Abstract
Colorectal cancer (CRC) is the third most common cause of cancer death in the US, accounting for ~51,000 deaths each year. We have previously shown in vitro chemopreventive effects of mixtures of aspirin, folic acid and calcium (AFAC) on colon cancer cell lines. The objective of the present study was to evaluate the in vivo effects of orally administered, colon targeted chemopreventive combination regimens on the inhibition of aberrant crypt foci (ACF) in a rat model of colon carcinogenesis using (1) unmodified (free drug) combinations of AFAC, and (2) nanoparticle-encapsulated combinations of the same agents. A 14-week animal study was conducted in three phases to determine an optimal effective dose from AFAC combinations and evaluate the efficacy of nanotechnology-based chemopreventive regimens administered in combined (mixtures), and individual (single entity) forms. ACF inhibition when compared to azoxymethane (AOM)-treated rat control group was significant in both, the unmodified and the modified nanoparticle-mediated chemopreventive regimens, demonstrating a range of 31 – 38% (p < 0.05) and 50 – 75% (p < 0.001) reduction respectively, in the number of ACFs. In addition, the nanoparticulate combination regimens of AFAC demonstrated a 2-fold increase in suppression of ACF compared to free drug mixtures. Individual administration of nanoparticle encapsulated drugs showed no significant effect on the reduction of ACF. Histochemical analysis provided further confirmation of chemopreventive effects, demonstrating a significant reduction in cell nuclear proliferation. Overall, our results provide a strong proof-of-concept using nanoparticle-mediated combination treatment in the chemoprevention of colon cancer.
Introduction
It was estimated that colon and rectal cancer would affect over 142,000 Americans in 2010 and result in the deaths of approximately 51,000 patients from this disease making it the third most common cancer in both men and women in the US (1). Lately, cancer chemoprevention has received significant attention as a growing body of evidence has emerged with the promise of chemopreventive agents being successfully used in the prevention of tumors (2–4). Cancer chemoprevention is an approach in which dietary or synthetic chemical agents are used to prevent cancer in normal and/or high-risk populations (5). Many compounds have been extensively studied for their chemopreventive effects. Among them, aspirin (ASP), folic acid (FA) and calcium (Ca) have shown promise when used on an individual basis in the prevention of colon cancer (6–8). ASP has demonstrated high effectiveness as a chemopreventive agent against colon cancer (9). The mechanism of inhibition involves the suppression of over-expressed Cox-2 enzyme in which leads to the suppression of colonic polyp formation (10–11). However, ASP is known to cause complications such as gastric mucosal injury, bleeding and anti-platelet effects in patients who frequently use high doses (12, 13). For this reason, physicians are reluctant to recommend this drug for regular use as a preventative towards colon cancer in the general population. Thus, further determination of the minimally effective dose, perhaps in combination with FA and Ca, is needed to balance benefits with potential toxic risks of long-term administration (14).
FA, is essential for DNA synthesis and DNA methylation (15). FA is provided in diet from major sources including citrus fruits, dark green vegetables and dried beans or by synthetic supplementation. Colorectal adenomas were shown to be decreased by 31% in epidemiological studies after prolonged folate supplementation of 400 μg /day. Maintaining adequate levels of FA ensures protection of genes from mutation leading to colon cancer (8, 16–18).
Among non-pharmacologic agents, Ca supplementation has emerged as a promising candidate for chemoprevention. Prior studies have suggested an inverse association between Ca intake and the occurrence of colorectal neoplasia (19). A mechanism by which it affects the intestinal epithelium is through activation of a Ca-sensing receptor with resultant growth inhibition and differentiation of transformed cells (20, 21). Due to the abundant availability of Ca, low cost and its potential effectiveness in preventing CRC, Ca is a good candidate for further studies in chemoprevention.
We have recently demonstrated that combinations of ASP, FA and Ca (AFAC) significantly reduced cell viability in HT-29 and SW-480 colon cancer cell lines (22). Other research groups have shown similar results whereby administering combinations of low doses of other chemopreventive agents provided increased efficacy and minimized toxicity (23–24). In addition, we were the first to report the development of novel nanotechnology-based formulations encapsulating ASP and FA with small particle sizes (average size 120 nm) and high encapsulation efficiencies (85%), for targeted delivery to the colon (22, 25). For the current study, using male Sprague-Dawley rats, we report AFAC, in unmodified (free drug) form and nanoparticle (NP) encapsulated combination forms, exerting significant effects in the colonic epithelia for the chemoprevention of colon cancer. To our knowledge, this is a first proof-of-concept report using nanotechnology-based chemopreventive regimens of AFAC combinations in the suppression of colon cancer.
Materials and Methods
Materials
ASP, FA, methylene blue, 10% buffered formalin and dichloromethane (DCM) were purchased from Sigma (St. Louis, MO) and calcium carbonate (Ca) was supplied by PCCA (Houston, TX). The carcinogen azoxymethane (AOM) was obtained from Spectrum Chemicals (Gardena, CA). Male Sprague-Dawley rats were supplied by Harlan (Indianapolis, IN) and disposable plastic cages for the rats were purchased from Innovive (San Diego, CA). H&E staining was performed at the Histo-Scientific Research Labs (Mt. Jackson, VA). Animal diet and water was provided ad libitum to the rats. The animal study protocol was approved by the Western University of Health Sciences Institutional Animal Care and Use Committee before the initiation of the experiments. For the preparation of nanotechnology-based chemopreventive regimens, the biodegradable polylactide-co-glycolide (PLGA 50:50) copolymer was purchased from Durect Corp. (Pelham, AL). Low molecular weight EudragitR S100, an enteric coating polymer, was gifted by Degussa (Parsippany, NJ).
Methods
Rat study design
Seven-week-old male Sprague-Dawley rats weighing approximately 225–300g were randomized in groups (n = 6) and placed in disposable plastic cages. At eight weeks old, AOM (15 mg/kg) was injected subcutaneously into rats once weekly for two consecutive weeks. For the next 14 weeks, rats were dosed every 24 h by oral gavage with freshly prepared chemopreventive regimens as per the following protocol:
Set 1
As indicated in Table 1, this set consisted of seven groups (G1–G7) of rats treated with high to low dose ranges of plain, unmodified mixtures AFAC) starting the day after the first AOM inoculation (G3–G7). The purpose of these studies was to determine a minimally effective chemopreventive dose for ACF suppression. Two groups of control animals, saline (G1) and AOM-treated (G2), serving as (+) and (–) controls respectively, were also included. The highest dose selected for ASP, FA and Ca regimen was 400, 32 and 650 mg/kg (G3), respectively. Based on previous studies in the literature, the highest dosage for ASP was determined to be 400 mg/kg (26). By drawing a correlation to cell line studies (22), the high dose levels for FA was calculated to be 32 mg/kg and Ca, 650 mg/kg. Other doses were calculated as 1/3 (G4), 1/10 (G5), 1/30 (G6) and 1/100 (G7) of the highest dose to obtain a wide range of doses (Table 1).
Table 1.
Treatment plan showing groups of Sprague-Dawley rats treated with unmodified and nanoparticle forms of chemopreventive agents aspirin (ASP), folic acid (FA) and calcium (Ca) at various doses.
| Set | Group (n=6) | Treatment Plan | Dose (mg/kg) |
|---|---|---|---|
| S 1 (unmodified combination) | G1 | Saline (control) | 0 |
| G2 | AOM (control) | 0 | |
| G3 | ASP + FA + Ca | 400 + 32 + 650 | |
| G4 | ASP + FA + Ca | 133 + 11 + 216 | |
| G5 | ASP + FA + Ca | 40 + 3.2 + 65 | |
| G6 | ASP + FA + Ca | 13 + 1.1 + 22 | |
| G7 | ASP + FA + Ca | 4 + 0.32 + 6.5 | |
| S 2 (unmodified and modified combination) | G8 | Saline (control) | 0 |
| G9 | AOM (control) | 0 | |
| G10 | ASP + FA + Ca | 133 + 11 + 216 | |
| G11 | ASP + FA + Ca | 80 + 6.4 + 130 | |
| G12 | ASP + FA + Ca | 40 + 3.2 + 65 | |
| G13 | ASP (NP) + FA (NP) + Ca | 133 + 11 + 216 | |
| G14 | ASP (NP) + FA (NP) + Ca | 80 + 6.4 + 130 | |
| G15 | ASP (NP) + FA (NP) + Ca | 40 + 3.2 + 65 | |
| S 3 (modified combination) | G16 | Saline (control) | 0 |
| G17 | AOM (control) | 0 | |
| G18 | ASP (NP) | 40 | |
| G19 | FA (NP) | 3.2 | |
| G20 | Ca | 65 | |
| G21 | ASP (NP) + FA (NP) + Ca | 40 + 3.2 + 65 |
Set 2
Based on results obtained from Set 1 studies and subsequent dose optimization, a comparative study between mixtures of unmodified free drugs and drugs encapsulated in nanoparticles was conducted (Set 2, Table 1) at lower doses. Three groups of rats (G10–G12) received unmodified combinations of AFAC whereas another three groups of rats (G13–G15) received combinations of drug-loaded NPs. Among the three chemopreventive agents, only ASP and FA were encapsulated within NPs. Ca was not encapsulated since it has previously been demonstrated to be a virtually non-toxic compound with no side effects even when ingested at very high doses (27).
Set 3
Animals in this final set of studies (G18–G20, Table 1) received single doses of low concentrations of chemopreventive agents encapsulated within polymer NPs. The last set (G21) received a combination of the three agents. This study was conducted to confirm our hypothesis that any inhibition response of NP drug combinations on ACF observed from the previous studies (Set 2) were based on a combined additive responses and not as a result of the effect of individual chemopreventive agents.
Colon harvesting and staining
At the end of each study, the colons were extracted from the sacrificed rats, cleaned with phosphate buffer solution (PBS) and cut into the proximal, middle and distal regions of the large intestine. A subjective examination of other organs (stomach, small intestine, liver and kidney) was conducted to observe for signs of toxicity from the chemopreventive regimen. To quantitate the aberrant crypt foci (ACF), methylene blue staining was conducted by dipping the colonic segments in 10% formalin buffer fixative solution for 24 h followed by methylene blue dye (0.1% w/v) staining for 20–30 min. A light microscope with a 40X magnification was used to quantitate ACFs on the colon. Aberrant crypts were identified as increased distance from the basal to lamina surface of cells, easily discernible pericryptal zone, split like opening in the centre, enlarged size and deeper staining. All colon segments were scored by the primary investigator responsible for these studies and subsequently by a trained laboratory assistant who was blinded to the study.
Dose response curve
Dose response curves were used to assess the inhibition of ACF per treatment dose after completion of studies from Set 1. A maximum response was defined as 100% inhibition of ACF’s while a minimum response consisted of the groups with most number of ACFs, when compared to the AOM-treated control group. The AOM-treated control group was expected to present the highest number of ACFs as a result of the carcinogenic effect of AOM on the colon. Subsequently, a probit analysis (28) was conducted to calculate the effective dose (ED50) from the range of treatment doses tested.
Histochemical analysis
Standard protocol for H&E staining was followed (29, 30). Four to five μm thick longitudinal sections of the distal colonic segments were cut from the paraffin block using a cryostat, and subsequently de-waxed in several baths of xylene and hydrated through graded alcohols to ultrapure water. Thereafter, the sectioned tissue was stained with hematoxylin and washed with distilled water. Tissues were clarified by a “blueing” step using 1% HCl and ammonium hydroxide to provide a better contrast. An eosin stain was applied and samples were dehydrated in 95% alcohol and absolute alcohol followed by clearing in xylene. Tissues were mounted on the slides and evaluated microscopically at 40X magnification.
Preparation of colon targeted novel nanotechnology-based chemopreventive formulations
ASP and FA were encapsulated within PLGA copolymer using a previously reported method (22). Briefly, ASP and FA suspended in distilled water were emulsified in organic solution of DCM containing PLGA 50:50 copolymers. The emulsion was probe sonicated and added drop wise to an outer aqueous 2% PVA solution. The DCM solvent was evaporated resulting in the precipitation of drug loaded NPs in the aqueous phase. Subsequently, ultracentrifugation at 10° C (34,500 g x 25 min) was conducted to recover the NPs. Finally, the NPs were freeze-dried and stored at 4° C until use. For the purposes of targeting to the colon, mixtures of freeze-dried ASP and FA NPs were coated with EudragitR S-100 (1% in methanol w/v). Particle sizing and drug encapsulation efficiency measurements were conducted and optimized (22).
Rat cecal content studies
To isolate the cecal contents, a previously published procedure was used (31). Briefly, Sprague-Dawley rats were sacrificed and the cecum was isolated and immediately transferring into a buffer medium at pH 6.8. The cecal bags were opened, contents weighed individually, then pooled and suspended in buffer continuously bubbled with carbon dioxide to maintain anerobic conditions. These were finally added to the dissolution media to give a final cecal dilution of 2%.
A 48-h drug release study was carried out in the presence of carbon dioxide. At different time intervals, 5 ml sample was withdrawn from the dissolution medium and analyzed by UV-Vis spectrophometry at wavelengths of 233 nm for ASP and 277 nm for FA. The sample was replaced by fresh dissolution medium. All runs were conducted in triplicate.
Statistical analysis
A 2-way ANOVA followed by a Bonferroni post hoc analysis using GraphpadR prism software was used for statistical comparison of results. Data was tested for significant difference on the frequency of ACFs along the different segments of colon. A p value of <0.05 was considered significant.
Results
General observations
A steady increase in mean body weight of the rats during the 14-week study was demonstrated. All animals were under continuous observation for signs of distress, weakness and other possible side effects. For rats in Set 1, the mean body weights were lower (243 g) at the start of the experiments, in comparison with Groups 2 (305 g) and 3 (317 g). By the end of the study, mean body weight for animals in their respective groups increased proportionately from their original weight (Set 1, 414 g; Set 2, 445 g; Set 3, 427 g). On average, the weight gain of approximately 50% was seen across all sets of rats tested, indicating overall good health of the rats during the study (see supplementary Figure A).
No signs of toxicity in organs (stomach, intestine, liver and kidney) from the AOM inoculation and chemopreventive regimens were evident after the 14-week duration of study.
Unmodified combinations of chemopreventive agents significantly inhibit ACF formation
In general, ACFs were more pronounced in the distal than in the proximal or the middle part of the colon, consistent with previous reports (32, 33). Thus, our results in this report focus primarily on the inhibition of ACF formation in the distal region of the rat colon.
Figure 1 shows AOM-treated rats (G2) from Set 1 which received unmodified combinations of AFAC in a wide range of doses (G3 – G7). Saline (G1) and AOM (G2) control groups were included for comparison. The objectives of this study were two-fold: (i) to demonstrate the effectiveness of combined treatment on AOM-treated rats when treated with unmodified forms of chemopreventive agents, AFAC, and (ii) to optimize the dose for subsequent design of drug-loaded PLGA NPs targeted to the colon.
Figure 1.
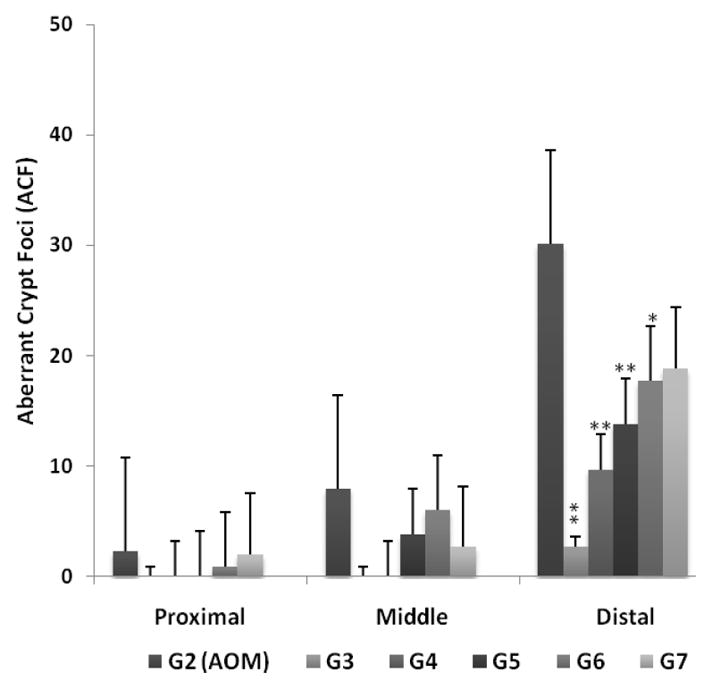
As shown in Figure 1, all treatment doses showed a wide range of effectiveness in the distal colon with significant inhibition of ACF formation observed in all treatment doses, except one. These observations provide first evidence of the effect of combined treatment using this particular regimen on the chemoprevention of colon cancer. As expected, the AOM control group (G2) showed the maximum number of ACFs (~30) in the distal colon. Rats treated with unmodified chemopreventive regimens exhibited a dose dependent response with strongest inhibition observed for the highest dose used (G3), progressively decreasing in effect as the dosing strengths were reduced (G4–G7). Animals in group G3, the highest dose regimen, showed maximum inhibition of ACFs with an average of 2.67 crypts, representing a 96% decrease in ACF formation when compared to the AOM control group. Similarly, groups G4 – G7 showed decrease in the ACF count to approximately 10 (G4), 14 (G5), 17 (G6) and 20 (G7) ACFs representing a decrease of 80%, 54%, 42% and 37%, respectively, thus demonstrating the dose-dependent inhibitory effect of the triple combination regimen. As such, all dose levels showed a reduction in ACF counts when compared to the AOM control group. The rat colonic tissues were tested for significant difference according to the position of aberrant crypts using a 2-way ANOVA where the results were compared with the AOM control group (G2) and confirmed via the post hoc Bonferroni test. None of the groups reflected significant differences along the proximal or middle sections of the colon, while significant differences were found towards the distal end. Rats in Set 1 showed the following results: G3 (t = 4.23, p < 0.001), G4 (t = 3.15, p < 0.01), G5 (t = 3.18, p < 0.01) and G6 (t = 2.49, p < 0.05) were significantly different from the G2 (AOM control group). However, G7 (t = 2.2, p > 0.05) was not significantly different from the AOM group despite showing a decrease in the number of ACFs when compared to control.
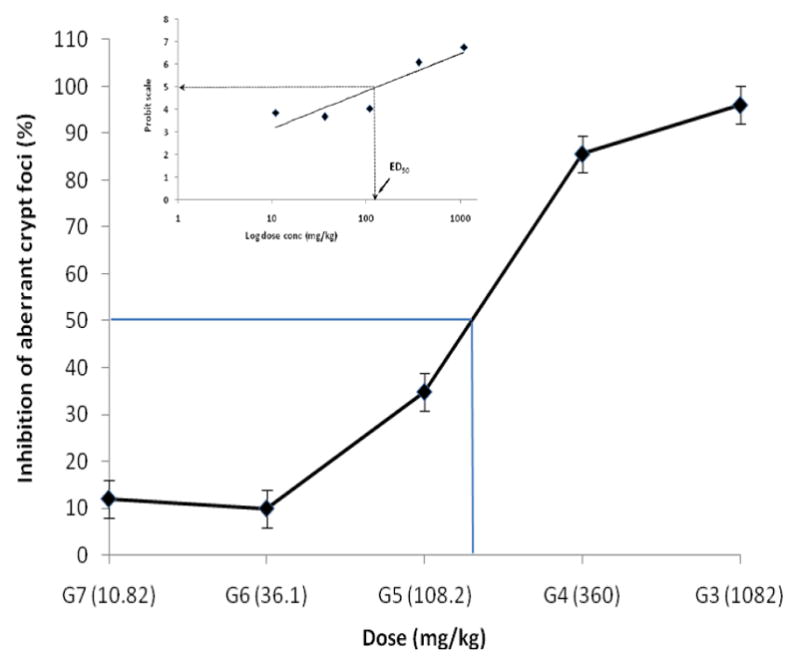
A plot of ACF inhibition (%) versus dose administered (mg/kg) for the unmodified chemopreventive combinations (Set 1) demonstrated a sigmoidal curve showing increased inhibition of ACF’s with increasing doses of chemopreventive regimens (Figure 2). An 8-fold difference in inhibition was observed when comparing the least responsive dose (12%, G7) to that of maximum response (96%, G3). A probit analysis was conducted to determine the effective dose (ED50) from the sigmoid dose-response curve and analyzed by regression either through least squares or maximum likelihood (Figure 2 inset). Percent inhibition response calculated previously was transformed into probits using Finney’s table (28), where a 50% response corresponded to a 5 on the probit scale (y axis). Extrapolating to the log dose values on the x axis obtained the corresponding dose value of ~120 mg/kg. This dosage value was deemed closest to the combined AFAC dose administered to rats in G5 of Set 1 studies (108 mg/kg).
Figure 2.
Both unmodified and NP mediated chemopreventive regimens significantly inhibit ACF formation in rat colon
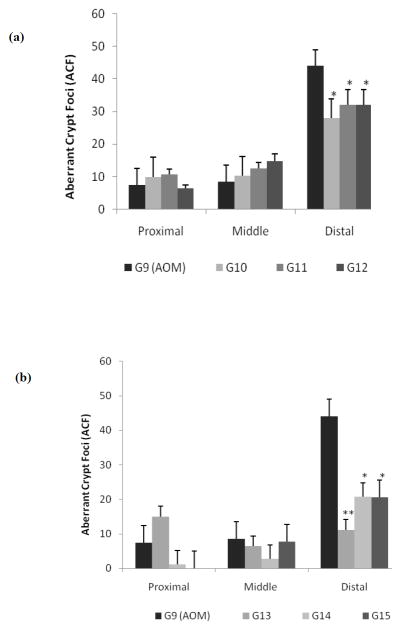
Based on results obtained from Set 1 studies and subsequent calculations of the ED50 dose, a new set of animal studies was conducted using optimized doses of unmodified chemopreventive combination regimens (Table 1, G10–G12). Two of these selected doses were similar to the previous studies in set 1 (G4 and G5). Additionally, an intermediate dose was selected to assess the effect of this new dosage regimen on colonic ACFs. Subsequently, three additional groups using modified, drug loaded nanoparticles (NPs) combination regimen (Table 1, G13–G15) at equivalent doses were used.
The purpose of these studies (Set 2) was (i) to further optimize the unmodified dosage regimens to enable the identification of the lowest dosing strength that would significantly decrease the incidence of colon ACFs in the rats, and (ii) to observe and report, for the first time, the effect of colon targeted nanotechnology-based formulations on the inhibition of ACFs in the colon. As before, a new saline (G8) and AOM treated control (G9) was maintained for this group of animals. As shown in Figure 3a, most of the ACFs were again present in the distal portion of the rat colon though there was some evidence of ACF formation in the proximal and middle colon regions. However, since no significant differences in the inhibition of ACF formation were apparent from the proximal and middle colon, only the distal colonic ACF’s were measured wherein the response of the treatment doses were all significant at the dose administered. As expected, the AOM treatment set (G9) showed the maximum number of ACFs in the distal colon (~45). Treatment with unmodified chemopreventive combinations resulted in 38% (G10), 31% (G11) and 31% (G12) reduction in the number of ACFs in the distal colon. This trend was similar to our observations from the previous set of studies in Set 1. However, the new intermediate dose (G11) did not show any difference in inhibition when compared to the low dose (G12) (Figure 3a) indicating that both doses have equivalent effect on the inhibition of ACF formation. A statistical 2-way ANOVA followed by the Bonferroni post hoc test revealed a significant treatment effect in the distal colon. The highest dose group G10 (360 mg/kg) was found to be the most effective chemopreventive combination in this group (Set 2). G10 with a difference of 16 ACFs (t = 3.33, p < 0.01) from the AOM control was most effective followed by G11 (216 mg/kg) and G12 (108 mg/kg) which were almost similar in effect with a difference of 12 crypts (t = 2.5, p < 0.05) each.
Figure 3.
In comparison, Figure 3b shows the effect of a modified chemopreventive regimen where ASP and FA were encapsulated in PLGA 50:50 polymer NPs (20) and mixed with free Ca and administered to rats in Set 2 (G13–G15). When compared to the AOM treated group (G9), significant differences were observed wherein treatment with the modified form of G13 (360 mg/kg) showed a significant inhibition of ACFs in the distal colon by approximately 75%. Similarly, the two other groups G14 (216 mg/kg) and G15 (108 mg/kg) each showed considerable ACF inhibition of over 50% when compared to the AOM control. These findings, reported here for the first time, show that the PLGA 50:50 polymer encapsulated NP regimens were significantly more effective in chemoprevention than their unmodified counterparts. The G13 dosage group which was most effective in the inhibition of ACF formation showed the maximum difference of 32.8 ACFs (t = 4.73, p < 0.001) when compared to G9 (AOM control). It was followed by G14 and G15 with a difference of 23.2 ACFs (t = 3.35, p < 0.01) and 23.4 ACFs (t = 3.38, p < 0.01) respectively from G9 (AOM control).
A comparison of dose responses from unmodified chemopreventive regimens (G10, G11 and G12) to that of the NPs set (G13, G14 and G15) showed that at the highest dose (360 mg/kg) for both groups, namely G10 (unmodified) and G13 (NP encapsulated) respectively, the average ACF count was observed as ~40 and ~25, respectively, representing a 1.6 fold increase in the efficacy of the NP modified regimen (Figure 3). Similarly, at 208 mg/kg dose of unmodified (G11) and modified (G14) regimens demonstrated similar results of ~48 and ~28 ACF, a 1.7 fold increase in efficacy again with the novel NP regimens. Finally, at the lowest dose (108 mg/kg) administered, G12 showed an average ACF count of ~ 59 whereas the modified G15 group demonstrated ~33 ACFs and hence an approximate increase of 1.8 fold over the unmodified regimen. Thus, the NP based treatment regimen showed better inhibition of ACF formation not only in comparison within its own group but also between treatment groups.
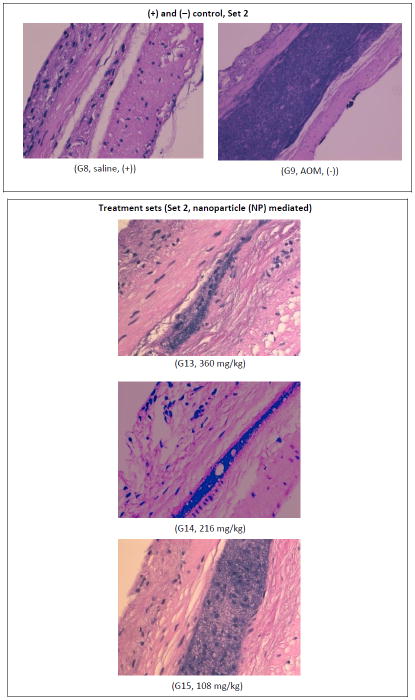
Histochemical analysis of distal colonic tissues samples qualitatively analyzed the cellular morphology of the saline and AOM treated samples from those that received a modified NP combined treatment regimen. The results obtained from these studies (Table 1, Set 2, G13–G15) provided further evidence of the effect of NP based combined treatment regimens of ASP, FA and Ca on inhibition of ACF formation. As shown in Figure 4, the AOM control group demonstrated a high concentration of cell nuclei stained by H&E as noted by the occurrence of a densely stained band. In comparison, the treatment sets (G13–G15) demonstrated decreasing levels of nucleus staining as the dosing strength reduced from a higher dose (360 mg/kg) to the lowest dose level (108 mg/kg). However, even at the lowest dose, significant reduction in nuclear proliferation was observed compared to the AOM control, thus confirming the positive effect of NP drug combinations on ACF of the rat distal colon. These results provided confirmation on the efficacy of the treatment regimen in controlling cellular proliferation.
Figure 4.
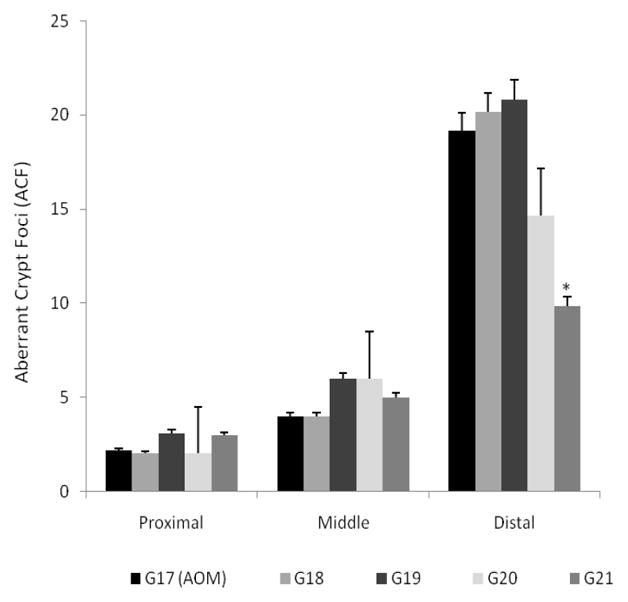
Individual administration of NP-mediated chemopreventive agents has no effect on ACF inhibition
The purpose of the final set of studies (Set 3, G16–G21) was (i) to study the effect of individually administered NP formulations of ASP and FA, and free Ca on rats treated with AOM and saline as control; and, (ii) to confirm that the inhibitory effect on ACF formation observed from previous studies was a result of an additive/synergistic effect of the triple combination and not due to the action of any one single chemopreventive agent used. The dosage regimen selection (G15) was based on the lowest combined dose showing a significant effect in the inhibition of ACF formation in the distal colon. Thus, individual concentrations of PLGA 50:50 encapsulated ASP (G18, 40 mg/kg) and FA (G19, 3.2 mg/kg) NPs and free Ca (G20, 65 mg/kg) were administered to AOM-treated rats. A combination dose (see Table 1, G21) was also administered to replicate the observations from the previous set of studies (Set 2). Individually, none of the chemopreventive agents (ASP, FA or Ca) showed any effect on the reduction of ACFs, though Ca seemed to have an effect in the distal colon however this was not statistically significant (Figure 5). When administered in combination, the distal colonic segments demonstrated significant inhibition of ACFs. The effect of the treatment combination was similar to that observed from previous studies wherein approximately 50% inhibition was evident when compared to the AOM-treated control group. These studies confirmed that individual administration of a chemopreventive drug at a low dose had no effect on ACF inhibition whereas a significant additive chemopreventive response was evident when administered in combination.
Figure 5.
Rat cecal content studies
ASP release from PLGA nanoparticles in rat cecal medium was significantly increased when compared to release of the same drug from a medium without cecal content (PBS). Over a 48-h period, approximately 80% of total aspirin was released from the cecal medium as compared to only 55% from the PBS dissolution medium. In case of FA release, there was not much difference in the extent (80%) however the rate of release from PLGA nanoparticles was increased when compared to release from the PBS dissolution medium.
Discussion
Recently, the term “nanochemoprevention” has been coined to demonstrate the novel approach of using nanotechnology-based regimens for the prevention of cancer (34–36). Successful encapsulation of a green tea extract and its subsequent increase in effectiveness over un-encapsulated treatment regimens for the chemoprevention of prostate cancer was reported. Other recent studies have shown the benefits of encapsulating curcumin (37, 38) and selenium (39) for anti-cancer therapy and prevention. Overall, the use of nanotechnology in cancer chemoprevention is still very limited. However, with more reports in this area being published this is expected to change.
There is increasing interest in the use of combinations of low doses of chemopreventive agents that differ in mode of action. This approach provides means of obtaining increased efficacy and minimized toxicity when a promising chemopreventive agent may demonstrate toxic effects at higher doses (40). Studies using Ca/vitamin D combination have shown to exert an additive effect leading to the conclusion that the two agents work together and not separately, in the reduction of the risk of CRC (41). Recently, the combined use of low dose mixtures of atorvastatin, ASP and celecoxib showed significant inhibition of colon carcinogenesis in comparison to individual doses of the same drug (42). Another study using piroxicam, another NSAID, combined with difluoroornithine showed increasing effectiveness in inhibiting incidence and multiplicity of colon adenocarcinomas, than administration of individual compounds at higher levels (43). More recently, combinations of Cox-2 inhibitors and oxaliplatin, an anticancer agent, showed increased growth inhibition and death in human colon cancer cells (44). The use of naproxen (an NSAID) and its combination with nitrous oxide (NO) has also been shown to be effective against colon and urinary bladder cancer (45).
Previously, no group has investigated the combined treatment effects of ASP with FA and Ca (AFAC) on the prevention of CRC. Our present study, an extension from our previous findings from in vitro studies (22), provides strong evidence for the successful use of nanotechnology-based formulations on the combined chemoprevention of colon cancer in the animal model. Since ASP, FA and Ca have different mechanisms of action, it was hypothesized that a combination effect of all these entities, administered in relatively low doses, would be additive for the prevention of CRC and would also be expected to exhibit minimal toxicity due to the targeting action creating a local effect with minimal systemic absorption. With the encouraging results from this study, we have established the importance of using a novel nanotechnology-based drug delivery system to ultimately target the human colon for chemopreventive treatment of colon cancer.
The PLGA 50:50 polymer is best known for its controlled release effect of drug over a prolonged period of time (46). Previous studies conducted in our lab (22, 25) measured the drug release kinetics of ASP and FA from PLGA based nanoparticles for a 72 h period indicating the controlled release of drugs encapsulated within polymer NPs. The use of EudragitR S-100, a colon targeted class of polymer imparts site specificity to the drug loaded nanoparticles due to a pH sensitive solubility profile. The polymer dissolves at the colonic pH (>7.0) but resists lower acidic pH (1–3) activity in the stomach or the small intestine (8). Upon transit into the colon, the Eudragit-S100 polymer dissolves thus exposing the drug encapsulated PLGA 50:50 nanoparticles within the colonic environment. From our studies on drug release from rat cecal contents, a significant difference in the extent of release of ASP at the end of the 48 h run was observed however FA release was very similar with or without rat cecal contents. The increased rate of release of both chemopreventive agents was probably due to the presence of cecal enzymes which rapidly hydrolyze the polymer backbone resulting in a faster release of drug from the matrix of the polymer shell (47).
The PLGA polymer encapsulated drug is released over time, initially due to simple diffusion effects and subsequently due to hydrolysis of the polymer backbone leading to degradation of the polymer, allowing the remainder of the encapsulated drugs to be released. This ensures a prolonged local presence of chemopreventive agents, and hence could explain the higher efficacy of these regimens compared to their unmodified counterparts. The controlled release of ASP and FA from polymer nanoparticles and the presence of Ca ensures a sustained, sub-toxic presence of chemopreventive agents to exhibit an additive chemopreventive effect. NPs due to their small size and large surface area are able to penetrate the mucous cell lining and improve bioavailability. Site specific drug loaded NPs were successfully demonstrated as chemopreventive regimens, increasing the amount of drug reaching the gut and demonstrating a significantly different effect than plain unmodified drugs.
The most compelling evidence for our hypothesis was the results from Set 2. Compared to AOM treated controls, both the unmodified and the encapsulated regimens administered at different dosage strengths displayed significant differences in ACF inhibition. However, the use of NP-based formulations proved to be more effective in their chemoprevetive action opening the possibility of optimizing the dosage strengths at lower concentrations. Results from the in vivo studies presented here corroborate our earlier findings (22, 25) that a combined regimen may be more effective in the chemoprevention of colon cancer than individual drug administration. For the first time, we have been able to demonstrate in vivo that specific chemopreventive agents (ASP, FA) when encapsulated within polymer based nanoparticles and administered in combination doses with Ca, are more effective in inhibition of ACF formation than when administered individually.
In summary, our study provides clear evidence of two important aspects in the chemoprevention of colon cancer: (1) combination treatment plays a significant role in the suppression of ACFs in the rat colon; and (2) the use of targeted nanotechnology-based combined regimens are more effective than the unmodified mixtures of chemopreventive agents in the chemoprevention of colon cancer. The drug delivery system used in this study has provided new information on the sustained release of chemopreventive agents in the colon and their effectiveness in CRC prevention and has potentially opened the doors for further research into these nano-scaled carriers for the chemoprevention of colon cancer. More studies are needed to determine the utility of this novel technology and its potential impact on chemoprevention as a whole. In addition, pharmacokinetic data on the release of ASP and FA from PLGA nanoparticles would be helpful in designing nanotechnology-based regimens for eventual use in humans. From the current study, this novel approach of delivering agents for chemoprevention of cancer appears to be promising.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by NIH National Cancer Institute grant CA121409 (to S. Prabhu).
References
- 1.American Cancer Society. Cancer Facts and Figures 2010. Atlanta (GA): 2010. [Google Scholar]
- 2.Clapper ML, Chang WC, Meropol NJ. Chemoprevention of colorectal cancer. Curr Opin Oncol. 2001;13:307–13. doi: 10.1097/00001622-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–80. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald P. Cancer Chemoprevention. BMJ. 2002;324:714–8. doi: 10.1136/bmj.324.7339.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporn MB. Prevention of Cancer in the Next Millennium. Cancer Res. 1999;59:4743–58. [PubMed] [Google Scholar]
- 6.Gustafson-Svard C, Lilja I, Hallbook O, Sjodahl R. Cyclo-oxygenase and colon cancer: clues to the aspirin effect? Ann Med. 1997;29:247–52. doi: 10.3109/07853899708999342. [DOI] [PubMed] [Google Scholar]
- 7.Cascinu S, Ligi M, Del Ferro E, et al. Effects of calcium and vitamin supplementation on colon cell proliferation in colorectal cancer. Cancer Invest. 2000;18:411–6. doi: 10.3109/07357900009032811. [DOI] [PubMed] [Google Scholar]
- 8.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 9.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki R, Kusunoki N, Matsuzaki T, Hashimoto S, Kawai S. Selective cyclooxygenase-2 inhibitors show a differential ability to inhibit proliferation and induce apoptosis of colon adenocarcinoma cells. FEBS Lett. 2002;531:278–84. doi: 10.1016/s0014-5793(02)03535-4. [DOI] [PubMed] [Google Scholar]
- 11.Sheng GG, Shao J, Sheng H, Hooton EB, Isakson PC, Morrow JD, et al. A selective cyclooxygenase 2 inhibitor suppresses the growth of H-ras-transformed rat intestinal epithelial cells. Gastroenterol. 1997;113:1883–91. doi: 10.1016/s0016-5085(97)70007-6. [DOI] [PubMed] [Google Scholar]
- 12.Chan AT, Giovannucci EL, Schernhammer ES, Colditz GA, Hunter DJ, Willett WC, et al. A prospective study of aspirin use and the risk for colorectal adenoma. Ann Intern Med. 2004;140:157–166. doi: 10.7326/0003-4819-140-3-200402030-00006. [DOI] [PubMed] [Google Scholar]
- 13.Roderick PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Clin Pharmacol. 1993;35:219–26. doi: 10.1111/j.1365-2125.1993.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan AT. Aspirin, non-steroidal anti-inflammatory drugs and colorectal neoplasia: future challenges in chemoprevention. Cancer Causes Contr. 2003;14:413–8. doi: 10.1023/a:1024986220526. [DOI] [PubMed] [Google Scholar]
- 15.Pufulete M, Al-Ghnaniem R, Leather AJ, Appleby P, Gout S, Terry C, et al. Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterol. 2003;124:1240–8. doi: 10.1016/s0016-5085(03)00279-8. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med. 1998;129:517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Baggott JE, Morgan SL, Ha T, Vaughn WH, Hine RJ. Inhibition of folate-dependent enzymes by non-steroidal anti-inflammatory drugs. Biochem J. 1992;282:197–202. doi: 10.1042/bj2820197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q, Williamson KE, O’rourke D, Rowlands BJ. The effect of L-arginine on crypt cell hyperproliferation in colorectal cancer. J Surg Res. 1999;81:181–8. doi: 10.1006/jsre.1998.5512. [DOI] [PubMed] [Google Scholar]
- 19.Terry P, Baron JA, Bergkvist L, Holmberg L, Wolk A. Dietary calcium and vitamin D and risk of colorectal cancer: a prospective cohort study in women. Nutr Cancer. 2002;43(1):39–46. doi: 10.1207/S15327914NC431_4. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs ET, Jurutka PW, Martinez ME, Alberts DS. Vitamin D, calcium and colorectal neoplasia: new insights on mechanisms of action. Cancer Prev Res. 2009;2:197–9. doi: 10.1158/1940-6207.CAPR-09-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1,25–dihydroxyvitamin D(3) Cancer Res. 2005;65:493–8. [PubMed] [Google Scholar]
- 22.Kanthamneni N, Chaudhary A, Wang J, Prabhu S. Nanoparticulate delivery of novel drug combination regimens for the chemoprevention of colon cancer. Int J Oncol. 2010;37:177–85. doi: 10.3892/ijo_00000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–70. [PubMed] [Google Scholar]
- 24.Torrance CJ, Jackson PE, Montgomery E, et al. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–8. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhary A, Wang J, Prabhu S. Development and validation of a high-performance liquid chromatography method for the simultaneous determination of aspirin and folic acid from nano-particulate systems. Biomed Chromat. 2010;24:919–25. doi: 10.1002/bmc.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Schut HA, Conran P, Kramer PM, Lubet RA, Steele VE, Hawk EE, Kelloff GJ, Pereira MA. Prevention by aspirin and its combination with alpha-difluoromethylornithine of azoxymethane-induced tumors, aberrant crypt foci and prostaglandin E2 levels in rat colon. Carcinogenesis. 1999 Mar;20(3):425–30. doi: 10.1093/carcin/20.3.425. [DOI] [PubMed] [Google Scholar]
- 27.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 28.Bailey M, Williams NA, Wilson AD, Stokes CR. Probit: weighted probit regression analysis for estimation of biological activity. J Immuno Meth. 1992;153:261–2. doi: 10.1016/0022-1759(92)90329-r. [DOI] [PubMed] [Google Scholar]
- 29.Ross MH, Romrell LJ, Kaye GI. Histology a text and atlas edition. 1995;3:1–17. [Google Scholar]
- 30.Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997;57:1301–5. [PubMed] [Google Scholar]
- 31.Tozaki H, Komoike J, Tada C, Maruyama T, et al. Chitosan capsules for colon specific drug delivery: Improvement of insulin absorption from the rat colon. J Pharm Sci. 1997;86 (9):1016–1021. doi: 10.1021/js970018g. [DOI] [PubMed] [Google Scholar]
- 32.Pretlow TP, Cheyer C, O’Riordan MA. Aberrant Crypt foci and colon tumors in F344 rats have similar increases in proliferative activity. Int J Cancer. 1994;56:599–602. doi: 10.1002/ijc.2910560422. [DOI] [PubMed] [Google Scholar]
- 33.Takahasi S, Ogawa K, Ohshima H, Esumi H, Ito N, Sugimura T. Induction of aberrant crypt foci in the large intestine of F344 rats by oral administration of 2-amido-1-methyl-6-phenylimidazol[4,5-b]pyridine. Jpn J Cancer Res. 1991;82:135–7. doi: 10.1111/j.1349-7006.1991.tb01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, Ahmad N, Cui H, Mousa SA, Mukhtar H. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui IA, Adhami VM, Ahmad N, Mukhtar H. Nanochemoprevention: sustained release of bioactive food components for cancer prevention. Nutr Cancer. 2010;62:883–90. doi: 10.1080/01635581.2010.509537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqui IA, Mukhtar H. Nanochemoprevention by bioactive food components: a perspective. Pharm Res. 2010;27:1054–60. doi: 10.1007/s11095-010-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta V, Aseh A, Rios CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin derived curcumin nanoparticles for cancer therapy. Int J Nanomed. 2009;4:115–22. doi: 10.2147/ijn.s5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A. Polymeric nanoparticle encapsulated curcumin (nanocurcumin): a novel strategy for human cancer therapy. J Nanobiotech. 2007;5:1–18. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T, Wong YS, Zheng W, Bai Y, Huang L. Selenium nanoparticles fabricated in Undaria pinnatifada polysaccharide solution induce mitochondria mediated apoptosis in A375 human melanoma cells. Colloids and Surface B: Biointerfaces. 2008;67:26–31. doi: 10.1016/j.colsurfb.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Reddy BN. Studies with Azoxymethane-Rate Preclinical Model for Assessing Colon Tumor Development and Chemoprevention. Environ. Mol Mutagen. 2004;44:26–35. doi: 10.1002/em.20026. [DOI] [PubMed] [Google Scholar]
- 41.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–71. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 42.Reddy BS, Wang CX, Kong AN, Khor TO, Zheng X, Steele VE, Kopelovich L, Rao CV. Prevention of azoxymethane–induced colon cancer by combination of low doses of atorvastatin, aspirin and clecoxib in F344 rats. Cancer Res. 2006;66:4542–6. doi: 10.1158/0008-5472.CAN-05-4428. [DOI] [PubMed] [Google Scholar]
- 43.Reddy BS, Rao CV. Chemoprevention of colon carcinogenesis by concurrent administration of piroxicam, a nonsteroidal anti-inflammatory drug, with D, L-alpha difluoromethyornithine, in diet. Cancer Res. 1990;50:2562–8. [PubMed] [Google Scholar]
- 44.Lin J, Hsiao PW, Chiu TH, Chao TJ. Combination of Cox-2 and oxaliplatin increases growth inhibition and death in human colon cancer cells. Biochem Pharmacol. 2005;70:658–67. doi: 10.1016/j.bcp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Steele VE, Rao CV, Zhang Y, Patlolla J, Boring D, Kopelovich L, Juliana MM, Grubbs CJ, Lubet R. Chemopreventive efficacy of naproxen and nonaproxen in rodent models of colon, urinary bladder, and mammary cancers. Cancer Prev Res. 2009;2:951–6. doi: 10.1158/1940-6207.CAPR-09-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hariharan S, Bhardwaj V, Bala I, Sitterberg J, Bakowsky U, Ravi Kumar MN. Design of estradiol loaded PLGA nanoparticulate formulations: a potential oral delivery system for hormone therapy. Pharm Res. 2006;23:184–95. doi: 10.1007/s11095-005-8418-y. [DOI] [PubMed] [Google Scholar]
- 47.Sinha VR, Mittal BR, Bhutani KK, Kumria R. Colonic delivery of 5-fluorouracil: an in vitro evaluation. Int J Pharm. 2004;269:101–8. doi: 10.1016/j.ijpharm.2003.09.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.