Abstract
The recurrent depth preference of three ciliate species (two prostomatids and one haptorid) in a transparent alpine lake indicates the existence of niche partitioning among them involving potential factors such as avoidance of high ultraviolet radiation levels and zooplankton predation, as well as competition for food resources.
Keywords: Askenasia chlorelligera, Balanion planctonicum, Cyclops, Urotricha, UV radiation
Ciliate assemblages in lowland lakes are characteristically composed of many species that change throughout the year in relation to food-availability, predation and abiotic environmental parameters (e.g. Müller et al., 1991; Sonntag et al., 2002, 2006). In contrast, high-altitude lakes are colonized by only few ciliate species (Wille et al., 1999). As in alpine lakes (i.e. located above the treeline), the concentration of chromophoric dissolved organic material is low, consequently transparency to photosynthetically active (PAR, 400–700 nm) and ultraviolet radiation (UVR, 280–400 nm) is high (Sommaruga and Psenner, 1997; Laurion et al., 2000; Sommaruga and Augustin, 2006). Up to now, the few studies available on the effect of UVR on ciliates indicate that sensitivity is species-specific (Giese et al., 1965; Wickham and Carstens, 1998; Mostajir et al., 1999; Sommaruga et al., 1999; Sanders et al., 2005). How ciliates cope with UVR in transparent alpine lakes is unknown; however, possible strategies include distribution in deep water layers during the day where most of the UV-B is attenuated, possession of efficient DNA repair mechanisms and accumulation of photoprotective compounds. Among photoprotective compounds mycosporine-like amino acids (MAAs) have been reported for several Chlorella-bearing ciliates (Tartarotti et al., 2004; Sonntag et al., 2007) including one species from an alpine lake (Summerer et al., 2008).
A previous study in the alpine Gossenköllesee reported that the ciliate community was dominated by three species, namely the dominant Balanion planctonicum found in deep water layers, the Chlorella-bearing Askenasia chlorelligera distributed in the whole water column and one Urotricha species present in the upper meters (Wille et al., 1999). Based on the observation that the distribution pattern of B. planctonicum in this alpine lake is different from that found in lowland less-transparent lakes, we hypothesized that not only food had a considerable influence on the depth preference of this algivorous species, but also UV sensitivity.
To test this hypothesis, we collected planktonic ciliates on 30 August 2005 from the oligotrophic Gossenköllesee (maximum depth = 9.9 m) situated at 2417 m above the sea level in the Austrian Central Alps (47°13′N, 11°00′E) by vertical net tows (10-μm mesh size) and placed them in a 10-L carboy after removal of zooplankton (100-μm mesh size net). After mixing the carboy several times, the water was distributed into 1-L-quartz tubes and exposed in situ at 0.30 m depth under sunny conditions for 7 h centred at solar noon. Three experimental conditions (each with three replicates) were tested: full sunlight (PAR +UVR, hereafter FULL), PAR only by excluding most of the UVR with a vinyl chloride foil (sharp cut-off: 0% transmittance at 390 nm, 50% at 405 nm) and darkness (DARK = control) by excluding radiation with aluminium paper and a black plastic foil. At T0 (from the carboy) and after 7 h of exposure, 250 mL samples were fixed with Bouin’s solution (5% final concentration) and quantitatively protargol stained (Skibbe, 1994; Pfister et al., 1999). The ciliates were identified following the taxonomic key of Foissner et al. (Foissner et al., 1999) and quantified using an Olympus BX50 microscope with bright field. As the normality test for this data set failed, statistical differences in the species-specific mortality were analysed by using a Kruskal–Wallis one-way ANOVA on ranks with Dunnett’s post hoc comparisons against the DARK—control (SigmaStat 3.5).
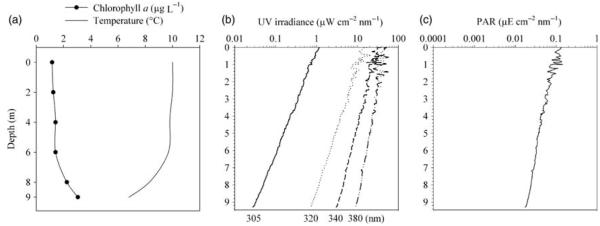
To further understand the depth preference of the ciliates, we assessed their vertical distribution during day and night, as well as their possible co-occurrence with predators and phytoplankton. Triplicate water samples were collected with a 5-L-Schindler-Patalas sampler (Uwitec) at 0, 2, 4, 6, 8 and 9 m depth on 10 August 2005 at 0100 h (T1), 0700 h (T2) and 1300 h (T3). Subsamples of 250 mL each were fixed, prepared and analysed as described. To statistically determine whether the species remained in certain depths through day and night, we first tested the precision of the data by re-sampling (10 000 bootstraps per replicate; Excel Add-In: Bootstrap), then calculated the weighted mean depth abundances and finally, applied a Friedman repeated measures ANOVA on ranks (SigmaStat 3.5). Additionally, the water temperature and chlorophyll a (Chl a) were measured at each sampling depth (Fig. 1a). Spearman rank order correlations were performed between the vertical profiles of ciliate abundance and depth, temperature and Chl a (Table I). Further, the downwelling irradiance was measured under sunny conditions within 2 h of solar noon with a profiling radiometer (PUV-501B, Biospherical Instruments) at 305, 320, 340 and 380 nm and between 400 and 700 nm (PAR; Figs 1b and c). The diffuse attenuation coefficients (Kd) were calculated after Laurion et al. (Laurion et al., 2000). The Kd and the respective 1% attenuation depth (Z1%) for PAR and UVR were: Kd PAR = 0.17 m−1 (Z1% = 27.7 m), Kd 380nm = 0.18 m−1 (Z1% = 26.1 m), Kd 340nm = 0.26 m−1 (Z1% = 17.4 m), Kd 320nm = 0.33 m−1 (Z1% = 14.0 m) and Kd 305nm = 0.44 m-1 (Z1% = 10.5 m).
Fig. 1.
Depth profiles of chlorophyll a (μg L−1) and temperature (°C) (a) and of UVR at 305, 320, 340 and 380 nm (μW cm−2 nm−1) (b), and PAR (μE cm−2 nm−1) (c) in Gossenköllesee on 10 August 2005.
Table I.
Spearman rank correlations between total ciliate abundance (Tot), Askenasia chlorelligera (Ask), Balanion planctonicum (Bal), Urotricha cf. castalia (Uro), depth (D), temperature (T) and chlorophyll a (Chl a) in Gossenköllesee on 10 August 2005 at T1 (0100 h), T2 (0700 h) and T3 (1300 h)
| T1 |
T2 |
T3 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ask | Bal | Uro | D | T | Chl a | Ask | Bal | Uro | D | T | Chl a | Ask | Bal | Uro | D | T | Chl a | |
| Tot | 0.143 | 1.000 | −0.886 | 0.886 | −0.794 | 0.870 | −0.143 | 0.943 | −0.600 | 0.771 | −0.516 | n.d. | −0.429 | 0.943 | −0.886 | 0.886 | −0.928 | 0.870 |
| 0.803 | 0.003 | 0.033 | 0.033 | 0.058 | 0.033 | 0.803 | 0.017 | 0.242 | 0.103 | 0.297 | 0.419 | 0.017 | 0.033 | 0.033 | 0.017 | 0.033 | ||
| Ask | 0.143 | 0.086 | −0.086 | 0.324 | −0.116 | −0.086 | 0.429 | −0.257 | 0.698 | n.d. | −0.486 | 0.543 | −0.543 | 0.522 | −0.522 | |||
| 0.803 | 0.919 | 0.919 | 0.497 | 0.803 | 0.919 | 0.419 | 0.658 | 0.136 | 0.356 | 0.297 | 0.297 | 0.297 | 0.297 | |||||
| Bal | −0.886 | 0.886 | −0.794 | 0.870 | −0.771 | 0.886 | −0.638 | n.d. | −0.943 | 0.943 | −0.928 | 0.928 | ||||||
| 0.033 | 0.033 | 0.058 | 0.033 | 0.103 | 0.033 | 0.175 | 0.017 | 0.017 | 0.017 | 0.017 | ||||||||
| Uro | −1.000 | 0.736 | −0.986 | −0.943 | 0.941 | n.d. | −1.000 | 0.986 | −0.986 | |||||||||
| 0.003 | 0.103 | 0.003 | 0.017 | 0.017 | 0.003 | 0.003 | 0.003 | |||||||||||
| D | −0.736 | 0.986 | −0.820 | n.d. | −0.986 | 0.986 | ||||||||||||
| 0.103 | 0.003 | 0.058 | 0.003 | 0.003 | ||||||||||||||
| T | −0.687 | n.d. | −0.971 | |||||||||||||||
| 0.136 | 0.003 | |||||||||||||||||
Correlation coefficients (rs, upper value) and P (lower value) are given (n = 6). Bold values indicate significant correlations (P < 0.05). n.d., not determined.
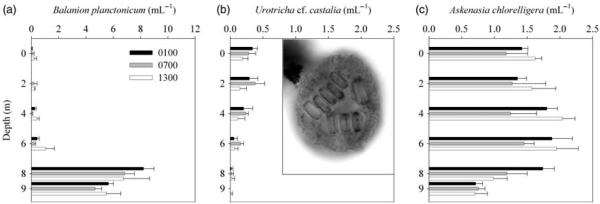
Irrespective of day or night time, the distribution pattern of the ciliates persisted (Fig. 2) suggesting the existence of factors constraining the colonization of specific water layers in this lake. As previously described in Wille et al. (Wille et al., 1999), A. chlorelligera, B. planctonicum and one Urotricha were the dominant species. Wille et al. (Wille et al., 1999) identified the Urotricha species as cf. pelagica; however, our re-analysis of their samples using protargol-staining of Lugol-formaldehyde-fixed samples indicated that it was the same Urotricha cf. castalia species as found in our study. Nevertheless, definite species identification was not possible in both cases as one important characteristic of Urotricha castalia, i.e. the somatic extrusomes, was not visible.
Fig. 2.
Abundance (mean number of cells mL−1 ± SD) along a depth gradient of Balanion planctonicum (a), Urotricha cf. castalia with insert showing a micrograph of a protargol-stained specimen taken at a magnification of ×1000 where several ingested centric diatoms are visible (b), and Askenasia chlorelligera (c) in Gossenköllesee on 10 August 2005 at T1 (0100), T2 (0700) and T3 (1300) local summer time. Note different bar scales.
Among the three ciliate species, B. planctonicum was the only one that showed a moderate UV sensitivity in the full sunlight treatment (P = 0.029) which is consistent with its avoidance of surface waters (Figs 2a and 3). Though, comparing the abundance at T0 (A. chlorelligera: 2.9 ± 0.3 mL−1, B. planctonicum: 2.7 ± 0.2 mL−1, U. cf. castalia: 0.3 ± 0.0 mL−1) and after 7 h incubation in the dark control, values for A. chlorelligera and U. cf. castalia were unchanged, but in B. planctonicum there was a decrease of 19%. Food depletion during exposure does not seem to explain the mortality of B. planctonicum because many cryptomonads were found at the end of the experiment (recognizable in the QPS preparations). However, reduced cell numbers of B. planctonicum in the control suggest that there was probably an effect caused by the experimental manipulation or sampling.
Fig. 3.
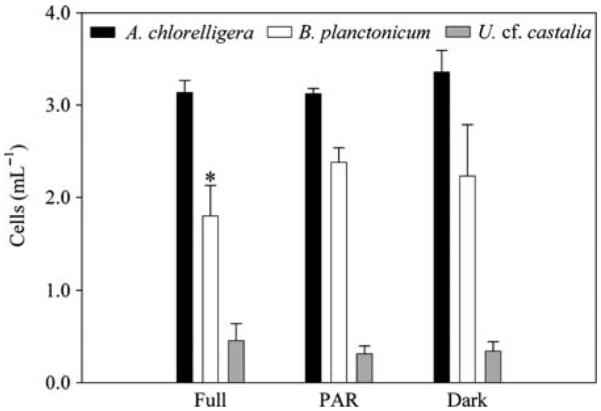
Abundance (cells mL−1) of Askenasia chlorelligera, Balanion planctonicum and Urotricha cf. castalia (mean ± SD) after 7 h of exposure at the lake surface. The ciliates were exposed to the full solar UVR (FULL), photosynthetically active radiation (PAR) or kept in the dark (DARK = control). *Indicates significant difference (P = 0.029).
The significant positive correlation of B. planctonicum with phytoplankton biomass (i.e. Chl a) suggests a strong dependence of this species on its main food source (Table I) as indicated by Wille et al. (Wille et al., 1999). Balanion planctonicum is known as an efficient algal feeder not able to survive longer starvation periods and to be dependent on a minimum density of 1500 algal cells mL−1 preferably cryptomonads (Müller, 1991; Weisse et al., 2001). In Gossenköllesee, cryptomonads are almost absent during day in the upper meters of the water column, whereas near the lake bottom up to 2000 cells mL−1 can be found (unpublished results).
Weisse et al. (Weisse et al., 2001) observed in experiments that B. planctonicum was a superior competitor when cryptomonad numbers were <3 × 104 mL−1. The same authors argued that with respect to the use of food resources, B. planctonicum probably had an advantage over other small algivorous prostomatids such as Urotricha farcta and Urotricha furcata in oligo- to mesotrophic lowland lakes, when food was not abundant (Weisse et al., 2001). The superior competitive ability of B. planctonicum for food resources could be an explanation for the distribution of prostomatids observed in Gossenköllesee. In this hypothesis, B. planctonicum out-competes U. cf. castalia and displaces it to the upper water layers where for most time of the day less phytoplankton is available. This in turn results in a reduced population size of the latter species, despite living at higher water temperatures than B. planctonicum (Figs 1a, 2b and 4). Interestingly, in the QPS preparations, we regularly observed individuals of U. cf. castalia with ingested centric diatoms (see insert in Fig. 2b). Thus, the water layers this species colonizes not only have lower phytoplankton biomass but probably also have less suitable algal food items compared with the zone of the deep chlorophyll maximum. In fact, there is evidence that prostomatid ciliates cannot survive on a diatom diet (Müller and Schlegel, 1999). Moreover, when a mixture of diatoms and cryptomonads has been offered to prostomatid ciliates such as B. planctonicum, they clearly selected for the latter (Müller and Schlegel, 1999).
Fig. 4.
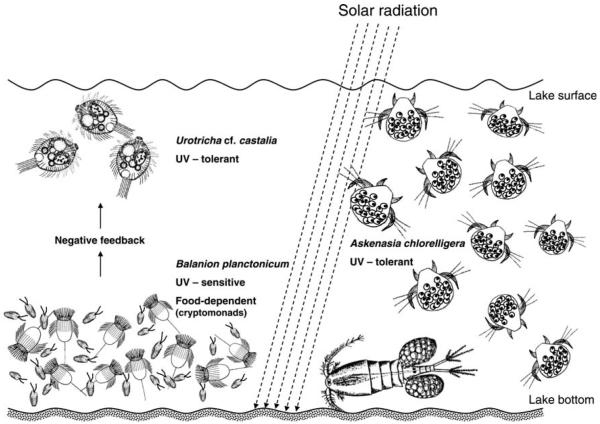
Scheme of the distribution of Askenasia chlorelligera, Balanion planctonicum and Urotricha cf. castalia in the alpine lake Gossenköllesee during the ice-free summer period indicating the main factors involved. Drawings modified from Remane et al. (1986), Streble and Krauter (1988) and Foissner et al. (1999). For details see text.
Urotricha cf. castalia was tolerant to short-term exposure to UVR (Fig. 3); however, in a mesocosm (1 m deep) experiment done in the same lake and lasting for 16 days, the net growth rate of urotrichs was slightly affected by UV-B radiation (Sommaruga et al., 1999). This suggests that, though tolerant, it may not be able to cope with UV-B in the upper 2 m of the water column for prolonged periods.
In lowland lakes, many mixotrophic ciliate species, which either bear algal symbionts or sequester algal plastids, prevail during the summer months in surface waters where they can account for more than 50% of the total ciliate abundance (e.g. Pace, 1982; Müller et al., 1991; Dolan, 1992; Carrias et al., 1998; Sonntag et al., 2006). Living in the epilimnion allows mixotrophic ciliates to optimally utilize nutrients and light to enable growth of host and symbionts (Wölfl and Geller, 2002; Modenutti et al., 2005). This nutritional advantage is an adaptation to living in oligotrophic environments when or where food is not abundant or not suitable (Dolan and Pérez, 2000). However, colonization of the upper part of the water column also means being exposed to potentially damaging levels of incident UVR. Summerer et al. (Summerer et al., 2008) recently reported the occurrence of MAAs in A. chlorelligera from Gossenköllesee that most likely allow this ciliate to exploit the upper layers, too (Figs 2c and 4). Interestingly, in a population of A. chlorelligera in a less UV transparent lake, no MAAs were detected (Summerer et al., 2008). Another advantage of hosting algal symbionts is the screening of UVR by several Chlorella layers that may reduce damaging effects on UV-sensitive cell compartments such as DNA-containing material (Sommaruga and Sonntag, 2009; Summerer et al., 2009).
Finally, another aspect that can influence the distribution of planktonic protists is predation by zooplankton (e.g. Wickham, 1995; Dobberfuhl et al., 1997). In Gossenköllesee, the only copepod species present is the cyclopoid Cyclops abyssorum tatricus that concentrates in deep water layers during daytime and migrates upwards at night (Tartarotti et al., 1999). In the presence of cyclopoid copepods, ciliate abundance can be significantly reduced and oligotrich and haptorid species (including Askenasia) in the size-range of 20–40 μm are known to be heavily grazed (Wiackowski et al., 1994; Dobberfuhl et al., 1997; Zöllner et al., 2003). In contrast, smaller ciliates such as Urotricha and Balanion species seem to cope well with grazing pressure by copepods because of their small size and their characteristic rapid jumps (Wiackowski et al., 1994; Dobberfuhl et al., 1997; Zöllner et al., 2003). Askenasia chlorelligera from Gossenköllesee also performs rapid jumps and responds to the pressure wave of an approaching object (personal observation). This behaviour appears to be in agreement with the lower concentration of A. chlorelligera at the greatest depths (Figs 2c and 4) and suggests avoidance of predation by C. abyssorum tatricus by jumping only when a predator approaches (Wickham, 1995).
Colonization by ciliates of extreme environments such as alpine lakes, where food is scarce and UVR levels are high, demands effective adaptation strategies. The present results indicate the existence of niche partitioning among the dominant ciliate species in this oligotrophic lake caused by several factors including adaptation to high UVR levels, competition for food resources and avoidance of zooplankton predation (Fig. 4).
ACKNOWLEDGEMENTS
We thank L. Alfreider, P. Hörtnagl, H. Peter and M. Tilg for help during field and laboratory work and A. Kloss-Brandstätter for statistical advice. Two anonymous reviewers are acknowledged for critical comments on an earlier version of the manuscript.
FUNDING This study was supported by the Austrian Science Fund (FWF), grants P16559-B06 to R.S. and P21013-B03 to B.S.
REFERENCES
- Carrias JF, Amblard C, Bourdier G. Seasonal dynamics and vertical distribution of planktonic ciliates and their relationship to microbial food resources in the oligomesotrophic Lake Pavin. Arch. Hydrobiol. 1998;143:227–255. [Google Scholar]
- Dobberfuhl DR, Miller R, Elser JJ. Effects of a cyclopoid copepod (Diacyclops thomasi) on phytoplankton and the microbial food web. Aquat. Microb. Ecol. 1997;12:29–37. [Google Scholar]
- Dolan J. Mixotrophy in ciliates: a review of Chlorella symbiosis and chloroplast retention. Mar. Microb. Food Webs. 1992;6:115–132. [Google Scholar]
- Dolan J, Pérez MT. Costs, benefits and characteristics of mixotrophy in marine oligotrichs. Freshwater Biol. 2000;45:227–238. [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1999;3/99:1–793. [Google Scholar]
- Giese AC, Mc Caw BK, Parker JW, et al. Comparative ultraviolet sensitivity of regeneration in the genus Blepharisma. J. Eukaryot. Microbiol. 1965;12:171–177. doi: 10.1111/j.1550-7408.1965.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Laurion I, Ventura M, Catalan J, et al. Attenuation of ultraviolet radiation in mountain lakes: factors controlling the among- and within-lake variability. Limnol. Oceanogr. 2000;45:1274–1288. [Google Scholar]
- Modenutti BE, Balseiro EG, Callieri C, et al. Effect of UV-B and different PAR intensities on the primary production of the mixotrophic planktonic Stentor araucanus. Limnol. Oceanogr. 2005;50:864–871. [Google Scholar]
- Mostajir B, Demers S, DeMora S, et al. Experimental test of the effect of ultraviolet-B radiation in a planktonic community. Limnol. Oceanogr. 1999;44:586–596. [Google Scholar]
- Müller H. Pseudobalanion planctonicum (Ciliophora, Prostomatida): ecological significance of an algivorous nanociliate in a deep meso-eutrophic lake. J. Plankton Res. 1991;13:247–262. [Google Scholar]
- Müller H, Schlegel A. Responses of three freshwater planktonic ciliates with different feeding modes to cryptophyte and diatom prey. Aquat. Microb. Ecol. 1999;17:49–60. [Google Scholar]
- Müller H, Schöne A, Pinto-Coelho RM, et al. Seasonal succession of ciliates in Lake Constance. Microb. Ecol. 1991;21:119–138. doi: 10.1007/BF02539148. [DOI] [PubMed] [Google Scholar]
- Pace ML. Planktonic ciliates: their distribution, abundance, and relationship to microbial resources in a monomictic lake. Can. J. Fish. Aquat. Sci. 1982;39:1106–1116. [Google Scholar]
- Pfister G, Sonntag B, Posch T. Comparison of a direct live count and an improved quantitative protargol stain (QPS) in determining abundance and cell volumes of pelagic freshwater protozoa. Aquat. Microb. Ecol. 1999;18:95–103. [Google Scholar]
- Remane A, Storch V, Welsch U, editors. Kurzes Lehrbuch der Zoologie. 3. Auflage Fischer Verlag; Stuttgart: 1986. [Google Scholar]
- Sanders RW, Macaluso AL, Sardina TJ, et al. Photoreactivation in two freshwater ciliates: differential responses to variations in UV-B flux and temperature. Aquat. Microb. Ecol. 2005;40:283–292. [Google Scholar]
- Skibbe O. An improved quantitative protargol stain for ciliates and other planktonic protists. Arch. Hydrobiol. 1994;130:339–347. [Google Scholar]
- Sommaruga R, Augustin G. Seasonality in UV transparency of an alpine lake is associated to changes in phytoplankton biomass. Aquat. Sci. 2006;68:129–141. [Google Scholar]
- Sommaruga R, Psenner R. Ultraviolet radiation in a high mountain lake of the Austrian Alps: air and underwater measurements. Photochem. Photobiol. 1997;65:957–963. [Google Scholar]
- Sommaruga R, Sonntag B. Photobiological aspects of the mutualistic association between Paramecium bursaria and Chlorella. In: Fujishima M, editor. Endosymbionts in Paramecium. Microbiology Monographs 12. Springer Verlag; Berlin, Heidelberg: 2009. pp. 111–130. [Google Scholar]
- Sommaruga R, Sattler B, Oberleiter A, et al. An in situ enclosure experiment to test the solar UVB impact on plankton in a high-altitude mountain lake. II. Effects on the microbial food web. J. Plankton Res. 1999;21:859–876. [Google Scholar]
- Sonntag B, Posch T, Klammer S, et al. Protozooplankton in the deep oligotrophic Traunsee (Austria) influenced by discharges of soda and salt industries. Water Air Soil Pollut: Focus. 2002;2:211–226. [Google Scholar]
- Sonntag B, Posch T, Klammer S, et al. Phagotrophic ciliates and flagellates in an oligotrophic deep alpine lake: contrasting variability with seasons and depths. Aquat. Microb. Ecol. 2006;43:193–207. [Google Scholar]
- Sonntag B, Summerer M, Sommaruga R. Sources of mycosporine-like amino acids in planktonic Chlorella-bearing ciliates (Ciliophora) Freshwater Biol. 2007;52:1476–1485. [Google Scholar]
- Streble H, Krauter D, editors. Das Leben im Wassertropfen. 8. Auflage Kosmos, Franckh’sche Verlagshandlung, W. Keller & Co; Stuttgart: 1988. [Google Scholar]
- Summerer M, Sonntag B, Sommaruga R. Ciliate-symbiont specificity of freshwater endosymbiotic Chlorella (Trebouxiophyceae, Chlorophyta) J. Phycol. 2008;44:77–84. doi: 10.1111/j.1529-8817.2007.00455.x. [DOI] [PubMed] [Google Scholar]
- Summerer M, Sonntag B, Hörtnagl P, et al. Symbiotic ciliates receive protection against UV damage from their algae: a test with Paramecium bursaria and Chlorella. Protist. 2009;160:233–243. doi: 10.1016/j.protis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Tartarotti B, Cabrera S, Psenner R, et al. Survivorship of Cyclops abyssorum tatricus (Cyclopoida, Copepoda) and Boeckella gracilipes (Calanoida, Copepoda) under ambient levels of solar UVB radiation in two high-mountain lakes. J. Plankton Res. 1999;21:549–560. [Google Scholar]
- Tartarotti B, Baffico G, Temporetti P, et al. Mycosporine-like amino acids in planktonic organisms living under different UVexposure conditions in Patagonian lakes. J. Plankton Res. 2004;26:753–762. doi: 10.1093/plankt/fbh073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisse T, Karstens N, Meyer VCL, et al. Niche separation in common prostome freshwater ciliates: the effect of food and temperature. Aquat. Microb. Ecol. 2001;26:167–179. [Google Scholar]
- Wiackowski K, Brett M, Goldman CR. Differential effects of zooplankton species on ciliate community structure. Limnol. Oceanogr. 1994;39:486–492. [Google Scholar]
- Wickham SA. Cyclops predation on ciliates: species-specific differences and functional responses. J. Plankton Res. 1995;17:1633–1646. [Google Scholar]
- Wickham SA, Carstens M. Effects of ultraviolet-B radiation on two arctic microbial food webs. Aquat. Microb. Ecol. 1998;16:163–171. [Google Scholar]
- Wille A, Sonntag B, Sattler B, et al. Abundance, biomass and size structure of the microbial assemblage in the high mountain lake Gossenköllesee (Tyrol, Austria) during the ice-free period. J. Limnol. 1999;58:117–126. [Google Scholar]
- Wölfl S, Geller W. Chlorella-bearing ciliates dominate in an oligotrophic North Patagonian lake (Lake Pirehueico, Chile): abundance, biomass and symbiotic photosynthesis. Freshwater Biol. 2002;47:231–242. [Google Scholar]
- Zöllner E, Santer B, Boersma M, et al. Cascading predation effects of Daphnia and copepods on microbial food web components. Freshwater Biol. 2003;48:2174–2193. [Google Scholar]