Abstract
Chronic human immunodeficiency virus (HIV) infection is associated with higher incidence of pulmonary complications including hypertension, vasculopathy, lymphocytic alveolitis, and interstitial pneumonitis not attributed to either opportunistic infections or presence of the virus. The Tat (TransActivator of Transcription) protein, a required transactivator for expression of full-length viral genes, is pleiotropic and influences expression of cellular inflammatory genes. Tat-dependent transactivation of cellular genes requires specific mediators, including NF-κB, widely recognized as sensitive to changes in cellular oxidant burden. We hypothesized that overproduction of Tat leads to increased oxidant burden and to alterations of basal inflammatory status as measured by NF-κB activation. We engineered transgenic mouse lines that express Tat (86-amino acid isoform) in the lung under the control of the surfactant protein C promoter (SP-C). Tat-transgenic mice exhibit increased pulmonary cellular infiltration, increased nitrotyrosine and carbonyl protein modifications, increased levels of NF-κB, MnSOD and thioredoxin interacting protein (TxNIP). These data indicate that Tat increases oxidant burden and resets the threshold for inflammation, which may increase susceptibility to secondary injuries.
Keywords: HIV, Tat, oxidative stress, NF-κB, inflammation, TxNIP
INTRODUCTION
Chronic human immunodeficiency virus (HIV) infection is associated with exaggerated inflammatory responses to pulmonary pathogens [1,2], suggesting a deregulation or imbalance between pro- and anti-inflammatory elements. Factors related to the virus itself, to the host and/or to environmental exposures probably account for this clinical manifestation. Reactive oxygen species (ROS) play a fundamental role in initiation, amplification and cell destruction during innate and adaptive inflammatory processes. Oxidative stress, characterized by increased production of cellular oxidants [e.g. superoxide, hydrogen peroxide, nitric oxide] and/or decreased antioxidants or antioxidant enzymes (e.g. glutathione, vitamin E, ascorbate, glutathione peroxidase, superoxide dismutases, catalase), is associated with nearly all pathological states, especially those involving inflammation [3]. AIDS patients are oxidatively stressed, with decreased glutathione (GSH) [4], selenium [5] and vitamin E [6], and increased lipid and protein oxidation products [7]. Alveolar macrophages from asymptomatic HIV-positive individuals release significantly more superoxide [8] and inflammatory cytokines [9]. Enhanced oxidative stress in these patients results in tissue damage accelerated by an exaggerated inflammatory response that contributes to the pulmonary pathologies observed.
The HIV protein Tat, a viral regulator required for efficient transcription of viral genes, is secreted from infected cells and taken up by uninfected by-stander cells where it can have pleiotropic effects on cellular gene expression [10,11]. Most relevantly, Tat influences the expression of key genes that amplify the inflammatory response. Analogous to HIV infected cells, TNF-α is elevated in cells exposed to Tat [12,13] and Tat-Tg mice, with global expression of tat, have altered cytokine levels [14]. Tat represses MnSOD expression in HeLa and Jurkat cells [15,16], and down-regulates glutathione (GSH) and GSH biosynthetic enzymes [17,18]. This deficiency of antioxidant enzymes probably contributes to the establishment and maintenance of increased baseline oxidant burden. Tat potentiates TNF-α-mediated NF-κB responses by altering the redox status [16]. We have demonstrated that Tat-dependent inhibition of MnSOD and glutathione peroxidase (GPx) expression induces an increase in basal redox status and leads to depletion of glutathione [15]. We have also demonstrated that one of the downstream effects of Tat-mediated oxidative stress in human endothelial cells is the activation of NF-κB [19], a potent inducer of pro-inflammatory molecules such as the leukocyte adhesion molecule ELAM (E-selectin) and the cytokines TNF-α, IL-1 and IL-6 and the Monocyte Chemoattractant Protein-1 (MCP-1) [20,21]. Therefore, we propose that Tat is partly responsible for the oxidative stress exhibited by AIDS patients and that this oxidative stress induces exaggerated inflammatory and other immune responses that initiate or potentiate pulmonary complications
In order to investigate the possible role of Tat in pulmonary pathologies, we generated a transgenic mouse where Tat expression is driven by the surfactant protein C (SPC) promoter active in Type II alveolar epithelial and bronchial club cells. We hypothesized that the local production of Tat would lead to basal increase in oxidant burden resulting in markedly enhanced inflammatory injury per se or in the setting of environmental oxidative stress. Thus, in the transgenic mouse, Tat initiates the injury.
MATERIALS AND METHODS
Cloning of SPC-tat
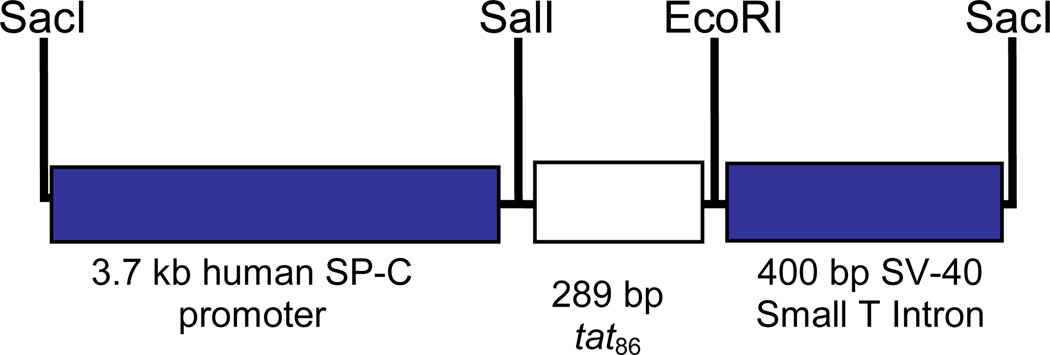
A 289 bp HindIII/EcoRI fragment containing the gene for Tat86 was generated from plasmid HIV-Tat Designer Gene (AIDS Research and Reference Reagent Program) and cloned into plasmid 3.7 hSP-C/SV-40 (generously provided by Jeffrey A. Whitsett, Children’s Hospital Medical Center, Cincinnati, Ohio, USA). This pUC18-based plasmid contains a 3.7 kb fragment of flanking sequence from the human SP-C promoter and SV40 small T-intron as a polyadenylation signal. This plasmid has been used to express transgenes in Type II alveolar epithelial cells and bronchial club cells [22,23]. The tat fragment was generated by digesting plasmid HIV-Tat Designer Gene with HindIII. The HindIII overhang was filled in with Klenow polymerase and the linear plasmid digested with EcoRI so as to generate a 289-bp tat fragment that had a blunt 5’-end and a 3’-EcoRI overhang. Similarly, the 3.7 hSP-C/SV-40 plasmid was initially digested with SalI and the overhang was filled-in with Klenow followed by digestion with EcoRI to generate a blunt 3’-end and a 5’-EcoRI overhang. The linear 3.7 hSP-C/SV-40 plasmid and the tat fragment were gel purified using a GenElute column (Sigma-Aldrich, St. Louis MO) and ligated using T4 DNA ligase (Invitrogen Life Technologies, Carlsbad CA). After transformation of E. coli Top10 cells (Invitrogen Life Technologies, Carlsbad CA) recombinants were selected by growth on ampicillin. Bacterial colonies harboring pSPC-Tat were screened by diagnostic digestion with SalI and EcoRI.
Generation of transgenic mice
pSPC-Tat was digested with SacI to generate a 4.4-kB fragment that contained the SPC-promoter, tat86 and the SV40 Small T-Poly A site (Figure 1); the construct was gel purified and dialyzed against endotoxin-free injection buffer composed of 5 mM Tris pH 7.4 and 0.1 mM EDTA. The purified fragment was used for pro-nuclear injections into FVB/N pseudopregnant females as described previously. The injections were performed at the University of Cincinnati Transgenic Core Facility (Cincinnati, Ohio, USA). A total of 63 pups were screened by PCR and 13 were positive for tat. The 13 founders were bred with wildtype FVB/N females and some of the resulting F1 offspring (at least 5 from each line) were sacrificed and tested for Tat expression in the lungs and heart by qPCR. Of the 13 DNA positive founders, 3 were determined to express tat, each at different levels (low, medium and high, see Figure 1). For maintenance of the various lines, positive males were bred with wildtype FVB/N females. Tail DNA from resultant litters was used to screen by PCR for the 289-bp tat fragment.
Figure 1. Recombinant genetic construct used to generate tat transgenic mice.
The recombinant construct used to generate the mice consisted of the tat86 upstream of the SV40 Small T-intron poly adenylation site and downstream of the human SP-C promoter. The SP-C promoter allows targeted expression to the lung epithelium.
Harvest and Culture of Lung Fibroblastic cells
Lung fibroblastic cells were harvested from Tat transgenic mice and cultured in RPMI (Invitrogen Life Technologies, Carlsbad CA). The mice were euthanized by intraperitoneal (IP) injection of sodium pentobarbital. The lungs were surgically removed and placed on a culture dish containing RPMI medium. The lungs were minced and 1mm pieces transferred to 25 mm culture flasks containing 5 ml of RPMI and incubated at 37°C in a humidified 6.5% CO2 incubator for 4 days until monolayers were evident in the area surrounding the lung segments. Large tissue pieces were removed, cell monolayers washed and incubated in fresh RPMI for 1 additional week. Cells were transferred to the cytogenetics lab for FISH analysis.
Fluorescence In-Situ Hybridization
Lung fibroblast cultures were harvested after incubation with Colcemid (Invitrogen Life Technologies, Carlsbad, CA) for 3 hrs at 37°C. Cells were detached with Trypsin-EDTA, hypotonized at 37°C, fixed and placed on clean microscope slides. The 4.4 kB SacI DNA fragment containing the SPC-promoter, Tat86 and the SV40 Small T-PolyA was labeled with SpectrumRed (SR) conjugated dUTPs using the Vysis Nick Translation Kit (Des Plaines, IL) according to the manufacturer’s protocol. The labeled probe was ethanol-precipitated with herring sperm DNA and resuspended in c-DenHyb (Insitus Biotechnologies, Albuquerque, NM). One slide of each sample was submitted to a single-target FISH assay per standard protocols. The mouse chromosome classification followed Nesbitt and Francke (1973, Chromosome, 41:155–158) and the guidelines of the International Committee on Standardized Genetic Nomenclature for mice (Rev. Jan 2005) [http://www.informatics.jax.org/mgihome/nomen/anomalies].
RNA isolation
Following euthanasia with sodium pentobarbital by IP injection, the chest and abdominal cavities were surgically opened and lungs removed. Approximately 100 mg of lung tissue were homogenized in 1 ml of Trizol Reagent (Invitrogen Life-Technnologies, Carlsbad CA). Total RNA was isolated according to the manufacturer’s instructions. The RNA pellet was resuspended in 55 µl of RNase-free water and incubated at 55°C for 10 minutes. The A260/A280 was measured using a Nanodrop-100 (Nanodrop Technologies Inc, Wilmington, DE) and the RNA concentration determined using an extinction coefficient of 1 A260 = 40 µg/ml.
RT-PCR
RT-PCR was performed using the One-Step RT enzyme mix from Qiagen (Valencia CA) with 250 ng of input template RNA. After reverse transcription, the RT was inactivated and simultaneously the Taq DNA Polymerase was activated by incubation at 95°C for 15 minutes. Reactions without RT (“No-RT”) were performed in order to account for contaminating genomic DNA. Amplicons were analyzed after electrophoresis on a 1% agarose gel. Digital images were acquired using a Kodak Image Station 440.
qPCR
Expression of the tat gene was quantified by quantitative PCR (qPCR) using standard techniques. Briefly, total RNA was extracted from lung tissue homogenized in Trizol reagent (Invitrogen Life Technologies, Carlsbad CA) according to the manufacturer’s instructions. The RNA samples were treated with DNaseI and cleaned-up using Qiagen RNeasy clean-up columns. qPCR was performed using a One-Step RT-PCR kit with SYBR green in an iCycler Thermal Cycler (both from BioRad Laboratories, Hercules CA) according to the manufacturer’s instructions. The tat primers amplify a 280 bp fragment of tat. The standard curve was generated by amplifying a product of similar size from the pSPC-tat plasmid. Statistical analysis was performed by calculating P values using an unpaired two-tailed Student t test between the groups; n = 4 animals per group. Values p<0.05 were considered statistically significant.
Lung protein isolation
Total protein was extracted from the right lung by homogenization and sonication in buffer containing 10 mM HEPES, 137 mM NaCl, 4.6 mM KCl, 1.1 mM KH2PO4, 0.6 mM MgSO4, 1.1 mM EDTA, 40 µg/ml PMSF, 0.1 mM DTT, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis MO). For Tat immunoblots, NaCl concentration was increased to 500 mM. To extract membrane proteins, triton X-100 was added to a final concentration of 0.2% and samples were incubated at 4°C for 1 hour. After centrifugation at 13,000×g for 20 minutes at 4°C to clarify the homogenate, the supernatant was stored at −70°C. Protein concentrations were determined with Bradford reagent (Bio-Rad Laboratories, Hercules CA). For detection of nitrotyrosine and carbonyl modifications, proteins were extracted by the same method except that the lung tissue was pulverized under liquid nitrogen into a fine powder prior to homogenization.
Immunoblots
Proteins were resolved by SDS polyacrylamide gel electrophoresis and electroblotted in Towbin buffer onto polyvinyl difluoride membrane (PVDF). Specific proteins were detected by standard detection techniques using a chemiluminescent HRP substrate (West-Femto ECL, Pierce Biotechnology, Rockford, IL). Images were captured on a Kodak Image Station 440 and immunoblots quantified by densitometry using the Kodak 1D Imaging software; signals were normalized to the β-actin signal. Anti-actin monoclonal was from Sigma Chemical Company (St. Louis MO); Anti-MnSOD and Anti-nitrotyrosine polyclonal antisera were from Millipore (Billerica MA); Anti-p65 was from Santa Cruz Biotechnology (Santa Cruz CA). Statistical analysis was performed by calculating P values using an unpaired two-tailed Student t test between the groups; n = 4 – 8 animals per group. Values p<0.05 were considered statistically significant.
Lung inflation for microscopic morphological analysis
Following euthanasia with sodium pentobarbital by IP injection the chest cavity was opened and the trachea was exposed. The lungs were perfused free of blood, the right lung lobes were tied off and a 0.5% solution of melted low-melting point Seaplaque agarose was injected slowly into the left lung through a nick in the trachea. The inflated lung was dissected and fixed in 4% paraformaldehyde ex vivo.
Immunohistochemistry
The inflated and fixed lungs were embedded in paraffin and serial sections prepared. Anti-Tat rabbit polyclonal antiserum was used to stain sections with a Zymed AEC (aminoethyl carbazole) immunohistochemical staining kit (Invitrogen Lifetechnologies, Carlsbad, CA) according to the manufacturer’s protocols. As negative controls, serial sections were stained with the secondary antibody alone.
Oxidative Stress qPCR panels
Real-time reverse transcription quantitative PCR was performed on all the RNA samples using RT2 Profiler PCR Array for Mouse Oxidative Stress and Antioxidant Defense (SABiosciences, Frederick MD) according to the manufacturer’s instructions on a BioRad iCycler. Statistical analysis was performed by calculating P values using an unpaired two-tailed Student t test between the groups; n = 4 – 8 animals per group. Values p<0.05 were considered statistically significant.
Nitrotyrosine ELISA
The presence and amount of 3-nitrotyrosine in the protein samples were measured with the OxiSelect Nitrotyrosine ELISA kit (Cell Biolabs, Inc. San Diego CA.) following the manufacturer’s instructions. Statistical analysis was performed by calculating P values using an unpaired two-tailed Student t test between the groups; n = 4 – 8 animals per group. Values p<0.05 were considered statistically significant.
NF-kB (p65) ELISA
Relative levels of activated NF-κB p65 (RelA) in the transgenic versus non-transgenic mice were determined using the p65-specific chemiluminescent TransAM Enzyme-Linked Immunoabsorbent Assay (ELISA) kit (Active Motive, Carlsbad, CA). Whole cell protein extracts were obtained from viable whole lung cell homogenates using Mammalian Protein Extraction Reagent (M-PER, Thermo Scientific, Rockford, IL) in the presence of protease and phosphatase inhibitors and DTT according to the manufacturer’s instructions. The ELISA was performed with 400 ng of protein extract as described by the manufacturer with the following minor modifications. Because the chemiluminescent signal from the positive control extract provided in the kit appeared to be saturated, we optimized the antibody dilutions so as to be in the linear range of the chemiluminescent signal. We determined that by increasing the antibody dilutions by 5-fold from those recommended by the manufacturer we could get the signals in the linear range. Therefore, for the ELISA from the mouse lung cell extract we used 5-fold less primary and secondary antibodies than recommended by the manufacturer. The sample size for each group was 4 – 8 animals, and p values were calculated using an unpaired two-tailed Student t test between the groups. Values p<0.05 were considered statistically significant.
Carbonyl Protein Immunobloting
Protein oxidation was analyzed by detecting carbonyl proteins using the Oxyblot protein oxidation detection kit (Millipore, Billerica MA). Derivatization of the protein lysates was done according to the manufacturer’s instructions (Millipore, Billerica MA). Carbonyl groups in the protein side chains were derivatized by reaction with 2,4-dinitrophenylhydrazone (DNP-hydrazone) and DNPH-protein adducts were detected through immunoblot analysis using an anti-DNPH antibody. Densitometric analysis was performed using Kodak 1D Imaging Software and the signals from the protein bands of interest were normalized to the β-actin signal. p values were calculated using an unpaired two-tailed Student t test between the groups. Values p<0.05 were considered statistically significant.
RESULTS
Generation and expression of tat-transgene
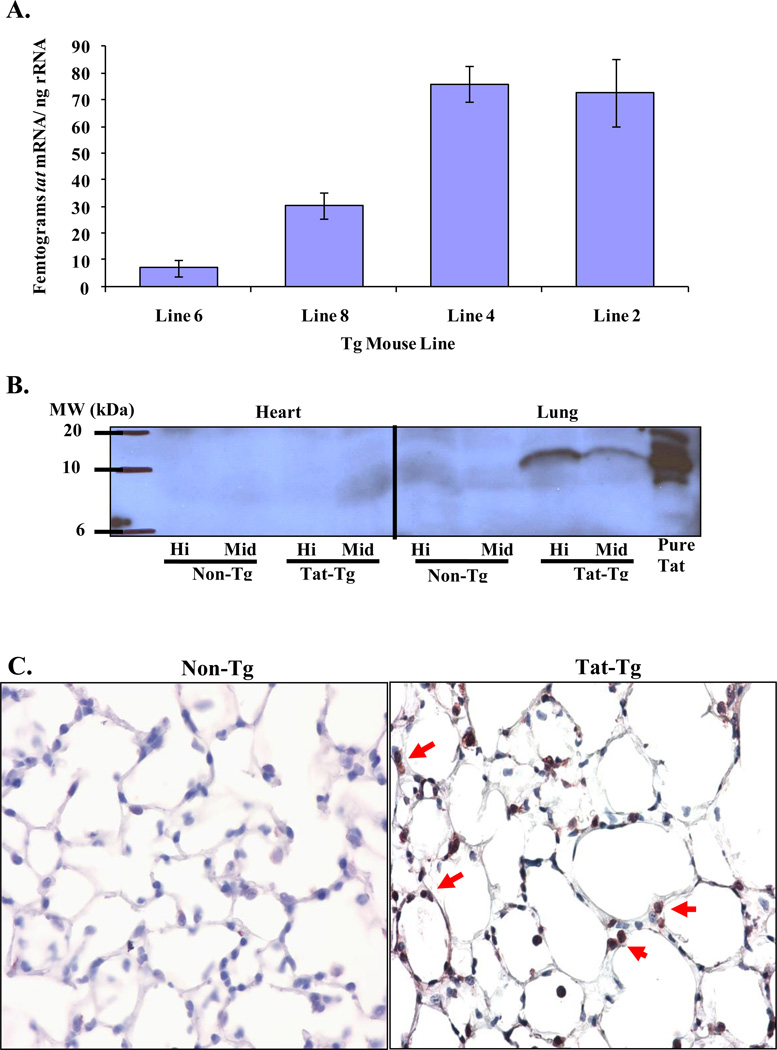
We tested several transgenic lines for expression of Tat. Figure 1 shows a schematic diagram of the plasmid construct used to generate the transgenic mice. Of thirteen founder lines, we selected 4 lines to analyze further on the basis of the quantitative PCR results. Figure 2A shows that line 6 had undetectable Tat, line 8 intermediate and lines 2 and 4 high levels of Tat, respectively. Tat protein was detected in the lungs of high Tat expressors by immunoblot (Figure 2B) as well as immunohistochemistry (Figure 2C).
Figure 2. The tat-transgene is expressed in the lungs.
Panel A. qPCR was utilized to determine the levels of mRNA in the various tat-transgenic lines. Lines with no expression (line 6), intermediate expression (line 8) and high expression (lines 2 and 4) were identified. Panel B. Tat protein was detected by western blot in the high and intermediate expressing lines in the lung but not in the heart. Panel C. Tat protein was also detected by immunohistochemical staining in lung sections from transgenic and non-transgenic mice. The arrows indicate alveolar epithelial cells that stained positive for Tat staining. There is no positive signal in the lungs from non-transgenic mice.
The tat gene inserted into different chromosome locations in each line
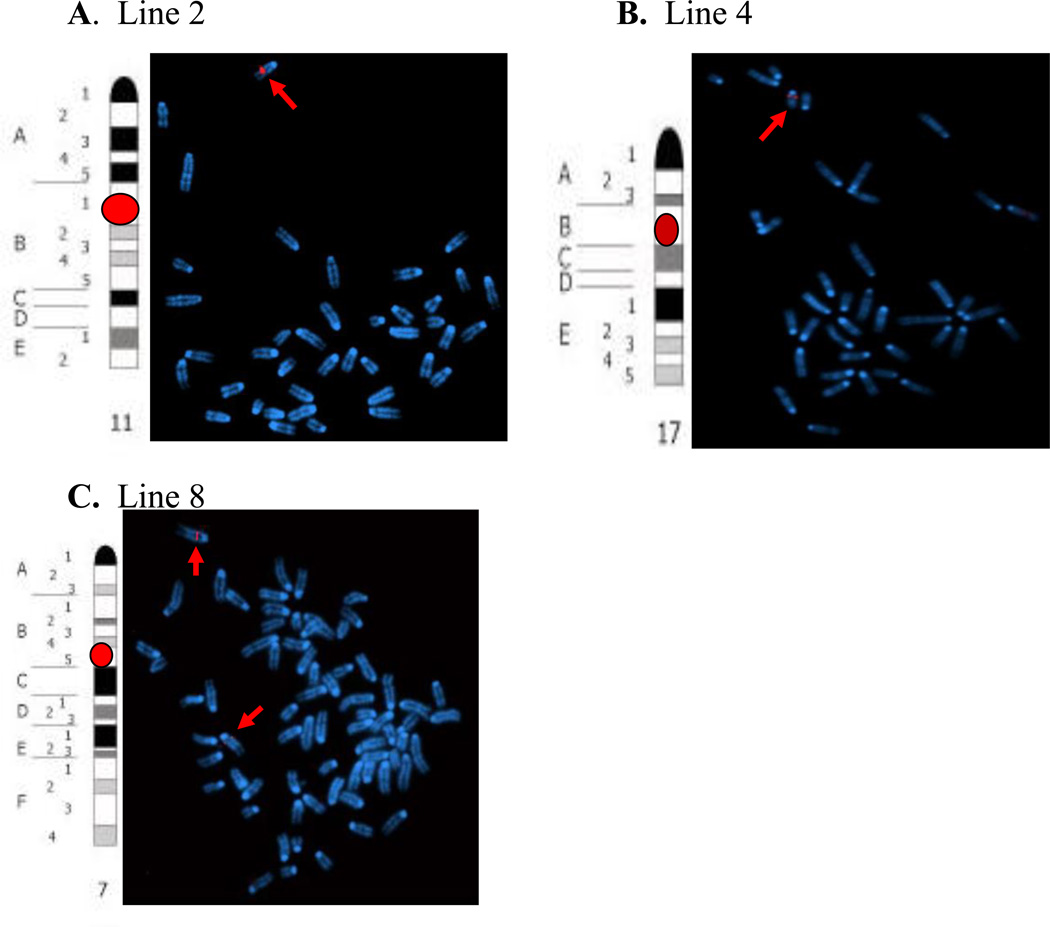
FISH analyses demonstrated that in line 4, the probe hybridized to chromosome 17, in a region located at the intersection of chromosome bands 17B and 17C. In Line 2 mice, the probe hybridized to chromosome 11 band B1 and to chromosome 7 band B2 or B3 in Line 8. These data are shown on Figure 3. All of the subsequent experiments were done with the positive lines, 2, 4 and 8 and non-transgenic littermates were used as negative controls.
Figure 3. FISH analysis of tat-transgene.
Panel A. The idiogram of mouse chromosome 11 shows the region homologous to the SacI probe (red dot) in line 2 mice. The merged DAPI and red images of chromosomes from a near-diploid metaphase cell hybridized with the SacI probe show chromosome 11 harboring SacI signals (indicated by arrow). Panel B. The idiogram of mouse chromosome 17 shows the region homologous to the SacI probe (red dot) in line 4 mice. The merged DAPI and red images of chromosomes from a diploid cell hybridized with the SacI probe show chromosome 17 harboring SacI signals (indicated by arrow). Panel C. The idiogram of mouse chromosome 7 shows the region homologous to the SacI probe (red dot) in line 8 mice. The merged DAPI and red images of chromosomes from a hypo-tetraploid metaphase cell show two chromosomes 7 harboring SacI signals (indicated by arrows) in line 8 mice.
Tat-tg mice display increased cellular infiltration in the lungs
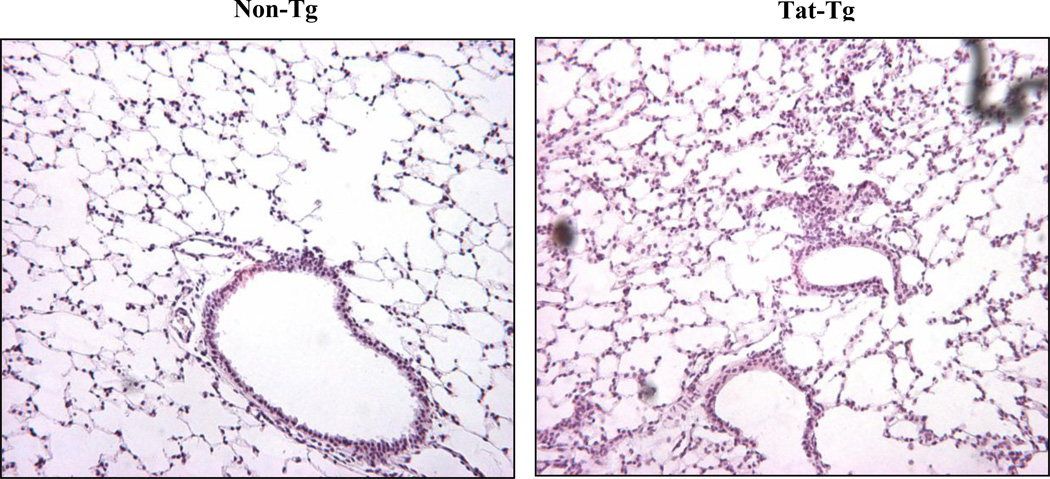
Figure 4 shows that although the lung architecture is not grossly affected by Tat, but there are more cellular infiltrates in the lungs from transgenic mice.
Figure 4. Hematoxylin and Eosin staining of tat-transgenic lungs.
Formalin-fixed paraffin-embedded lung sections from Tat transgenic and non-transgenic mice were stained and examined by light microscopy. A representative photomicrograph of each is shown. While the gross morphology and architecture of the lungs does not appear to be affected by the transgene, Tat transgenic mice exhibited more cellular infiltration than non-transgenic mice.
Tat-transgenic mice exhibit increased indices of oxidative stress
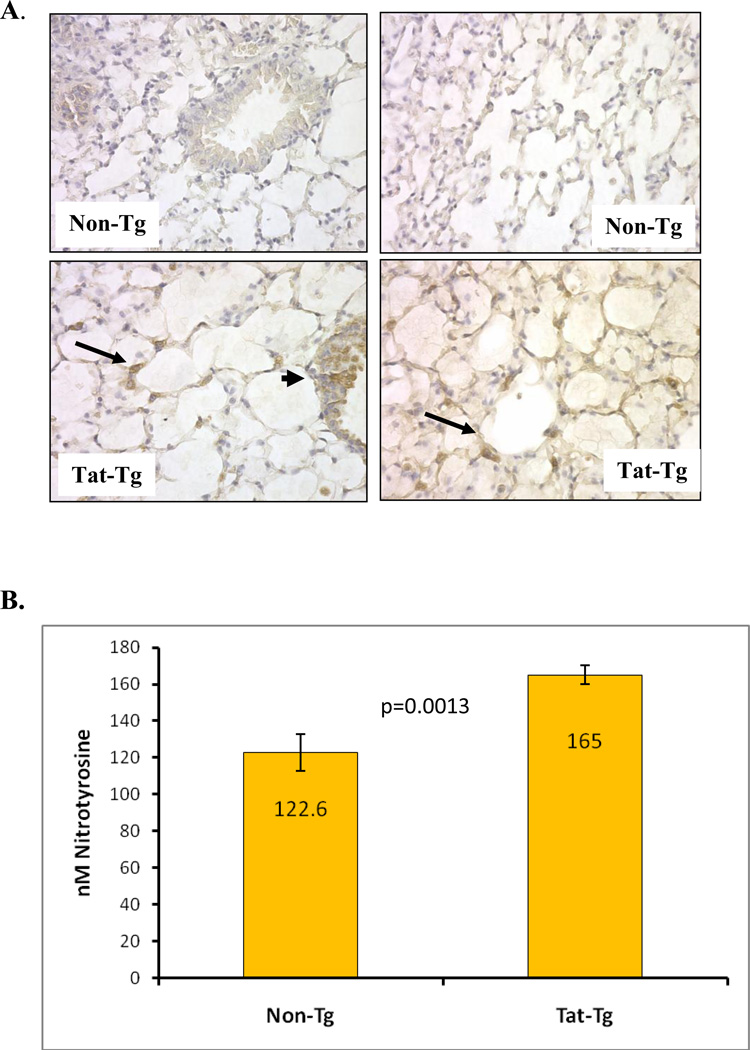
To assess oxidative stress, we measured protein nitration in several ways. First, we histologically analyzed protein nitration of paraffin-embedded lung sections. Figure 5A shows that lungs of tat-transgenic mice have more nitrotyrosine than non-transgenic littermates. Furthermore, the pattern of nitrotyrosine staining is very similar to the pattern of Tat staining (Figure 2C), which appears to be localized to the junctional Type II epithelial cells within the alveolar spaces (arrows).
Figure 5. Modification of proteins by nitrotyrosine is evident in tat-transgenic but not in non-transgenic littermates.
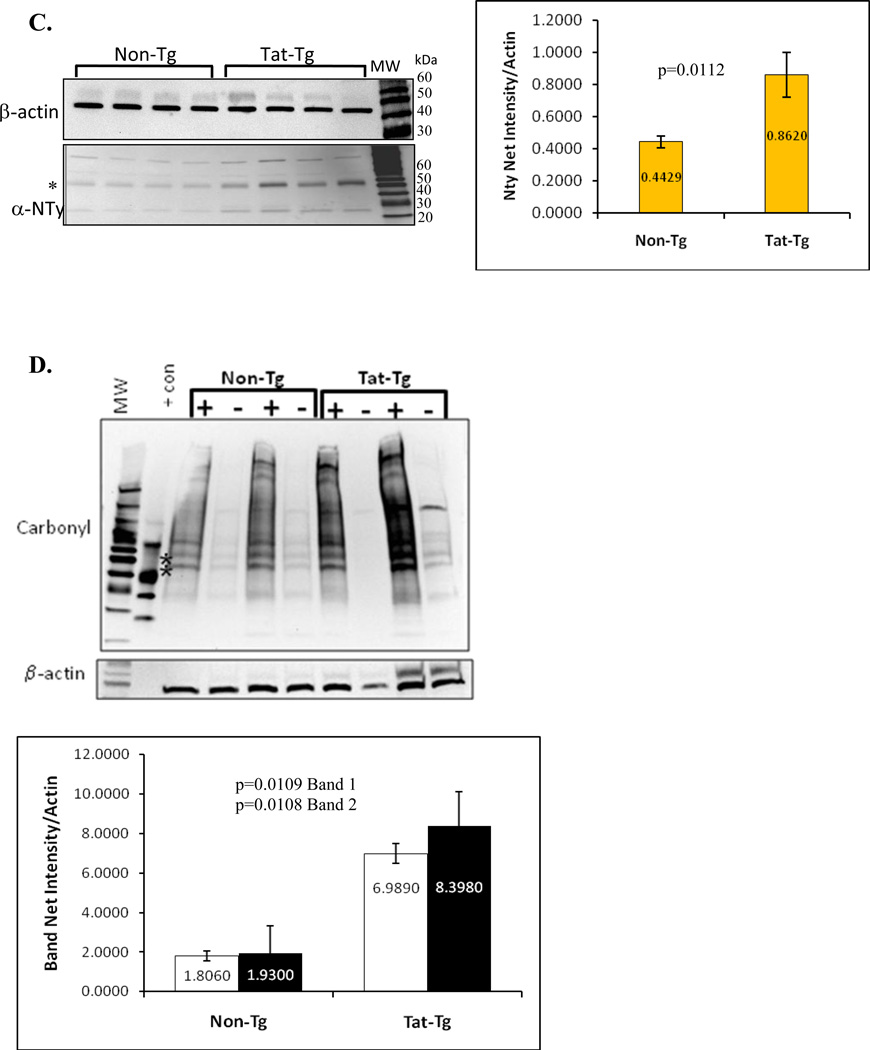
Panel A. Immunohistochemical detection of nitrotyrosine in lung sections from tat transgenic mice (bottom images) and non-transgenic controls (top images). All images shown were taken at 200X magnification. The images on the left show bronchial sections and those on the right show alveolar regions. Arrows point to areas of intense alveolar nitrotyrosine staining while arrowheads point to bronchial staining. Panel B. Levels of nitrated tyrosine in proteins from lungs of Tat transgenic mice and non-transgenic controls were quantified by ELISA. The numbers inside the bars represent the mean nitrotyrosine concentration (in nM) per group. The differences between tat-transgenic lines compared to the non-transgenics were statistically significant (p=0.0013). Panel C. Nitrated proteins from total lung extracts were detected by immublotting using anti-nitrotyrosine polyclonal antibody. To measure the relative intensity of one of the nitrated bands, indicated by the *, we performed densitometric analysis using Kodak 1D Imaging Software and normalized to the β-actin signal. There was a significant increased in the Tat-Tg mice. Panel D. Carbonyl modification of whole lung proteins was detected by immunoblotting using the Oxyblot system. Proteins were either derivatized (+) or not (−) with DNPH prior to electrophororesis, and following immunoblotting they were detected with an anti-DNPH antibody. To measure the relative effect of Tat on carbonyl protein formation we performed densitometric analysis using Kodak 1D Imaging Software of two of the bands, indicated by the * (band 1 is the top band and band 2 is the lower band), and normalized to the β-actin signal. The quantification results shown by the graph indicate a significant increase in carbonyl modifications in the Tat-Tg mice. In all cases, p values were calculated using an unpaired two-tailed Student t test between the groups, and the sample size (n) was 4 – 8 animals per group.
Secondly, we measured the overall level of nitrated proteins in lung lysates by ELISA. Figure 5B shows that lungs from transgenic mice had higher concentrations of nitrotyrosine (p=0.0013). The presence of nitrotyrosine was also confirmed via western blotting (Fig. 5C). Clear differences in the level of nitration were noted: several protein bands were detected, all of which were higher in intensity in the lysates from transgenic mice as compared to the non-transgenic controls. The relative intensity of one of the nitrated bands, indicated by the *, was significantly increased in Tat-Tg mice compared to non-transgenic littermates. These data are consistent with the data from the nitrotyrosine ELISA (Fig 5B).
Lastly, we measured the levels of carbonyl protein modification by immunoblot detection of the carbonyl adducts formed after derivatization with DNPH. The representative blot in figure 5D clearly shows that there is more carbonyl protein modification in the transgenic mouse samples than the non-transgenic. The relative intensity of the two bands, labeled with the *, showed that both proteins are increased by approximately 4-fold in the transgenic mice (p values < 0.01).
Tat-transgenic mice have increased total Mn-SOD and NF-κB levels
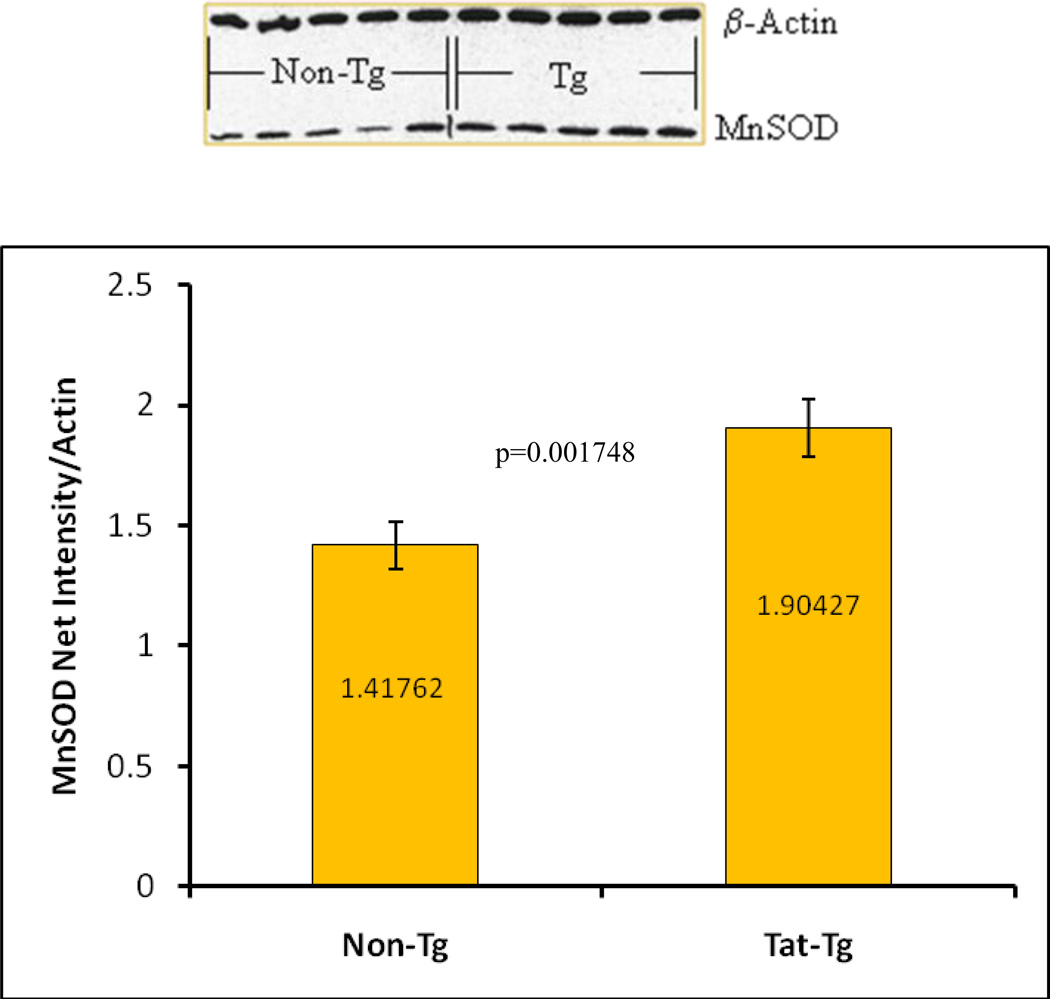
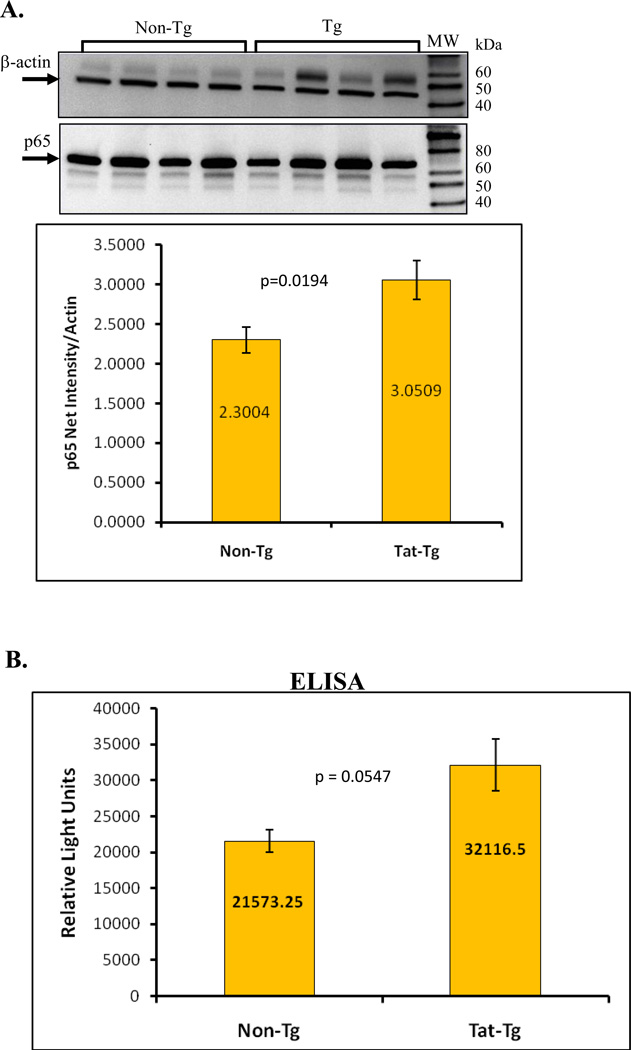
We measured MnSOD levels via immunoblot analyses and found that transgenic mice had a significant increase in MnSOD protein (p = 0.0017) (Figure 6). RelA protein (p65), detected by western blot, was also higher in transgenic mice (Figure 7). Furthermore, to confirm the results of the semi-quantitative p65 immunoblot, we performed ELISA to detect activated p65 in whole cell protein extracts from viable whole lung cells. The graph in figure 7B shows the significant increase in activated p65 levels in the transgenic mice compare to non-transgenic animals.
Figure 6. Semi-quantitative Detection of MnSOD.
MnSOD protein levels from total lung protein extracts were detected by immunoblotting. A representative immunoblot is shown. The relative intensity of the MnSOD signal was measured densitometrically using Kodak 1D Imaging Software and normalized to the β-actin signal. There was a significant increase in MnSOD levels in Tat-Tg mice. p values were calculated using an unpaired two-tailed Student t test between the groups. n = 4 – 8 animals per group.
Figure 7. Detection of NF-kB p65.
Total p65 protein levels from whole lung protein extracts were detected by immunoblotting and activated p65 was measured by ELISA. Panel A. A representative immunoblot is seen along with the semi-quantitative results which show a significant increase in the Tat-Tg mice. The relative intensity of the p65 signal was measured densitometrically using Kodak 1D Imaging Software and was normalized to the β-actin signal. Panel B. ELISA of activated p65 indicates a significant increase in the levels of active p65 in the transgenic mice. In all cases, p values were calculated using an unpaired two-tailed Student t test between the groups. n = 4 – 8 animals per group.
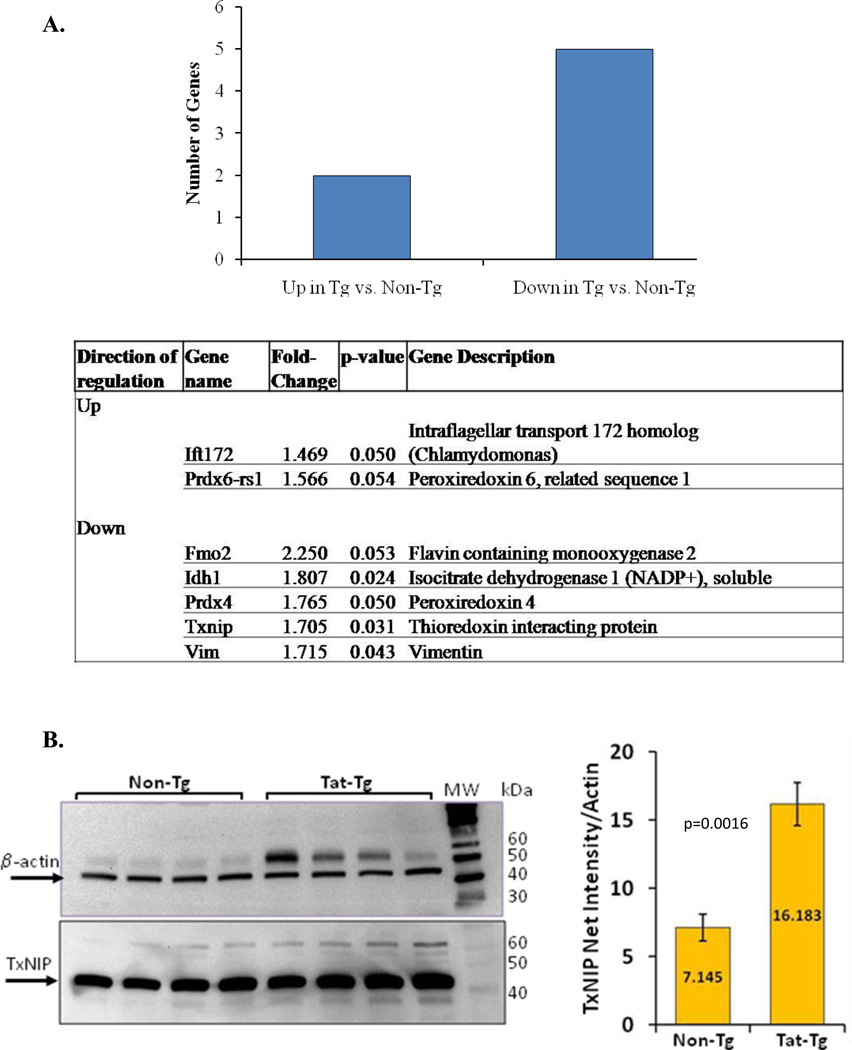
Oxidative stress genes are elevated in Tat-transgenic mice
Using a targeted array panel (Super Array), we measured 84 oxidative-stress and antioxidant defense-related genes by qPCR (Figure 8). The number of genes with as statistically significant 1.5-fold change in expression (higher or lower) compared to non-transgenic mice was determined. The data show that 8.3% (7/84) of the genes represented on the array were differentially expressed in the transgenic vs non-transgenic mice. Of those, 2 genes were significantly up-regulated and 5 were significantly downregulated.
Figure 8. Dysregulated expression of oxidative stress and antioxidant defense genes in tat-transgenic mice.
Panel A. Expression levels of a targeted Super Array panel of 84 oxidative stress and antioxidant defense genes were measured in Tat transgenic and non-transgenic mice. The relative fold differences between the groups of transgenic mice and the non-transgenic control mice were analyzed. The number of genes with significant 1.5 fold change in expression, either higher or lower, in transgenic mice compared to non-transgenic controls was determined. In total, 7 out of 84 genes (8.3%) were differentially expressed, 2 were increased and 5 were decreased, compared to non-transgenic controls. The table lists the names, descriptions, fold-regulation and p values of the 7 differentially expressed genes. Panel B. TxNIP protein from total lung protein extracts was detected by immunoblotting. The relative intensity of the TxNIP band was measured densitometrically using Kodak 1D Imaging Software and was normalized to the β-actin signal. There was a significant increase in TxNIP protein the Tat-Tg mice. p values were calculated using an unpaired two-tailed Student t test between the groups. n = 4 – 8 animals per group.
Thioredoxin interacting protein (TxNIP) is significantly increased in line 4 mice
The thioredoxin interacting protein, TxNIP, inhibits the reducing activity of thioredoxin by forming a disulfide bond with the redox active cysteine of thioredoxin. Our array data show that TxNIP was down-regulated in line 4 Tat-Tg mice. However, Western blot analysis demonstrated a significant 2.3-fold increase (p=0.0016) in TxNIP in the transgenic mice compared to the control, Non-Tg, group (Figure 8).
DISCUSSION
Oxidants damage tissues either by direct oxidation of key biological molecules (i.e., lipid peroxidation, DNA damage) or by alterations in transcriptional factors such as those of the NF-κB, Sp or AP families. Expression of adhesion molecules such as E-selectin, and inflammatory cytokines such as TNF-α are regulated by these transcriptional factors, particularly NF-κB p65 [24–28]. MCP-1, a potent chemokine for monocytes that induces their transendothelial migration, is regulated by reactive oxidant species (ROS) [29] through NF-κB [30]. O2 therapy (or hyperoxia), used in intensive care situations such as in respiratory distress syndrome, can cause lung injury via [31,32] increased ROS generation. In fact, hyperoxia has been used as a model of acute lung injury [33]. The oxidants formed cause further damage by inducing the secretion of inflammatory cytokines such as TNF-α, IL-1 or IL-6. Furthermore, dietary insufficiency of antioxidants (i.e. vitamin E) increases the susceptibility of rats to hyperoxia-induced lung damage and this damage can be mitigated by antioxidant enzyme induction [34,35]. These reports highlight the role of oxidative stress in the regulation of the inflammatory response and suggest that dysregulation of oxidant/antioxidant homeostasis can result in increased sensitivity to pro-inflammatory stimuli, in essence re-setting the threshold for inflammation.
Oxidative stress in HIV infection might occur either through increased production of ROS or decreased antioxidant defenses [36,37]. Chronic inflammation in HIV infection is also associated with high levels of inflammatory cytokines. The accessory proteins of HIV-1 play a key role in pathogenesis, but the specific contribution of Tat has not been not fully elucidated. In order to study the in vivo effects of Tat, we constructed a Tat-transgenic mouse with targeted expression of tat. The perturbations of Tat to cellular metabolism have occurred at doses as low as 1.6 ng/ml, which is the measured level of Tat in the circulation of HIV-infected patients [38], but it is likely that local concentrations of Tat in tissues harboring HIV-infected cells is much higher than in blood. Figure 2 shows that the transgenic lines had varying degrees of transgene transcription, with lines 2 and 4 being high expressors. Detection of Tat protein was accomplished both by immunoblot and by immunohistochemical staining but quantification of the protein levels was not possible with either of these techniques.
We found that the overall lung morphology is not affected by the presence of Tat, but did find more cellular infiltrates in the air spaces of transgenic animals (Figure 4). Although we do not know the identity of these cells, this observation suggests that there is increased recruitment/migration of cells into the lungs of transgenic mice. This could be due to increased secretion of chemotactic factors and/or decreased clearance/efferocytosis of resident or infiltrating cells [39]. This phenotype is consistent with a report by Kim et al in which they observed increased infiltration of activated monocytes and T lymphocytes into the brains of transgenic mice with astrocyte-specific expression of tat [40]. Since oxidative stress can induce aberrant expression of chemotactic cytokines, we decided to investigate whether oxidative balance was also influenced by the presence of Tat in these mice. The presence and severity of oxidative stress can be detected by biochemical indicators including the chemical protein adducts nitrotyrosine and carbonylation. Both of these indices were elevated in the transgenic animals. Neither the identity of the modified proteins nor whether the modifications have any effect on function is known at this time; nonetheless these data support the existence of an oxidative imbalance in the lungs of the tat-transgenic mice.
Changes in expression or activity of cellular superoxide dismutase enzymes are also indicative of oxidative imbalance. For example, in most cases in vitro, cells respond to oxidative stress by increasing levels of MnSOD in order to combat the accumulation of reactive oxygen species or the depletion of other antioxidants. We measured the levels of MnSOD in the Tat transgenic mice by semi-quantitative immunoblotting, to determine the in vivo effect of Tat on MnSOD. The immunoblots indicate a significant increase in MnSOD. Interestingly, we have observed that Tat can have differential effects on cultured cells depending on the exposure route. We previously published that Tat expressed intracellularly decreases MnSOD expression [15]. However, we have also observed that Tat given exogenously increases MnSOD both at the transcriptional and protein levels (unpublished results). This suggests that intracellular and extracellular Tat activate different MnSOD regulatory pathways. Thus, in the lungs of these transgenic mice, the type 2 epithelial cells could secrete Tat into their microenvironment and exert paracrine effects. In such an environment, it is difficult to determine which response would have a greater effect on the overall balance. Unfortunately, there are no published reports on the state of MnSOD per se in HIV patients or even in other transgenic models. Furthermore, reports on total SOD activity appear to differ depending on the cellular compartment tested. Several groups have reported a decrease in SOD activity in plasma and/or mononuclear cells of HIV+ patients [41–43]. In contrast, Gil et al reported an increase in SOD activity in erythrocytes of HIV+ individuals [44]. Nevertheless, an ample number of reports describe that Tat and/or HIV infection induce an oxidative state in animal models, cultured cells and patients [5,45–47]. The effects of Tat and/or HIV infection specifically on MnSOD have been addressed only in cultured cells and in animal models. As with the clinical studies the results differ depending on several parameters including cell type and method of infection or exposure to the Tat protein. For example, it has been shown that HIV infection of CD4+ lymphocytes represses MnSOD levels, but it upregulates MnSOD transcription in human monocytes-derive macrophages [48]. Thus, given the documented contradictory effects of Tat on MnSOD expression, further experiments will be required to fully determine the mechanisms for the observed effects of Tat on MnSOD in these mice.
NF-κB is a critical transcription factor required for expression of inflammatory response genes. Tat modulates the activity the NF-κB p65 subunit in vitro; we therefore measured p65 protein in the transgenic mice compared to non-transgenic littermates. We detected a statistically significant difference in NF-kB p65 levels between transgenic mice and non-transgenic littermates. Importantly, the translocation of p65 into the nucleus is the crucial step in downstream activation of inflammatory genes. We did not fractionate the lysates into cytoplasmic and nuclear compartments and therefore are unable to establish whether the levels of nuclear versus cytoplasmic p65 are altered in the mice. However, we did measure levels of activated p65 by the chemiluminescent p65 TransAM ELISA kit. This assay quantitatively detects the relative amount of p65 in protein extracts that can bind to the p65-consensus oligonucleotide that is immobilized on the ELISA plate. By that measurement, the transgenic mice had higher levels of active p65 in the lungs, corroborating the results from the semi-quantitative immunoblots. It should be noted that p65 transcription was not determined in the SuperArray panel because this gene was not included. Nevertheless, these results indicate that the Tat-Tg mice have elevated levels of total and activated p65.
A qPCR SuperArray panel of oxidative stress-related genes was used to substantiate the findings from the nitrotyrosine and carbonyl data. Of the 84 genes present in this array, 7.1 % were differentially expressed in the combined lines, supporting the existence of an overall state of oxidative imbalance. Two of those genes were up-regulated and 5 were down-regulated in the transgenic mice. Taken together, these data are consistent with previous reports by several groups which demonstrated that targeted or generalized expression of tat decreases glutathione levels [17] and leads to a condition of generalized oxidative stress. It should be noted that while the superoxide dismutase genes (sod1 and sod2) were on the targeted array, a significant change in the mRNA levels of either of them was not detected. This observation is paradoxical given the results of the nitrotyrosine and carbonyl protein modification which strongly indicate the presence of a state of oxidative stress. This result is also unsupported by the immunoblot analysis of MnSOD which showed an increase of MnSOD protein in the transgenic mice. Although the Tat transgene did not appear to affect MnSOD transcription it did correlate with higher total MnSOD protein. While we cannot affirm that the effect of Tat on MnSOD is at the transcriptional level, the nitrotyrosine and carbonyl protein data strongly support the presence of oxidative stress in the lungs of these Tat-Tg mice.
One of the oxidant balance genes notably down-regulated in the targeted SuperArray was the gene for thioredoxin interacting protein (TxNIP). Thioredoxin facilitates the reduction of thiols in proteins, thus inhibition of thioredoxin activity would increase overall oxidant burden. TxNIP reacts with reduced thioredoxin via a disulfide exchange reaction between Cys73 of thioredoxin and Cys247 of TxNIP and inhibits its reducing activity [49]. Interestingly, TxNIP protein levels were two-fold higher in Tat-transgenic mice. One possible explanation for the conflicting data between the SuperArray and the immunoblots is that TxNIP is regulated via post-transcriptional mechanisms. Currently not much is known about the regulation of TxNIP expression. Recent reports have implicated TxNIP in the oxidant-mediated activation of the Nod-like receptor protein 3 (NLRP3) inflammasome. Zhou et al observed that at high concentrations of ROS-generators that activate NLRP3, such as H2O2, Imiquimod (R-837) and monosodium urate (MSU), TxNIP dissociates from thioredoxin, associates with NLRP3 and mediates inflammasome activation [50]. Thus, TxNIP directly links oxidative stress with inflammasome activation.
We have demonstrated here that Tat transgenic mice have increased cellular infiltration to the lungs, are under increased oxidative burden and have higher levels of the most important pro-inflammatory transcription factor, NF-κB. Oxidative stress and chronic inflammation are part of the pathogenesis of chronic HIV-1 infection. Although other HIV accessory proteins may aid in the severity of oxidative stress and inflammation in HIV-1 infection, it is indisputable that Tat contributes significantly to the oxidative stress seen in these mice and could reset their lungs to a heightened state of sensitivity for inflammatory stimuli. Our data further suggest that TxNIP may be a molecular link between the oxidative stress detected in these mice and the lower inflammatory threshold as TxNIP has been shown to activate the NLRP3 inflammasome in a redox-dependent manner in other systems.
Highlights.
We constructed transgenic mice that express HIV-tat in the lungs. Tat-Tg mice have more cellular infiltration in the lungs. Tg mice have more NF-κB, MnSOD, TxNIP and oxidative stress. Tat increases the oxidant burden and resets the inflammatory threshold.
ACKNOWLEDGEMENTS
This work was funded by grant number 5R01HL059785 from the National Heart Lung and Blood Institute (SCF). The authors wish to thank Dr. Jeffrey A. Whitsett for providing the plasmid 3.7 hSP-C/SV-40, and Drs. Mark Geraci and Norbert Voelkel for support during the generation and establishment of the mouse colony. "The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 Tat Designer Gene™ from Dr. Marty Kissil.”
Abbreviations
- HIV
Human immunodeficiency virus
- Non-Tg
non-transgenic
- MnSOD
manganese superoxide dismutase
- NF-κB
nuclear factor kappa B
- ROS
reactive oxygen species
- Tat
transactivator of transcription
- Tg
transgenic
- SP-C
surfactant protein C
- TNF-α
tumor necrosis factor alpha
- TxNIP
thioredoxin interacting protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mwandumba HC, Squire SB, White SA, Nyirenda MH, Zijlstra EE, Molyneux ME, Russell DG, Rhoades ER. Alveolar macrophages from HIV-infected patients with pulmonary tuberculosis retain the capacity to respond to stimulation by lipopolysaccharide. Microbes. Infect. 2007;9:1053–1060. doi: 10.1016/j.micinf.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Steele C, Shellito JE, Kolls JK. Immunity against the opportunistic fungal pathogen Pneumocystis. Med. Mycol. 2005;43:1–19. doi: 10.1080/13693780400015360. [DOI] [PubMed] [Google Scholar]
- 3.McCord JM. Superoxide Radical: Controversies, Contradictions and Paradoxes. Proc. Soc. Exptl. Med. Biol. 1995:1–6. doi: 10.3181/00379727-209-43885c. [DOI] [PubMed] [Google Scholar]
- 4.Eck HP, Gmunder H, Hartmann M, Petzoldt D, Daniel V, Droge W. Low concentrations of acid soluble thiol (cysteine) in blood plasmas of HIV-1-infected patients. Biol. Chem. Hoppe. Seyler. 1989;370:101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- 5.Favier A, Sappey C, Leclerc P, Faure P, Micoud M. Antioxidant status and lipid peroxidation in patients infected with HIV. Chem. Biol. Interact. 1994;91:165–180. doi: 10.1016/0009-2797(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang YJ, Watson RR. Potential therapeutics of vitamin E (Tocopherol) in AIDS and HIV. Drugs. 1994;48:327–338. doi: 10.2165/00003495-199448030-00001. [DOI] [PubMed] [Google Scholar]
- 7.Revillard JP, Vincent CMA, Favier AE, Richard MJ, Zittoun M, Kazatchkine MD. Lipid peroxidation in human immunodeficiency virus infection. J. Acq. Immun. Defic. Syndrome. 1992;5:637–638. [PubMed] [Google Scholar]
- 8.Buhl R. Imbalance between oxidants and antioxidants in the lungs of HIV-seropositive individuals. Chem. -Biol. Interactions. 1994;91:147–158. doi: 10.1016/0009-2797(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 9.Yoo J, Chen H, Kraus T, Hirsch D, Polyak S, George I, Sperber K. Altered cytokine production and accessory cell function after HIV-1 infection. Journal. of Immunology. 1996;157:1313–1320. [PubMed] [Google Scholar]
- 10.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type-1 tat protein on cell growth and viral transactivation. J. Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noonan D, Albini A. From the outside in: extracellular activities of HIV Tat. Adv. Pharmacol. 2000;48:229–250. doi: 10.1016/s1054-3589(00)48008-7. [DOI] [PubMed] [Google Scholar]
- 12.Buonaguro L, Barillari G, Chang HK, Bohan CA, Kao V, Morgan R, Gallo RC, Ensoli B. Effects of the human immunodeficiency virus type-1 TAT protein on the expression of inflammatory cytokines. J. Virol. 1992;66:7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sastry KJ, Reddy RHR, Pandita R, Totpal K, Aggarwal BB. HIV-1 tat gene induces tumor necrosis factor-beta(lymphotoxin) in a human B-lymphoblastoid cell line. J. Biol. Chem. 1990;265:20091–20093. [PubMed] [Google Scholar]
- 14.Brady HJ, Abraham DJ, Pennington DJ, Miles CG, Jenkins S, Dzierzak EA. Altered cytokine expression in T lymphocytes from human immunodeficiency virus Tat transgenic mice. Journal. of Virology. 1995;69:7622–7769. doi: 10.1128/jvi.69.12.7622-7629.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores SC, Marecki JC, Harper KP, Bose SK, Nelson SK, McCord JM. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc. Natl. Acad. Sci. USA. 1993;90:7632–7636. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westendorp MO, Shatrov VA, Schulzeosthoff K, Frank R, Kraft M, Los M, Krammer PH, Droge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J, Liu RM, Kundu RK, Sangiorgi F, Wu W, Maxson R, Forman HJ. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J. Biol. Chem. 2000;275:3693–3698. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- 18.Richard MJ, Guiraud P, Didier C, Seve M, Flores SC, Favier A. Human immunodeficiency virus type 1 Tat protein impairs selenoglutathione peroxidase expression and activity by a mechanism independent of cellular selenium uptake: consequences on cellular resistance to UV-A radiation. Arch. Biochem. Biophys. 2001;386:213–220. doi: 10.1006/abbi.2000.2197. [DOI] [PubMed] [Google Scholar]
- 19.Cota-Gomez A, Flores NC, Cruz C, Casullo A, Aw TY, Ichikawa H, Schaack J, Scheinman R, Flores SC. The human immunodeficiency virus-1 Tat protein activates human umbilical vein endothelial cell E-selectin expression via an NF-kappa B-dependent mechanism. J. Biol. Chem. 2002;277:14390–14399. doi: 10.1074/jbc.M108591200. [DOI] [PubMed] [Google Scholar]
- 20.Lafrenie RM, Wahl LM, Epstein JS, Yamada KM, Dhawan S. Activation of monocytes by HIV-Tat treatment is mediated by cytokine expression. J. Immunol. 1997;159:4077–4083. [PubMed] [Google Scholar]
- 21.Park IW, Wang JF, Groopman JE. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood. 2001;97:352–358. doi: 10.1182/blood.v97.2.352. [DOI] [PubMed] [Google Scholar]
- 22.Glasser SW, Korfhagen TR, Bruno MD, Dey C, Whitsett JA. Structure and expression of the pulmonary surfactant protein SP-C gene in the mouse. J. Biol. Chem. 1990;265:21986–21991. [PubMed] [Google Scholar]
- 23.Glasser SW, Korfhagen TR, Wert SE, Bruno MD, McWilliams KM, Vorbroker DK, Whitsett JA. Genetic element from human surfactant protein SP-C gene confers bronchiolar-alveolar cell specificity in transgenic mice. Am. J. Physiol. 1991;261:L349–L356. doi: 10.1152/ajplung.1991.261.4.L349. [DOI] [PubMed] [Google Scholar]
- 24.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B - an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radical Res. Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Lin J-X, Vilcek J. Interleukin-6 Induction by Tumor Necrosis Factor and Interleukin-1 in Human Fibroblasts Involves Activation of a Nuclear Factor Binding to a kB-Like Sequence. Molecular. &. Cellular. Biology. 1990;10(7):3818–3823. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle EM, Jr, Kovacich JC, Canty TG, Jr, Morgan EN, Chi E, Verrier ED, Pohlman TH. Inhibition of nuclear factor-kappa B nuclear localization reduces human E-selectin expression and the systemic inflammatory response. Circulation. 1998;98:II282–II288. [PubMed] [Google Scholar]
- 27.Wrighton CJ, Hofer-Warbinek R, Moll T, Eytner R, Bach FH, de Martin R. Inhibition of endothelial cell activation by adenovirus-mediated expression of I kappa B alpha, an inhibitor of the transcription factor NF-kappa B. J. Exp. Med. 1996;183:1013–1022. doi: 10.1084/jem.183.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB. Inhibition of proinflammatory molecule production by adenovirus- mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J. Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- 29.Aukrust P, Berge RK, Ueland T, Aaser E, Damas JK, Wikeby L, Brunsvig A, Muller F, Forfang K, Froland SS, Gullestad L. Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. J. Am. Coll. Cardiol. 2001;37:485–491. doi: 10.1016/s0735-1097(00)01110-4. [DOI] [PubMed] [Google Scholar]
- 30.Martin T, Cardarelli PM, Parry GC, Felts KA, Cobb RR. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur. J. Immunol. 1997;27:1091–1097. doi: 10.1002/eji.1830270508. [DOI] [PubMed] [Google Scholar]
- 31.Aerts C, Wallaert B, Voisin C. Invitro effects of hyperoxia on alveolar type-II pneumocytes - inhibition of glutathione synthesis increases hyperoxic cell injury. Exp. Lung Res. 1992;18:845–861. doi: 10.3109/01902149209031711. [DOI] [PubMed] [Google Scholar]
- 32.Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S. Cellular oxygen toxicity. Oxidant injury without apoptosis. J. Biol. Chem. 1996;271:15182–15186. doi: 10.1074/jbc.271.25.15182. [DOI] [PubMed] [Google Scholar]
- 33.He L, Chang S-W, Ortiz de Montellano P, Burke TJ, Voelkel NF. Lung injury in Fischer but not Sprague-Dawley rats after short-term hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 1990;259:L451–L458. doi: 10.1152/ajplung.1990.259.6.L451. [DOI] [PubMed] [Google Scholar]
- 34.Clerch LB, Massaro D. Tolerance of rats to hyperoxia - lung antioxidant enzyme gene expression. J. Clin. Invest. 1993;91:499–508. doi: 10.1172/JCI116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaoka S, Kim HS, Ogihara T, Oue S, Takitani K, Yoshida Y, Tamai H. Severe Vitamin E deficiency exacerbates acute hyperoxic lung injury associated with increased oxidative stress and inflammation. Free Radic. Res. 2008;42:602–612. doi: 10.1080/10715760802189864. [DOI] [PubMed] [Google Scholar]
- 36.Coaccioli S, Crapa G, Fantera M, Del GR, Lavagna A, Standoli ML, Frongillo R, Biondi R, Puxeddu A. Oxidant/antioxidant status in patients with chronic HIV infection. Clin. Ter. 2010;161:55–58. [PubMed] [Google Scholar]
- 37.Wanchu A, Rana SV, Pallikkuth S, Sachdeva RK. Short communication: oxidative stress in HIV-infected individuals: a cross-sectional study. AIDS Res. Hum. Retroviruses. 2009;25:1307–1311. doi: 10.1089/aid.2009.0062. [DOI] [PubMed] [Google Scholar]
- 38.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV- 1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 39.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 40.Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suresh DR, Annam V, Pratibha K, Prasad BV. Total antioxidant capacity--a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J. Biomed. Sci. 2009;16:61. doi: 10.1186/1423-0127-16-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibeh BO, Obidoa O, Uzoegwu PN. High plasma activity of endogenous antioxidants protect CD4+ T-cells in HIV-serodiscordant heterosexual partners in a Nigerian population. Int. J. STD AIDS. 2008;19:536–540. doi: 10.1258/ijsa.2008.008031. [DOI] [PubMed] [Google Scholar]
- 43.Treitinger A, Spada C, Verdi JC, Miranda AF, Oliveira OV, Silveira MV, Moriel P, Abdalla DS. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur. J. Clin. Invest. 2000;30:454–459. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- 44.Gil L, Martinez G, Gonzalez I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tapanes R, Perez J, Leon OS. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol. Res. 2003;47:217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 45.Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17 beta-estradiol. J. Neurochem. 2001;78:1315–1324. doi: 10.1046/j.1471-4159.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- 46.Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci. Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- 47.Buhl R. Imbalance between oxidants and antioxidants in the lungs of HIV-seropositive individuals. Chem. Biol. Interact. 1994;91:147–158. doi: 10.1016/0009-2797(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 48.Raoul H, Le NR, Blond D, Dormont D. HIV type 1 infection of human macrophages induces an upregulation of manganese superoxide dismutase gene that may protect cells from death. AIDS Res. Hum. Retroviruses. 1998;14:427–434. doi: 10.1089/aid.1998.14.427. [DOI] [PubMed] [Google Scholar]
- 49.Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]