Abstract
OBJECTIVE
This study tests the hypothesis that the use of a semantic organizational strategy, during the free recall phase of a verbal memory task predicts remission of geriatric depression.
METHODS
65 elderly patients with major depression participated in a 12-week escitalopram treatment trial. Neuropsychological performance was assessed at baseline after a 2-week drug washout period. The Hopkins Verbal Learning Test-Revised (HVLT-R)(Brandt 2001) was used to assess verbal learning and memory. Remission was defined as a Hamilton Depression Rating Scale Score (HDRS) less than or equal to 7 for two consecutive weeks and no longer meeting DSM-IV-TR criteria for major depression. The association between the number of clusters used at the final learning trial (Trial 3) and remission was examined using Cox’s proportional hazards survival analysis. The relationship between the number of clusters utilized in the final learning trial and words recalled after a 25 minute delay was examined in a regression with age and education as covariates.
RESULTS
Greater number of clusters utilized predicted remission rate (Hazard ratio (95% CI) = 1.26 (1.04–1.54), χ2 =4.23, df=3, p=0.04). There was a positive relationship between the total number of clusters used by the end of the third learning trial and the total number of words recalled at the delayed recall trial (F(3,58)=7.93;p<.001)
CONCLUSIONS
Effective semantic strategy use at baseline on a verbal list learning task by elderly depressed patients was associated with greater rate of remission with antidepressant treatment. This result provides support for previous findings indicating that measures of executive functioning at baseline are useful in predicting antidepressant response.
Keywords: Executive function, geriatric, depression, remission, semantic strategy, Hopkins Verbal Learning Test-Revised (HVLT)
Introduction
Executive dysfunction, a neuropsychological expression of frontal systems impairment, is common in geriatric depression. Approximately 42% of elderly depressed patients have executive deficits (Alexopoulos et al. 2002b). Abnormal performance on some tests of executive function predicts both poor and unstable antidepressant response in late-life depression (Alexopoulos et al. 2000; Kalayam and Alexopoulos 1999; Sneed et al.; Sneed et al. 2008; Sneed et al. 2007), although some disagreement exists (Butters et al. 2004a). Structural neuroimaging studies document that reduced fractional anisotropy in cortico-striatal-limbic pathways predicts both poor executive performance and failure to remit with antidepressant treatment (Alexopoulos et al. 2008; Murphy et al. 2007). A critical question is which among the many executive functions is critical in predicting remission with antidepressant treatment.
In addition to executive dysfunction, episodic memory impairment has been commonly reported in geriatric depression (Beats et al. 1996; Hickie et al. 2005; Kramer-Ginsberg et al. 1999; Story et al. 2008). Memory encoding, a process that leads to new memory traces, may be influenced by the use of strategies (an executive function) (Tulving 1983), and is a multifaceted process that likely involves several brain areas (Miotto et al. 2006; Savage et al. 2001). One important strategic process is semantic organization, whereby verbal learning is improved when relationships among items are processed and then reordered into semantic categories. Imaging studies point to a role of the prefrontal cortex in episodic memory encoding, particularly when strategic processes are initially engaged (Savage et al. 2001). We recently reported that semantic organizational strategy use on a verbal fluency measure was the specific executive function that predicted remission rates of geriatric depression (Morimoto et al. 2010). Therefore, we hypothesized that decrements in semantic organizational strategy may exert a “top down” negative effect on verbal episodic memory performance, and that use of this strategy may predict remission rates of geriatric depression. We also hypothesize that this finding would be independent of psychomotor speed, though psychomotor speed has been suggested to mediate decrements in executive function by others (Butters et al. 2004b; Nebes et al. 2000).
The objective of this study was to examine whether the use of strategy, an executive function, during the process of verbal episodic memory encoding was predictive of remission rates of geriatric depression. This study tests the hypothesis that the initiation of verbal strategy during the learning phase of a verbal memory task (HVLT-R) will be more impaired in elderly depressed patients at baseline who remain symptomatic following treatment with escitalopram relative to those patients who remit from geriatric depression. This hypothesis is based on findings suggesting that verbal strategy initiation requires integrity of frontal systems (Gold et al. 2006; Gold et al. 2005) and on observations that structural and functional abnormalities of frontal and frontal-subcortical systems are associated with poor response to antidepressants (Aizenstein et al. 2009; Alexopoulos et al. 2002a; Alexopoulos et al. 2008; Gunning-Dixon et al.; Simpson et al. 1998). We also anticipated that increased use of strategy would enhance both verbal learning and long term episodic memory retrieval.
PARTICIPANTS AND METHODS
Participants
Participants included 65 depressed, elderly (> 60 years) patients from a university-based geriatric psychiatry clinic who were recruited for an escitalopram treatment trial. A total of 65 patients met eligibility criteria, had complete neuropsychological batteries and entered the 2-week single-blind, placebo lead in period. Of these 65, 56 completed the 12 week treatment trial and 9 exited prior to completion. Of the 9 subjects, 2 had 11 weeks of treatment (both exited because they found the treatment ineffective), 1 had 10 weeks of treatment (exited because she found the treatment ineffective) 2 had 9 weeks of treatment (one was lost to follow-up and one exited because of worsening depression), 2 had 8 weeks of treatment (both exited because they found the treatment ineffective), 1 had 7 weeks of treatment (one exited because he found the treatment ineffective), and 1 had 6 weeks of treatment (no longer wanted to participate in research. Neuropsychological tests were performed during a 2-week single blind psychotropic drug wash out/placebo lead-in phase. Participants met DSM-IV-TR criteria and Research Diagnostic Criteria for unipolar major depression and had a score > 19 on the 24-item Hamilton Depression Rating Scale(HDRS) (Davies et al. 2004). Exclusion criteria were 1) major depression with psychotic features (according to DSM-IV-TR); 2) history of other psychiatric disorders (except personality disorders) before the onset of depression; 3) severe medical illness (i.e., metastatic cancer, brain tumors, unstable cardiac, hepatic, or renal disease, myocardial infarction, or stroke) within the 3 months preceding the study; 4) neurological disorders (i.e., dementia or delirium according to DSM-IV criteria, history of head trauma, Parkinson’s disease, and multiple sclerosis); 5) conditions often associated with depression (i.e., endocrinopathies other than diabetes, lymphoma, and pancreatic cancer); 6) drugs causing depression (i.e., steroids, α-methyl-dopa, clonidine, reserpine, tamoxifen, and cimetidine); and 7) Mini-Mental State Examination (Folstein et al. 1975) score < 25; 8) Current psychotherapy. These criteria resulted in a group of elderly patients with non-psychotic unipolar major depression without a diagnosable dementing disorder. Depressive symptoms were assessed using the 24-item HDRS. Side effects of escitalopram were monitored with the Udvalg for Kliniske Undersøgelser (UKU) side effect scale (Lingjaerde et al. 1987). Disability was measured with the World Health Organization Disability Assessment Schedule-II (WHODAS-II). Baseline gross cognitive status was rated with the Mini-Mental State Examination (Folstein et al. 1975).
The Weill Cornell Medical College Institutional Review Board approved all procedures. After a complete description of the study to subjects, written informed consent was obtained.
Treatment
Patients were informed that they would receive placebo at some point during their 14-week trial. After a 2-week psychotropic drug wash-out and single blind placebo lead-in, subjects who still met DSM-IV-TR criteria for major depression and had a 24-item HDRS score of 19 or greater received controlled treatment with escitalopram 10 mg daily for 12 weeks. Patients were instructed to take a single dose of escitalopram in the morning, and were administered medication in one-week supply blisters that permitted dispensation of their daily dosage separately.
The treatment phase consisted of weekly follow up sessions beginning with the placebo lead-in, continuing until the 12th week of treatment with escitalopram. During each follow-up meeting, a research assistant administered the HDRS, the UKU, obtained vital signs, questioned the subjects about medication adherence, and counted the remaining tablets. This meeting was followed by a brief session with a research psychiatrist to assess the risk of continuing the treatment trial and to clinically confirm any remission. The session followed a medication clinic model consisting of a review of symptoms, explanations related to the need for treatment, and encouragement of treatment adherence. No subject received psychotherapy during the study. The subjects were considered in remission if they no longer met DSM-IV-TR criteria for major depression and had an HDRS score of 7 or below for two consecutive weeks.
Measures
Neuropsychological instruments were administered by research assistants at the end of the 2-week, placebo lead-in drug wash out phase and consisted of the Hopkins Verbal Learning Test-Revised (HVLT-R) as well other neuropsychological measures including Trails A to assess psychomotor speed; and the Mattis Dementia Rating (MDRS) scale to assess for cognitive impairment.
The Hopkins Verbal Learning Test-Revised (HVLT-R)(Brandt 2001) was administered to assess verbal learning and memory. The HVLT-R was chosen due to its shorter duration than, and high correlation with the California Verbal Learning Test (CVLT) (Lacritz et al. 2001), as well as its suitability for a geriatric population (Woods et al. 2005). The HVLT-R, presents subjects with a list of 12 words containing an embedded semantic structure (four categories of three words each). The patient is read the list by an examiner, and then asked to repeat as many words as they can remember in any order (free recall). This process is repeated three times, which represents the three learning trials. After a 25-minute delay, the patient is asked again to remember as many of the words from the word list in any order. Patients’ semantic strategy can be evaluated by examining the degree to which words are semantically clustered during the three learning trials. In the standard administration, no items from the same category are presented together, and subjects are not informed of the semantic organization. Research assistants followed standard administration procedures and were trained and supervised by a clinical neuropsychologist.
Scoring
The HVLT-R’s three learning trials, and delay recall trial were scored separately. The three learning trial scores (number of correct words) were then summed to yield a total score. Each of the three learning trials was rescored for number of clusters. Clusters were scored using criterion first utilized by Echemendia et. al. 2001 (Echemendia and Julian 2001), and detailed in Gaines et al, 2006 (Gaines et al. 2006). If a participant recalled a correct word followed by another correct word from the same category, (e.g.“emerald”; “sapphire”), it was counted as one cluster. If a semantically related intrusion or perseveration separated two correct words from the same category, the two correct words were counted as a cluster (eg. “emerald”; “diamond”; “pearl”). Adjusted semantic ratios were formed for each learning trial to account both for chance and for varying maximum clustering values(Stricker et al. 2002).
Statistical Analysis
First, a repeated measures ANCOVA with remission as the between subjects variable, number of clusters in each trial as the within subjects variable and age and education as covariates, was performed to assess difference in semantic strategy learning slopes between remitters and non-remitters. Next, the relationship between total number of clusters used in the final learning trial and the occurrence of remission was examined using Cox’s proportional hazards survival analysis. The third learning trial was selected to allow adequate trials for participants to initiate the semantic strategy. To examine the relationship between use of strategy and the number of words recalled after the delay, adjusted semantic ratio of clusters utilized in the final learning trial and the total number of words recalled after a 25 minute delay was examined in a regression with age and education as covariates. A Mann-Whitney U test was conducted to examine differences in psychomotor speed (measured by Trails A) at baseline between remitters and non-remitters.
RESULTS
At baseline, there were no differences in the severity of depression, number of previous episodes, length of current episode, level of disability, or age of onset between remitters and non-remitters (Table 1). Likewise, there was no difference between remitters and non-remitters with respect to baseline psychomotor speed (Table 1).
Table 1.
Baseline Demographic and Clinical Data of 65 Elderly Patients with Major Depression Treated with Escitalopram 10 mg Daily.
| Remitter | Non-R | Wilcoxon | Statistic | |
|---|---|---|---|---|
| Variable | Mean(SD) | Mean(SD) | Z | p |
| Age (years) | 70.1(5.8) | 70.4(7.1) | −.22 | 0.8 |
| Education (years) | 15.7(3.5) | 16.1(3.6) | −.73 | 0.5 |
| Baseline HDRS | 21.8(4.1) | 22.4(3.7) | −1.1 | 0.3 |
| Age of Onset (years) | 58.1(16.4) | 52.5(21.0) | −.92 | 0.4 |
| Length of current episode (months) | 35.9(92.0) | 31.3(32.6) | −1.35 | 0.2 |
| Number of Previous Episodes | 3.3(3.4) | 2.7(2.2) | −.92 | 0.4 |
| WHODAS-II Total | 35.4(10.4) | 39.5(12.3) | −1.71 | 0.8 |
| Mini Mental State Exam | 28.5(1.5) | 27.9(1.5) | −1.69 | 0.1 |
| Psychomotor Speed (Trails A) | 44.2(15.3) | 48.6(44.4) | −.38 | 0.7 |
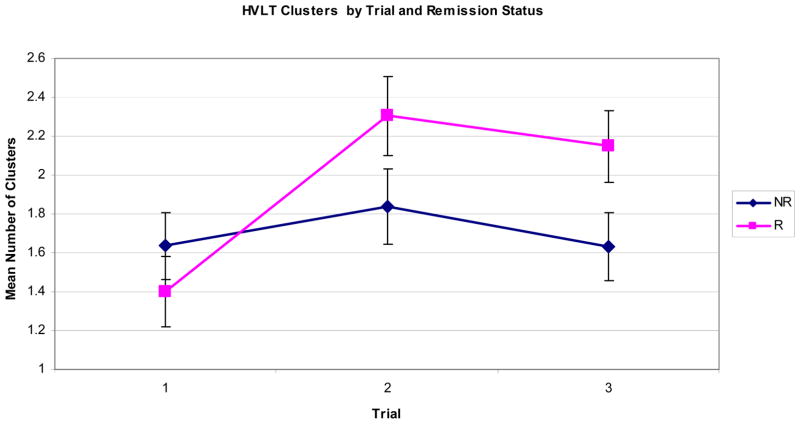
In order to assess differences in semantic strategy learning slopes between remitters and non-remitters, a repeated measures ANCOVA with remission as the between subjects variable and number of clusters over three trials as the within subjects variable with age and education as covariates was performed. The results showed a significant effect of trial (Wilks’ lambda =0.85, F(2)=5.15, p=0.009) across the three trials. There was also a significant difference of number of clusters achieved over the three trials by remission status, (F(2) =11.78, p=0.001). Post-hoc paired samples t-tests revealed that there was no significant increase in the number of clusters from trial one to trial two (t (33) =−1.23, p=0.23), or from trial two to trial three (t(33)=1.19, p=0.242), for non-remitters indicating that the clustering strategy of non-remitters remained unchanged. In contrast, remitters utilized a higher number of clusters in trial two compared to trial one (t(30)=−5.78, p<0.001) and maintained the same level of clustering strategy utilization from trial two to trial three (t(30)=0.961; p=0.34). Therefore, unlike non-remitters, remitters initiated a semantic clustering strategy and maintained it throughout the learning trials.
To explore the contribution of verbal memory to the relationship between verbal strategy and remission, a Mann Whitney U test was conducted. There was no statistically significant difference in the number of words recalled after the delay between remitters and non-remitters (Mann Whitney U (65),z= −.088, p=.93), suggesting the relationship to remission was specific to strategy use, and not to verbal memory.
A regression controlling for age and education conducted on the full sample revealed that there was a positive relationship between the total number of clusters used by the end of the third learning trial and the total number of words recalled at the delayed recall trial (F(3,58)=7.93; p<.001).
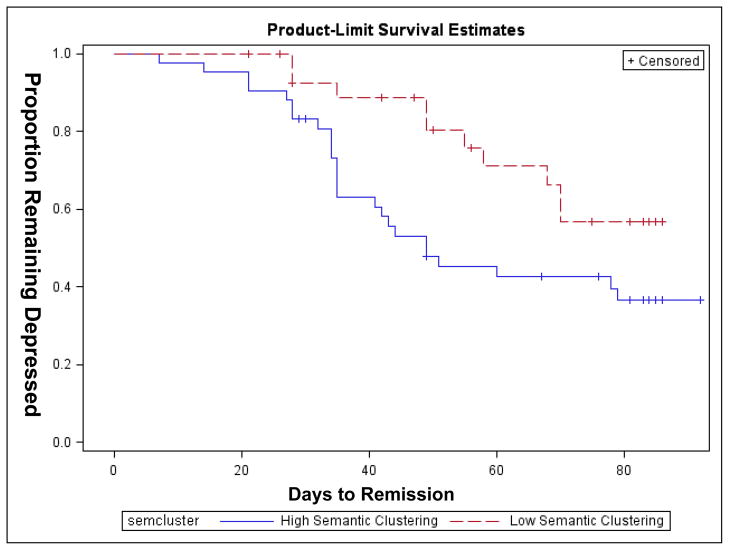
Finally, there was a significant association between the number of adjusted clusters used at trial three and remission rate such that greater number of clusters predicted remission when age and education were taken into consideration (Hazard ratio (95% CI) = 1.26 (1.04–1.54), χ2 =4.23, df=3, p=0.04.)
DISCUSSION
The principal finding of this study is that use of semantic organizational strategy on an episodic memory task predicted remission rates in geriatric major depression. This finding was specific to the initiation of verbal strategy as remitters and non-remitters did not differ in their use of semantic clustering at the first learning trial. Only remitters increased their strategy use by the second learning trial, and then maintained that strategy for the third learning trial, whereas non-remitters’ clustering scores remained both constant and significantly lower across the second and third trials. Adjusted clustering ratios were positively associated with number of words recalled after a long delay, indicating that the strategy, if implemented, was successful in aiding retrieval. This finding was also independent of psychomotor speed, which has been previously implicated in the relationship of neurobehavioral measures and treatment remission (Butters et al. 2004b; Nebes et al. 2000). This is the first study, to our knowledge that suggests successful semantic organizational strategy initiation during an episodic memory test is associated with remission of geriatric depression.
The findings of this study extend our recent report which indicated that deficits in semantic strategy use on a verbal fluency task predicted remission rate in geriatric depression (Morimoto et al. 2010). Furthermore, our findings complement reports that semantic clustering indices differentiate depressed elderly patients from controls, and that patients’ executive functioning mediates episodic memory performance on list learning tasks (Elderkin-Thompson et al. 2007). Deficient strategy utilization may occur through ineffective inhibition of automatic actions, and mobilization of other goal-directed, strategic behavior (Deckersbach et al. 2005; Elderkin-Thompson et al. 2008; Nobre et al. 1999; Schoenbaum et al. 1998).
An important assumption of this study is that the cognitive deficits of geriatric depression are indirect indicators of neurobiological function and are, therefore, biologically meaningful. Previous research with healthy adults demonstrates that effective verbal strategy during encoding is correlated with activations in the left dorsolateral prefrontal cortex (DLPFC), and that spontaneous strategy initiation is correlated with blood flow to the orbital frontal cortex (OFC)(Savage et al. 2001). In geriatric depression, studies suggest that abnormalities in neural systems related to executive functions are associated with poor remission rate of late-life depression (Alexopoulos et al. 2004; Alexopoulos et al. 2008; Gunning-Dixon et al.; Kalayam and Alexopoulos 1999; Simpson et al. 1998). Structural and functional neuroimaging have documented both frontostriatal impairment and the relationship between frontostriatal impairment and executive dysfunction in geriatric depression (Aizenstein et al. 2005; Aizenstein et al. 2009; Murphy et al. 2007). A recent study found significant associations between fractional anisotropy and Stroop Color Word Interference performance in multiple frontostriatal limbic regions, providing evidence for the association of these areas with the executive dysfunction often accompanying geriatric depression (Murphy et al. 2007).
One interpretation of the relationship of defective strategic initiation to remission rate is that abnormal activity of fronto-striatal limbic networks implicated in the pathophysiology of geriatric depression, contribute to both behaviorally expressed difficulties in episodic memory and with patients’ ability to benefit from antidepressant treatment. The current findings replicate the results from our previous study suggesting pre-treatment strategy utilization predicts remission rate, and may be a short, yet useful baseline indicator of treatment response potential in these patients.
There are several limitations to this study. One limitation is the lack of a placebo control group. In addition, given that remission status was based on both a fixed dose of escitalopram and 12-week course of treatment, it is possible that some subjects may have remitted with higher dosages of escitalopram or if longer treatment were offered. However, 8 of 9 subjects who exited the trial had 7–11 weeks of treatment. As this is a preliminary study, we did not complete a more complex analysis of semantic clustering. More refined analysis of semantic organization can include analysis of cluster size, level of switches between clusters, as well as serial organizational strategies. These scoring strategies may be examined in future studies to further delineate the specific executive functions integral to the course of geriatric depression. Last, there was no statistically significant difference in the number of words recalled after the long delay between remitters and non-remitters. Though this result is unexpected, our focus for this paper was the identification of executive functions associated with remission. There are many possible explanations for this result, as the influence of executive functioning on memory performance can be affected by many factors including depression. In this case we propose that during encoding, strategy initiation engages cerebral networks relevant for remission of depression, independent of networks involved in memory. For example, it is possible that working memory of both remitters and non-remitters is sufficiently impaired by the depressed state at baseline, to diminish the positive effects of strategy use. It is also possible that in these depressed patients memory consolidation in both remitters and non-remitters is impaired, or susceptible to interference, eliminating the benefits of strategy on learning trials after a 25-minute delay.
In conclusion, geriatric depressed patients who failed to initiate an effective semantic organizational strategy on a verbal episodic memory task showed both poorer escitalopram treatment response, and poorer memory recall rates than those who used strategy on this measure.
Figure 1.
Figure 2.
Acknowledgments
This work was supported by National Institute of Mental Health grants P30 MH68638 (GSA), R01 MH079414 (GSA), T32 MH019132 (GSA) K23 MH74818 (FMG), K23 MH067702 (CFM) the Sanchez Foundation, the TRU Foundation, and Forest Pharmaceuticals.
References
- Aizenstein HJ, Butters MA, Figurski JL, et al. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58(4):290–6. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Choi SJ, et al. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002a;159(11):1929–32. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002b;10(1):98–106. [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Murphy C, et al. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29(12):2278–84. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57(3):285–90. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165(2):238–44. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26(3):591–603. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins verbal learning test: revised. Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- Butters MA, Bhalla RK, Mulsant BH, et al. Executive functioning, illness course, and relapse/recurrence in continuation and maintenance treatment of late-life depression: is there a relationship? Am J Geriatr Psychiatry. 2004a;12(4):387–94. doi: 10.1176/appi.ajgp.12.4.387. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004b;61(6):587–95. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Davies RR, Graham KS, Xuereb JH, et al. The human perirhinal cortex and semantic memory. Eur J Neurosci. 2004;20(9):2441–6. doi: 10.1111/j.1460-9568.2004.03710.x. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, Dougherty DD, et al. Spontaneous and directed application of verbal learning strategies in bipolar disorder and obsessive-compulsive disorder. Bipolar Disord. 2005;7(2):166–75. doi: 10.1111/j.1399-5618.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- Echemendia RJ, Julian LJ. Mild traumatic brain injury in sports: neuropsychology’s contribution to a developing field. Neuropsychol Rev. 2001;11(2):69–88. doi: 10.1023/a:1016651217141. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Hellemann G, Pham D, et al. Prefrontal brain morphology and executive function in healthy and depressed elderly. Int J Geriatr Psychiatry. 2008 doi: 10.1002/gps.2137. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Mintz J, Haroon E, et al. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol. 2007;22(2):261–70. doi: 10.1016/j.acn.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaines JJ, Shapiro A, Alt M, et al. Semantic clustering indexes for the Hopkins Verbal Learning Test-Revised: initial exploration in elder control and dementia groups. Appl Neuropsychol. 2006;13(4):213–22. doi: 10.1207/s15324826an1304_2. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, et al. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26(24):6523–32. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, et al. Common and dissociable activation patterns associated with controlled semantic and phonological processing: evidence from FMRI adaptation. Cereb Cortex. 2005;15(9):1438–50. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Walton M, Cheng J, et al. MRI signal hyperintensities and treatment remission of geriatric depression. J Affect Disord. doi: 10.1016/j.jad.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie I, Naismith S, Ward PB, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56(8):713–8. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- Kramer-Ginsberg E, Greenwald BS, Krishnan KR, et al. Neuropsychological functioning and MRI signal hyperintensities in geriatric depression. Am J Psychiatry. 1999;156(3):438–44. doi: 10.1176/ajp.156.3.438. [DOI] [PubMed] [Google Scholar]
- Lacritz LH, Cullum CM, Weiner MF, et al. Comparison of the hopkins verbal learning test-revised to the California verbal learning test in Alzheimer’s disease. Appl Neuropsychol. 2001;8(3):180–4. doi: 10.1207/S15324826AN0803_8. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors U, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, et al. Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Hum Brain Mapp. 2006;27(4):288–95. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto SS, Gunning FM, Murphy CF, et al. Executive Function and Short-Term Remission of Geriatric Depression: The Role of Semantic Strategy. Am J Geriatr Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181e751c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CF, Gunning-Dixon FM, Hoptman MJ, et al. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61(8):1007–10. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med. 2000;30(3):679–91. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Frith CD, et al. Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nat Neurosci. 1999;2(1):11–2. doi: 10.1038/4513. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, et al. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain. 2001;124(Pt 1):219–31. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1(2):155–9. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Simpson S, Baldwin RC, Jackson A, et al. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28(5):1015–26. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- Sneed JR, Culang ME, Keilp JG, et al. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 18(2):128–35. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Keilp JG, Brickman AM, et al. The specificity of neuropsychological impairment in predicting antidepressant non-response in the very old depressed. Int J Geriatr Psychiatry. 2008;23(3):319–23. doi: 10.1002/gps.1889. [DOI] [PubMed] [Google Scholar]
- Sneed JR, Roose SP, Keilp JG, et al. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry. 2007;15(7):553–63. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- Story TJ, Potter GG, Attix DK, et al. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry. 2008;16(9):752–9. doi: 10.1097/JGP.0b013e31817e739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker JL, Brown GG, Wixted J, et al. New semantic and serial clustering indices for the California Verbal Learning Test-Second Edition: background, rationale, and formulae. J Int Neuropsychol Soc. 2002;8(3):425–35. doi: 10.1017/s1355617702813224. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Clarendon Press; Oxford University Press; 1983. [Google Scholar]
- Woods SP, Scott JC, Conover E, et al. Test-retest reliability of component process variables within the Hopkins Verbal Learning Test-Revised. Assessment. 2005;12(1):96–100. doi: 10.1177/1073191104270342. [DOI] [PubMed] [Google Scholar]