Abstract
Advanced stages of epithelial carcinogenesis involve the loss of intercellular adhesion, but it remains unclear how proteins that regulate alterations in cell-cell and cell-matrix adhesion are deregulated to promote the early stages of cancer development. To address this, a three-dimensional human tissue model that mimics the incipient stages of Squamous Cell Carcinoma (SCC) was used to study how E-cadherin suppression promotes tumor progression in Ras-expressing human keratinocytes. We found that E-cadherin suppression triggered elevated mRNA and protein expression levels of Focal Adhesion Kinase (FAK), and increased FAK and Src activities above the level seen in Ras-expressing E-cadherin-competent keratinocytes. sh-RNA-mediated depletion of FAK and Src restored E-cadherin expression levels by increasing its stability in the membrane, and blocked tumor cell invasion in tissues. Surface transplantation of these tissues to mice resulted in reversion of the tumor phenotype to low-grade tumor islands in contrast to control tissues that manifested an aggressive, high-grade SCC. These findings suggest that the tumor-promoting effect of E-cadherin suppression, a common event in SCC development, is exacerbated by enhanced E-cadherin degradation induced by elevated FAK and Src activities. Furthermore, they imply that targeting FAK or Src in human epithelial cells with neoplastic potential may inhibit the early stages of SCC.
Keywords: Squamous cell carcinoma, E-cadherin, Focal adhesion kinase, Src kinase, 3D tissues
INTRODUCTION
Squamous Cell Carcinoma (SCC) is initiated as a foci of cells with neoplastic potential selectively undergo intraepithelial (IE) expansion, degrade the basement membrane, and invade into the stroma in the transition from precancer to malignancy (Dlugosz, et al, 2002). While altered tissue structure linked to loss of intercellular adhesion is a morphologic hallmark of the IE stage of SCC (Bissell & Radisky, 2001), cellular events that promote the conversion of a premalignant lesion to carcinoma, and control its biological potential are not well understood.
Signals directed by cadherin-mediated cell-cell adhesions and integrin-mediated cell-matrix interactions regulate normal homeostasis of stratified squamous epithelia, while perturbations of intercellular contacts can alter cell motility, survival, and growth. E-cadherin that integrates cell-cell adhesion in adherens junctions with mitogenic signaling is known to play a critical role as a suppressor of invasiveness (Cavallaro & Christofori, 2004; Yap et al., 2007). Loss of E-cadherin function is most commonly associated with advanced stages of SCC progression (Behrens, 1999; Conacci-Sorrell et al., 2002; Birchmeier & Behrens, 1994), and is linked to metastasis (Bissell & Radisky, 2001; Cavallaro & Christofori, 2004) and poor clinical prognosis (Bagutti et al., 1998). Integrins that link matrix components to the cytoskeleton in focal adhesions, transmit signals between the cells and the extracellular matrix to regulate cell cycle, motility, and survival in normal and tumorigenic cell contexts (Chen, et al., 2006). However, it remains unclear how E-cadherin-mediated cell-cell and integrin-mediated cell-matrix adhesions work in concert to modulate and promote the early stages of SCC development.
Focal Adhesion Kinase (FAK) and Src kinase are linked to cadherins, growth factors receptors, and integrin-associated signaling complexes (Mitra & Schlaepfer, 2006; Playford & Schaller, 2004; Zhao & Guan, 2009). FAK recruitment to focal adhesions results in FAK autophosphorylation, and consequently activation of Src (Sieg et al., 2000; Roskoski, 2005). FAK hyper-phosphorylation by Src directs focal adhesion turnover, and cell motility (Zhao & Guan, 2009; Schlaepfer et al., 2004; Mitra et al., 2005). Src can also increase cell motility by phosphorylating cadherin-catenin complexes and regulators of adherense junctions, resulting in E-cadherin internalization and cell spreading (Avizienyte et al., 2002; Frame, 2004; Fujita et al., 2002). FAK and Src up-regulation has been correlated with the progression of a variety of human cancers. Elevated FAK expression and activity has been linked to breast (Oktay et al., 2003), colon (Withers et al, 1996), prostate (Tremblay et al., 1996) and oral (Schneider et al., 2002) cancers, while increased Src activity have been shown in bladder carcinoma (Boyer et al., 2002), head and neck (Mandal et al., 2008), colon (Aligayer et al., 2002) and breast (Reissig et al., 2001) cancers. Nevertheless, how FAK and Src regulate the early stages of SCC remains unclear.
This study provides new insights into the functional consequences of altered E-cadherin-mediated cell-cell adhesion in the early stages of SCC development. In particular, we found that tumor progression in a human three-dimensional tissue model of SCC induced by oncogenic Ras and E-cadherin suppression was associated with and required the up-regulation of FAK and Src activities that magnify E-cadherin loss by increasing its turnover. Furthermore, preventing the increase in FAK and Src activity associated with loss of E-cadherin led to a dramatic decrease in tumor growth and reverted tumor phenotype from high- to low-grade carcinoma in vivo. These findings demonstrate a critical mechanism by which E-cadherin loss drives the early stages of SCC development through enhanced FAK and Src kinase activities.
RESULTS
E-cadherin suppression in Ras-transformed keratinocytes is associated with elevated FAK expression, and FAK and Src tyrosine phosphorylation that promote enhanced cell motility
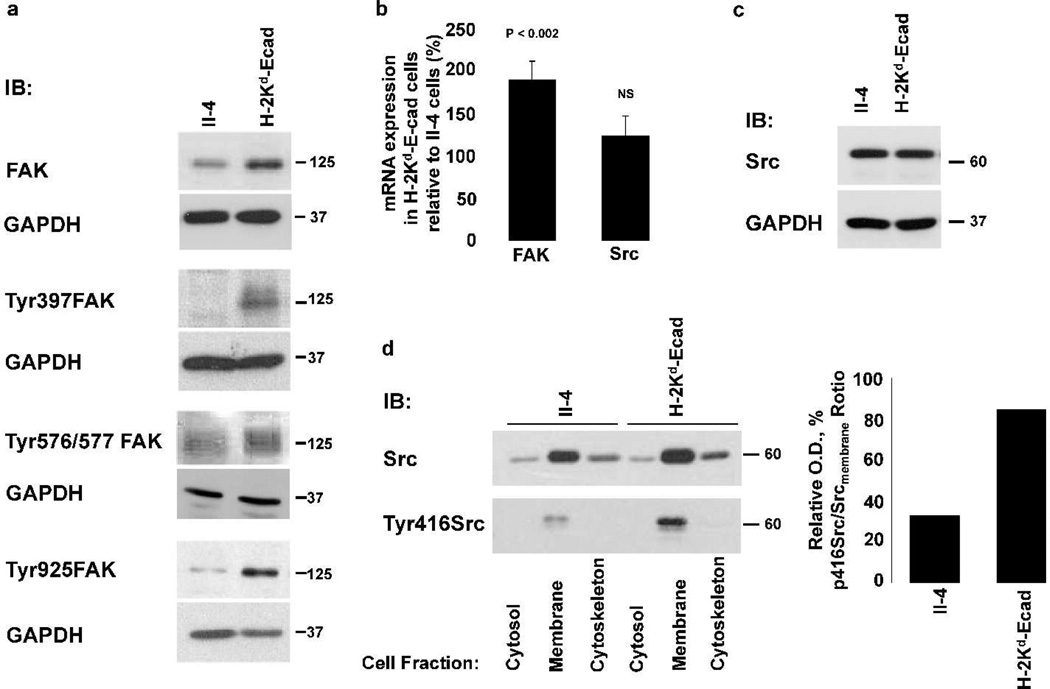
To better understand how E-cadherin suppression and FAK and Src activities are related during early stages of SCC development, we studied FAK and Src function when Ras-expressing HaCaT-II-4 keratinocytes (II-4 cells) are converted to invasive cells (H-2Kd-Ecad-II-4 cells). This occurs by an overexpression of a dominant-negative E-cadherin fusion protein (H-2Kd-Ecad), which through cytoplasmic sequestration of β-catenin leads to destabilization of E-cadherin-β-catenin complexes and the loss of cadherin-mediated intercellular adhesion (Zhang et al., 2006). Since elevated FAK activity is linked to increased phosphorylation at its autophosphorylation site (Tyr397), activation loop (Tyr576/577), and FAT domain (Tyr925) (Katz et al., 2003), we compared FAK expression and phosphorylation in both cell lines. Immunoblotting revealed that compared to non-invasive II-4 cells, invasive H-2Kd-Ecad-II-4 cells showed ~2-fold increase in FAK expression and elevated FAK phosphorylation at tyrosine Tyr397, Tyr576/577 and Tyr925 (Figure 1a). Furthermore, qRT-PCR showed that the increase in FAK expression was associated with a significant up-regulation of FAK mRNA level (Figure 1b). Next, we explored if the increase in FAK expression and activity was associated with changes in Src kinase. qRT-PCR did not show an increase in Src mRNA level (Figure 1b), and immunoblotting showed similar expression of Src in both cell lines (Figure 1c). Since Src recruitment to focal adhesions is associated with its phosphorylation at Tyr416 (Roskoski, 2005), cell fractionation was performed (Figure 1d). While Src was detected in all cell fractions it was highest in the membrane fraction of both cell lines, and its phosphorylation at Tyr416 was 3-fold higher in H-2Kd-Ecad-II-4 cells than in II-4 cells (Figure 1d). Thus, E-cadherin suppression in SCC tumor cells resulted in up-regulation of FAK mRNA and protein levels that contributed to increased FAK and Src activities.
Figure 1. Loss of E-cadherin function is associated with up-regulation of FAK and Src expression and/or phosphorylation.
WB analysis of lysates of H-2Kd-Ecad-II-4 cells revealed an increase in the expression and specific tyrosine phosphorylation of FAK (a). qRT-PCR analysis revealed a significant increase in FAK mRNA expression in H-2Kd-Ecad-II-4, relative to its level in II-4 cells. The mean ± SD of FAK or Src mRNA expression levels in H-2Kd-Ecad-II-4 relative to II-4 cells are shown (b). WB analysis showed similar expression level of Src protein in both cell lines (c). WB and ratio analysis of cell fractions of the two cell lines revealed increased tyrosine phosphorylation of Src at Tyr416 in the membrane fraction of H-2Kd-Ecad-II-4 cells (d). NS, non-significant.
To determine if elevated FAK and Src activities increase the motility of H-2Kd-Ecad-II-4 cells, cell motility was analyzed in scrape-wounded II-4- and H-2Kd-Ecad-II-4 cultures in the presence of the inhibitors Tyrphostin AG1007 or PP2 (Supplementary Figure S1). While augmented cell motility was observed in DMSO-treated H-2Kd-Ecad-II-4 cultures relative to DMSO-treated-II-4 cultures, inhibitor-treated cultures showed inhibition of wound closure (Supplementary Figure S1) that was reversible upon inhibitor removal from the media. Although Tyrphostin AG1007 and PP2 are not specific only for FAK and Src, and can affect other kinases, inhibition of the augmented motility of H-2Kd-Ecad-II-4 cells was linked, at least in part, to decreased FAK and Src phosphorylation to levels similar to those found in DMSO-treated II-4 cells (Supplementary Figure S2).
Prevention of the elevated activity of FAK or Src blocks augmented motility and invasiveness of H-2Kd-Ecad-II-4 tumor cells in vitro, and reverses SCC tumor phenotype in vivo
To determine if up-regulation of FAK or Src activity in response to decreased E-cadherin levels is necessary for the invasive phenotype of H-2Kd-Ecad-II-4 cells we used lentivirus-mediated shRNA to decrease FAK or Src levels in these cells by generating stable sh-FAK- and sh-Src-H-2Kd-Ecad-II-4 cell lines. Immunoblotting revealed that FAK expression in sh-FAK-H-2Kd-Ecad-II-4 cells and Src expression in sh-Src-H-2Kd-Ecad-II-4 cells were reduced ~70% and ~50% respectively, when compared to their levels in sh-Scrambled-H-2Kd-Ecad-II-4 cells (Figure 2a). In contrast, the expression level of another Src family member, Fyn, was similar in these cell lines, demonstrating specific down regulation of Src kinase in sh-Src-H-2Kd-Ecad-II-4 cells (Supplementary Figure S3). Next, scrape-wounding assay was performed to compare the effect of FAK and Src shRNA-mediated depletion with the pharmacological inhibition described earlier. In comparison to the wound closure seen in sh-Scrambled-H-2Kd-Ecad-II-4 cultures (Figure 2c), cell motility was inhibited in sh-FAK- or sh-Src-H-2Kd-Ecad-II-4 cultures (Figure 2e, and 2g, respectively), and was similar to that seen in sh-Scrambled-II-4 cultures (Figure 2i). While sh-Scrambled-H-2Kd-Ecad-II-4 cultures showed an average of 85% of wound closure at 24hr, wound closure was significantly reduced to 58% and 40%, in sh-FAK- or sh-Src-H-2Kd-Ecad-II-4 cultures, respectively (Figure 2j). These findings show that the increase in FAK and Src activities present in H-2Kd-Ecad-II-4 cells are necessary for their augmented motility.
Figure 2. Decreased FAK or Src expression in H-2Kd-Ecad-II-4 cells is associated with inhibition of tumor cell motility.
WB analysis of lysates of sh-FAK- and sh-Src-H-2Kd-Ecad-II-4 cells showed a decrease in FAK or Src levels, respectively, when compared to their levels in sh-Scrambled-H-2Kd-Ecad-II-4 cells (a). Scrape-wounded cultures of sh-Scrambled- (b, c), sh-FAK- (d, e) and sh-Src-H-2Kd-Ecad-II-4 cells (f, g), and of sh-Scrambled-II-4 cells (h, i) were imaged at 0 and 24hr following injury. Lines indicate the wound gaps. The mean ± SD of wound closure for each cell line are indicated and show the average change in the gaps at 24hr relative to time 0 (j). P values indicate significant changes in wound closure of sh-Scrambled-II-4, sh-FAK- and sh-Src-H-2Kd-Ecad-II-4 cells in comparison to sh-Scrambled-H-2Kd-Ecad-II-4 cells.
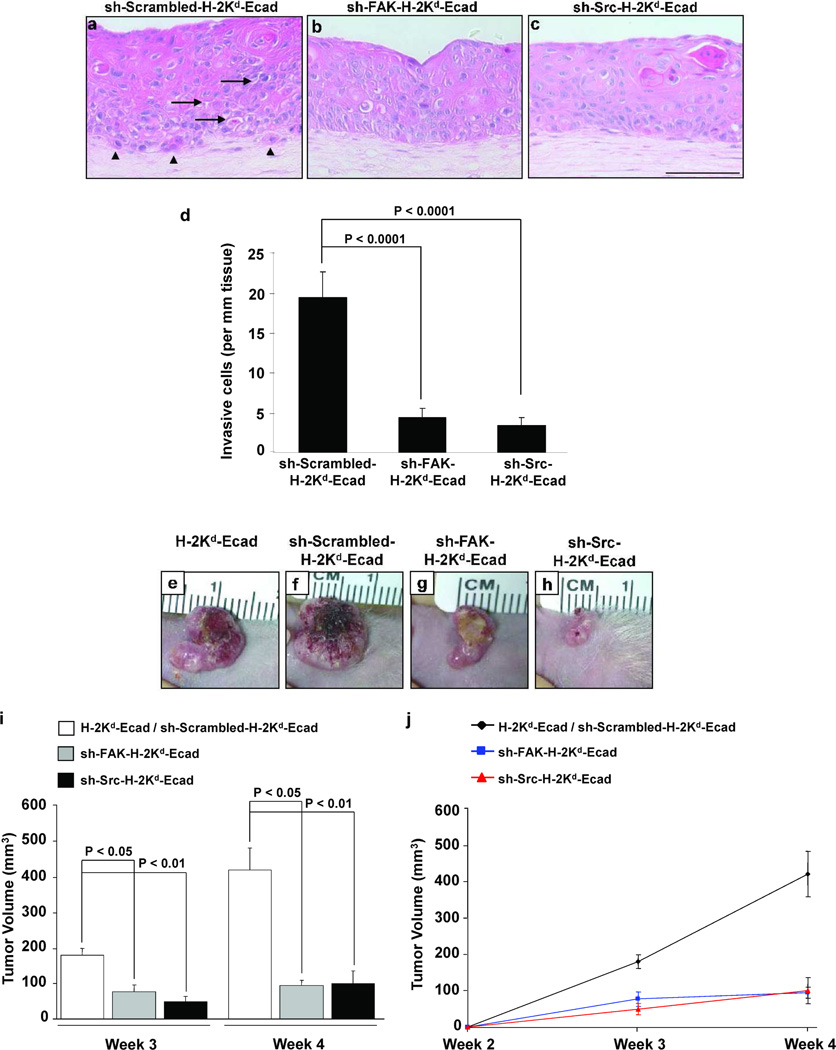
To test the concepts revealed using monolayer cultures in a more physiologically relevant setting, an engineered human skin tissue model was used. This three-dimensional (3D) tissue model closely mimics the features of the premalignant stages of SCC in human stratified squamous epithelium (Alt-Holland et al., 2005; Margulis et al., 2005). Tissues generated with sh-Scrambled-H-2Kd-Ecad-II-4 cells showed separation between epithelial cells (Figure 3a, arrows), and tumor cells that invaded into the underlying matrix (Figure 3a, arrowheads). In contrast, tissues harboring sh-FAK- (Figure 3b) or sh-Src-H-2Kd-Ecad-II-4 cells (Figure 3c) revealed a greater degree of basal cell layer organization, and a significant decrease of tumor cell invasion into the matrix (Figure 3d). These results suggested that upon E-cadherin loss increased FAK and Src activities in H-2Kd-Ecad-II-4 cells, above the level seen in II-4 cells, is necessary for their invasive behavior in 3D tissues.
Figure 3. FAK or Src down-regulation in H-2Kd-Ecad-II-4 cells is linked to inhibition of tumor cell invasion in 3D tissues and suppression of tumor growth in vivo.
H&E staining of sh-Scrambled- (a), sh-FAK- (b) and sh-Src-H-2Kd-Ecad-II-4 (c) tissue sections. sh-Scrambled-H-2Kd-Ecad-II-4 tissues showed intracellular gaps (arrows) and cell invasion into the matrix (arrowheads). Bar, 100µm. The mean ± SD of cell invasion in each of the tissues are indicated (d). Representative images of the clinical appearance of H-2Kd-Ecad- (e), sh-Scrambled-H-2Kd-Ecad- (f), sh-FAK-H-2Kd-Ecad- (g) and sh-Src-H-2Kd-Ecad-II-4 (h) tumors at week 4. The mean ± SD of tumor volumes are shown (i). Growth curve of tumor volume demonstrated rapid development of H-2Kd-Ecad- and sh-Scrambled-H-2Kd-Ecad-II-4 tumors and growth inhibition of sh-FAK- and sh-Src-H-2Kd-Ecad-II-4 tumors (j). n≥3 for each cohort.
To determine how preventing the increase in FAK and Src activities induced by E-cadherin loss might alter tumor progression in vivo, tissues comprised of H-2Kd-Ecad-II-4 cells, sh-Scrambled-, sh-FAK- or sh-Src-H-2Kd-Ecad-II-4 cells were transplanted to the dorsum of nude mice. After 4 weeks, grafts harboring H-2Kd-Ecad-II-4 cells (Figure 3e) or sh-Scrambled-H-2Kd-Ecad-II-4 cells (Figure 3f) demonstrated large erythematous tumors with raised irregular borders and central areas of ulceration. In contrast, grafts harboring sh-FAK-H-2Kd-Ecad-II-4 cells (Figure 3g) slowly enlarge, while sh-Src-H-2Kd-Ecad-II-4 tumors remained as small exophytic nodular tumors (Figure 3h). Tumor volume measurements showed that H-2Kd-Ecad-II-4 or sh-Scrambled-H-2Kd-Ecad-II-4 tumors had ~4-fold increase in their volume when compared to sh-FAK- or sh-Src-H-2Kd-Ecad-II-4 tumors (Figure 3i). Tumor growth curves showed accelerated development of H-2Kd-Ecad-II-4 and sh-Scrambled-H-2Kd-Ecad-II-4 tumors compared to slower growth of sh-FAK- and sh-Src-H-2Kd-Ecad-II-4 tumors (Figure 3j).
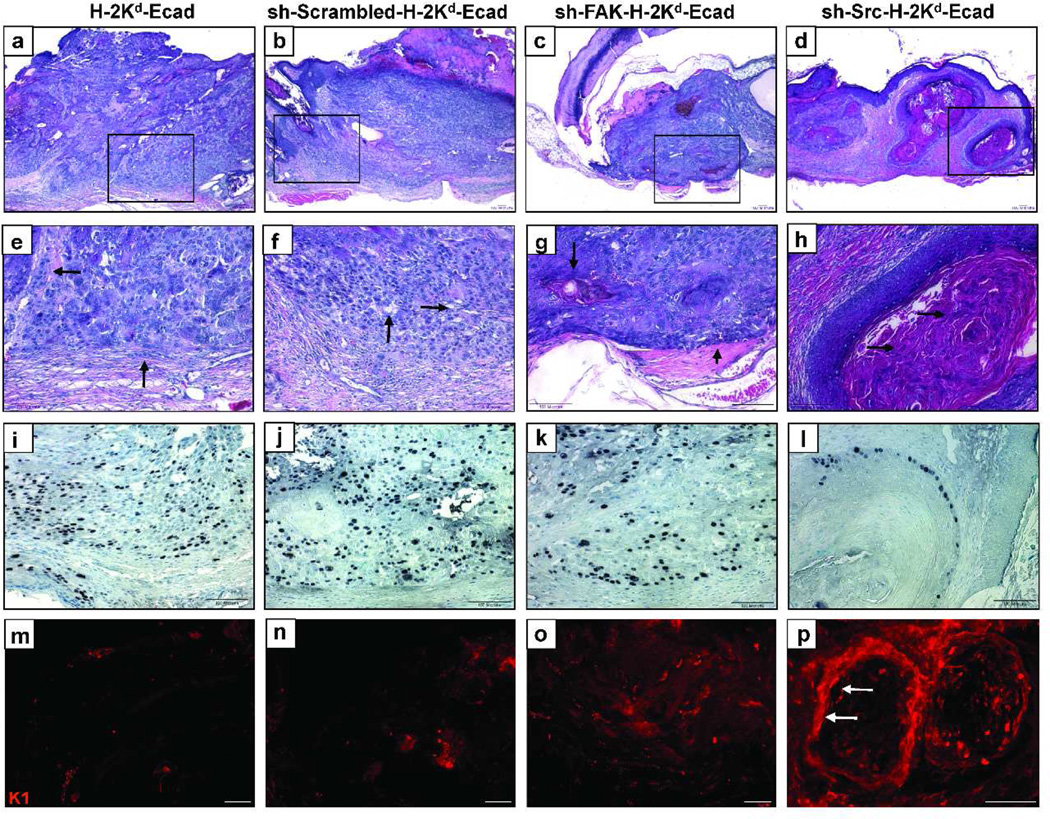
Next, tumor histology was analyzed by H&E staining, while tumor cell proliferation and the degree of tumor differentiation were determined using anti-Ki67 or anti-cytokeratin K1 antibodies, respectively. H-2Kd-Ecad-II-4 (Figure 4a, e) or sh-Scrambled-H-2Kd-Ecad-II-4 (Figure 4b, f) tumors revealed sheets of pleiomorphic poorly-differentiated cells, areas of widened intercellular gaps (Figure 4f, arrows), and cells that had separated from the tumor edge and infiltrated into the surrounding stroma (Figure 4e, arrows). This aggressive pattern of invasion was associated with the presence of Ki-67-positive tumor cells throughout the tumor cell masses (Figure 4i, j), and the absence of K1 (Figure 4m, n) indicated lack of cell differentiation in these tumors. In contrast, sh-FAK-H-2Kd-Ecad-II-4 tumors showed tumor cell sheets with well-defined margins opposing the stroma (Figure 4c, g, arrowhead). An increased degree of differentiation was evidenced by keratin pearls (Figure 4g, arrow), a more limited distribution of Ki-67-positive cells to the periphery of the tumors (Figure 4k), and scattered K1 staining in the inner area of the tumors (Figure 4o). sh-Src-H-2Kd-Ecad-II-4 tumors were affected to a greater degree as they revealed a switch to low-grade tumors showing demarcated, well-differentiated tumor islands with defined margins and central masses of keratin (Figure 4d, h, arrows). Ki-67-positive tumor cells were restricted to a basal position at the periphery of these tumor islands (Figure 4l), associated with a strata-specific K1 staining (Figure 4p, arrows). This tumor phenotype was similar to that previously described for E-cadherin-competent II-4 tumors (Margulis et al, 2005, 2006). These data show that E-cadherin suppression in Ras-expressing keratinocytes drives SCC development towards an aggressive tumor phenotype, at least in part, through up-regulation of FAK and of Src, which in turn, promote tumorigenesis.
Figure 4. E-cadherin suppressed H-2Kd-Ecad-II-4 cells exhibit a switch from high-grade to low-grade tumors upon down-regulation of FAK or Src.
Images of H&E (panels a–d and in higher magnification panels e–h), Ki67 immunohistochemistry (i–l), and K1 immunofluorescence staining (m–p) of tumor sections. H-2Kd-Ecad- (a, e) and sh-Scrambled-H-2Kd-Ecad-II-4 tumors (b, f) showed poorly differentiated, invasive cells (e, f, arrows) and Ki67-positive tumor cells (i, j), while K1 was undetected (m, n). sh-FAK-H-2Kd-Ecad-II-4 tumors (c, g) showed tumor cell differentiation (g, arrow), disperse K1 (o), and Ki67-positive cells that were more limited to the tumor invasive edge (k). Well-differentiated sh-Src-H-2Kd-Ecad-II-4 tumor islands (d, h) showed keratin pearls (h, arrows), stratum-specific K1 (p, arrows), and Ki67-positive tumor cells at the periphery of the tumor clusters (l). Bar, 100µm.
Prevention of the elevated activity of FAK or Src in H-2Kd-Ecad-II-4 cells restores E-cadherin expression and its stability in the plasma membrane
To reveal how prevention of the increased FAK and Src activities reverses the tumorigenic potential of H-2Kd-Ecad-II-4 cells, immunofluorescence analysis of E-cadherin in tissue sections was performed. sh-Scrambled-H-2Kd-Ecad-II-4 tissues showed a faint pattern of E-cadherin staining and cell invasion into the underlying matrix (Figure 5a, arrow, inset). While K1 was absent (Figure 5d), Ki-67-positive cells were seen throughout the epidermis (Figure 5g, arrowheads). In contrast, tissues harboring sh-FAK-H-2Kd-Ecad-II-4 cells showed increased E-cadherin staining at cell-cell borders (Figure 5b). K1 expression was found in suprabasal layers of the epidermis (Figure 5e), and proliferating Ki67-positive cells were more restricted to the basal and immediate suprabasal layers (Figure 5h), suggesting normalization of tissue phenotype. A more dramatic effect was seen in sh-Src-H-2Kd-Ecad-II-4 tissues that demonstrated restoration of E-cadherin localization at cell-cell borders (Figure 5c), increased K1 expression (Figure 5f), and restricted cell proliferation to the basal layer of the epidermis (Figure 5i). These findings link FAK and Src to the capacity of H-2Kd-Ecad-II-4 cells to acquire invasive properties and to undergo a transition from non-invasive to invasive tumor cell phenotype in 3D tissues. They also show that down regulation of FAK or Src in invasive epithelial tumor cells can block this process in 3D tissues by restoring a normalized tissue phenotype. These results indicate that the decrease in E-cadherin expression associated with the increase in FAK and Src activities plays a critical mechanistic role in directing the fate of H-2Kd-Ecad-II-4 cells and tumor progression in this tissue model system. To further investigate the effect of FAK and Src depletion on E-cadherin in sh-FAK- or sh-Src-H-2Kd-Ecad-II-4 cells, the protein and mRNA levels of E-cadherin in these cells were analyzed. E-cadherin expression was markedly decreased in sh-Scrambled-H-2Kd-Ecad-II-4 cells compared to sh-Scrambled-II-4 cells (Figure 5j). However, sh-RNA-targeting of FAK or of Src in H-2Kd-Ecad-II-4 cells resulted in restoration of E-cadherin expression to the level seen in sh-Scrambled-II-4 cells (Figure 5j), while no significant changes in E-cadherin mRNA levels were observed (Figure 5k).
Figure 5. Decreased FAK or Src in H-2Kd-Ecad-II-4 cells is associated with up-regulation and stabilization of E-cadherin protein leading to normalization of tissue phenotype.
Immunofluorescence staining of sh-Scrambled-H-2Kd-Ecad-II-4 tissue sections showed inconsistent E-cadherin staining and cell invasion (a, arrow, inset), and while K1 was not detected (d), Ki-67-positive cells appeared throughout the epidermis (g, arrowheads). sh-FAK-H-2Kd-Ecad-II-4 tissues showed intense E-cadherin staining (b), K1 expression (e) and Ki67-positive cells at the basal and immediate suprabasal layers (h). sh-Src-H-2Kd-Ecad-II-4 tissues revealed intense E-cadherin staining (c), elevated K1 (f), and Ki67-positive cells at the basal layer (i). Bar, 100µm. While an increase in E-cadherin protein was detected in sh-FAK- or sh-Src-H-2Kd-Ecad-II-4 cells (j), E-cadherin mRNA level was not significantly changed (k). Representative WB of E-cadherin in Cycloheximide-treated cultures (l), and the mean ± SD of the remaining E-cadherin in these cultures in 5 independent experiments (m).
Upon its removal from the cell membrane, E-cadherin is either targeted to degradation or recycled back to the cell membrane (Jeanes et al., 2008). To elucidate the possible mechanism underling the restoration of E-cadherin expression in sh-FAK- or sh-Src-H-2Kd-Ecad-II-4 cells, E-cadherin turnover was assayed by treating cultures with 10µM Cycloheximide in order to block de novo protein synthesis and to test if FAK and Src depletion affected E-cadherin stabilization at the plasma membrane. Immunoblotting revealed that compared to Cycloheximide-treated sh-Scrambled-II-4 cells, Cycloheximide-treated sh-Scrambled-H-2Kd-Ecad-II-4 cells showed an accelerated degradation of E-cadherin over 24 hours (Figure 5l). In contrast, E-cadherin was considerably more stable in Cycloheximide-treated sh-FAK-, or sh-Src-H-2Kd-Ecad-II-4 cells, and under these conditions its levels were higher than those of sh-Scrambled-II-4 cells (Figure 5l). Figure 5m illustrates the percentages of the remaining E-cadherin in the Cycloheximide-treated cultures over 24 hours relatively to the corresponding Cycloheximide-treated cultures at time 0. At the 24 hour time point, when compared to the remaining 71% of E-cadherin in sh-Scrambled-II-4 cells, E-cadherin level in sh-Scrambled-H-2Kd-Ecad-II-4 cells decreased to 41%, while its levels in sh-FAK-, or sh-Src-H-2Kd-Ecad-II-4 cells were 72% and 70%, respectively. Under these conditions the exogenous H-2Kd-Ecad fusion protein decreased similarly in sh-Scrambled-, sh-FAK- and sh-Src-H-2Kd-Ecad-II-4 cell lines (Supplementary Figure 4). These data indicate that down regulation of FAK and Src in sh-FAK-, and sh-Src-H-2Kd-Ecad-II-4 cells, respectively, contribute at least in part, to a decrease in E-cadherin degradation and an increase in E-cadherin stability in these cells.
DISCUSSION
This study reveals that loss of the suppressor of invasiveness, E-cadherin, drives the early stages of Ras-induced SCC progression through increased activation of FAK and Src that in turn, further directs destabilization of E-cadherin at the plasma membrane, thus enhancing its degradation. Elevated FAK and Src activities magnify the effect of E-cadherin suppression, promote tumor cell invasion in engineered tissues, and result in the progression to an aggressive carcinoma in vivo. Preventing FAK or Src up-regulation resulted in increased E-cadherin expression by enhancing its stability in cell-cell contacts, which at least in part, blocked the invasiveness of H-2Kd-Ecad-II-4 cells and directed them to a more normalized cell fate in 3D tissues. In vivo, these tissues showed reversion of an aggressive high-grade tumor phenotype to a low-grade, indolent tumor behavior. These data suggest a critical new mechanistic role for E-cadherin suppression in directing FAK and Src up-regulation as an important component of early SCC development.
We have previously shown that loss of E-cadherin-mediated adhesion in H-2Kd-Ecad-II-4 cells was linked to increased integrin-mediated adhesion (Zhang et al., 2006) and enhanced cell motility (Alt-Holland et al., 2008). This interdependence between loss of cell-cell- and gain of cell-matrix adhesions suggested a co-activation of regulatory events that could orchestrate and augment H-2Kd-Ecad-II-4 tumorigenic potential. FAK and Src that are linked to cadherins and integrins (Zhao & Guan, 2009; Mitra & Schlaepfer, 2006) and are involved in tumor cell adhesion, migration, and invasion (Summy & Gallick, 2003; Schlaepfer et al., 2004; Siesser & Hanks, 2006) emerged as candidates to regulate the behavior of these cells. FAK or Src up-regulation has been correlated with advanced stages of cancer progression (Ishizawar & Parsons, 2004; Jones, 2000). For example, elevated expression and activation of FAK has been linked to the progression of breast, colon, prostate, and oral cancers (Withers et al., 1996; Tremblay et al., 1996; Oktay et al., 2003; Schneider et al., 2002), and amplification of the FAK gene has been shown in invasive SCC (Agochiya et al., 1999). Increased Src activation has been associated with head and neck SCC, and breast cancers (Mandal et al., 2008; Reissig et al., 2001). In colon cancer, increased Src expression has been found in preneoplastic lesions and activating mutations are seen in metastatic foci (Irby et al., 1999). In this study, the use of in vitro 3D tissues and in vivo transplants that closely mimic the features of early stages of SCC in the human skin enabled us to analyze the fate of E-cadherin-suppressed tumor cells in which FAK or Src were either up-regulated or suppressed. Here we report that E-cadherin suppression in the incipient stages of SCC drives the up-regulation of FAK mRNA and protein levels and of FAK and Src activities above the level seen in E-cadherin-competent II-4 cells. This increase is necessary for the invasiveness of H-2Kd-Ecad-II-4 cells in 3D tissues, and consequently for the progression of premalignant tissues to aggressive carcinomas in vivo. A modest decrease of FAK or Src was sufficient to inhibit the invasiveness of these cells by restoration of E-cadherin at cell-cell borders and normalization of tissue phenotype. This, in turn, resulted in reversion of the aggressive tumor phenotype and inhibition of tumor growth in vivo. These data demonstrate the critical regulatory role of elevated FAK and Src activity in exacerbating the tumor-promoting effect of decreased E-cadherin expression during the early stages of SCC development.
How does increased activity of FAK and Src affect the motility of early stage E-cadherin-suppressed SCC tumor cells? H-2Kd-Ecad-II-4 cells showed increased phosphorylation of the autophosphorylation site of FAK (Tyr397), the activation domain of FAK (Tyr576/577), and FAK C-terminus domain (Tyr925). FAK phosphorylation at Tyr925 has been linked to focal adhesion turnover, cell spreading and motility (Katz et al., 2003). Thus, E-cadherin suppression in concert with increased FAK activity and FAK phosphorylation on Tyr925 can facilitate, at least in part, the augmented motility of H-2Kd-Ecad-II-4 cells. In fibroblasts, depletion of the β1 integrin (Raghavan et al., 2003) or of FAK (Ilic et al., 1995) resulted in increased numbers of focal contacts and inhibition of cell motility, whereas stable reconstitution of FAK in FAK-null fibroblasts promoted focal adhesion turnover and enhanced cell motility (Sieg, et al., 1999). In carcinoma cells, increased FAK expression was correlated with enhanced cell motility (Hauck et al., 2001), while depletion of FAK in keratinocytes inhibited tumor formation (McLean et al., 2004). Our study has extended these findings to show that E-cadherin loss in H-2Kd-Ecad-II-4 cells led to increased FAK and Src activity that consequently resulted in Src-mediated hyper-phosphorylation of FAK leading to enhance motility of these cells. In supporting this, exposure of H-2Kd-Ecad-II-4 cells to FAK or Src inhibitors blocked their augmented motility, and led to decreased phosphorylation of FAK and Src to a similar level seen in II-4 cells. Nevertheless, these findings do not exclude the possible activation and involvement of other Src family kinases, such as Fyn, in the augmented neoplastic phenotype of H-2Kd-Ecad-II-4 cells (Yadav et al., 2010).
How can up-regulation of Src and FAK exacerbate E-cadherin loss during the early stages of SCC? It has been shown that overexpression of active Src in the colorectal cancer cell line, KM12C, resulted in disruption of E-cadherin-mediated adhesion that was dependent on Src-mediated FAK phosphorylation (Avizienyte et al., 2002). Our study expands these observations by showing that suppression of E-cadherin function can also promote elevated Src activity and lead to similar consequences of Src overexpression. Active Src has also been shown to induce tyrosine phosphorylation of cadherin-catenin complexes or regulators of adherense junctions, resulting in E-cadherin endocytosis and disruption of cadherin-mediated cell-cell adhesions thus further enhancing tumor cell motility (Frame, 2004; Irby & Yeatman, 2002; Fujita et al., 2002; Jeanes et al, 2008). However, an alternative possibility to increased E-cadherin endocytosis in H-2Kd-Ecad-II-4 is that degradation and turnover of E-cadherin can occur also via MMP9-mediated E-cadherin ectodomain shedding from the plasma membrane that can be promoted by FAK or Src as seen in ovarian carcinoma cell lines (Cowden et al., 2008; Symowicz et al., 2007). Our findings suggest that as a result of E-cadherin suppression, elevated Src activity can advance the early stages of SCC by hyper-phosphorylation of FAK, and by further deregulating E-cadherin-mediated adhesions. The fact that Src suppression limits its accessibility to phosphorylate FAK and to further decrease E-cadherin stability in the plasma membrane can explain, at least in part, why sh-Src-H-2Kd-Ecad-II-4 tissues showed a more profound degree of normalization in vitro and a shift to a low-grade behavior in vivo compared to sh-FAK-H-2Kd-Ecad-II-4 tissues. The findings that E-cadherin suppression led to simultaneous activation of FAK and Src in H-2Kd-Ecad-II-4 cells suggest that the interaction between both kinases can amplify the tumorigenic potential of E-cadherin-suppressed tumor cells. They also imply that these kinases may exert complementary roles in regulating tumor cell invasiveness during the early stages of SCC development and the course of progression of this disease.
Collectively, our findings demonstrate an important new role for E-cadherin in the early stages of SCC development. Abrogation of E-cadherin-mediated adhesion in Ras-expressing early-stage human epithelial tumor cells induces elevated expression and/or activation of FAK and Src that work in concert to promote aggressive tumor cell behavior during incipient SCC development. Preventing the increase in FAK and of Src activities profoundly altered tumor outcome in vivo. As FAK and Src evolved as therapeutic targets for cancer invasion and metastasis (Brunton and Frame, 2005; McLean et al, 2005; Rucci et al, 2008) our findings suggest that the premalignant stages of SCC development may be inhibited by targeting these kinases in human epithelial cells with neoplastic potential. Moreover, by further understanding the events occurring in the progression of precancer to malignancy in clinically relevant, in vivo-like human tissues, new therapeutic approaches designed to block these events may be formulated to impair early cancer invasion thus preventing SCC development or reoccurrence.
MATERIALS AND METHODS
Cells
Human foreskin fibroblasts (HFF) were derived from newborn foreskins and grown in DMEM with 10% fetal bovine serum (FBS, HyClone, Thermo Scientific, Rockford, Il). HaCaT-II-4 keratinocytes (33) were grown in DMEM (Invitrogen, Carlsbad, CA) with 5% FBS. H-2Kd-Ecad-II-4 cells were generated by retroviral infection of HaCaT-II-4 cells as described (Margulis et al, 2005). II-4 and H-2Kd-Ecad-II-4 cells were infected with sh-Scrambled-, sh-FAK- or sh-Src-lentiviruses at a multiplicity of infection between 0.5 and 1. Stable cell lines were selected with Blasticidin (7µg/ml) (Invitrogen). Cells were grown at 37°C in 7.5% CO2. FAK or Src depletion was confirmed by WB analysis at the end of the selection process, during cell passages and before the fabrication of 3D tissues.
Construction of sh-FAK and sh-Src-mediated lentiviruses
Previously published siRNA sequences to knock down FAK (Tilghman et al., 2005; McLean et al, 2004) or Src (Trevino et al., 2006; Wu et al., 2008) were designed into oligonucleotides (IDT, San Diego, CA) with a loop sequence of tcaagag. These oligonucleotides were ligated into hairpin vectors using the BLOCK-iT Vector Kit (Invitrogen) and recombined into pLenti6-DEST (Invitrogen). Lentiviruses expressing shRNA against FAK, Src, or a scrambled sequence were titered using an end-point dilution assay.
Three-dimensional cell culture
Tissues harboring H-2Kd-Ecad-II-4 cells or sh-Scrambled-, sh-FAK or sh-Src-H-2Kd-Ecad-II-4 cells were generated as previously described (Margulis et al., 2005). Briefly, 6×105 tumor cells were seeded onto HFF-populated Type I collagen gels, in deep-well polycarbonate tissue culture inserts (Organogenesis, Canton, MA, USA). Cultures were maintained submerged for 3 days in low-calcium epidermal growth medium (EGM), and for 2 days in normal-calcium EGM before they were raised to an air-liquid interface for 7 days.
In vivo tissue transplantation
All animal experiments were performed according to a protocol approved by the Tufts University Institutional Animal Care and Use Committee. Briefly, a 1.3cm skin section from the dorsum surface of 6-week-old male Swiss nude mice (N:NIHS-nuf DF; Taconic farms, Germantown, NY, USA) was removed from groups of n≥3 per cell line, and tissues were transplanted at the excision site. Following bandages removal tumor volume was estimated using the formula Volume = Length × Width × Height. Mice were sacrificed 4 weeks after grafting, and tumors were excised and processes as described (Margulis et al., 2006).
Quantification of cell invasion
For each fabricated tissue, events of tumor cell invasion into the matrix were counted and averaged from >100 microscope fields in >20 sections from multiple experiments. Averages represent ≥ 3 independent experiments ± S.D. P-values were calculated by Mann-Whitney test.
E-cadherin protein turnover
Cell cultures were treated with either 10µM DMSO (vehicle) or Cycloheximide (Sigma, St Louis, MO, USA) for 0, 4, 8, 16 and 24hr, and cell lysates were subjected to WB analysis. Immunoblots of 3 independent experiments were quantified by densitometry, using Image J software (NIH). The percentage of the remaining E-cadherin was measured relative to treatment with DMSO and reported as a value relative to time 0.
Supplementary Material
Acknowledgements
We thank J. Edwards, Drs. M.W. Carlson and S. Dong for their assistance, Dr. N. Fusenig for HaCaT cells and their derivatives, Dr. F. Watt for the H-2Kd-Ecad vectors, and Dr. D. Jay for Fyn antibodies. This work was supported by NIDCR grant 2RO1DE011250-06 and DE017413 (to J.A.G) and NIGMS grant GM47717 (to L.A.F).
Footnotes
Conflict of Interest: The authors state no conflict of interest.
REFERENCE LIST
- Agochiya M, Brunton VG, Owens DW, et al. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene. 1999;18:5646–5653. doi: 10.1038/sj.onc.1202957. [DOI] [PubMed] [Google Scholar]
- Aligayer H, Boyd DD, Heiss MM, et al. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- Alt-Holland A, Shamis Y, Riley KM, et al. E-cadherin suppression directs cytoskeletal rearrangement and intraepithelial tumor cell migration in 3D human skin equivalents. J Invest Dermatol. 2008;128:2498–2507. doi: 10.1038/jid.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt-Holland A, Zhang W, Margulis A, et al. Microenvironmental control of premalignant disease: The role of intracellular adhesion in the progression of squamous cell carcinoma. Semin Cancer Biol. 2005;15:84–96. doi: 10.1016/j.semcancer.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Avizienyte E, Wyke AW, Jones RJ, et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nat Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- Bagutti C, Speight PM, Watt FM. Comparison of integrin, cadherin, and catenin expression in squamous cell carcinomas of the oral cavity. J Pathol. 1998;86:8–16. doi: 10.1002/(SICI)1096-9896(199809)186:1<8::AID-PATH156>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15–30. doi: 10.1023/a:1006200102166. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer B, Bourgeois Y, Poupon MF. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 2002;21:2347–2356. doi: 10.1038/sj.onc.1205298. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Stanbridge EJ, Foo DY, et al. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990;50:2840–2847. [PubMed] [Google Scholar]
- Brunton VG, Frame MC. Src and focal adhesion kinase as therapeutic target in cancer (2008) Curr Opin Pharmaco. 2008;8:427–432. doi: 10.1016/j.coph.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Cowden Dahl KD, Symowicz J, Ning Y, et al. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent E-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68:4606–4613. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Crosstalk between different adhesion molecules. Curr Opin Cell Biol. 2006;18:572–578. doi: 10.1016/j.ceb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Zhurinsky J, Ben Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz A, Merlino G, Yuspa SH. Progress in cutaneous cancer research. J Investig Dermatol Symp Proc. 2002;7:17–26. doi: 10.1046/j.1523-1747.2002.19631.x. [DOI] [PubMed] [Google Scholar]
- Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Sieg DJ, Hsia DA, et al. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 2001;61:7079–7090. [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Irby RB, Mao W, Coppola D, et al. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- Irby RB, Yeatman TJ. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer Res. 2002;62:2669–2674. [PubMed] [Google Scholar]
- Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Brunton VG, Frame MC. Adhesion-linked kinases in cancer; emphasis on src, focal adhesion kinase and PI 3-kinase. Eur J Cancer. 2000;36:1595–1606. doi: 10.1016/s0959-8049(00)00153-2. [DOI] [PubMed] [Google Scholar]
- Katz BZ, Romer L, Miyamoto S, et al. Targeting membrane-localized focal adhesion kinase to focal adhesions: roles of tyrosine phosphorylation and SRC family kinases. J Biol Chem. 2003;278:29115–29120. doi: 10.1074/jbc.M212396200. [DOI] [PubMed] [Google Scholar]
- Mandal M, Myers JN, Lippman SM, et al. Epithelial to mesenchymal transition in head and neck squamous carcinoma: association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer. 2008;112:2088–2100. doi: 10.1002/cncr.23410. [DOI] [PubMed] [Google Scholar]
- Margulis A, Zhang W, Alt-Holland A, et al. E-cadherin suppression accelerates squamous cell carcinoma progression in three-dimensional, human tissue constructs. Cancer Res. 2005;65:1783–1791. doi: 10.1158/0008-5472.CAN-04-3399. [DOI] [PubMed] [Google Scholar]
- Margulis A, Zhang W, Alt-Holland A, et al. Loss of intercellular adhesion activates a transition from low- to high-grade human squamous cell carcinoma. Int J Cancer. 2006;118:821–831. doi: 10.1002/ijc.21409. [DOI] [PubMed] [Google Scholar]
- McLean GW, Carragher NO, Avizienyte E, et al. The role of focal adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- McLean GW, Komiyama NH, Serrels B, et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Oktay MH, Oktay K, Hamele-Bena D, et al. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum Pathol. 2003;34:240–245. doi: 10.1053/hupa.2003.40. [DOI] [PubMed] [Google Scholar]
- Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Vaezi A, Fuchs E. A role for alphabeta1 integrins in focal adhesion function and polarized cytoskeletal dynamics. Dev Cell. 2003;5:415–427. doi: 10.1016/s1534-5807(03)00261-2. [DOI] [PubMed] [Google Scholar]
- Reissig D, Clement J, Sanger J, et al. Elevated activity and expression of Src-family kinases in human breast carcinoma tissue versus matched non-tumor tissue. J Cancer Res Clin Oncol. 2001;127:226–230. doi: 10.1007/s004320000197. [DOI] [PubMed] [Google Scholar]
- Roskoski R. Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Rucci N, Susa M, Teti A. Inhibition of protein kinase C-Src as a therapeutic approach for cancer and bone metastasis. Anticancer Agents Med Chem. 2008;8:342–349. doi: 10.2174/187152008783961905. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Schneider GB, Kurago Z, Zaharias R, et al. Elevated focal adhesion kinase expression facilitates oral tumor cell invasion. Cancer. 2002;95:2508–2515. doi: 10.1002/cncr.10992. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res. 2006;12:3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- Symowicz J, Adley BP, Gleason KJ. Engagement of collagen binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2008;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- Tilghman RW, Slack-Davis JK, Sergina N, et al. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J Cell Sci. 2005;118:2613–2623. doi: 10.1242/jcs.02380. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Hauck W, Aprikian AG, et al. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68:164–171. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Trevino JG, Gray MJ, Nawrocki ST, et al. Src activation of Stat3 is an independent requirement from NF-kappaB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis. 2006;9:101–110. doi: 10.1007/s10456-006-9038-9. [DOI] [PubMed] [Google Scholar]
- Withers BE, Hanks SK, Fry DW. Correlations between the expression, phosphotyrosine content and enzymatic activity of focal adhesion kinase, pp125FAK, in tumor and nontransformed cells. Cancer Biochem Biophys. 1996;15:127–139. [PubMed] [Google Scholar]
- Wu L, Bernard-Trifilo JA, Lim Y, et al. Distinct FAK-Src activation events promote alpha5beta1 and alpha4beta1 integrin-stimulated neuroblastoma cell motility. Oncogene. 2008;27:1439–1448. doi: 10.1038/sj.onc.1210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V, Denning MF. Fyn is induced by Ras/PI3K/Akt signaling and is required for enhanced invasion/migration. Mol Carcinog. 2011;50:346–352. doi: 10.1002/mc.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Crampton MS, Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol. 2007;19:508–514. doi: 10.1016/j.ceb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Alt-Holland A, Margulis A, et al. E-cadherin loss promotes the initiation of squamous cell carcinoma invasion through modulation of integrin-mediated adhesion. J Cell Sci. 2006;119:283–291. doi: 10.1242/jcs.02738. [DOI] [PubMed] [Google Scholar]
- Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.