Abstract
The matricellular SPARC-family member hevin (Sparc-like 1/SPARCL-1/SC1/Mast9) contributes to neural development and alters tumor progression in a range of mammalian models. Based on sequence similarity, we hypothesized that proteolytic digestion of hevin would result in SPARC-like fragments (SLF) that affect the activity and/or location of these proteins. Incubation of hevin with matrix metalloproteinase-3 (MMP-3), a protease known to cleave SPARC, produced a limited number of peptides. Sequencing revealed the major proteolytic products to be SPARC-like in primary structure. In gliomas implanted into murine brain, a SLF was associated with SPARC in the neovasculature but not with hevin, the latter prominent in the astrocytes encompassed by infiltrating tumor. In this model of invasive glioma that involves MMP-3 activity, host-derived SLF was not observed in the extracellular matrix adjacent to tumor cells. In contrast, it occurred with its homolog SPARC in the angiogenic response to the tumor. We conclude that MMP-3-derived SLF is a marker of neovessels in glioma, where it could influence the activity of SPARC.
Keywords: SPARC, HEVIN, SPARC-LIKE FRAGMENT, MATRIX METALLOPROTEINASE-3, PLASMIN, THROMBIN, GLIOMA, ANGIOGENESIS
INTRODUCTION
Matricellular proteins exhibit a range of biological activities. As secreted, largely nonstructural proteins, they have been shown to mediate interactions between cells and components of the extracellular matrix (ECM) (reviewed in [Bornstein and Sage, 2002]). This cell-ECM interaction is crucial for most basic cellular functions including proliferation, migration, and differentiation [Brekken and Sage, 2001]. Proteolytic processing has been demonstrated as one of several mechanisms that influence the ECM environment, especially with respect to matricellular proteins and collagens. Previous studies indicate that extracellular protease activity can result in the creation of biologically functional peptides [Sage, 1997]. The prototypic matricellular protein SPARC is a substrate for several extracellular proteases, and its cleavage produces unique bioactive peptides with specific cellular functions. For example, proteolysis of SPARC by matrix metalloproteinase (MMP)-3 generates peptides with angiogenic activity [Sage et al., 2003].
The secreted matricellular protein hevin, also known as SC1, MAST9, and SPARC-like 1, was originally identified in high endothelial venules [Girard and Springer, 1995]; reviewed in [Sullivan and Sage, 2004]. Its occurrence in various other tissues is more limited than that of SPARC. Hevin reaches its highest level of expression in neural organs and can also be found in lung, kidney and heart [Hambrock et al., 2003]. It has been proposed that expression by an alternative transcript or post-translational modification yields a fragment, seen in some tissues, of Mr 55,000 (approximately half the expected size of the intact protein) [Brekken et al., 2004]. Subsequently, hevin has been discovered to undergo proteolysis by A Disintegrin and Metalloproteinase with Thrombospondin Motifs 4 (ADAMTS4). The major proteolytic product of hevin, which is highly similar to SPARC and of about the same size, was found to be necessary for normal development of murine brain [Weaver et al., 2010].
Expression of SPARC is readily apparent in cultured cells and increases following various forms of stress. In contrast, despite the continued presence of hevin mRNA, hevin protein is rarely found in cultured cells or their conditioned media. Its absence in vitro indicates that post-transcriptional control mechanisms, including translational inhibition or protein degradation, might regulate the levels of secreted hevin.
SPARC and hevin (in both human and mouse) contain a series of highly conserved motifs comprising ~220 residues that occur within the C-terminal half of the proteins: follistatin-like, Kazal-like, and EF-hand [Hohenester et al., 1997]. The N-termini of the two proteins consist of poorly characterized, acidic sequences of ~50 residues in SPARC and ~400 residues in hevin. Cleavage of recombinant murine hevin by ADAMTS4 [Weaver et al., 2010] and by thrombin, plasmin, and MMP-3 (as reported here) eliminated the major part of the unique N-terminal segment of hevin and left intact the motifs common to both SPARC and hevin in the truncated protein.
A comparison of the effects of targeted deletion of hevin, SPARC, or both in a foreign-body-response model showed that hevin and SPARC compensated for each other in the inhibition of angiogenesis, but that they differed in their effects on inflammation and encapsulation of the implant [Barker et al., 2005]. We considered the possibility that a SPARC-like fragment (SLF), which is produced by proteolysis of hevin by MMP-3, could compensate for the activity of SPARC in loci where MMP-3 and hevin are present. To test this proposal we utilized the human U87 glioma cell line that is negative for glial fibrillary acidic protein (GFAP). Parental U87 cells and U87 cells transfected with control or SPARC-expressing vectors were implanted in murine brains. Tumors were examined with human- and mouse-specific antibodies to study the locations of tumor-derived MMP-3 and host-derived hevin, SPARC, and the SLF. Although SPARC, hevin, and the SLF might compensate for each other under some circumstances, the reality is more complex than initially anticipated. Depending on the physiological and anatomical context, the proteins/peptide can function independently, in concert, or in opposition with regard to their effect on, e.g., development, wound healing, or tumorigenesis. However, the SLF, against which we have produced specific antibodies, is a marker of brain neovessels in the setting of glioma.
MATERIALS AND METHODS
CELL CULTURE
CHO-S cells were grown as stationary cells in CD CHO chemically-defined medium containing HT Supplement and GlutaMAX™-1 (Invitrogen, Carlsbad, CA). HEK 293 cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) (Invitrogen) containing 10% fetal bovine serum (FBS) (Fisher Scientific, Hampton, NH).
PRODUCTION OF POLYCLONAL ANTIBODY AGAINST HEVIN
Antiserum was collected from rabbits that had been injected with recombinant murine hevin expressed in Sf9 cells [Brekken et al., 2004]. The antiserum was affinity-purified on hevin coupled to Sepharose 4 Fast Flow (GE Healthcare Life Sciences, Pittsburgh, PA). The resulting antibody solution was cleared of SPARC-reactive species by passage through a SPARC affinity column. Specificity of the antibody was tested by ELISA and immunoblot with recombinant SPARC and hevin proteins, by immunoblot with wild-type (WT), SPARC-null, and hevin-null mouse tissue lysates, and by immunohistochemistry on the respective tissue sections.
PROTEINASE DIGESTION
Two µg of recombinant murine hevin was combined with plasmin or thrombin (both from human plasma) (Sigma, St. Louis, MO) at an enzyme-to-substrate molar ratio of 1:500. The irreversible thrombin inhibitor D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) was added to some samples at a PPACK-to-enzyme molar ratio of 10:1. The samples were incubated 30 min to 24 h at 37°C, separated under reducing conditions by SDS-PAGE, and stained with Coomassie brilliant blue R. For immunoblotting, 40 ng of hevin was combined with plasmin or thrombin at the above ratio or with 1% FBS (v/v), incubated 30 min or 24 h, separated as above, electro-transferred to an Immobilon-P membrane (Millipore, Billerica, MA), and probed with rabbit anti-hevin IgG.
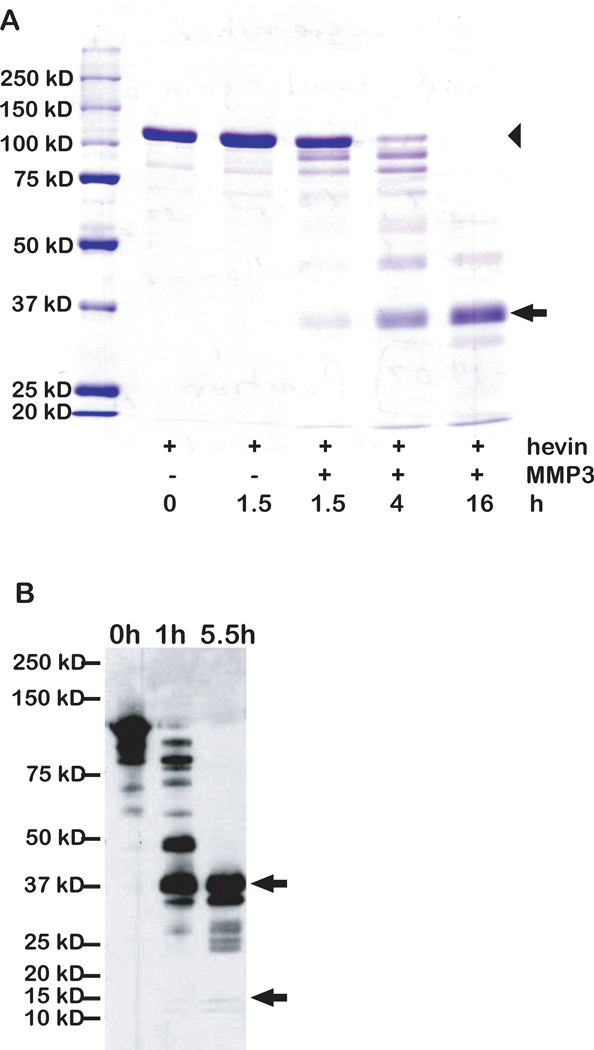
100 ng of recombinant murine MMP-3 (R&D Systems, Minneapolis, MN) was activated according to the manufacturer’s instructions and was incubated with 10 µg of hevin. After 4.5 hr at 37°C, the proteins were separated under nonreducing conditions, electro-transferred to an Immobilon-P membrane, and lightly stained with Ponceau S (Sigma). Two bands, a doublet at ~37 kDa and the top band of a triplet at ~10 kDa, were excised from the membrane and submitted to the Stanford PAN Facility for Edman sequencing (Stanford University, Palo Alto, CA). These fragments, among others, were confirmed as hevin breakdown products by immunoblots probed with rabbit anti-hevin IgG.
EXPRESSION CONSTRUCTS FOR PROTEIN PRODUCTION
The expression constructs for full-length murine hevin and the SLF were described in Weaver et al., 2010. The expression construct for alkaline phosphatase (AP) was from GenHunter (Nashville, TN). The constructs each have the coding sequence for a 6xHis tag at the C-terminus.
PROTEIN PRODUCTION
CHO-S or HEK 293 cells, cultured as monolayers, were transfected to express murine hevin, the SLF, or AP with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After incubation for 6 to 16 h, cells were rinsed with phosphate-buffered saline (PBS) and grown for 2 to 3 days in serum-free media. The proteins were purified by affinity chromatography on nickel-nitrilotriacetic acid agarose (Invitrogen) according to the manufacturer’s instructions.
CELL LINES
U87 glioma cells (American Type Culture Collection, Manassas, VA) were transfected for constitutive SPARC expression (A2b2) or to express empty vector (C2a2), as previously reported [Golembieski et al., 1999]. The A2b2 cell line expresses and secretes approximately 5-fold more SPARC than the C2a2 cell line, as estimated by ImageJ analysis.
BRAIN XENOGRAFT MODEL
In accordance with protocols approved by the Henry Ford Hospital Institutional Animal Care and Use Committee, cells were injected into the brains of athymic nude mice (NCI-Frederick, MD) as previously described [Schultz et al., 2002] or manually. Briefly, the tumor cells (5 × 105/5 µl PBS) were slowly injected 2.5 mm to the right of bregma at a depth of 2.5 mm with a 10-µl Hamilton syringe. Animals (A2b2- and C2a2-implanted) were injected daily with 200 µl of 10% DMS0 beginning at 3 days post-implantation and were sacrificed at 18 days post-injection. Untreated U87-implanted animals were sacrificed on day 19 along with control animals with normal brains. The removed brains were fixed in 10% formalin overnight, placed in a coronal brain matrix (Activational Systems, Inc., Warren, MI), and sliced into 2 mm blocks. The blocks were processed routinely, paraffin-embedded, and serially sectioned at 5 µm. Three to 6 animals per cell line were used.
IMMUNOHISTOCHEMISTRY
Brains sections were dehydrated in a series of ethanol solutions and were embedded in paraffin. Sections were next deparaffinized and rehydrated. Antigen unmasking was performed by heating of the slides for 10 min in 10 mM citrate buffer (pH 6.0). Nonspecific binding sites of all sections were blocked with 20% Aquablock (East Coast Bio, North Berwick, ME) in PBS with 0.2% Tween for 1 h. Sections were incubated with varying combinations of rabbit anti-MMP-3, rabbit anti-GFAP, mouse anti-VWF, goat anti-murine hevin, goat anti-murine SPARC, and rat anti-SLF IgGs for 15–18 h at 4°C, washed in PBS-Tween, and exposed to goat anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC) or tetramethyl rhodamine isothiocyanate (TRITC) and/or goat anti-rat IgG conjugated to FITC or TRITC (Invitrogen) and/or donkey anti-goat IgG conjugated to FITC or TRITC (Invitrogen) for 1 h. All co-staining antibodies described above were used at concentrations according to the manufacturers' protocols. Cell nuclei were stained with Hoechst 33258 fluorochrome (4 µg/ml; Invitrogen). Five-mm sections of normal brains and U87 parental tumors were placed on charged slides, heated at 60°C for 40 min, subsequently deparaffinized through xylene and graded solutions of ethanol, and finally rinsed in distilled water. The sections were immersed in 3% H2O2 for 5 min and next rinsed in distilled water. The sections were immersed in preheated (95–97°C) Target Retrieval Solution (S1700) (Dako, Carpinteria, CA) and were steamed for 12 min. After cooling for 20 min, the slides were rinsed in distilled water, immersed in Tris-buffered saline with Tween 20 ([TBS-T] TWB945, Biocare Medical, Concord, CA), and rinsed for 10 min. Sections were incubated with anti-murine hevin at a 1:1000 dilution in a 1:1 mixture of antibody diluent (Biocare Medical DaVinci diluent PD900) and Sniper blocking reagent (Biocare Medical) for 40 min at room temperature. Sections were rinsed with TBS-T and were incubated in biotinylated anti-rat secondary antibody (1:200 dilution for 30 min), followed by rinsing in TBS-T and 10 min in 4+-HRP (Biocare Medical). Sections were developed in 3,3'-diaminobenzidine (DAB) for 4 min, rinsed well in distilled water, and counterstained in hematoxylin (Biocare Medical) for 8 sec, after which they were dehydrated in alcohol, cleared in xylene, and coverslipped. Negative controls included substitution of primary antibody by non-immune rabbit, goat, and/or rat IgG. Routine sample analysis and identification of astrocytes/microglial cells were performed on serial sections of brain tissue stained with hematoxylin and eosin (H&E). All images were compiled into figures in Adobe Illustrator or Adobe Photoshop CS3 (San Jose, CA).
RESULTS
PROTEOLYSIS OF HEVIN IN VITRO
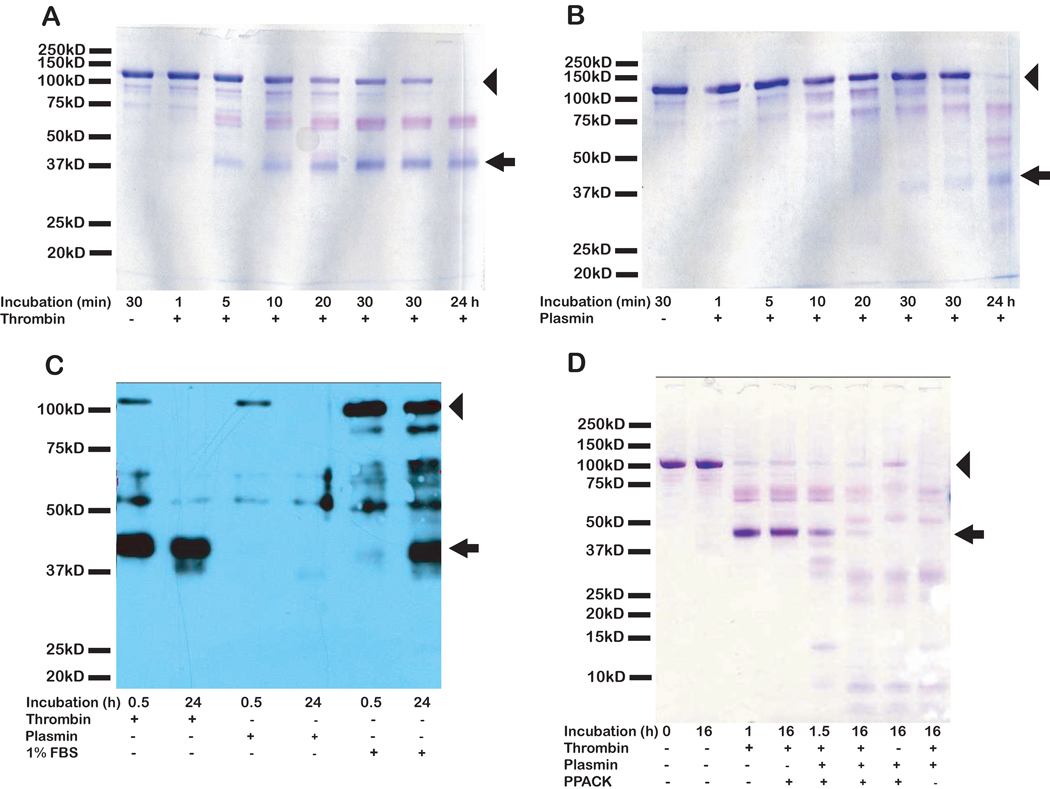
SPARC and hevin have been identified in a variety of tissues [Brekken and Sage, 2001; Brekken et al., 2004]. SPARC has also been found in vitro as a protein secreted by both primary and immortalized cell lines from diverse tissues and organisms. In contrast, hevin mRNA can be detected in cultured cells, but only the earliest passages of cultured primary cells produce hevin protein. It was therefore of interest to determine whether proteolysis was responsible for the elimination of hevin that was secreted into the conditioned media. The serum proteases thrombin and plasmin were tested first for their capacity to cleave hevin in vitro. Both thrombin (Fig. 1A) and plasmin (Fig. 1B) digested hevin in a time-dependent manner. The cleavage patterns of hevin generated by these two enzymes exhibited limited similarity to that of hevin digested by incubation with FBS alone (Fig. 1C). Similar cleavage patterns for hevin were observed when mouse serum was used as the source of serum proteases (data not shown). The specificity of this cleavage was determined by immunoblotting with rabbit anti-hevin IgG, which revealed an immunoreactive band of comparable size to SPARC. The combination of thrombin and plasmin produced a unique cleavage pattern for hevin (Fig. 1D) that was comparable to that seen after hevin was incubated with FBS alone (Fig. 1C). The thrombin inhibitor PPACK, which was added to hevin in combination with thrombin and/or plasmin (Fig. 1D), did not preserve full-length hevin in the presence of thrombin (with or without plasmin), but it did protect the SPARC-sized fragment of hevin from complete proteolysis during an incubation of 16 h. In contrast, in the absence of PPACK, plasmin and thrombin eliminated all visible evidence of the SPARC-sized fragment in the same period of time (as assessed by SDS-PAGE and Coomassie blue stain).
Figure 1. Proteolysis of hevin by thrombin, plasmin, and fetal bovine serum.
2 µg or 40 ng (for stain or immunoblot, respectively) of hevin was incubated at 37°C, +/− active thrombin or plasmin (at an enzyme-to-substrate molar ratio of 1:500) or 1% FBS (v/v) for the indicated times. The digests were resolved by SDS-PAGE, and proteins were stained with Coomassie blue (A, B, D), or electro-transferred and immunoblotted with rabbit anti-hevin IgG (C). A) Thrombin digestion of hevin, 1 to 24 h. B) Plasmin digestion of hevin, 1 to 24 h. C) Immunoblot of hevin digested by thrombin, plasmin, or FBS. D) Double digestion of hevin by plasmin and thrombin +/− the thrombin inhibitor PPACK. Molecular mass standards (in kD) indicated on the left. Arrow indicates the SLF. Arrowhead indicates full-length hevin. Hevin at 0 min appeared the same as at 30 min and is not shown (A–C). Results are representative of at least three independent experiments.
CLEAVAGE OF HEVIN BY MMP-3
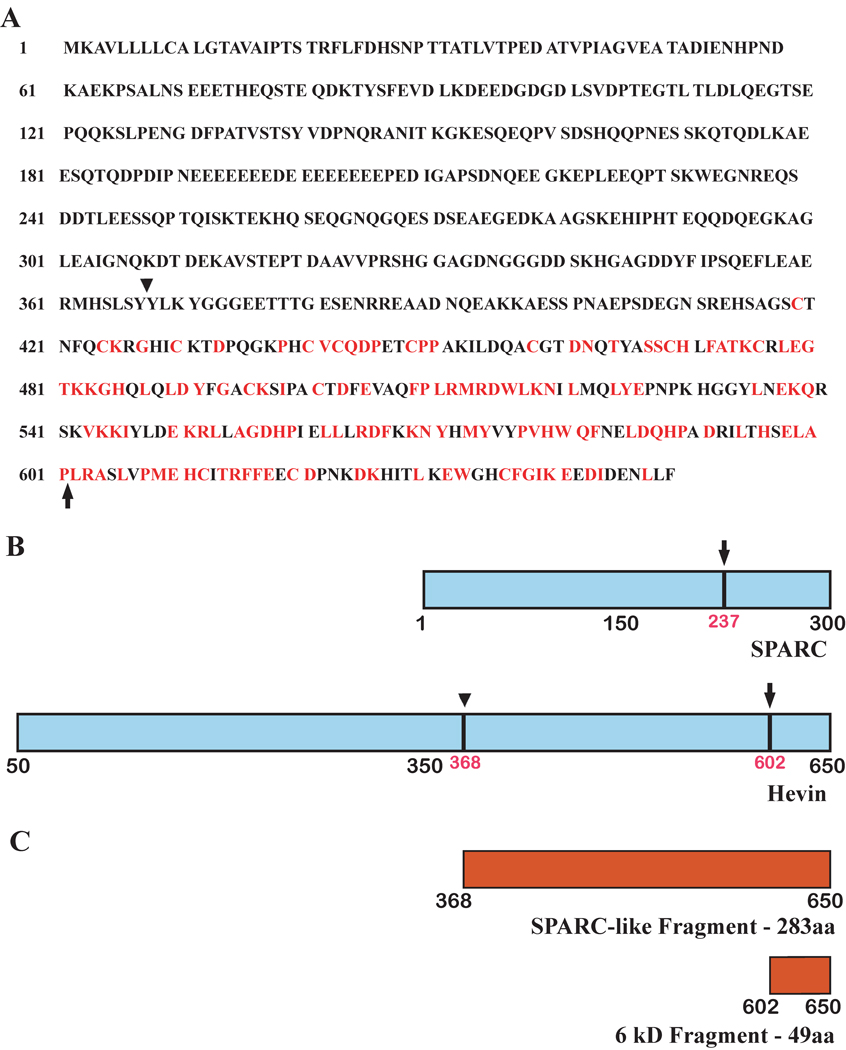
The lability of secreted hevin in vitro, as well as the production of a fragment approximately the size of SPARC by two serum-derived proteases, prompted us to test other proteinases that might exhibit specificity for hevin. Previous work demonstrated the cleavage of SPARC by MMP-3 and the subsequent release of bioactive peptides that regulate several cellular functions [Sage et al., 2003]. When MMP-3 was incubated with hevin, proteolysis occurred in a time-dependent manner (Fig. 2A and 2B). Similar to the pattern observed after thrombin/plasmin digestion (Fig. 1), MMP-3 produced a hevin peptide of an apparent molecular size similar to that of intact SPARC. Additionally, a smaller fragment was observed that resembled in size the C-terminal SPARC peptide previously shown to be released by MMP-3 [Sage et al., 2003]. These two peptides were sequenced and found to be derived from the C-terminus of hevin. The SLF (Mr ~37,000) includes the C-terminal portion of hevin with the highest level of identity to SPARC (Fig. 3A and 3B). The cleavage site giving rise to the smaller hevin peptide is located within an amino acid sequence homologous to SPARC and identical to the MMP-3 cleavage site previously found in SPARC. Comparison of the relative locations of MMP-3 cleavage sites in SPARC and hevin is shown in Fig. 3B. A construct was made for the expression of the SLF for use in subsequent experiments (Fig. 3C).
Figure 2. Hevin is a substrate for MMP-3 in vitro.
2.5 µg hevin was incubated at 37°C +/− active MMP-3 (at an enzyme-to-substrate molar ratio of 1:150) for the indicated times. The digests were resolved by SDS-PAGE and proteins were stained with Coomassie blue (A) or were electro-transferred and immunoblotted with rabbit anti-hevin IgG (B). A) MMP-3 digestion of hevin from 1.5 to 16 h. Arrow indicates the SLF. Arrowhead indicates full-length hevin. B) Immunoblot of MMP-3-derived hevin fragments. Arrows indicate the sizes of bands extracted from a similar blot and subjected to N-terminal sequencing. Molecular mass standards (in kD) are indicated on the left. Results are representative of at least three independent experiments.
Figure 3. MMP-3-derived hevin fragments exhibit sequence similarity to murine SPARC.
N-terminal sequencing was performed on fragments of digested hevin. The resulting sequences were compared for identity with murine SPARC and were used to derive vectors expressing SPARC-related hevin fragments. A) Hevin amino acid sequence. Residues that are identical to those of SPARC are highlighted in red. B) Diagram of hevin and SPARC sequences. Arrowhead (amino acid 368) indicates unique cleavage site in hevin determined by N-terminal sequencing. Arrows indicate homologous cleavage sites in both hevin and SPARC determined by N-terminal sequencing. aa, amino acid. C) Diagram of products of MMP-3 digestion of hevin.
HEVIN IN GLIOMAS
Gliomas are characterized by a high degree of neovascularization and infiltration by immune cells from the host tissue [Schiffer et al., 2010; Yang et al., 2010] (reviewed in both), and have been associated with the expression of SPARC [Rempel et al., 1998]. Additionally, gliomas exhibit increased proteolytic activity, hypothesized to be responsible, in part, for their highly invasive phenotype [Lakka et al., 2005; Levicar et al., 2003; Nuttall et al., 2003]. SPARC has been shown to increase the expression of MMP-3 [Rich et al., 2003]. To investigate the effects of SPARC and MMP-3 on hevin proteolysis in host cells, we obtained sections from mouse brains that had been implanted with clones of a noninvasive glioma cell line that had been stably transfected with either a plasmid for the expression of SPARC (A2b2) or a plasmid with a nonexpressing vector as a control. Previous work has shown that the A2b2 cells are highly invasive in a xenograft rodent model [Schultz et al., 2002; Thomas et al., 2010].
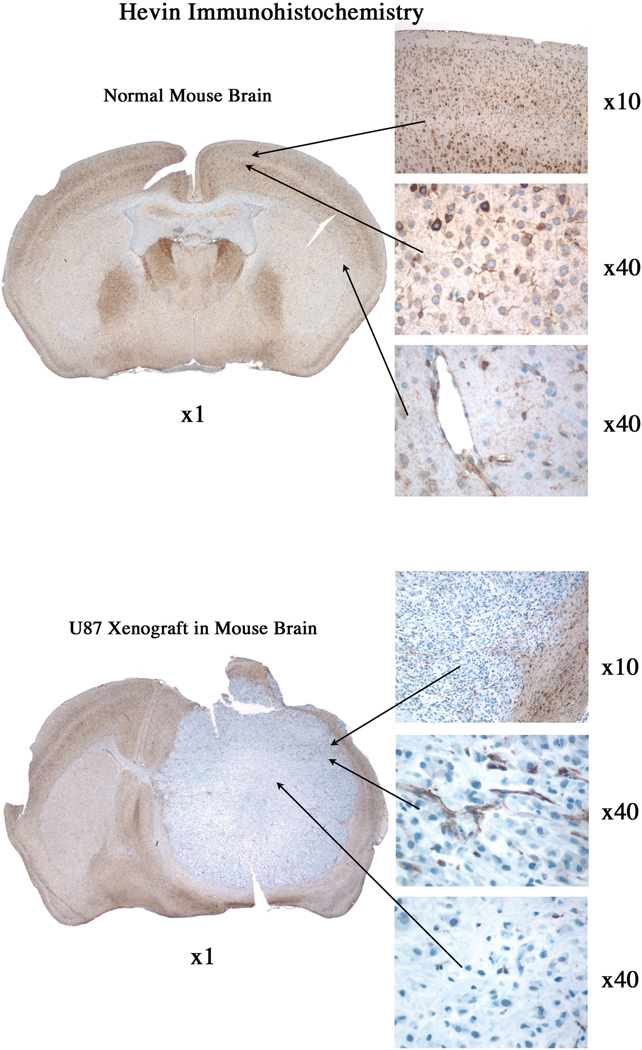
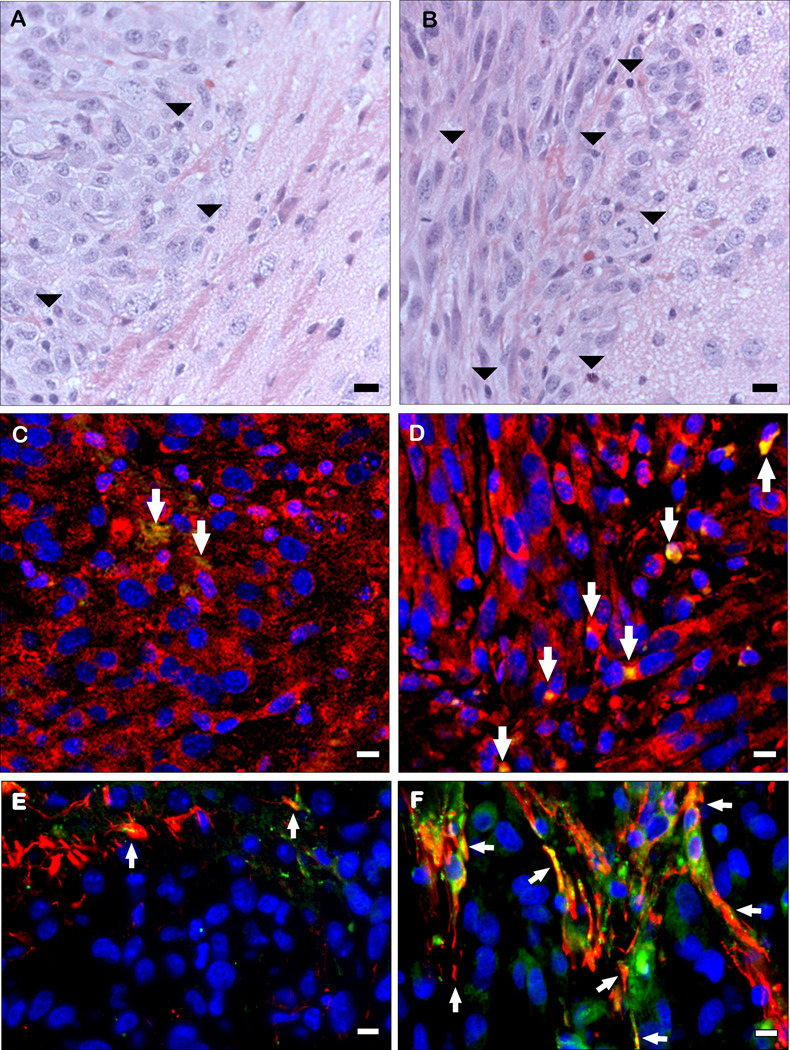
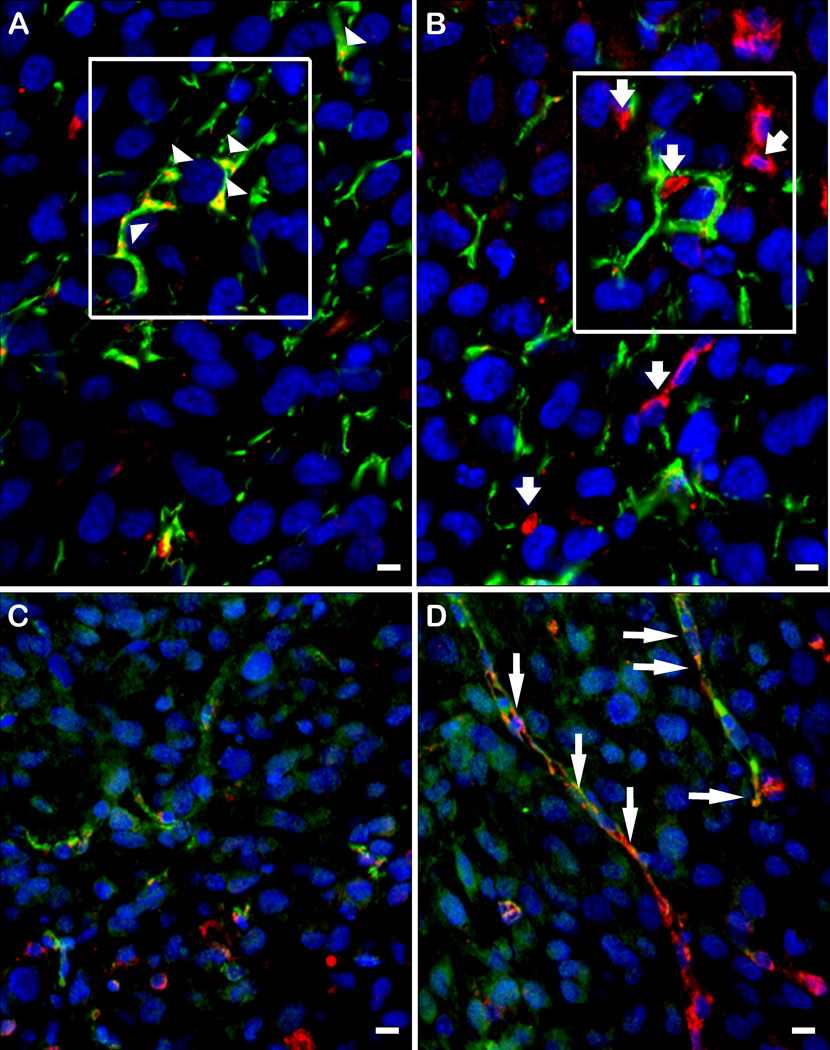
Sections of brain tumors that developed following implantation of U87 parental, A2b2, or C2a2 control cells were analyzed after H&E staining and hevin immunohistochemistry. Initial comparison between control, non-cell-injected brains and those injected with the U87 parental cells revealed hevin in host tissues that was undetectable in the U87 parental cell tumors (Fig. 4). Tumors seeded with the control cells were characterized by a well-circumscribed border with adjacent brain tissue (Fig. 5A), whereas the SPARC-expressing tumors invaded adjacent brain and had higher levels of infiltrating host-derived cells (Fig. 5B). Sections were also examined by immunohistochemistry for the expression of hevin and MMP-3. Increased expression of SPARC by the tumor cells was accompanied by higher levels of MMP-3, relative to control cells, but no detectable hevin. However, the host immune cells that infiltrated the gliomas were associated with an enhanced co-localization of hevin and MMP-3 (Fig. 5, C and D). Tumors were also evaluated for the astrocyte marker GFAP and hevin to evaluate expression of the latter protein in astrocytes encompassed by infiltrating tumor cells. Hevin was observed in host astrocytes at the periphery of the control tumors (Fig. 5E) and in astrocytes engulfed by the invasive tumors (Fig. 5F). SPARC-expressing A2b2 tumors were co-stained with antibodies against either SPARC or hevin in combination with antibodies against the vascular marker von Willebrand factor (VWF) or GFAP (Fig 6, A–D). Hevin was closely associated with the encompassed host astrocytes (Fig. 6A), whereas SPARC was associated with neovasculature (Fig. 6D).
Figure 4. Hevin is expressed in normal mouse brain but not in U87 glioma cells.
5 µm sections of normal mouse brain (A) and mouse brain implanted with U87 glioma cells (B) were subjected to immunohistochemistry to detect hevin. A) Hevin was observed regionally in normal brain (×1 magnification), with high levels in neurons and astrocytes (×10, ×40 magnifications; top and middle panels) and blood vessels (×40 magnification; bottom panel). B) Hevin was undetectable in glioma U87 tumor cells (×1, ×40 magnification, bottom panel), but was present in surrounding normal brain (×10 magnification; top panel) and blood vessels (×40 magnification; middle panel).
Figure 5. Host cells that infiltrate gliomas express hevin that is co-localized with MMP-3.
Non-invasive (C2a2) and invasive (A2b2) glioma cells were implanted in nude mouse brains. After 18 days, animals were sacrificed and the brains were removed, formalin fixed, and paraffin-embedded. Sections were stained with H&E (A, B), and immunohistochemistry was performed with goat anti-hevin IgG (green) and either rabbit anti-MMP-3 IgG (red) (C, D) or rabbit anti-GFAP IgG (red) (E, F). A) Non-invasive control-expressing C2a2 tumor. B) invasive SPARC-expressing A2b2 tumor. Arrowheads indicate host-derived infiltrative cells. C) Hevin and MMP-3 in noninvasive C2a2 tumor. D) Hevin and MMP-3 in invasive A2b2 tumor. Arrows indicate hevin+/MMP-3+ cells. Scale bars, 10 µm. E) C2a2 tumor. F) A2b2 tumor. Arrows indicate hevin+/GFAP+ cells. Scale bars, 1 µm.
Figure 6. Hevin is located in glial infiltrates, whereas SPARC is found in microvasculature.
Gliomas that arose from A2b2 (SPARC-overexpressing) cells were subjected to immunohistochemistry with a combination of rabbit anti-GFAP IgG (A, B) or mouse anti-VWF IgG (C, D) (red), and goat anti-murine hevin IgG (A, C) or goat anti-murine SPARC IgG (green) (B, D). A) Co-localization of hevin (green) and GFAP (red) staining. B) Disparate localization of SPARC (green) and GFAP (red) staining. Boxed areas in A and B represent identical tumor locations in the serial sections. C) Disparate localization of hevin (green) and VWF (red). D) Co-localization of SPARC (green) and VWF (red). Arrowheads (A) indicate hevin+/GFAP+ cells. Short arrows (B) indicate SPARC (green) independent from GFAP (red). Long arrows (D) indicate SPARC+/VWF+ cells. Scale bars, 10 µm.
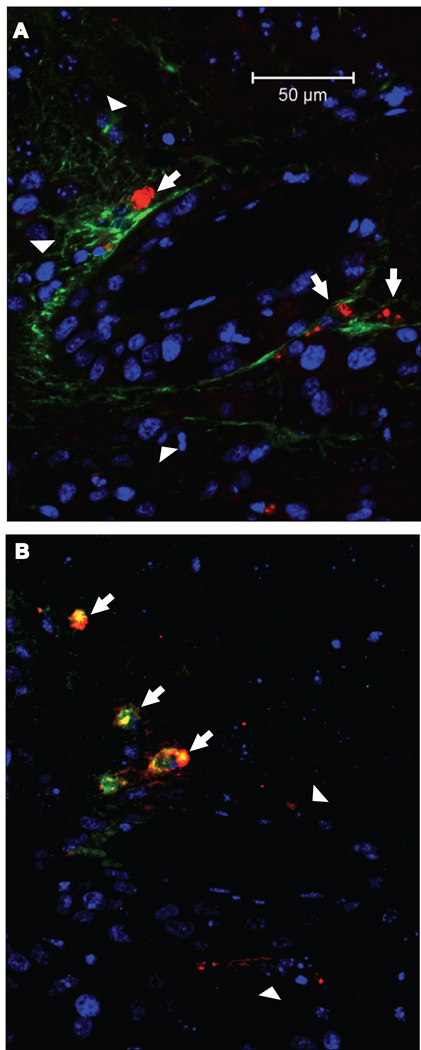
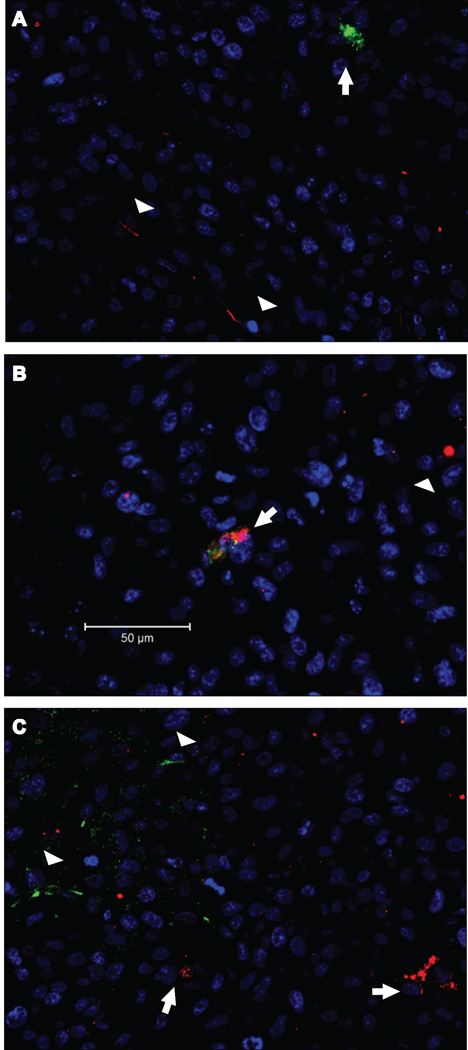
In another experiment, the SLF, which we expected would mimic the activity of SPARC, was located in serial sections of the tumors by the simultaneous detection of the SLF with either VWF or GFAP. The SLF was associated with neovascular cells (VWF-positive) and not with astrocytes (GFAP-positive) (Fig. 7). As shown in Figure 8, not only do neovascular cells in the glioma express SPARC, they can also be characterized by the presence of the SLF (Fig. 8B). In none of the sections was the SLF detected in astrocytes (Fig. 8A), nor was SPARC seen in association with hevin (Fig. 8C).
Figure 7. The SLF of hevin is associated with the microvasculature.
Serial sections from gliomas seeded by A2b2 (SPARC-overexpressing) cells were subjected to immunohistochemistry with a combination of rabbit anti-GFAP IgG (A) or mouse anti-VWF IgG (B), and rat anti-SLF IgG (A, B). A) Disparate localization of GFAP (green) and SLF (red). Arrows indicate SLF (red) independent from GFAP (green, arrowheads). B) Co-localization of SLF (green) and VWF (red). Arrows indicate SLF+/VWF+ cells. Arrowheads indicate additional VWF+ cells. Scale bars, 50 µm.
Figure 8. SLF is present in cells that produce SPARC but not full-length hevin.
Gliomas from A2b2 (SPARC-overexpressing) cells were subjected to immunohistochemistry with a combination of rabbit anti-hevin (full-length) IgG (A, C), mouse anti-SPARC IgG (B, C), and rat anti-SLF IgG (A, B). A) Arrows indicate SLF (green) independent from full-length hevin (red, arrowheads). B) Co-localization of SLF (green) and SPARC (red). Arrows indicate SLF+/SPARC+ cells. Arrowheads indicate additional SPARC+ cells. C) Disparate localization of hevin (green) and SPARC (red). Arrowheads indicate hevin+ cells and arrows indicate SPARC+ cells. Scale bars, 50 µm.
DISCUSSION
That SPARC and its family members play indispensable roles in development, wound healing, and angiogenesis is amply supported by their prevalence in mammalian tissues and by accumulating evidence that in some cases one family member can compensate for the absence of another. Mice in which the SPARC gene has been silenced develop normally in most respects, an observation supporting the supposition that similar proteins can assume its functions. In tissues lacking such compensatory molecules, abnormalities would be expected to occur. Indeed, the early cataractogenesis characteristic of these animals is accompanied by little if any increase in the constitutively low level of the SPARC-like protein hevin seen in the lenses of WT mice [Yan et al., 2005]. In contrast, in brain, the tissue in which hevin is the most prevalent, deprivation of SPARC results in no known phenotypic abnormalities [Eroglu, 2009].
The question, whether hevin might compensate for the absence of SPARC in the brain, touches nicely on the phenomenon of the apparent lack of hevin protein in cultured cells that express hevin mRNA. Could proteolysis of hevin explain both its disappearance in cell culture and the apparent redundancy of SPARC and hevin in vivo? To address this question, we first established that plasmin and thrombin, the most common of the plasma proteases, digest hevin efficiently and virtually eliminate the full-length protein in one hour. Although a number of other proteases are present in both serum and brain, we chose MMP-3 as our next candidate for proteolysis of hevin because it acts on SPARC to produce discrete fragments with defined biological activity and therefore might have a similar effect on hevin. Indeed, digestion of hevin by MMP-3 yielded two stable peptides, the larger of which is composed of a C-terminal portion of hevin that is highly similar to SPARC. The smaller peptide that appeared upon MMP-3 digestion was similar to a product of the proteolysis of SPARC by MMP-3, also at the C-terminus. We did not characterize this peptide (amino acids 602–650, Mr 6000) as it proved refractory to production in mammalian cells.
We investigated whether SPARC and the SLF have similar effects on endothelial cells in vitro by the use of two classic assays: a test for the inhibition of proliferation and a migration assay to assess the invasive potential of the cells. Our results showed only slight and inconsistent differences among the effects on cells of SPARC, the SLF, and full-length hevin (data not shown). The reason for these results became clear when we compared the relative locations of the three proteins in a highly invasive tumor implant model. Gliomas are characterized by the abundant expression of SPARC and proteolytic enzymes, the rapid incursion of neovascular cells, and the trapping of adjacent normal brain tissue as tumors invade [Rempel et al., 1998; Schiffer et al., 2010]. We found that SPARC and the SLF occur together in the neovasculature and might thus augment rather than compensate for each other’s activity. In studies of hepatocellular carcinoma, Lau et al. reported correlation of angiogenesis with the expression of SPARC and hevin [Lau et al., 2006]. However, it is also possible that hevin-derived SLF could act as a SPARC antagonist/competitor. Because the anti-hevin and anti-SLF antibodies are specific for the murine protein, we cannot rule out the possibility that the human tumor cells used in this model do not produce hevin, subsequent cleavage of which into the SLF would modify angiogenesis and tumor invasion, functions previously described for the parent protein in other systems [Barker et al., 2005; Sullivan et al., 2008].
Hevin was found in astrocytes and was not co-localized with either SPARC or the SLF. Although hevin was co-localized with MMP-3 in apparently infiltrative cells, the lack of the SLF poses the possibility that cleavage of hevin might not take place at the tissue sites occupied by these two proteins and that another enzyme might be responsible for the generation of the SLF in this model. For example, brain endothelial cells have been reported to express MMP-3 [Hummel et al., 2001] and could conceivably process hevin into the SLF at this site.
Our results indicate that 1) the SLF can be considered a unique and non-redundant polypeptide in WT animals, 2) it can be detected independently of full-length hevin, and 3) it marks neovasculature in gliomas. Its role in diseases such as glioma deserves further study, especially in animal models, in which the differences among the functions of SPARC, hevin, and the SLF can be resolved.
ACKNOWLEDGMENTS
This work was supported by grants from National Institutes of Health (GM40711 to E.H.S. and CA86997 to S.A.R.).
We are grateful to Dr. Virginia Green for her assistance with the preparation of this manuscript.
REFERENCES
- Barker TH, Framson P, Puolakkainen PA, Reed M, Funk SE, Sage EH. Matricellular homologs in the foreign body response: hevin suppresses inflammation, but hevin and SPARC together diminish angiogenesis. Am J Pathol. 2005;166:923–33. doi: 10.1016/S0002-9440(10)62312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sullivan MM, Workman G, Bradshaw AD, Carbon J, Siadak A, Murri C, Framson PE, Sage EH. Expression and characterization of murine hevin (SC1), a member of the SPARC family of matricellular proteins. J Histochem Cytochem. 2004;52:735–748. doi: 10.1369/jhc.3A6245.2004. [DOI] [PubMed] [Google Scholar]
- Eroglu C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J Cell Commun Signal. 2009;3:167–176. doi: 10.1007/s12079-009-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard JP, Springer TA. Cloning from purified high endothelial venule cells of hevin, a close relative of the antiadhesive extracellular matrix protein SPARC. Immunity. 1995;2:113–123. doi: 10.1016/1074-7613(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Golembieski WA, Ge S, Nelson K, Mikkelsen T, Rempel SA. Increased SPARC expression promotes U87 glioblastoma invasion in vitro. Int J Dev Neurosci. 1999;17:463–472. doi: 10.1016/s0736-5748(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Hambrock HO, Nitsche DP, Hansen U, Bruckner P, Paulsson M, Maurer P, Hartmann U. SC1/hevin. An extracellular calcium-modulated protein that binds collagen I. J Biol Chem. 2003;278:11351–11358. doi: 10.1074/jbc.M212291200. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Maurer P, Timpl R. Crystal structure of a pair of follistatin-like and EF-hand calcium-binding domains in BM-40. EMBO J. 1997;16:3778–3786. doi: 10.1093/emboj/16.13.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel V, Kallmann BA, Wagner S, Fuller T, Bayas A, Tonn JC, Benveniste EN, Toyka KV, Rieckmann P. Production of MMPs in human cerebral endothelial cells and their role in shedding adhesion molecules. J Neuropathol Exp Neurol. 2001;60:320–327. doi: 10.1093/jnen/60.4.320. [DOI] [PubMed] [Google Scholar]
- Lakka SS, Gondi CS, Rao JS. Proteases and glioma angiogenesis. Brain Pathol. 2005;15:327–341. doi: 10.1111/j.1750-3639.2005.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CP, Poon RT, Cheung ST, Yu WC, Fan ST. SPARC and Hevin expression correlate with tumour angiogenesis in hepatocellular carcinoma. J Pathol. 2006;210:459–468. doi: 10.1002/path.2068. [DOI] [PubMed] [Google Scholar]
- Levicar N, Nuttall RK, Lah TT. Proteases in brain tumour progression. Acta Neurochir (Wien) 2003;145:825–838. doi: 10.1007/s00701-003-0097-z. [DOI] [PubMed] [Google Scholar]
- Nuttall RK, Pennington CJ, Taplin J, Wheal A, Yong VW, Forsyth PA, Edwards DR. Elevated membrane-type matrix metalloproteinases in gliomas revealed by profiling proteases and inhibitors in human cancer cells. Mol Cancer Res. 2003;1:333–345. [PubMed] [Google Scholar]
- Rempel SA, Golembieski WA, Ge S, Lemke N, Elisevich K, Mikkelsen T, Gutierrez JA. SPARC: a signal of astrocytic neoplastic transformation and reactive response in human primary and xenograft gliomas. J Neuropathol Exp Neurol. 1998;57:1112–1121. doi: 10.1097/00005072-199812000-00002. [DOI] [PubMed] [Google Scholar]
- Rich JN, Shi Q, Hjelmeland M, Cummings TJ, Kuan CT, Bigner DD, Counter CM, Wang XF. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. J Biol Chem. 2003;278:15951–15957. doi: 10.1074/jbc.M211498200. [DOI] [PubMed] [Google Scholar]
- Sage EH. Pieces of eight: Bioactive fragments of extracelular proteins as regulators of angiogenesis. Trends Cell Biol. 1997;7:182–186. doi: 10.1016/S0962-8924(97)01037-4. [DOI] [PubMed] [Google Scholar]
- Sage EH, Reed M, Funk SE, Truong T, Steadele M, Puolakkainen P, Maurice DH, Bassuk JA. Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J Biol Chem. 2003;278:37849–37857. doi: 10.1074/jbc.M302946200. [DOI] [PubMed] [Google Scholar]
- Schiffer D, Annovazzi L, Caldera V, Mellai M. On the origin and growth of gliomas. Anticancer Res. 2010;30:1977–1998. [PubMed] [Google Scholar]
- Schultz C, Lemke N, Ge S, Golembieski WA, Rempel SA. Secreted protein acidic and rich in cysteine promotes glioma invasion and delays tumor growth in vivo. Cancer Res. 2002;62:6270–6277. [PubMed] [Google Scholar]
- Sullivan MM, Puolakkainen PA, Barker TH, Funk SE, Sage EH. Altered tissue repair in hevin-null mice: inhibition of fibroblast migration by a matricellular SPARC homolog. Wound Repair Regen. 2008;16:310–319. doi: 10.1111/j.1524-475X.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- Sullivan MM, Sage EH. Hevin/SC1, a matricellular glycoprotein and potential tumor-suppressor of the SPARC/BM-40/Osteonectin family. Int J Biochem Cell Biol. 2004;36:991–996. doi: 10.1016/j.biocel.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Thomas SL, Alam R, Lemke N, Schultz LR, Gutierrez JA, Rempel SA. PTEN augments SPARC suppression of proliferation and inhibits SPARC-induced migration by suppressing SHC-RAF-ERK and AKT signaling. Neuro Oncol. 2010;12:941–955. doi: 10.1093/neuonc/noq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver MS, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Sage EH. Processing of the matricellular protein hevin in mouse brain is dependent on ADAMTS4. J Biol Chem. 2010;285:5868–5877. doi: 10.1074/jbc.M109.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Perdue N, Blake D, Sage EH. Absence of SPARC in murine lens epithelium leads to increased deposition of laminin-1 in lens capsule. Invest Ophthalmol Vis Sci. 2005;46:4652–4660. doi: 10.1167/iovs.05-0460. [DOI] [PubMed] [Google Scholar]
- Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]