Abstract
Low vitamin D (VD) status may increase prostate cancer risk but experimental evidence for this relationship is modest. We tested whether low VD status or VD receptor (VDR) deletion influences prostate epithelial cell (PEC) biology using intact mice, castrated mice, or castrated mice treated with testosterone propionate (TP, 2.5 mg/kg BW). PEC proliferation (Ki-67 staining) and apoptosis (TUNEL method) were determined in the anterior prostate (AP). In study 1, wild-type (WT) and TgAPT121 mice (a model of prostate intraepithelial neoplasia) were fed diets with 25, 200 (reference diet), or 10000 IU VD/kg diet (as vitamin D3) prior to castration/repletion. Serum 25 hydroxyvitamin D levels were 26, 78, and 237 nmol/L in the three diet groups, respectively. Castration reduced proliferation and increased apoptosis in the AP while TP reversed these effects. Low VD diet increased proliferation in WT (+82%) and TgAPT121 (+24%) mice while it suppressed apoptosis in WT (−29%) and TgAPT121 (−37%) mice. This diet also increased the severity of PIN lesions in the AP of intact TgAPT121 mice. In study 2, mice with PEC-specific VDR deletion (PEC VDR KO) were examined after castration/repletion. TUNEL staining was 60% lower in castrated PEC VDR KO mice compared to castrated WT mice. In castrated mice given TP, Ki-67 staining was 2-fold higher in PEC VDR KO compared to WT mice. Our data show that low diet VDR or VDR deletion provide a prostate environment that is permissive to early procarcinogenic events that enhance prostate cancer risk.
Keywords: androgen, stroma, epithelium, carcinogenesis, diet
Introduction
High serum 25 hydroxyvitamin D3 (25OH D) levels have been proposed to reduce prostate cancer risk (1), a finding observed in some (2, 3), but not other (4) epidemiologic studies. While the epidemiologic evidence is equivocal, studies on cultured prostate cells supports this hypothesis. 25OH D has low affinity for the vitamin D receptor (VDR) but is further metabolized to 1α,25 dihydroxyvitamin D3 (1,25(OH)2 D), a hormone that binds the VDR and regulates gene transcription (5). The VDR has been detected in the secretory-epithelial and stromal cells of the human prostate (6) and treatment of cells from the healthy prostate, from benign prostatic hypertrophy, and from prostate cancer with 1,25(OH)2 D induces growth inhibition that is dependent upon the presence of the VDR (7, 8). In vivo, pharmacologic treatment with 1,25(OH)2D suppresses prostate tumor growth in models like Nkx3.1+/−; Pten+/− mice (9) and in mice with LNCaP (10) or PC3 (11) xenographs. However, the impact of dietary-induced alterations in serum 25OH D on prostate biology and cancer is less certain. Critically, except during frank vitamin D deficiency, serum 1,25(OH)2D levels are not modulated by elevated serum 25OH D levels (12). As a result, studies using 1,25(OH)2D treatment do not address whether variations in serum 25OH D can alter prostate cell biology or prostate cancer in vivo.
Prostate carcinogenesis is a multi-step process with a long latency period. As such, there are many points where agents could provide a preventative benefit (13). One potentially vulnerable period is during adolescence where testosterone induces events that increase the size of the prostate gland by several-fold. Prostate expansion is followed by the appearance of low grade prostate intraepithelial neoplastic (PIN) lesions (14) suggesting that prostate gland expansion permits molecular events necessary to initiate prostate cancer. With this in mind, we hypothesize that high vitamin D status may exert its beneficial effect early in life when the prostate is more susceptible to cancer initiating genetic or epigenetic events.
Here we examined the impact of disrupting vitamin D signaling in mice using castration-repletion to model the rapid expansion of prostate mass (15) and in TgAPT121 mice that model the early stages of cancer where prostate epithelial cell (PEC) hyperproliferation and low grade PIN is evident (16). Our data show that low dietary vitamin D intake or deletion of VDR in PECs create an environment in the prostate characterized by high proliferation and low apoptotic rates that may be permissive to events that enhance subsequence prostate carcinogenesis. This is the first direct evidence that links vitamin D status to the modulation of prostate biology.
Materials and Methods
Animal and diet information
Experiments were approved by the Purdue Animal Care and Use Committee. Mice were housed with a 12 h light/12 h dark photoperiod and lights were covered with a UVB filter to prevent skin vitamin D synthesis (Pegasus Associates Lighting, Beaver, PA). AIN93G-based (17) diets were purchased from Research Diets (New Brunswick, NJ). Diets and water were provided ad libitum.
Probasin promoter-Cre recombinase mice were obtained from the NCI Mouse Models of Human Cancers Consortium (PB-Cre, Frederick, MD; strain number 01XF5, genetic background C57BL/6N). Mice with loxP sites flanking exon 2 of the VDR gene (VDRfl/fl, genetic background C57BL/6 × TT2) were from Dr. S. Kato (University of Tokyo, Japan). TgAPT121 mice were from Dr. Terry Van Dyke (NCI, Frederick, MD, C57BL/6 × DBA/2J genetic background) (16). Female TgAPT121 mice were bred to male B6D2F1 mice (Jackson Laboratories, Bar Harbor, ME) to perpetuate the line. Non-transgenic mice from these breedings were used as wild-type controls in experiment 1.
Mice with prostate epithelium-specific VDR ablation (VDRfl/fl; PB-Cre+/− mice; PEC VDR KO) were made by breeding VDRfl/fl mice to PB-Cre mice and crossing the offspring back to VDRfl/fl mice. Mice from these litters lacking the PB-Cre transgene were used as controls.
Genotyping
Genomic DNA was prepared by using the Qiagen DNeasy kit (Qiagen, Valencia, CA). The APT121 transgene was identified by PCR (16). Other PCR primers for genotyping were: PB-Cre transgene Cre #1, 5′ACCAGCCAGCTATCAACTCG3′ 5′TTACATTGGTCCAGCCACC3′ (199 bp product); floxed or wild type VDR allele (see Figure 5A): VDR #1, 5′ TCTGACTCCCACAAGTGTACCACGG3′, and VDR #2, 5′ATGGACAGGAACACACAGCATCA3′ (WT = 256 bp product; floxed VDR allele = 337 bp product); deleted exon 2 allele of VDR: VDR #1, and VDR #3, 5′CCAGGTGAGTTTACCTACCACTTCCC3′ (348 bp product). The amplification protocol used was 94°C/10 min (1 cycle), 94°C/1 min; 60°C/1 min; 72°C/1 min (35 cycles), 75°C/10 min (1 cycle).
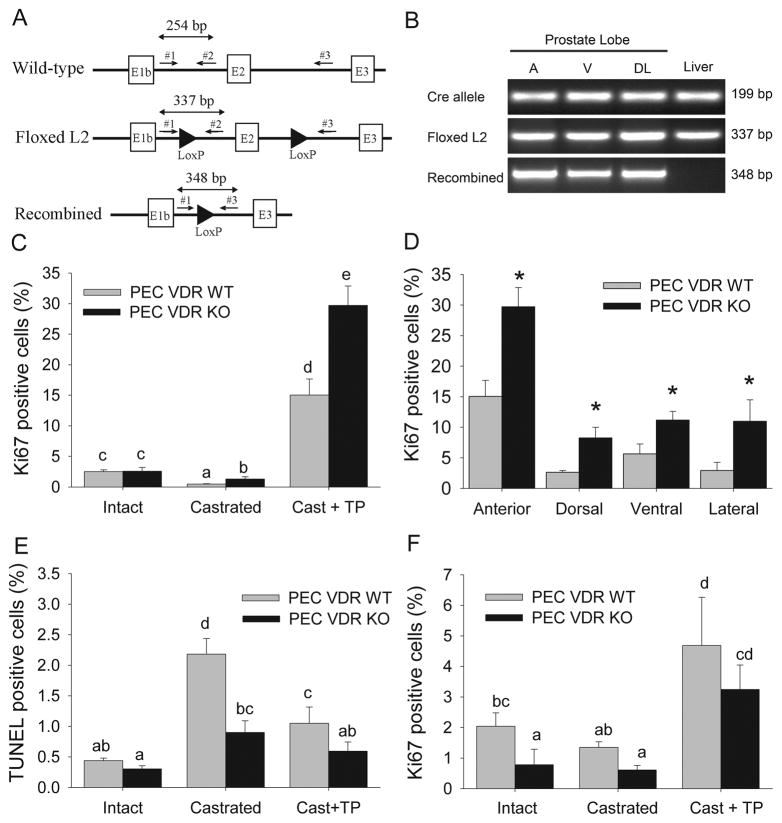
Figure 5. Prostate epithelial cell deletion of VDR regulates prostate cell proliferation and apoptosis.
Mice with prostate epithelial cell-specific deletion of the VDR (PEC VDR KO) or their wild-type littermates (PEC VDR WT) were subjected to castration (Cast) and testosterone repletion (Cast + TP). (A) Schematic of wild-type, floxed (L2), and Cre-recombined VDR alleles showing exons (boxes), LoxP sites (arrowheads) and PCR primers (arrows). (B) PCR analysis of VDR alleles. Cre-recombinase transgene = Cre allele, the floxed VDR L2 allele = Floxed L2, Cre-recombined VDR allele = Recombined. (C) Ki67 labeled prostate epithelial cells in the anterior lobe; (D) Ki67 labeled prostate epithelial cells in each of the lobes of the Cast + TP group; (E) TUNEL stained prostate epithelial cells in the anterior lobe; (F) Ki67 labeled prostate stromal cells in the anterior prostate. Bars are the mean±SE, n=6. In (C, E, F) bars without a common letter superscript are significantly different (p<0.05). In (D) * = significantly different from the PEC VDR WT group (p<0.05).
Experimental design
Effects of dietary vitamin D level on androgen-dependent proliferation and apoptosis in wild-type and TgAPT121 mouse prostate
At 15 d of age pups and dams of were switched from commercial chow diets to AIN93G diets modified to contain 200 IU vitamin D3/kg diet. At weaning, 54 male wild type (WT) and 54 male TgAPT121 littermates were randomized to one of 9 groups in a 3 × 3 factorial design experiment modulating dietary vitamin D3 (final levels were 25 IU (0.625μg), 200 IU (5 μg, reference diet group), or 10,000 IU (250 μg)/kg of diet) and androgen status (intact, castrated, castrated plus testosterone propionate (TP)) (n=6 mice per group). AIN-93G diets (17) were modified to contain increasing amounts of vitamin D3. At 9 wks, 36 mice per genotype were weighed and surgically castrated. After 1 wk, 6 castrated mice per genotype and diet group received an osmotic pump (subcutaneous implantation, Alzet Corp., Cupertino, CA) containing TP dissolved in a 4:1 mixture of dimethy sulfoxide and ethanol (2.5 mg/kg/d, Sigma-Aldrich, St. Louis, MO). The dose of TP was determined to restore the proliferative rate of PECs in preliminary studies (data not shown). 5 d after pump implantation, all animals were sacrificed. At harvest blood was collected by cardiac puncture and serum was isolated and saved at −80°C. Prostate, bladder and seminal vesicles were harvested en block and processed for immunohistochemical analysis.
Role of VDR level on androgen dependent proliferation and apoptosis in mouse prostate
At 15 d of age pups and their dams of were switched from commercial chow diets to AIN93G diet containing 200 IU vitamin D3/kg diet. At weaning 23 male WT and 23 male PEC VDR KO littermates were randomized into three androgen status groups: intact, castrated, or castrated + TP treatment groups (n=7–8 per genotype, 2 × 3 factorial design). At 9 wks of age mice underwent the castration-TP repletion protocol and all mice were euthanized 5 d after the start of TP repletion (75 d of age) for tissue harvests as described above.
Immunohistochemistry
The urogenital tract was fixed in 10% neutral buffered formalin for 24 h and stored in 75% ethanol for 3–5 d. It was then divided into a portion containing the anterior prostate lobes and a portion containing the ventral, dorsal and lateral lobes according to the recommendations of the Bar Harbor Classification of Mouse Prostate Pathology (18). These portions were individually processed and embedded into paraffin. Step sections (4.0 μm thick) were prepared from all of the blocks and stained with hematoxylin and eosin. Immunohistochemical analysis for cell proliferation was done by staining for Ki67 antigen. Antigen retrieval was done by boiling the slides in citrate buffer (Biogenex, San Ramon, CA) for 15 min. Endogenous peroxidase activity was quenched with 10-min incubation in 3% H2O2 in methanol. Sections were incubated with anti-Ki67 primary antibody (1:200, M7249, DAKO Corp, Carpinteria, CA), for 30 min at room temperature, followed by 30-min incubation with a horseradish peroxidase (HRP) labeled secondary antibody. Detection for specific Ki67 staining was done using the rodent IHC Detection Systems Vector (Biogenex, San Ramon, CA) and sections were countertained with hematoxylin. Apoptosis was evaluated by TUNEL staining with the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International, Billerica, MA) and were counterstained with methyl green.
Image capture, quantification and grading
Tissue sections were reviewed without knowledge of experimental group. For proliferation and apoptotic index, 5 representative, non-overlapping images without artifacts (400x magnification) were digitally recorded for each anterior lobe and 3 images were collected for the other prostatic lobes using bright field microscopy (Olympus BX-51, Tokyo, Japan). The captured images were analyzed using Image-Pro Plus 5.5 image analysis software (Media Cybernetics, Silver Spring, MD).
Proliferation index (PI) was the percentage of Ki67 stained nuclei in 5 (anterior lobe) or 3 (other lobes) independent fields; PI (%) = L × 100/(L + C), where L = labeled cells and C = counterstained, unlabeled cells. The rate of apoptosis in prostatic epithelium was the percentage of TUNEL stained nuclei. Apoptotic index (AI %)= L × 100/(L + C).
For grading, Ki67 and hematoxylin stained sections of the anterior prostate from intact 11 wks ld TgAPT121 mice were examined using the 6 grades established for the lesions of TRAMP mice (19). TgAPT121 mice do not express the advanced grade 6 lesions seen in TRAMP mice and they express grade 5 lesions only after 4–6 months of age. Lesions were grouped into two categories: normal/low grade (normal, grade 1 and 2) or high grade (grade 3 and 4). Data were evaluated as number of lesions in each grade, area of each grade, and % total area accounted for by each grade.
Serum Calcium and vitamin D metabolite analysis
Serum 1,25(OH)2D and 25OH D were determined by radioimmunoassay (IDS Inc, Fountain Hills, AZ). Serum calcium concentrations were measured using the QuantiChrom™ Calcium Assay Kit (BioAssay Systems, Hayward, CA).
Statistical analysis
Prior to analysis, all data were checked to ensure they fit a normal distribution using the Shapiro-Wilk test. Skewed or non-normally distributed data was log transformed prior to analysis and the correction to a normal distribution was confirmed. Values are reported as means ± SE of the non-transformed data.
Treatment effects for castration-repletion studies were assessed by 2-way ANOVA within each genotype group (main effects for study 1 = castration state, diet; for study 2 = castration state, genotype) using the SAS statistical software package (SAS 9.1.2 Cary, NC). For study 1 orthogonal contrasts for diet effects were conducted (25 IU vs. the other two vitamin D groups; 200 IU vs. 10k IU group). For study 2, pair-wise comparisons using Fisher’s protected least significance difference were utilized and differences between genotype groups were assessed within the castration + TP treatment group using a two-tailed Student’s t-test for: body weight, reproductive system weight, and Ki67 staining. Differences between means were considered significant at p<0.05.
When assessing the impact of diet on prostate histology in TgAPT121 mice a non-parametric equivalent of one-way ANOVA, the Kruskal-Wallis test, was used. When a significant diet effect was detected (p < 0.05), we performed two orthogonal contrasts (25 IU vs the other two vitamin D groups; 200 IU vs 10k IU group). The groups in a contrast were considered significantly different when p < 0.05.
Results
Effects of vitamin D and androgen status on growth and serum parameters
There was no effect of dietary vitamin D or androgen status on body weight (data not shown). As expected, castration significantly reduced the weight of the reproductive system (seminal vesicles, prostate, and bladder) by 64% (p<0.05) in both WT and TgAPT121 mice and TP repletion partially reversed this effect in both genotype groups.
The impact of dietary vitamin D or androgen status on serum vitamin D metabolite and calcium levels were not different between WT and TgAPT121 mice so these data are reported as pooled across genotype groups.
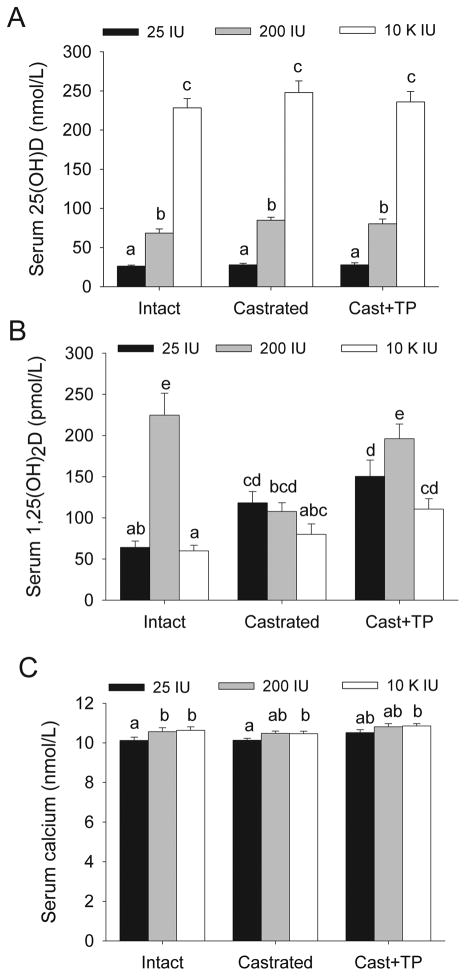
Raising dietary vitamin D level increased serum 25OH D from deficient (26.4±1.2 nmol/L at 25 IU), to adequate (73.0±4.1 nmol/L at 200 IU), or to supraphysiologic levels (245.1±13.1 nmol/L at 10,000 IU) (Figure 1A). Compared to the other diet groups, serum calcium levels were lower in mice fed the 25 IU vitamin D diet (p = 0.014 in intact mice, Figure 1C). Neither serum calcium nor serum 25OH D were influenced by changes in androgen status (Figure 1A, C). Consistent with our earlier report (20), feeding intact mice either the 25 IU diet or the 10k IU diet resulted in lower serum 1,25(OH)2D compared to the levels seen in intact mice fed the 200 IU diet (i.e. by 74.9% and 74.8%, respectively, p<0.05) (Figure 1B). The effect of androgen status on serum 1,25(OH)2D level was influenced by dietary vitamin D level. In the 200 IU group serum 1,25(OH)2D was 53% lower after castration (p<0.001) and TP repletion restored it to the level seen in intact mice. In the 25 IU diet group, serum 1,25(OH)2D level was higher after castration (p=0.02) but TP infusion did not reverse this effect.
Figure 1. Regulation of serum vitamin D metabolites and calcium levels by dietary vitamin D and castration/repletion.
Wild type and TgAPT121 mice were fed diets with increasing levels of vitamin D from weaning followed by castration (Cast) and testosterone repletion (Cast + TP). Serum was analyzed for (A) 25OH D, (B) 1,25(OH)2 D, and (C) calcium. Bars are the mean ± SE of the combined wild type and TgAPT121 mice data, n = 12. Bars without a common letter are significantly different, P<0.05.
Dietary vitamin D modulates proliferation and apoptosis in the epithelial cells of anterior prostate of wild type and TgAPT121 mice
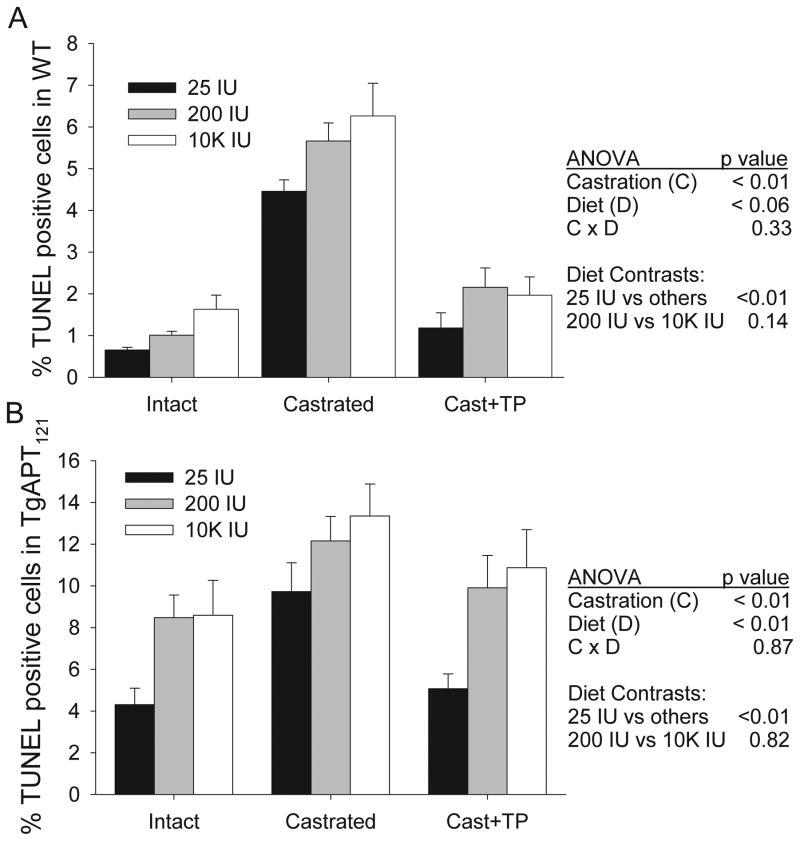
As expected, Ki67 (> 15-fold) and TUNEL staining (5 fold) were higher in the PEC of anterior prostate in intact TgAPT121 mice compared to intact WT mice (genotype effect, p<0.01)(see Supplemental figure for representative staining). In both lines castration significantly reduced, and TP infusion stimulated, PEC proliferation (castration main effect, p<0.01, Figure 2). Also, castration significantly increased, and TP infusion suppressed PEC apoptosis (castration main effect, p<0.01, Figure 3). However, there was no statistical interaction between androgen status and dietary vitamin D level on PEC proliferation or apoptosis in either WT or TgAPT121 mice.
Figure 2. Dietary vitamin D and castration/repletion regulate prostate epithelial cell proliferation.
Mice were fed diets with increasing levels of vitamin D from weaning followed by castration (Cast) and testosterone repletion (Cast + TP). Proliferation was quantified in Ki67 stained sections of the anterior prostate lobe from (A) wild type or (B) TgAPT121 mice. Bars are the mean ±SE, n=6. Results from 2-way ANOVA are presented along with the two orthogonal contrasts for diet.
Figure 3. Dietary vitamin D and castration/repletion regulate prostate epithelial cell apoptosis index.
Mice were fed diets with increasing levels of vitamin D from weaning followed by castration (Cast) and testosterone repletion (Cast + TP). Apoptosis was quantified in TUNEL stained sections of the anterior prostate lobe from (A) wild type or (B) TgAPT121 mice. Bars are the mean ±SE, n=6. Results from 2-way ANOVA are presented along with are presented along with the two orthogonal contrasts for diet.
Dietary vitamin D level influenced PEC proliferation in both WT and TgAPT121 mice (main effect of diet, p<0.001). Mice fed the 25 IU diet had the highest proliferation index in all three androgen status groups, although the impact of low vitamin D intake was proportionally greater in the WT group. Suppression of proliferation was maximal in the 200 IU group and no additional benefit was observed by raising serum 25OH D to supraphysiologic levels with the 10k IU diet. The apoptotic index was lowest in mice fed the 25 IU diet compared to the other diet groups (50% lower in intact and castrated + TP groups, 25% lower in castrated mice, p<0.05, Figure 3) and there was no additional impact of the 10k IU diet on TUNEL staining beyond that seen in the 200 IU diet group in either WT or TgAPT121 mice.
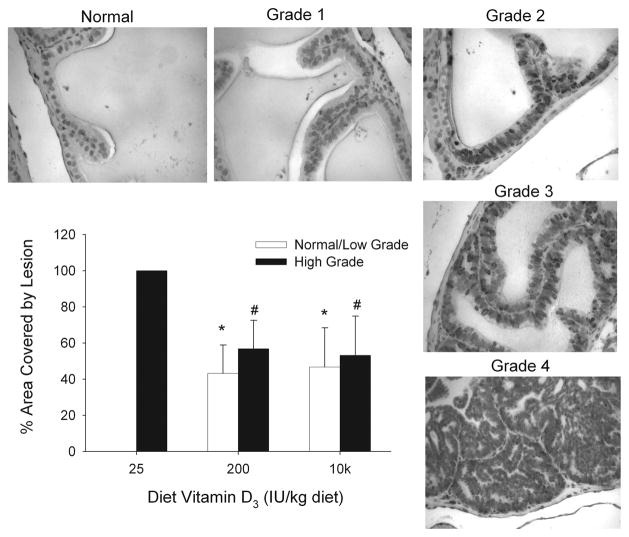
Dietary vitamin D level modulates the PIN phenotype of TgAPT121 mice
The TgAPT121 has prostate epithelial cell-specific expression of a truncated SV40 Large T antigen protein leading to a hyperproliferative phenotype in all lobes of the prostate. The model has strong histologic similarities to the earlier stages of human PCa, i.e. demonstrating hyperplasia by 5 wks, various grades of PIN starting at 7–8 wks, and adenocarcinoma starting at 12 wks of age (unpublished data from our lab and (16)). During the 11 wk study period all intact TgAPT121 mice developed epithelial cell abnormalities. However, TgAPT121 mice fed the 25 IU vitamin D diet had no areas of normal epithelium, simple hyperplasia, or low grade PIN; their prostates were filled with high grade PIN (Figure 4). In contrast, there was an equal distribution of high grade lesions and normal/low grade lesions in TgAPT121 mice fed the reference or high vitamin D diet. This suggests that the combination of high cell proliferation and low apoptosis seen in the prostate epithelial cells of TgAPT121 mice fed the low vitamin D diet contributes to an acceleration of the early prostate cancer phenotype in this model.
Figure 4. Dietary vitamin D3 level influences the severity of prostate lesions in the anterior prostate of TgAPT121 mice.
TgAPT121 mice were fed diets with increasing levels of vitamin D from weaning mice until 11 wks of age. Histological evaluation of the anterior prostate was conducted on Ki67 and hematoxylin stained tissue sections and classified as low grade (normal, grade 1, grade 2) or high grade (grade 3, grade 4) lesions. Representative images for the grades are presented. Bars are the mean ±SE, n=6. * Significantly different from the 25 IU diet, low grade group; # significantly different from the 25 IU diet, high grade group (p<0.05).
VDR is critical for the control of PEC proliferation and apoptosis in wild-type mice
Recombination of the VDR floxed region in PEC VDR KOanimals was confirmed by PCR in the prostate lobes of 8 wk old mice whereas only the floxed VDR allele was observed in the liver where the probasin promoter is not active (Figure 5B). The presence of the intact floxed VDR allele in prostate lobes reflects the multiple cell types in the prostate and the fact that only the epithelial cells express Cre recombinase from the probasin promoter (21).
At the end of the study, body weight was not different among the treatment or genotype groups (data not shown). Androgen status had expected effects on the weight of the reproductive tract, Ki67 staining, and TUNEL staining, and there was no effect of the VDR deletion on the weight of the reproductive system.
The PEC proliferation index was 97% higher in the anterior prostate of PEC VDR KO mice compared to WT mice in both the castration group and in the castration + TP group (p<0.05, Figure 5C). A similar effect of VDR deletion on PEC proliferation was seen in the dorsal, lateral, and ventral prostate lobes of mice from the castration + TP group (Figure 4D). The apoptotic index was significantly lower in mice from two PEC VDR KO groups: castrated (40% of WT values, p<0.05) and castrated + TP (57% of WT values, p<0.05). Although the apoptotic index was 31% lower in intact PEC VDR KO mice, this difference was not statistically significant (p = 0.09).
PEC VDR deletion affects proliferation of fibromuscular stroma cells
Others have demonstrated that communication between PECs and the cells of the stroma contributes to prostate carcinogenesis (22). Consistent with the existence of androgen receptor-mediated signaling in prostate stromal cells, castration and androgen repletion significantly regulated stromal cell proliferation index (p= 0.003). There was also a significant main effect of PEC VDR deletion on the Ki67 staining (−43%, p=0.005) (Figure 4F) but not TUNEL staining of stromal cells.
Discussion
There is growing evidence that high vitamin D status may reduce the risk of various cancers, including prostate cancer (23). Unfortunately, the direct evidence linking vitamin D status to prostate health is modest. Our data clearly show that low vitamin D status increased PEC proliferation and reduced PEC apoptosis and that these vitamin D-regulated events are not lost during the early hyperproliferative and dysplastic transition to PIN. In addition, we found that a greater proportion of the prostate epithelium was characterized by advanced PIN phenotypes in TgAPT121 mice fed the low vitamin D diet. As such, our study is the first to directly link diet-induced and human-relevant levels of serum 25OH D to the control of testosterone-driven prostate biology and the early stages of prostate cancer.
A consequence of low vitamin D status for normal prostate biology was previously suggested by Xue et al. (24). They found that PEC proliferation in the anterior and dorsal prostate lobes were elevated by 100% after feeding weanling mice a high fat diet with low folate, methionine, choline, and cysteine levels that is deficient in calcium (1/10th requirement) and had low vitamin D levels (100 IU). However, the weakness of this study is that neither serum Ca nor vitamin D metabolites were measured and the dietary calcium restriction was at a level others have coupled to low dietary vitamin D intake to deplete vitamin D status (25). As a result, in the Xue et al. study it is unclear how proliferation relates to levels of vitamin D status being discussed as protective against prostate cancer in humans.
Cell-based research has shown that 25OH D and 1,25(OH)2 D inhibit the growth of normal PECs (26), immortalized but non-transformed PECs (27), and prostate cancer cells (28). Several other cell studies show 1,25(OH)2 D or its analogs can induce apoptosis in prostate cancer cells (29, 30). These phenomena have also been seen in animal studies after treatment with vitamin D analogs, e.g. daily oral doses of BXL-628 for one month reduced prostate cell proliferation by 40% in castrated rats treated with testosterone (31), vitamin D analogs induced apoptosis in the prostate of rats (31) and mice (32). Unfortunately, treatment with 1,25(OH)2 D and its analogs often results in hypercalcemia (31, 33) making the results from these studies challenging to interpret regarding risks and benefits. Our data are consistent with these earlier findings and they extend them by directly demonstrating that these effects can be elicited by dietary vitamin D alone and by demonstrating that the protective effects of high vitamin D status can be extended to the cellular environment of early stage prostate cancer where growth control and cell survival are dysregulated.
The enzyme responsible for 1,25(OH)2 D production (CYP27B1) is expressed in PECs and treatment of cultured PEC with 25OH D leads to 1,25(OH)2 D production and cell growth arrest (34, 35). As a result it has been hypothesized that vitamin D-mediated effects on the prostate are due to increased local production of 1,25(OH)2 D from 25OH D. Our data are enlightening, but equivocal in this regard. On one hand, the 25 IU diet caused moderate vitamin D deficiency (i.e. normal growth, reduced serum 1,25(OH)2 D and calcium), suggesting the prostate cell effects seen in intact mice are a reflection of low serum 1,25(OH)2 D. On the other hand, the effects of vitamin D status on PEC dynamics was seen in castrated mice where serum 1,25(OH)2 D levels are not different between the dietary vitamin D groups, suggesting the differences in the 25 IU group are driven by low serum 25OH D levels. Regardless, our data do not support the hypothesis that there are benefits to increasing serum 25OH D beyond the value we observed in our 200 IU group (73 nmol/L).
Our data show that the VDR is crucial to protecting the prostate microenvironment. This is consistent with early studies showing that 1,25(OH)2 D-induced growth arrest of ALVA-31 cells was reduced by antisense RNA against the VDR (7) and that VDR over-expression enhanced 1,25(OH)2 D-mediated growth arrest in PC-3 and DU-145 cells (8). Others previously reported that VDR knockout mice have higher cell proliferation rates in the colon (36) and in mammary tissue (37) but our data are the first to show that the targeted deletion of VDR from PEC alters their growth in vivo. However, the gene targets for VDR-regulation of prostate cell proliferation and apoptosis are not known with certainty (27) and our in vivo approach cannot determine this mechanism. Some potential mechanisms include induction of cyclin-dependent kinase inhibitor p21 gene transcription (38), disruption of Wnt-signaling through direct VDR-β catenin interactions (27, 39), repression of genes for anti-apoptotic proteins (29, 40), and suppression of Iroquois homeobox gene 5 (Lrx5) expression (a negative regulator of p21 and p53 that inhibits apoptosis) (41). Future studies will be needed to gain greater insight into the molecular mechanisms for the effects of vitamin D status that we saw on PEC proliferation and apoptosis.
An unanticipated finding from our study is that while the deletion of VDR from PEC increased their proliferation index, it also reduced the proliferation index of the prostate stromal cells. There is an emerging body of research showing that autocrine and paracrine cross-talk between the prostate epithelial and stromal compartments is a component of prostate carcinogenesis (22). While both prostate epithelial and stromal cells have been reported to contain the VDR and respond to treatment with 1,25(OH)2 D (27, 42), our data are the first to show that disruption of VDR signaling in the epithelial cell may modulate the communication between epithelium and stroma. Epithelial cells are known to secrete a large number of growth factors that could influence stromal cell biology through paracrine signaling (43). However, it is not clear how 1,25(OH)2 D or VDR deletion influences these pathways in vivo.
Earlier studies in LNCaP cells showed that androgens increase VDR mRNA levels (44) and that androgens are needed for 1,25(OH)2 D-induced cell growth arrest (45). In contrast, we found that the impact of low vitamin D status or VDR content on the PEC was consistently observed regardless of androgen status (i.e. the main effects of androgen and vitamin D were statistically independent). This is consistent with studies showing that the anti-androgen effects of Casodex are independent of the antiproliferative activity of 1,25(OH)2 D in human cell lines derived from a bone metastasis of prostate cancer (46) or in several prostate cancer cells lines that express the androgen receptor (47). Thus, our data suggest that while androgen is the primary regulator of PEC growth, maintaining high vitamin D status and signaling through the VDR is an important, androgen-independent means to suppress cell growth in the normal prostate and in the early hyperproliferative stage of prostate cancer.
Another unanticipated finding from our study was that serum 1,25(OH)2 D levels were regulated by castration and androgen repletion in the reference 200 IU vitamin D diet group (i.e. reduced 53% after castration and restored by testosterone repletion). This suggests that androgens play an important role in regulating vitamin D metabolism and therefore calcium metabolism. Studies have shown that antiandrogen therapy in men is associated with osteoporosis and risk of fracture (48). Consistent with a role for androgens in the control of vitamin D and calcium metabolism, Francis et al. (49) found that serum 1,25(OH)2 D levels and intestinal calcium absorption increased significantly after testosterone treatment in hypogonadal men. However, additional research is needed to determine if the reduction in serum 1,25(OH)2 D levels we observed is simply a short term adjustment to castration or whether androgen depletion has a persistent impact on vitamin D metabolism.
In summary, our data clearly demonstrate that low vitamin D status and reduced signaling through the VDR disrupt the balance between proliferation and apoptosis in the epithelial cell of the normal prostate. The effect of low vitamin D intake also occurs in the prostate of TgAPT121 mice with premalignant prostate lesions and these vitamin D regulated effects on cell dynamics are associated with an increased severity of prostate cancer lesions. The effect of vitamin D signaling is consistent and can influence the PEC independent of androgen status. We believe that low vitamin D status/signaling creates a microenvironment in the prostate that is permissive to early procarcinogenic, testosterone-stimulated events that enhance subsequent prostate cancer risk.
Supplementary Material
Acknowledgments
This research was supported by an American Institute for Cancer Research grant and NCI award CA101113 to JCF and SKC.
The authors thank Ms. Kate Barzan and Ms. Rebecca McCreedy-Replogle for technical assistance with the animal studies.
Abbreviations
- 1,25(OH)2 D
1,25 dihydroxyvitamin D3
- 25OH D
25 hydroxyvitamin D3
- BW
body weight
- IU
international unit
- PEC
prostate epithelial cell
- PIN
prostate intraepithelial neoplasia
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- TP
testosterone proprionate
- VDR
vitamin D receptor
- WT
wild-type
Reference List
- 1.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11:847–52. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 3.Corder EH, Guess HA, Hulka BS, Friedman GD, Sadler M, Vollmer RT, et al. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomarkers Prev. 1993;2:467–72. [PubMed] [Google Scholar]
- 4.Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, et al. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolis RN, Christakos S. The nuclear receptor superfamily of steroid hormones and vitamin D gene regulation. An update. Ann N Y Acad Sci. 2010;1192:208–14. doi: 10.1111/j.1749-6632.2009.05227.x. [DOI] [PubMed] [Google Scholar]
- 6.Kivineva M, Blauer M, Syvala H, Tammela T, Tuohimaa P. Localization of 1,25-dihydroxyvitamin D-3 receptor (VDR) expression in human prostate. Journal of Steroid Biochemistry and Molecular Biology. 1998;66:121–7. doi: 10.1016/s0960-0760(98)00054-5. [DOI] [PubMed] [Google Scholar]
- 7.Hedlund TE, Moffatt KA, Miller GJ. Vitamin D receptor expression is required for growth modulation by 1 alpha,25-dihydroxyvitamin D3 in the human prostatic carcinoma cell line ALVA-31. J Steroid Biochem Mol Biol. 1996;58:277–88. doi: 10.1016/0960-0760(96)00030-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang SH, Schwartz GG, Cameron D, Burnstein KL. Vitamin D receptor content and transcriptional activity do not fully predict antiproliferative effects of vitamin D in human prostate cancer cell lines. Mol Cell Endocrinol. 1997;126:83–90. doi: 10.1016/s0303-7207(96)03974-3. [DOI] [PubMed] [Google Scholar]
- 9.Banach-Petrosky W, Ouyang X, Gao H, Nader K, Ji Y, Suh N, et al. Vitamin D inhibits the formation of prostatic intraepithelial neoplasia in Nkx3. 1;Pten mutant mice. Clin Cancer Res. 2006;12:5895–901. doi: 10.1158/1078-0432.CCR-06-1039. [DOI] [PubMed] [Google Scholar]
- 10.Guns ES, Xie X, Fedoruk M, Madera C, Cowell S, Mayer LD, et al. pH modulation using CsCl enhances therapeutic effects of vitamin D in LNCaP tumor bearing mice. Prostate. 2005;64:316–22. doi: 10.1002/pros.20257. [DOI] [PubMed] [Google Scholar]
- 11.Yin Y, Ni J, Chen M, Guo Y, Yeh S. RRR-alpha-vitamin E succinate potentiates the antitumor effect of calcitriol in prostate cancer without overt side effects. Clin Cancer Res. 2009;15:190–200. doi: 10.1158/1078-0432.CCR-08-0910. [DOI] [PubMed] [Google Scholar]
- 12.Need AG, O’Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D Metabolites and Calcium Absorption in Severe Vitamin D Deficiency. J Bone Miner Res. 2008 doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 13.Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer. 2002;2:537–43. doi: 10.1038/nrc844. [DOI] [PubMed] [Google Scholar]
- 14.Sakr WA, Sarkar FH, Sreepathi P, Drozdowicz S, Crissman JD. Measurement of cellular proliferation in human prostate by AgNOR, PCNA, and SPF. Prostate. 1993;22:147–54. doi: 10.1002/pros.2990220207. [DOI] [PubMed] [Google Scholar]
- 15.Colombel MC, Buttyan R. Hormonal control of apoptosis: the rat prostate gland as a model system. Methods Cell Biol. 1995;46:369–85. doi: 10.1016/s0091-679x(08)61936-6. [DOI] [PubMed] [Google Scholar]
- 16.Hill R, Song Y, Cardiff RD, Van Dyke T. Heterogeneous tumor evolution initiated by loss of pRb function in a preclinical prostate cancer model. Cancer Res. 2005;65:10243–54. doi: 10.1158/0008-5472.CAN-05-1579. [DOI] [PubMed] [Google Scholar]
- 17.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: Final report of the american institute of nutrition Ad Hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 18.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 19.Suttie A, Nyska A, Haseman JK, Moser GJ, Hackett TR, Goldsworthy TL. A grading scheme for the assessment of proliferative lesions of the mouse prostate in the TRAMP model. Toxicol Pathol. 2003;31:31–8. doi: 10.1080/01926230390173842. [DOI] [PubMed] [Google Scholar]
- 20.Fleet JC, Gliniak C, Zhang Z, Xue Y, Smith KB, McCreedy R, et al. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr. 2008;138:1114–20. doi: 10.1093/jn/138.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–9. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 22.Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76:578–86. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 23.Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29:388–96. doi: 10.1016/j.mam.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue L, Yang K, Newmark H, Lipkin M. Induced hyperproliferation in epithelial cells of mouse prostate by a Western-style diet. Carcinogenesis. 1997;18:995–9. doi: 10.1093/carcin/18.5.995. [DOI] [PubMed] [Google Scholar]
- 25.Vieth R, Fraser D, Kooh SW. Low dietary calcium reduces 25-hydroxycholecalciferol in plasma of rats. J Nutr. 1987;117:914–8. doi: 10.1093/jn/117.5.914. [DOI] [PubMed] [Google Scholar]
- 26.Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. 1994;54:805–10. [PubMed] [Google Scholar]
- 27.Kovalenko PL, Zhang Z, Cui M, Clinton SK, Fleet JC. 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genomics. 2010;11:26. doi: 10.1186/1471-2164-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller GJ, Stapleton GE, Hedlund TE, Moffat KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1 alpha, 25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995;1:997–1003. [PubMed] [Google Scholar]
- 29.Guzey M, Kitada S, Reed JC. Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667–77. [PubMed] [Google Scholar]
- 30.Berkovich L, Ben-Shabat S, Sintov AC. Induction of apoptosis and inhibition of prostate and breast cancer growth by BGP-15, a new calcipotriene-derived vitamin D3 analog. Anticancer Drugs. 2010;21:609–18. doi: 10.1097/CAD.0b013e328337f3e9. [DOI] [PubMed] [Google Scholar]
- 31.Crescioli C, Ferruzzi P, Caporali A, Scaltriti M, Bettuzzi S, Mancina R, et al. Inhibition of prostate cell growth by BXL-628, a calcitriol analogue selected for a phase II clinical trial in patients with benign prostate hyperplasia. Eur J Endocrinol. 2004;150:591–603. doi: 10.1530/eje.0.1500591. [DOI] [PubMed] [Google Scholar]
- 32.Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, Sanvito F, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504–11. doi: 10.4049/jimmunol.177.12.8504. [DOI] [PubMed] [Google Scholar]
- 33.Oades GM, Dredge K, Kirby RS, Colston KW. Vitamin D receptor-dependent antitumour effects of 1,25-dihydroxyvitamin D3 and two synthetic analogues in three in vivo models of prostate cancer. BJU Int. 2002;90:607–16. doi: 10.1046/j.1464-410x.2002.02964.x. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7:391–5. [PubMed] [Google Scholar]
- 35.Barreto AM, Schwartz GG, Woodruff R, Cramer SD. 25-Hydroxyvitamin D3, the prohormone of 1,25-dihydroxyvitamin D3, inhibits the proliferation of primary prostatic epithelial cells. Cancer Epidemiol Biomarkers Prev. 2000;9:265–70. [PubMed] [Google Scholar]
- 36.Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, et al. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–35. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 37.Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoidtissues. J Steroid Biochem Mol Biol. 2005;97:153–64. doi: 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 38.Boyle BJ, Zhao XY, Cohen P, Feldman D. Insulin-like growth factor binding protein-3 mediates 1 alpha,25-dihydroxyvitamin d(3) growth inhibition in the LNCaP prostate cancer cell line through p21/WAF1. J Urol. 2001;165:1319–24. [PubMed] [Google Scholar]
- 39.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blutt SE, McDonnell TJ, Polek TC, Weigel NL. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology. 2000;141:10–7. doi: 10.1210/endo.141.1.7289. [DOI] [PubMed] [Google Scholar]
- 41.Myrthue A, Rademacher BL, Pittsenbarger J, Kutyba-Brooks B, Gantner M, Qian DZ, et al. The iroquois homeobox gene 5 is regulated by 1,25-dihydroxyvitamin D3 in human prostate cancer and regulates apoptosis and the cell cycle in LNCaP prostate cancer cells. Clin Cancer Res. 2008;14:3562–70. doi: 10.1158/1078-0432.CCR-07-4649. [DOI] [PubMed] [Google Scholar]
- 42.Lou YR, Miettinen S, Kagechika H, Gronemeyer H, Tuohimaa P. Retinoic acid via RARalpha inhibits the expression of 24-hydroxylase in human prostate stromal cells. Biochem Biophys Res Commun. 2005;338:1973–81. doi: 10.1016/j.bbrc.2005.10.178. [DOI] [PubMed] [Google Scholar]
- 43.Niu YN, Xia SJ. Stroma-epithelium crosstalk in prostate cancer. Asian J Androl. 2009;11:28–35. doi: 10.1038/aja.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ting HJ, Bao BY, Hsu CL, Lee YF. Androgen-receptor coregulators mediate the suppressive effect of androgen signals on vitamin D receptor activity. Endocrine. 2005;26:1–9. doi: 10.1385/ENDO:26:1:001. [DOI] [PubMed] [Google Scholar]
- 45.Zhao XY, Ly LH, Peehl DM, Feldman D. 1alpha,25-dihydroxyvitamin D3 actions in LNCaP human prostate cancer cells are androgen-dependent. Endocrinology. 1997;138:3290–8. doi: 10.1210/endo.138.8.5328. [DOI] [PubMed] [Google Scholar]
- 46.Zhao XY, Peehl DM, Navone NM, Feldman D. 1alpha,25-dihydroxyvitamin D3 inhibits prostate cancer cell growth by androgen-dependent and androgen-independent mechanisms. Endocrinology. 2000;141:2548–56. doi: 10.1210/endo.141.7.7549. [DOI] [PubMed] [Google Scholar]
- 47.Murthy S, Agoulnik IU, Weigel NL. Androgen receptor signaling and vitamin D receptor action in prostate cancer cells. Prostate. 2005;64:362–72. doi: 10.1002/pros.20251. [DOI] [PubMed] [Google Scholar]
- 48.Serpa NA, Tobias-Machado M, Esteves MA, Senra MD, Wroclawski ML, Fonseca FL, et al. A systematic review and meta-analysis of bone metabolism in prostate adenocarcinoma. BMC Urol. 2010;10:9. doi: 10.1186/1471-2490-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis RM, Peacock M, Aaron JE, Selby PL, Taylor GA, Thompson J, et al. Osteoporosis in hypogonadal men: role of decreased plasma 1,25-dihydroxyvitamin D, calcium malabsorption, and low bone formation. Bone. 1986;7:261–8. doi: 10.1016/8756-3282(86)90205-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.