | (0.86,1.32) | 1.04 | (0.83,1.29) | ||
| 4 | 503/6235 | 1.10 | (0.87,1.37) | 1.05 | (0.84,1.33) | ||
| 5 | 174/2244 | 1.05 | (0.81,1.36) | 0.99 | (0.75,1.29) | ||
| ≥6 | 73/989 | 1.03 | (0.74,1.42) | 0.95 | (0.68,1.34) | ||
| Per 1-unit increase | 1.01 | (0.97,1.05) | 0.58 | 0.99 | (0.95,1.04) | 0.82 |
Models multivariate adjusted for age, family history of colorectal cancer, time period of endoscopy, number of reported endoscopies during the study period (one endoscopy vs. ≥2 endoscopies), reason for most recent endoscopy, height (continuous, except for models for height categories), packyears of smoking (0, 0-10,>10-20, >20-40,>40), current physical activity (quintiles), aspirin use (never, past, current 1 day/week, 2-3 days/week, 4-5 days/week, ≥6 days/week), current cumulative average alcohol intake (quintiles), current cumulative average dietary intake of red meat, processed meat, folate and calcium (quintiles)
additionally adjusted for current cumulative average body mass index (continuous)
Table 3. Association between height, body shape at ages 5, 10 and 20 years and colorectal adenoma by subsite, stage and age at diagnosis.
| Adenoma subsite | Adenoma stage | Age at diagnosis c | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proximal (1037 cases) |
Distal (1119 cases) |
Rectal (403 cases) |
Early a (1037 cases) |
Advanced b (680 cases) |
< 50 years (1098 cases) |
≥ 50 years (1229 cases) |
||||||||
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Height (inches) | ||||||||||||||
| 50-<62 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| 62-<65 | 0.98 | (0.77,1.25) | 1.24 | (0.95,1.64) | 0.94 | (0.64,1.38) | 1.10 | (0.86,1.41) | 1.13 | (0.81,1.57) | 1.14 | (0.86,1.52) | 1.02 | (0.80,1.29) |
| 65-<68 | 0.99 | (0.77,1.26) | 1.48 | (1.13,1.94) | 0.95 | (0.64,1.39) | 1.21 | (0.95,1.55) | 1.25 | (0.90,1.74) | 1.15 | (0.87,1.52) | 1.14 | (0.90,1.44) |
| ≥68 | 1.06 | (0.81,1.39) | 1.71 | (1.28,2.29) | 0.98 | (0.64,1.52) | 1.24 | (0.95,1.63) | 1.44 | (1.00,2.07) | 1.38 | (1.01,1.88) | 1.20 | (0.93,1.56) |
| ptrend | 0.47 | <0.0001 | 0.74 | 0.09 | 0.02 | 0.04 | 0.05 | |||||||
| Pictogram at age 5 years | ||||||||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| 2 | 1.07 | (0.90,1.28) | 1.06 | (0.89,1.26) | 1.08 | (0.82,1.43) | 1.18 | (0.99,1.39) | 0.94 | (0.76,1.18) | 0.94 | (0.77,1.14) | 1.17 | (0.99,1.38) |
| 3 | 1.18 | (0.99,1.42) | 1.11 | (0.93,1.34) | 1.14 | (0.85,1.54) | 1.29 | (1.08,1.53) | 0.99 | (0.79,1.26) | 1.12 | (0.92,1.37) | 1.18 | (0.99,1.40) |
| 4 | 0.96 | (0.76,1.20) | 1.19 | (0.96,1.47) | 1.02 | (0.71,1.47) | 0.95 | (0.76,1.18) | 1.06 | (0.81,1.40) | 0.93 | (0.72,1.19) | 1.11 | (0.90,1.37) |
| ≥5 | 1.12 | (0.85,1.46) | 1.40 | (1.09,1.79) | 1.12 | (0.73,1.72) | 1.23 | (0.95,1.58) | 1.29 | (0.94,1.78) | 1.20 | (0.89,1.61) | 1.40 | (1.11,1.77) |
| ptrend | 0.43 | 0.01 | 0.57 | 0.35 | 0.14 | 0.27 | 0.01 | |||||||
| Pictogram at age 10 years | ||||||||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| 2 | 1.08 | (0.90,1.31) | 1.05 | (0.87,1.27) | 0.90 | (0.67,1.21) | 1.08 | (0.90,1.30) | 0.95 | (0.74,1.21) | 0.94 | (0.76,1.17) | 1.06 | (0.89,1.27) |
| 3 | 1.10 | (0.90,1.35) | 1.07 | (0.88,1.31) | 0.93 | (0.68,1.28) | 1.14 | (0.94,1.38) | 0.98 | (0.76,1.26) | 1.06 | (0.85,1.32) | 1.06 | (0.88,1.28) |
| 4 | 1.05 | (0.84,1.30) | 1.15 | (0.93,1.42) | 0.97 | (0.69,1.37) | 0.95 | (0.77,1.18) | 1.13 | (0.86,1.49) | 0.95 | (0.75,1.22) | 1.13 | (0.92,1.38) |
| ≥5 | 1.12 | (0.89,1.41) | 1.23 | (0.98,1.53) | 0.82 | (0.56,1.19) | 1.09 | (0.87,1.36) | 1.18 | (0.89,1.57) | 1.07 | (0.83,1.38) | 1.23 | (1.00,1.52) |
| ptrend | 0.45 | 0.04 | 0.53 | 0.93 | 0.12 | 0.61 | 0.03 | |||||||
small/tubular
large or tubulovillous or villous or highgrade dysplasia
for controls: age at most recent endoscopy
Models for pictogram at age 5 years or 10 years multivariate adjusted for age, family history of colorectal cancer, time period of endoscopy, number of reported endoscopies during the study period (one endoscopy vs. ≥2 endoscopies), reason for most recent endoscopy, height (continuous), packyears of smoking (0, 0-10,>10-20, >20-40,>40), current physical activity (quintiles), aspirin use (never, past, current 1 day/week, 2-3 days/week, 4-5 days/week, ≥6 days/week), current cumulative average alcohol intake (quintiles), current cumulative average dietary intake of red meat, processed meat, folate and calcium (quintiles)
Models for height adjusted for all variables mentioned above excluding height, but including current cumulative updated average BMI (continuous)
We also investigated the association between height and early life body shape and colorectal adenoma classified by location (proximal, distal, rectal adenoma), stage (early, advanced adenoma) or age at diagnosis (<50 years, ≥ 50 years). We formally compared associations by adenoma subtypes using polytomous logistic regression models (28).
To evaluate whether the association between height or body fatness during early life and colorectal adenoma differed by number of endoscopies during follow-up, we investigated whether associations changed when analyses were restricted to participants who had reported only one endoscopy during follow-up (n=16,807).
To investigate whether associations between body fatness at young ages and colorectal adenoma were independent of adult body shape we also adjusted for adult body mass index (BMI) (calculated as average BMI using information on BMI from all available follow-up questionnaires up to the start of the 2-year interval before the most recent endoscopy), waist to hip ratio (WHR) (available for a subset, i.e. n=17,045) and waist circumference (n=17,111). We examined the joint effect of early life body shape and adult BMI or height by cross-classifying participants by both variables. Associations between height, body shape at ages 5, 10 and 20 years were also examined after stratification by birth weight, physical activity, age at menarche, and family history of colorectal cancer (CRC). We tested for statistical interaction by adding a cross-product term of the body shape variable and the respective stratification variable to a model along with the main effects of body shape and stratification variable.
All statistical tests were two-sided. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SAS software package, version 9.2 (SAS Institute, Cary, NC, USA)
Results
Current smoking at baseline was more frequent among women in the highest body fatness category (body shape # 6 or higher) at ages 5, 10 and 20 years than among women in the leanest body shape category (#1), but the percentage of current smokers did not differ between women who were of small stature vs. those of tall stature (Table 1). BMI at baseline as well as BMI at age 18 years was higher among participants who reported higher body fatness at ages 5, 10 or 20 years than among those who reported lean body shapes at these ages. Other baseline characteristics including menopausal status at baseline, family history of colorectal cancer, reason for most recent endoscopy as well as aspirin use did not differ appreciably according to adult height or body shapes at ages 5, 10 or 20 years.
Table 1. Baseline characteristics by categories of height and body shapes at ages 5, 10 and 20 years.
| Height (inches) | Pictogram at age 5 years | Pictogram at age 10 years | Pictogram at age 20 years | |||||
|---|---|---|---|---|---|---|---|---|
| 50-<62 | ≥68 | #1 | ≥#6 | #1 | ≥#6 | #1 | ≥#6 | |
| N | 2721 | 5154 | 8174 | 532 | 6219 | 1043 | 1664 | 1062 |
| Age at baseline (not standardized) | 38.1 | 37.9 | 38.4 | 38.8 | 38.4 | 38.7 | 39 | 37.7 |
| Age at most recent endoscopy (not standardized) | 48.7 | 48.5 | 48.9 | 49.5 | 49.0 | 49.1 | 49.8 | 48 |
| Smoking status | ||||||||
| Never smoker (%) | 65 | 62 | 65 | 54 | 66 | 53 | 63 | 55 |
| Past smoker (%) | 24 | 27 | 24 | 30 | 24 | 30 | 25 | 28 |
| Current smoker (%) | 11 | 11 | 11 | 16 | 10 | 16 | 12 | 17 |
| Packyears of smoking at baseline | 4 | 5 | 4 | 7 | 4 | 7 | 5 | 7 |
| Premenopausal (%) | 95 | 95 | 94 | 94 | 94 | 93 | 95 | 92 |
| Family history of colorectal cancer (%) | 15 | 16 | 15 | 15 | 15 | 16 | 14 | 16 |
| Reason for most recent endoscopy is screening (%) | 48 | 49 | 48 | 45 | 48 | 45 | 46 | 43 |
| Height (inches) | 60 | 69 | 65 | 65 | 65 | 65 | 65 | 65 |
| BMI at baseline (kg/m2) | 25 | 25 | 24 | 29 | 23 | 28 | 22 | 32 |
| BMI at age 18 years (kg/m2) | 22 | 21 | 20 | 26 | 19 | 25 | 18 | 29 |
| Physical activity at baseline (MET-hours/week) | 24 | 23 | 23 | 25 | 24 | 23 | 24 | 23 |
| Physical activity between ages 12 and 22 MET-hours/week) | 43 | 48 | 47 | 43 | 48 | 39 | 47 | 36 |
| Aspirin use at baseline 2 or more times/week (%) | 12 | 13 | 13 | 14 | 13 | 13 | 13 | 14 |
| Total Calories kcal | 1738 | 1816 | 1781 | 1781 | 1791 | 1786 | 1781 | 1759 |
| Alcohol gm | 2.6 | 3.4 | 3.2 | 2.5 | 3.3 | 2.9 | 3.2 | 2.5 |
| Protein gma | 87 | 87 | 86 | 91 | 85 | 90.9 | 84 | 91.3 |
| Carbohydrates gm a | 226 | 225 | 226 | 220 | 227 | 219 | 230 | 217 |
| Total Fat gm a | 63 | 63 | 63 | 63.6 | 63 | 63.8 | 62 | 64.9 |
| Fiber gm a | 18 | 19 | 18 | 19.2 | 18 | 19.3 | 18 | 19 |
| Calcium mg a | 992 | 1059 | 1000 | 1100 | 995 | 1079 | 984 | 1066 |
| Folate mcg a | 456 | 481 | 459 | 504 | 460 | 485 | 459 | 473 |
Dietary intake variables adjusted for energy intake by residual method; BMI, body mass index; MET, metabolic equivalent
Higher adult height was significantly associated with higher risk of colorectal adenoma (Table 2) with each 2-inch increment in height corresponding to a 5% increase in risk of colorectal adenoma. We observed a significant positive association between higher body fatness at age 5 years and risk of colorectal adenoma. Women who were in the highest body fatness category at the age of 5 years had a 44% greater risk for colorectal adenoma compared to women who were lean at age 5 years. There was a positive association between body shape at age 10 years and risk of colorectal adenoma (p-trend 0.05). Body fatness at age 20 years was not associated with risk of colorectal adenoma. Associations between adult height and body fatness at ages 5, 10, or 20 years and risk of adenoma were not changed substantially after additional further adjustment for current BMI, WHR (data not shown) or waist circumference (data not shown). However, after adjustment for current BMI, the p-value for trend relating body fatness at age 10 years to risk of colorectal adenoma was no longer significant (p=0.16).
BMI at age 18 years was not associated with risk of colorectal adenoma, and for adult BMI, compared to BMI <25 kg/m2 ORs were 1.14, 95% CI 1.02-1.09 for BMI of 25- 29.9 kg/m2 and 1.09, 95% CI 0.96-1.23 for a BMI of ≥30 kg/m2. WHR (OR highest versus lowest quintile 1.14, 95% CI 0.94-1.40) and waist circumference (OR highest versus lowest quintile 1.17, 95% CI 0.94-1.46) were not significantly associated with colorectal adenoma risk.
In models mutually adjusting for body shapes at ages 5, 10, 20 years and at baseline in 1989 (two body shape variables were included in the models simultaneously), body shape at age 5 years remained significantly positively associated with risk of colorectal adenoma after adjustment for body shape at age 20 years (OR highest versus lowest category: 1.50, 95%CI 1.07-2.11, p-trend: 0.02), and at baseline (OR highest versus lowest category: 1.39, 95% CI 1.00-1.93, p-trend: 0.06), while associations were not significant after adjustment for body shape at age 10 years (OR highest versus lowest category: 1.50, 95% CI 0.97-2.30, p-trend: 0.15). After adjustment for body shape at age 5, body shape at age 10 years was not associated with colorectal adenoma, but it remained non-significantly positively associated with colorectal adenoma after adjustment for body shape at age 20 (OR highest versus lowest category: 1.25, 95% CI 0.94-1.65, p-trend: 0.06).
Adult height and body fatness at ages 5 and 10 years were positively associated with risk of distal adenoma, but not with proximal or rectal adenoma (Table 3). We observed statistically significant differences in the associations between adult height and risks of distal versus proximal adenoma (pdifference: 0.002). None of the other site-specific associations showed significant differences. Body shape at age 20 years was non-significantly positively associated with risk of distal adenoma (data not shown).
Associations were not changed appreciably when participants with multiple adenomas at different sites (n=227) were excluded. Comparing the highest versus lowest category, ORs (95% CI, p-trend) for distal only adenoma were 1.70 (1.23-2.34, <0.0001) for height, 1.44 (1.10-1.89, 0.02) for body shape at age 5 years and 1.28 (1.00-1.63, 0.04) for body shape at age 10 years. Body shapes at ages 5, 10 or 20 years and adult height were not statistically significantly associated with risk of multiple adenoma at different sites, but sample size was limited (data not shown).
Higher adult height was associated with a 44% increased risk of advanced adenoma comparing the highest with the lowest height category, while non-significant positive associations were seen for non-advanced adenoma (pdifference: 0.45). No remarkable differences in the associations between body shape at ages 5 and 10 years and early versus advanced adenoma were observed. Body fatness at ages 5 and 10 years was significantly positively associated with colorectal adenoma diagnosed at or after the age of 50 years, while no significant positive associations were observed with colorectal adenoma diagnosed before the age of 50 years (pdifference: 0.34 for body shape at age 5 years and 0.44 for body shape at age 10 years).
The positive associations between height, body shape at age 5 and 10 years and risk of total, distal, early and advanced adenoma were slightly stronger when the study population was restricted to women who had only one endoscopy during follow-up (data not shown).
When we examined joint effects of body shape at age 5 years and adult height, participants with a combination of tall stature and body shape of ≥5 at the age of 5 years had an 75% increased risk of developing colorectal adenoma compared with participants who were lean at age 5 and of small stature (OR 1.75, 95% CI 1.31-2.34, p-interaction: 0.08, Figure 2). Risk of distal adenoma was highest among women who had a body shape ≥ 5 at age 5, and were overweight (OR 1.44, 95% CI 0.97-2.13) or obese (OR 1.40, 95% CI 0.97-2.02) as adults compared to those who were lean at age 5 and as adults (p-interaction=0.09).
Figure 2.
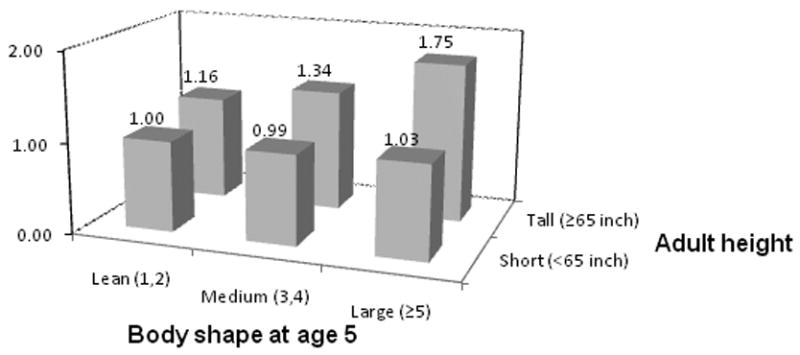
ORs of distal adenoma by different levels of body shape at age 5 and adult attained height. P for interaction = 0.08.
The associations between height or body shape at age 5, 10 or 20 years and colorectal adenoma were not significantly modified by birth weight, physical activity, or age at menarche (data not shown). There was a slight suggestion of effect modification by family history of CRC: positive associations between body shape at age 5 and 10 were restricted to women without a family history of CRC while not seen in women who reported a first-degree relative with a colorectal cancer diagnosis (p-interaction=0.04 for body shape at age 5 and 0.10 for body shape at age 10).
Discussion
In this large prospective study, higher body fatness at age 5 years was significantly associated with increased risk of colorectal adenoma, independent of adult body fatness. Body shape at age 10 years was non-significantly positively associated with colorectal adenoma risk, while no association was observed for body shape at age 20 years. Higher adult height was associated with increased risk of colorectal adenoma. The positive associations for body fatness at ages 5 and 10 years as well as adult height were restricted to distal adenoma, and were not seen for proximal or rectal adenoma.
Childhood and adolescence are time periods characterized by major changes in body composition and hormone levels. A positive association between obesity and colorectal cancer or adenoma is widely assumed to be explainable by obesity-related changes in hormonal pathways. Obesity is associated with higher circulating insulin concentrations, which, in turn, lead to increased bioactivity of IGF-1 (29). Both insulin and IGF-1 act as tissue growth factors but also as hormones regulating energy metabolism and can enhance tumor development by stimulating cell proliferation, and by inhibiting apoptosis (30). Childhood obesity may thus contribute to lifetime insulin exposure, as obesity in pre-adolescent and adolescent girls is associated with higher basal insulin levels (6).
The association between body fatness in early life and colorectal cancer later in life has been addressed in several small epidemiological studies (8-15). To our knowledge, our study is the first prospective analysis relating body fatness during childhood and adolescence to the risk of colorectal adenoma, a precursor for colorectal cancer (16). The only study we are aware of that investigated obesity during childhood in relation to later risk of colorectal cancer is a historical cohort study based on a survey in pre-war Britain (1937-1939) (10). After 50 years of follow-up, participants in the highest quartile of body fatness during childhood (mean age 8 years) had a non-significantly by 36% increased risk of colorectal cancer when compared to those in the lowest quartile. Other studies have investigated the association between body fatness in adolescents (age range 13-19 years) (8, 11, 13) or young adults attending college or university (9, 12, 15) and colorectal cancer or colorectal cancer mortality later in life. Three of these studies observed positive associations between higher BMI during adolescence and colorectal cancer incidence (12) or death (11, 13) in men. Positive associations between adolescent BMI and colon cancer mortality in both men and women were observed in one study (8) and no association was observed in another study (15). Of note, most of the above mentioned studies (9-12, 15) date back to time periods before or shortly after the Second World War, characterized by shortages in food availability and children in poorer families might have suffered from undernutrition. Therefore, observed associations between body fatness and colorectal cancers in these studies may reflect different relationships between body fatness and colorectal cancers than would be observed during more wealthy time periods characterized by overnutrition and poor quality nutrition (31).
In the present study, the positive association between body fatness at age 5 and 10 years was restricted to distal adenomas, supporting the hypothesis that proximal colon, distal colon and rectum represent three distinct anatomic subsites with possibly different molecular pathways leading to neoplasia (32, 33). Associations between adult obesity and colorectal cancer have been stronger for colon than for rectal cancer (34, 35), whereas no differences were observed by anatomic subsite in a recent meta-analysis (7). With respect to colorectal adenomas, among the studies that specifically examined anatomic subsites (25, 26, 36-41), three studies observed stronger positive associations of adult BMI with distal than with proximal or rectal adenoma (26, 37, 41).
The positive association between body shape at age 5 and 10 years observed in the present study was not attenuated after adjustment for adult BMI, WHR or waist circumference. Similarly, in the Harvard Growth Study, the positive association between higher BMI during adolescence and colorectal cancer was not substantially altered after adjustment for adult BMI (13).
We observed a positive association between adult attained height and colorectal adenoma. This is in line with previously observed positive associations between adult height and risk of colorectal cancer (1) and two studies on colorectal adenoma (26, 42), whereas two other studies on colorectal adenoma did not observe an association with height (36, 37). IGF-1 levels during childhood and adolescence influence linear growth (17) and positive associations between height and colorectal adenomas may be explained by the cancer promoting effects of IGF-1 (29). Alternately, it has been suggested that height is associated with the total number of cells in the body (43) or with the length of the intestines (44), thereby increasing the chance of malignant cell-transformation. In our study, higher height was associated with risk of distal but not proximal or rectal adenoma. In contrast, in another study which examined adult height in relation to adenoma subsites, positive associations were found for proximal and rectal, but not distal adenoma. Further studies to elucidate the site-specific effects of adult height on colorectal adenoma are warranted.
Strengths of our study include the ability to control for a variety of potential confounders including BMI during adulthood and to specifically investigate adenomas by anatomic subsite or histological stage. Our study also has several limitations. In estimating body fatness during childhood and adolescence we relied on the participant's memory and ability to correctly classify their body shape according to the 9-level figure drawing, which also has the limitation of being age-neutral, i.e., the same figure drawing was used for estimation of body shape at ages 5, 10, and 20 years. In two validation studies using the same figure drawing, high correlations between childhood body shape recalled among elderly and measured BMI during childhood were observed (23, 45). The accuracy of recall did not differ by adult BMI, but there is concern of systematic underestimation, i.e., the tendency to underestimate body fatness at young ages increased with increasing BMI at the recalled age (45). However, in our study we were able to detect a significant trend across all body fatness levels providing support for the validity of body fatness estimation. Furthermore, misclassification of recalled body fatness is more likely to occur between adjacent categories than extreme categories and we observed significant positive associations when comparing extreme categories of body fatness, i.e. women who were most lean versus women who were overweight.
Another potential source of error in our study is that some women had more than one endoscopy during the follow-up. However, this is not likely to be related to childhood obesity and our observed positive associations between body fatness and adenoma were slightly stronger when restricting the study population to women who had only one endoscopy during follow-up.
In conclusion, our study confirms previous findings of a positive association between adult attained height and risk of colorectal adenoma and suggests that body fatness during childhood increases risk of colorectal adenoma later in life, independent of adult BMI. These findings suggest that certain risk factors for colorectal cancer such as body fatness may act very early in life and if confirmed by other studies could have important public health implications.
Acknowledgments
Grant support
The work in this manuscript was supported by the American Institute for Cancer Research (Investigator initiated grant (II) to K.W.), National Cancer Institute (R01 CA50385 to W.C.W.), and the Entertainment Industry Foundation's National Colorectal Cancer Research Alliance (NCCRA to W.C.W.).
K.N. is recipient of a scholarship within the Postdoc-Program of the German Academic Exchange Service (DAAD).
References
- 1.World Cancer Research Fund / American Institute for Cancer R Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 2.Lee WW. An overview of pediatric obesity. Pediatric diabetes. 2007;8 9:76–87. doi: 10.1111/j.1399-5448.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 3.Cali AM, Caprio S. Obesity in children and adolescents. The Journal of clinical endocrinology and metabolism. 2008;93(11 Suppl 1):S31–6. doi: 10.1210/jc.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uauy R, Solomons N. Diet, nutrition, and the life-course approach to cancer prevention. The Journal of nutrition. 2005;135(12 Suppl):2934S–45S. doi: 10.1093/jn/135.12.2934S. [DOI] [PubMed] [Google Scholar]
- 5.Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28(26):4052–7. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caprio S, Hyman LD, Limb C, McCarthy S, Lange R, Sherwin RS, et al. Central adiposity and its metabolic correlates in obese adolescent girls. The American journal of physiology. 1995;269(1 Pt 1):E118–26. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- 7.Harriss DJ, Atkinson G, George K, Cable NT, Reilly T, Haboubi N, et al. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Dis. 2009;11(6):547–63. doi: 10.1111/j.1463-1318.2009.01766.x. [DOI] [PubMed] [Google Scholar]
- 8.Bjorge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. American journal of epidemiology. 2008;168(1):30–7. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 9.Burton A, Martin R, Galobardes B, Davey Smith G, Jeffreys M. Young adulthood body mass index and risk of cancer in later adulthood: historical cohort study. Cancer Causes Control. 2010 doi: 10.1007/s10552-010-9625-3. [DOI] [PubMed] [Google Scholar]
- 10.Jeffreys M, Smith GD, Martin RM, Frankel S, Gunnell D. Childhood body mass index and later cancer risk: a 50-year follow-up of the Boyd Orr study. International journal of cancer. 2004;112(2):348–51. doi: 10.1002/ijc.20423. [DOI] [PubMed] [Google Scholar]
- 11.Le Marchand L, Wilkens LR, Mi MP. Obesity in youth and middle age and risk of colorectal cancer in men. Cancer Causes Control. 1992;3(4):349–54. doi: 10.1007/BF00146888. [DOI] [PubMed] [Google Scholar]
- 12.Lee IM, Paffenbarger RS., Jr Quetelet's index and risk of colon cancer in college alumni. Journal of the National Cancer Institute. 1992;84(17):1326–31. doi: 10.1093/jnci/84.17.1326. [DOI] [PubMed] [Google Scholar]
- 13.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. The New England journal of medicine. 1992;327(19):1350–5. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 14.Okasha M, McCarron P, McEwen J, Smith GD. Body mass index in young adulthood and cancer mortality: a retrospective cohort study. Journal of epidemiology and community health. 2002;56(10):780–4. doi: 10.1136/jech.56.10.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittemore AS, Paffenbarger RS, Jr, Anderson K, Lee JE. Early precursors of site-specific cancers in college men and women. Journal of the National Cancer Institute. 1985;74(1):43–51. [PubMed] [Google Scholar]
- 16.Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. The British journal of surgery. 2002;89(7):845–60. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 17.Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. The Journal of clinical endocrinology and metabolism. 1994;78(3):744–52. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- 18.Engeland A, Tretli S, Austad G, Bjorge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control. 2005;16(8):987–96. doi: 10.1007/s10552-005-3638-3. [DOI] [PubMed] [Google Scholar]
- 19.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Journal of the National Cancer Institute. 2006;98(13):920–31. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 20.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, et al. Comparison of risk factors for colon and rectal cancer. International journal of cancer. 2004;108(3):433–42. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. AmJEpidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 22.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. In: Kety SS, Rowland LP, Sidman SQ, Mathysee SW, editors. The Genetics of Neurological and Psychiatric Disorders. New York City: Raven Press; 1983. pp. 115–20. [PubMed] [Google Scholar]
- 23.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. American journal of epidemiology. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology (Cambridge, Mass. 1990;1(6):466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Hermann S, Rohrmann S, Linseisen J. Lifestyle factors, obesity and the risk of colorectal adenomas in EPIC-Heidelberg. Cancer Causes Control. 2009;20(8):1397–408. doi: 10.1007/s10552-009-9366-3. [DOI] [PubMed] [Google Scholar]
- 26.Morois S, Mesrine S, Josset M, Clavel-Chapelon F, Boutron-Ruault MC. Anthropometric Factors in Adulthood and Risk of Colorectal Adenomas: The French E3N-EPIC Prospective Cohort. American journal of epidemiology. 2010 doi: 10.1093/aje/kwq258. [DOI] [PubMed] [Google Scholar]
- 27.Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7(3):R314–25. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 29.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. The Proceedings of the Nutrition Society. 2001;60(1):91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 30.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nature reviews. 2008;8(12):915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 31.Barnard ND. Trends in food availability, 1909-2007. The American journal of clinical nutrition. 91(5):1530S–6S. doi: 10.3945/ajcn.2010.28701G. [DOI] [PubMed] [Google Scholar]
- 32.Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. Journal of surgical oncology. 2004;88(4):261–6. doi: 10.1002/jso.20156. [DOI] [PubMed] [Google Scholar]
- 33.Iacopetta B. Are there two sides to colorectal cancer? International journal of cancer. 2002;101(5):403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 34.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 35.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11(1):19–30. doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 36.Bird CL, Frankl HD, Lee ER, Haile RW. Obesity, weight gain, large weight changes, and adenomatous polyps of the left colon and rectum. American journal of epidemiology. 1998;147(7):670–80. doi: 10.1093/oxfordjournals.aje.a009508. [DOI] [PubMed] [Google Scholar]
- 37.Boutron-Ruault MC, Senesse P, Meance S, Belghiti C, Faivre J. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutrition and cancer. 2001;39(1):50–7. doi: 10.1207/S15327914nc391_7. [DOI] [PubMed] [Google Scholar]
- 38.Chung YW, Han DS, Park YK, Son BK, Paik CH, Lee HL, et al. Association of obesity, serum glucose and lipids with the risk of advanced colorectal adenoma and cancer: a case-control study in Korea. Dig Liver Dis. 2006;38(9):668–72. doi: 10.1016/j.dld.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs ET, Ahnen DJ, Ashbeck EL, Baron JA, Greenberg ER, Lance P, et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: a pooling study. American journal of epidemiology. 2009;169(6):657–66. doi: 10.1093/aje/kwn401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen IK, Grotmol T, Almendingen K, Hoff G. Lifestyle as a predictor for colonic neoplasia in asymptomatic individuals. BMC gastroenterology. 2006;6:5. doi: 10.1186/1471-230X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honjo S, Kono S, Shinchi K, Wakabayashi K, Todoroki I, Sakurai Y, et al. The relation of smoking, alcohol use and obesity to risk of sigmoid colon and rectal adenomas. Jpn J Cancer Res. 1995;86(11):1019–26. doi: 10.1111/j.1349-7006.1995.tb03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauret KG, Bostick RM, Matthews CE, Hussey JR, Fina MF, Geisinger KR, et al. Physical activity and reduced risk of incident sporadic colorectal adenomas: observational support for mechanisms involving energy balance and inflammation modulation. American journal of epidemiology. 2004;159(10):983–92. doi: 10.1093/aje/kwh130. [DOI] [PubMed] [Google Scholar]
- 43.Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. Epidemiologic reviews. 2001;23(2):313–42. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 44.Ahrens EH, Jr, Blankenhorn DH, Hirsch J. Measurement of the human intestinal length in vivo and some causes of variation. Gastroenterology. 1956;31(3):274–84. [PubMed] [Google Scholar]
- 45.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? American journal of epidemiology. 2002;155(7):672–9. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]


