Abstract
Objectives
The intestinal mucosal barrier is important to protect the body from the large numbers of microbes that inhabit the intestines and the molecules they release. Intestinal barrier function is impaired in humans with cystic fibrosis (CF), including reduced activity of the lipopolysaccharide detoxifying enzyme intestinal alkaline phosphatase (IAP) and increased permeability. The objective of this study was to determine the suitability of using the CF mouse to investigate intestinal barrier function, and whether interventions that are beneficial for the CF mouse intestinal phenotype (antibiotics or laxative) would improve barrier function. Also tested were the effects of exogenous IAP administration.
Methods
The Cftrtm1UNC mouse was used. IAP expression (encoded by the murine Akp3 gene) was measured by qRT-PCR and enzyme activity. Intestinal permeability was assessed by measuring rhodamine dextran plasma levels following gavage.
Results
CF mice had 40% Akp3 mRNA expression and 30% IAP enzyme activity, as compared to wild type mice. Oral antibiotics and laxative treatments normalized Akp3 expression and IAP enzyme activity in the CF intestine. CF mice had a 5-fold greater transfer of rhodamine dextran from gut lumen to blood. Antibiotic and laxative treatments reduced intestinal permeability in CF mice. Administration of exogenous purified IAP to CF mice reduced intestinal permeability to WT levels and also reduced small intestinal bacterial overgrowth by more than 80%.
Conclusions
The CF mouse intestine has impaired mucosal barrier function, similar to human CF. Interventions that improve other aspects of the CF intestinal phenotype (antibiotics and laxative) also increased IAP activity and decreased intestinal permeability in CF mice. Exogenous IAP improved permeability and strongly reduced bacterial overgrowth in CF mice, suggesting this may be a useful therapy for CF.
Keywords: permeability, intestinal alkaline phosphatase, Akp3, Akp6, antibiotics, laxative, bacterial overgrowth
INTRODUCTION
Cystic fibrosis (CF) is one of the most common life-shortening genetic diseases in Caucasians (www.CFF.org). In the absence of functional CFTR anion channel, affected epithelial surfaces are poorly hydrated and more acidic than normal (1). The altered luminal environment of the affected epithelial organs impairs turnover and clearance of the normally protective mucus layer (2) and this situation fosters abnormal bacterial colonization (3). The major cause of mortality in CF is chronic airway infection that leads to progressive damage and eventual respiratory failure. In addition, CF affects other organs with important consequences for health and longevity, particularly the gastrointestinal system.
The intestinal tract is involved early in life in CF, and long term gut dysfunction in CF is apparent as malnutrition. Poor nutrition in CF is strongly associated with airway disease severity and progression (4-7). A major factor of malnutrition in CF is exocrine pancreatic insufficiency. However, even with optimal pancreatic enzyme therapy, nutrition is often not fully corrected (8; 9). Also, despite the fact that gene targeted mouse models of CF are pancreatic sufficient (10; 11) their major phenotype is in the intestines and they exhibit poor body weight gain (12). These facts suggest there are functional defects in the CF small intestine where digestion and absorption occur.
The CF small intestine exhibits mild inflammation and structural changes to the mucosa (13-15) which may affect digestive and absorptive functions. Such changes may be due to dysbiosis of the normal enteric microbiota [altered bacterial composition and/or small intestinal bacterial overgrowth (SIBO)]. Microbial dysbiosis is a likely consequence of abnormal mucus clearance in the CF intestine. In CF mice, SIBO occurs with colonic type bacteria that colonize the accumulated mucus (16; 17), and CF mice are more susceptible to colonization with pathogenic bacteria (18). Although the evidence is less direct, microbial dysbiosis is likely also common in human CF (19-22). Additionally, the mucosal barrier function of the intestine is compromised in CF patients as shown by enhanced urinary excretion of orally administered permeability markers, and elevated levels of serum albumin in the intestinal lumen (15; 23-26). Increased permeability is expected to allow passage of danger signals such as the bacterial component lipopolysaccharide (LPS) from the intestinal lumen into the mucosa, which can trigger inflammatory reactions (27).
Besides the physical barrier comprised of the lining epithelium and its mucus covering, there are other mechanisms that contribute to the mucosal barrier function. One of these is the enzyme intestinal alkaline phosphatase (IAP) which protects against LPS from Gram negative bacteria. IAP dephosphorylates and thereby detoxifies LPS (28). IAP activity in biopsies of human CF duodenum is decreased by 20-60% of normal levels (29; 30). This is expected to reduce the ability of the CF intestine to detoxify LPS.
We used the Cftr knockout mouse (Cftrtm1unc, CF mouse) to determine if this mouse is a good model to investigate intestinal mucosal barrier function in CF. We also tested the hypothesis that interventions known to ameliorate the CF intestinal phenotype would improve the barrier function. The CF mouse has many similarities to human CF with respect to effects on the small intestine. These include excessive luminal mucus accumulation (31; 32), SIBO (16; 17; 20; 31), altered innate defenses (18; 33), and poor weight gain (9; 16). The CF intestinal phenotype in mice is significantly improved by eradication of SIBO using oral administration of broad spectrum antibiotics (16; 31) or by improving the hydration of the gut lumen with oral osmotic laxative (34). In this work we tested whether the intestinal mucosal barrier function could be improved by antibiotic or laxative treatments, and we also investigated the effects of inhibiting endogenous IAP or supplementation with exogenous IAP on permeability and bacterial load in the small intestine.
MATERIALS AND METHODS
Materials
Unless otherwise specified, all reagents were from Sigma (St. Louis MO USA).
Animals
Cftrtm1UNC+/- mice were originally obtained from the Jackson Laboratories (Bar Harbor ME USA). These mice have been bred onto the C57BL/6J background until congenic. They are periodically backcrossed with wild type (WT) C57BL/6J mice to prevent genetic drift of our colony and mice used in this study were at generation 31 of backcrossing. Cftrtm1UNC+/- mice were bred to obtain Cftrtm1UNC-/- (CF) and Cftrtm1UNC+/+ (WT) mice. Mice aged 6-12 weeks and of both genders were used; no gender differences in the measured parameters were observed in this study. Cftrtm1UNC+/- mice are phenotypically normal and were used occasionally when needed as WTs; none of the parameters measured in this study were different between Cftr homozygous wild type and Cftr heterozygous mice. Unless otherwise indicated, WT and CF mice were fed a liquid diet (Peptamen, Nestle Nutrition, Florham Park NJ USA) from weaning which prevents lethal intestinal obstruction in CF mice. Some mice received broad spectrum antibiotics added to the liquid diet (ciprofloxacin, 0.05 mg/ml; metronidazole, 0.5 mg/ml) as previously described (16). Some mice received purified calf intestinal alkaline phosphatase (Lee Biosolutions, St. Louis MO USA) (35-38), at 13.3 U/ml in the liquid diet. Another group of mice received the AP selective inhibitor L-phenylalanine (L-Phe) (39), at 10 mM in the liquid diet. Another group of mice was maintained on standard mouse chow and given an osmotic laxative (Colyte® formulation) in their drinking water (40). Before sacrifice, all mice were fasted overnight (<16 hr) with free access to water (supplemented with L-Phe as appropriate) or laxative solution as appropriate. All animal use was submitted to and approved by the University of Kansas Medical Center IACUC.
IAP histochemistry
Intestinal tissue was fixed in 4% paraformaldehyde overnight followed by paraffin embedding, sectioning, deparaffinization, and rehydration in saline. For conventional histochemistry of IAP, slides were incubated in 0.1 M Tris-HCl, pH 9.5, 5 mM MgCl2, 0.1M NaCl containing 0.19 mg/ml 5-bromo-4-chloro-3-indolyl-phosphate and 0.5mg/ml nitroblue tetrazolium. WT and CF samples were processed in parallel using identical conditions and times of incubation. For histochemistry using LPS as substrate, slides were processed according to (28). Briefly, slides were incubated with 50 μg/ml LPS and lead nitrate at pH 7.6, plus or minus the selective inhibitor of IAP L-Phe (10 mM) (28). The lead precipitate was converted to a visible product with ammonium sulfide.
qRT-PCR
The entire small intestine was flushed with ice cold saline and the mesentery was trimmed off. The tissue was then processed with TRIzol (Invitrogen, Carlsbad, CA USA) to isolate total RNA as previously described (16). Real time qRT-PCR was performed with an iCycler instrument (Bio-Rad, Hercules CA USA) with a one-step RT-PCR kit (Qiagen, Valencia, CA USA). The following primers were used for Akp3, the mouse IAP gene: forward 5'-CAT GGA CCG CTT CCC ATA-3' and reverse 5'-CTT GCA CTG TCT GGA ACC TG-3', product = 72 bp; and for Akp6: forward 5'-AGG ATC CAT CTG TCC TTT GGT-3' and reverse 5'-CAG CTG CCT TCT TGT TCC A-3', product = 73 bp. The mRNA for ribosomal protein L26 (Rpl26) was used as a housekeeping gene for normalization as previously described (34). Expression levels were calculated using the ΔΔCt method after correcting for differences in PCR efficiencies, and were expressed relative to WT control levels.
Enzyme activity measurement
Tissues were homogenized by sonication on ice in 10 mM Tris, pH 7.0, plus protease inhibitors at 10 ml buffer per gram wet weight of tissue. Alkaline phosphatase activity was measured using di-Tris p-nitrophenyl phosphate (18 mM final) as substrate in 0.1M Tris-HCl, pH 10, 0.1 mM MgCl2, 0.1M NaCl buffer (41). The reaction was measured at 405 nm using zero order kinetics on a Synergy HT microplate reader (BioTek, Winooski VT USA) at 30°C. Where indicated, the IAP selective inhibitor L-Phe was included in the assay. Alkaline phosphatase activity data are presented as μmol product (p-nitrophenol) per min, normalized to DNA content of the homogenates. DNA was measured using a fluorometric assay (42).
Western blot of serum albumin
Mice were fasted overnight with free access to water. The next morning mice were sacrificed, the entire small intestine was removed and lavaged with 5 ml ice cold saline. The lavaged fluid was centrifuged to pellet debris and the supernatant was saved. Equal volumes of supernatant (12 μl) were separated on 7.5% SDSPAGE and transferred to PVDF membrane. The membranes were probed with an antibody to mouse serum albumin (#ab19194; Abcam.com).
Intestinal permeability measured in vivo
Mice were fasted overnight with free access to water or laxative solution as appropriate. In the morning, they were gavaged with 0.1 ml solution of 1.5% methylcellulose (to mimic the viscosity of digesta) in saline with 25 mg/ml rhodamine-dextran (70 kDa). Ninety minutes later mice were sacrificed and blood collected in EDTA-tubes. The samples were centrifuged and plasma collected. The fluorescence in plasma samples was measured on the plate reader and concentrations of rhodamine dextran in plasma was determined using a standard curve of known concentrations of rhodamine-dextran.
Estimation of bacterial load
The bacterial 16S rRNA gene was used as an estimate of bacterial load in the small intestine as previously described (34). Briefly, mice were fasted overnight with free access to water. The small intestine was resected and flushed with PBS containing the mucolytic agent dithiothreitol (10 mM). The flushed material was centrifuged and the pellet was processed to extract bacterial DNA, using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA, USA) with minor modifications as previously reported (16). The DNA was used to amplify the bacterial 16S rRNA gene with universal primers by real-time PCR and quantified by comparison to a standard curve of known amounts of a cloned PCR 16S product (16).
Data analysis
Data are expressed as means ± SE and the number of animals used for each group is given in the figure legends. Statistical analysis was by ANOVA with posthoc Tukey's test using Systat software (SPSS Inc, Chicago IL USA).
RESULTS
IAP activity on the brush border membrane is decreased in the CF intestine
IAP is a brush border enzyme, most heavily expressed in the proximal small intestine (43). We performed histochemical staining for IAP using conventional reaction conditions (5-bromo-4-chloro-3-indolyl-phosphate as substrate at pH 9.5, which when cleaved by alkaline phosphatase precipitates nitroblue tetrazolium). In the WT mouse the reaction product was strong in the duodenum (Fig.1A) on the brush border surface (Fig.1A'), as expected. Under identical reaction conditions and time of development using CF tissue, the reaction product was still localized to the brush border surface but was noticeably weaker (Fig.1B, B') as compared to WT control (Fig.1A). To verify others’ work that IAP can use LPS as a substrate, we also performed the reaction at physiological pH (7.6) using LPS as substrate. Again, the product in WT intestine was on the brush border surface (Fig.1C, C'). We also used the selective IAP inhibitor L-Phe (10 mM) (44) to demonstrate specificity of the reaction using LPS as substrate. As shown in Fig.1D, L-Phe totally inhibited formation of reaction product in WT intestinal tissue with LPS as substrate.
1. Histochemistry of alkaline phosphatase activity in WT and CF duodenum.
(A) WT duodenum with bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium as substrate at pH 9.5. (A') Inset showing brush border surface of villus. (B) CF duodenum with bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium as substrate at pH 9.5. (B') Inset showing brush border surface of villus. WT and CF samples were processed in parallel using identical conditions and times of incubation. (C) WT duodenum with LPS and lead nitrate as substrate at pH 7.6. (D) WT duodenum with LPS and lead nitrate as substrate at pH 7.6 plus 10 mM L-Phe (IAP-selective inhibitor).
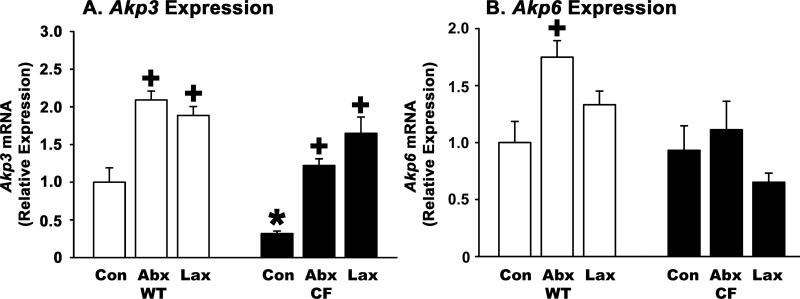
CF mice have decreased IAP (Akp3) gene expression and interventions that improve the CF phenotype increase IAP expression
The gene encoding intestinal alkaline phosphatase is Akp3 and we measured its mRNA levels by qRT-PCR. As shown in Fig.2A, CF control mice express less than a third as much Akp3 as do WT controls. To further test whether Akp3 expression is associated with the CF intestinal phenotype we used interventions previously shown to improve intestinal function in CF mice. One of these is oral administration of broad spectrum antibiotics which eradicates SIBO and improves several aspects of the CF phenotype (16; 31). When CF mice were treated with antibiotics, there was a 3.8-fold increase in Akp3 expression as compared to CF controls (Fig.2A). When WT mice were treated with antibiotics there was a 2.1-fold increase in Akp3 mRNA expression as compared to WT controls (Fig.2A).
2. Akp3 and Akp6 gene expression levels in WT and CF small intestine and effects of interventions.
Total RNA was used for qRT-PCR using (A) Akp3 and (B) Akp6 gene specific primers. The ribosomal protein 26 (Rpl26) mRNA was used as a housekeeping gene and data were calculated by the ΔΔCt method with correction for differential PCR efficiencies (see Methods and Materials). Con: Control, mice were fed the liquid diet; Abx: Mice were fed the liquid diet supplemented with antibiotics; Lax: Mice were fed standard solid chow and given laxative solution to drink. (*) p=0.009 CF control vs. WT control; (+) p<0.04 treated vs. control of same genotype. (n=9 WT Con, 11 WT Abx, 12 WT Lax, 9 CF Con, 8 CF Abx, 6 CF Lax)
Another intervention is use of oral osmotic laxative (Colyte® formulation) that better hydrates the gut lumen, preventing intestinal obstruction and allowing CF mice to be maintained on standard solid chow (40). Laxative treatment was shown to improve several aspects of the CF intestinal phenotype (34), so we tested its effects on Akp3 gene expression. Treatment of CF mice with laxative increased Akp3 expression more than 5-fold as compared to CF controls (Fig.2A). When WT mice were maintained on laxative there was a 1.9-fold increase in Akp3 expression as compared to WT controls (Fig.2A).
Recently, there has been discovered a second intestine specific alkaline phosphatase gene in mice, Akp6, whose expression is increased in Akp3 knockout mice (43). To see if there are any compensatory changes in Akp6 expression in control CF mice or after experimental interventions, we measured its expression levels by qRT-PCR. In control WT mouse small intestine, Akp6 expression was 6.5-fold less than that of Akp3, based on comparison of Ct values; the PCR efficiencies for the two genes were identical (data not shown). There was no difference in Akp6 expression comparing control CF to control WT mice (Fig.2B). After treatment with antibiotics, there was a small but significant increase in Akp6 expression in treated WT mice as compared to control (Fig.2B). Antibiotic treatment did not change Akp6 expression in CF mice (Fig.2B). In mice treated with laxative, there was not a significant change in Akp6 expression in either WT or CF mice (Fig.2B).
CF mice have decreased IAP enzyme activity and interventions that improve the CF phenotype increase IAP activity
We next measured IAP enzyme activity to compare expression levels of Akp3 mRNA to actual enzyme activity. Specific IAP activity was considered to be that which was sensitive to inhibition by 10 mM L-Phe (44). As expected, IAP activity was strongest in the proximal small intestine, with very little activity in the WT mouse after the first tenth of the intestine (Fig.3A). IAP activity in the CF intestine was also similarly localized to the first tenth of the intestine, but it was less than a third of the WT control activity (Fig.3A), essentially the same as the respective mRNA levels. In CF mice treated with antibiotics, IAP enzyme levels were now the same as in WT control mice (Fig.3B), consistent with the increase in Akp3 mRNA in antibiotic treated CF mice (Fig.2A). Antibiotic treatment of WT mice did not significantly affect IAP enzyme levels as compared to WT controls (Fig.3B). Laxative treatment of CF mice increased IAP activity to a level comparable to that of control WT mice (Fig.3C), similar to the effect of laxative on Akp3 mRNA in the CF intestine (Fig.2A). When WT mice were treated with laxative, there was a small decrease in IAP activity as compared to WT controls (Fig.3C), in contrast to the significant increase observed in Akp3 mRNA levels in laxative treated WT mice (Fig.2A).
3. IAP enzyme activity distribution in WT and CF small intestine and effects of interventions.
The small intestine was divided into 10 equal segments from (1) duodenum to (10) ileum. Homogenates were prepared and used to measure IAP activity (alkaline phosphatase activity sensitive to 10 mM L-Phe). (A) Control WT and CF samples; mice were fed the liquid diet. (B) WT and CF samples from antibiotic treated mice; mice were fed the liquid diet supplemented with antibiotics. (C) WT and CF samples from laxative treated mice; mice were fed standard solid chow and given laxative solution to drink. (*) p=0.023 CF control vs. WT control for the first segment of the small intestine. (+) p=0.0025 CF Abx vs. CF control for the first segment of the small intestine. (n=6 mice for each group and genotype)
To see if a non-IAP alkaline phosphatase (AP) activity was increased in the control CF intestine or after the specific interventions, we also calculated the L-Phe insensitive activity. As shown in Fig.4, there was very little L-Phe insensitive activity in either WT or CF small intestine, under either control or experimental conditions. Because the enzyme encoded by Akp6 has not been characterized biochemically, it is not known if this enzyme is L-Phe sensitive, and we could not investigate its enzymatic activity in WT and CF mice.
4. Non-IAP alkaline phosphatase enzyme activity distribution in WT and CF small intestine and effects of interventions.
The small intestine was divided into 10 equal segments from duodenum to ileum. Homogenates were prepared and used to measure non-IAP activity (alkaline phosphatase activity that was insensitive to 10 mM L-Phe). (A) Control WT and CF samples; mice were fed the liquid diet. (B) WT and CF samples from antibiotic treated mice; mice were fed the liquid diet supplemented with antibiotics. (C) WT and CF samples from laxative treated mice; mice were fed standard solid chow and given laxative solution to drink. (n=6 mice for each group and genotype)
CF mice have increased intestinal permeability and interventions that improve the CF phenotype decrease permeability
As an indication of increased intestinal permeability, the presence of serum albumin in the luminal content of the small intestine was assessed by Western blot. As shown in Fig.5, there was more immunoreactive serum albumin in intestinal lavage fluid from CF mice as compared to WT. Note that there were several reactive bands smaller than the expected size of intact serum albumin, indicating proteolytic activity in the lumen of the intestine of both WT and CF mice, which are pancreatic sufficient. When this antibody was used on mouse plasma a single immunoreactive band at the expected location was observed (data not shown). To determine intestinal permeability more quantitatively, we measured passage of a nondigestible fluorescent tracer (rhodamine-dextran) from the intestine into the blood circulation. Mice were fasted overnight to empty the gastrointestinal tract followed by gavage of the tracer into the stomach. After 90 min the mice were sacrificed and blood was collected to measure levels of fluorescence as an indicator of intestinal permeability. Fluorescence in the plasma of CF mice was about 5-fold greater than wild type (WT) mice (Fig.6).
5. Serum albumin in the small intestinal lumina of WT and CF mice.

The small intestines of 3 WT and 3 CF mice were lavaged with 5 ml saline and 12 μl of the lavage fluids were separated on 7.5% SDS-PAGE and transferred to PVDF. The membrane was probed with an antibody to mouse serum albumin. (*) Size of intact serum albumin (66 kDa). There is more serum albumin immunoreactivity in the CF samples. (n=3 WT and 3 CF mice).
6. Intestinal permeability of WT & CF mice and effects of interventions.
Mice were fasted overnight and in the morning gavaged with the indigestible tracer rhodaminedextran. After 90 min the mice were killed and the concentration of rhodamine in blood plasma was determined. Con: Control, mice were fed the liquid diet; Abx: Mice were fed the liquid diet supplemented with antibiotics; Lax: Mice were fed standard solid chow and given laxative solution to drink; IAP: Mice were fed the liquid diet supplemented with 13.3 U/ml purified calf IAP; L-Phe: Mice were fed the liquid diet supplemented with 10 mM L-Phe. (*) p=0.00001 CF Con vs WT Con; (+) p=0.040 CF Lax vs CF Con; (+) p= 0.00025 CF IAP vs CF Con; (*) p=0.024 CF L-Phe vs WT L-Phe (n=16 WT Con, 6 WT Abx, 6 WT Lax, 6 WT IAP, 9 WT L-Phe, 15 CF Con, 7 CF Abx, 5 CF Lax, 6 CF IAP, 4 CF L-Phe)
When CF mice were treated with broad spectrum antibiotics to eradicate SIBO, plasma fluorescence after gavage was reduced as compared to CF controls and this difference was borderline significant (p=0.07 vs CF control). The plasma fluorescence of antibiotic treated CF mice was not significantly different compared to WT mice (p=0.22 vs antibiotic treated WT) (Fig.6). Antibiotic treatment of WT mice did not affect plasma fluorescence after gavage compared to WT control (Fig.6).
We next tested the effect of laxative, which aids normal hydration of the CF gut lumen and improves several aspects of the CF intestinal phenotype, for potential effects on intestinal permeability. When CF mice were treated with laxative, the amount of plasma fluorescence after gavage was less than one half that of CF controls (p=0.045; Fig.6). This value was not significant as compared to WT plasma fluorescence (p=0.73 vs. laxative treated WT; Fig.6). When WT mice were treated with oral laxative solution there was no change in plasma fluorescence as compared to WT control (Fig.6).
IAP is not directly related to intestinal permeability
To gain insight into whether IAP has a direct role in intestinal permeability, two experimental manipulations were used. First, exogenous purified calf IAP was added to the liquid diet for 3 weeks followed by measurement of intestinal permeability. Exogenous IAP has been shown to be protective in experimental colitis (35; 37) and necrotizing enterocolitis (38), as well as in human patients with inflammatory bowel disease (36). In WT mice exogenous IAP caused a non-significant decrease in permeability (Fig.6). In CF mice, there was a significant decrease in permeability after IAP treatment, and the level of fluorescence in the plasma was not significantly different from the WT group (p=0.79 vs WT IAP treated) (Fig.6).
Second, to test whether loss of IAP activity results in increased permeability, endogenous IAP activity was inhibited by addition of the IAP-selective inhibitor L-Phe to the liquid diet for 3 weeks. Previous work showed that oral L-Phe inhibited IAP activity and increased the severity of experimental colitis (39). In mice treated with L-Phe there was not a significant change in permeability in either WT or CF mice (Fig.6). Interestingly, 2 of the 6 CF mice administered L-Phe died within 1 week of starting the treatment, whereas none of the 9 L-Phe treated WT mice died. The postweaning death rate of CF control mice on Peptamen is less than 1/5 that observed in the L-Phe treated mice; only 2 of 31 CF control mice in recent litters died after weaning to Peptamen, and no WT mice died.
Exogenous IAP affects bacterial load
Because it was recently reported that the fecal microbiota is altered in IAP-deficient Akp3 knockout mice (45) and CF mice have small intestinal bacterial overgrowth, it was of interest to measure bacterial load in the small intestine after experimentally manipulating IAP levels. In WT mice treated with oral exogenous IAP, there was not a significant effect on the normal low bacterial load in the small intestine (Fig.7). In contrast, in IAP treated CF mice, where was more than 80% decrease in bacterial load (Fig.7). Administration of the IAP inhibitor L-Phe had no significant effect on bacterial load in either WT or CF mice (Fig.7).
7. Bacterial overgrowth of the CF mouse small intestine and effects of exogenous IAP and L-Phe.
The small intestine was resected and flushed with PBS containing a mucolytic (10 mM dithiothreitol). The flushed fluid was centrifuged and the pellets processed to extract bacterial DNA for realtime PCR of the bacterial 16S gene. The copy numbers of the bacterial 16S rRNA gene were used to estimate bacterial load per intestine. Con: Control, mice were fed the liquid diet; IAP: Mice were fed the liquid diet supplemented with 13.3 U/ml purified calf IAP; L-Phe: Mice were fed the liquid diet supplemented with 10 mM L-Phe. (*) p<0.0001 CF vs corresponding WT; (+) p=0.00027 CF L-Phe vs CF Con (n=5 WT Con, 6 WT IAP, 10 WT L-Phe, 6 CF Con, 6 CF IAP, 4 CF L-Phe)
DISCUSSION
In this work we set out to determine if the CF mouse was a suitable model to investigate altered mucosal barrier function of the CF intestine; and whether interventions known to improve the CF intestinal phenotype would also improve barrier function. We demonstrate that CF mice have impairments of the intestinal mucosal barrier similar to that reported in human CF patients. We show that oral broad spectrum antibiotics or osmotic laxative, interventions that improve the CF intestinal phenotype, also improve mucosal barrier function. Also, administration of exogenous IAP to CF mice improved intestinal permeability as well as reducing small intestinal bacterial overgrowth more than 80%.
Investigation of the LPS detoxifying enzyme IAP showed that IAP activity was correctly localized to the brush border of villus enterocytes in the CF mouse but the strength of the reaction was reduced as compared to WT. Expression of the murine IAP gene (Akp3) in the CF mouse intestine was also reduced, to less than a third of WT. This was paralleled by an equal decrease in IAP enzyme activity. The regulation of IAP expression is complex and is affected by many conditions in the gut [for review see (46)]. It needs to be pointed out that changes in IAP expression are not directly linked to Cftr. IAP levels can be brought back to normal in the CF mouse by antibiotics or laxative, so the decrease in IAP in CF is not a direct consequence of loss of Cftr, but rather is secondary to its loss. A common result in the CF intestine to oral antibiotics and laxative treatments is that both eradicate bacterial overgrowth. Hence, it is proposed that as part of the intestinal response to bacterial overgrowth that IAP expression is decreased in the CF intestine.
Because a second intestine specific alkaline phosphatase gene, Akp6, was recently discovered whose expression is increased in Akp3 knockout mice (43), we also measured RNA levels for this gene in CF mice with and without the interventions used here. There was a modest increase in Akp6 mRNA levels in antibiotic treated WT mice (175% of WT control) but no changes in CF mice under any conditions. Because the Akp6 encoded enzyme has not been characterized, it is not known if it is L-Phe sensitive and it was not possible to measure its enzymatic activity. In any case, unlike the Akp3 knockout mouse, Akp6 gene expression is not significantly different in the CF mouse which has a deficiency in Akp3 expression and activity.
Two situations that may be relevant to the CF phenotype that have been shown to reduce IAP expression are inflammation and severe malnutrition. The CF intestine has a mild inflammation which could contribute to decreased IAP expression. It is known that inflammation of the gut decreases IAP expression, and this can be mediated by the inflammatory cytokines IL-1β and TNFα (47). When CF mice are treated with antibiotics to eradicate SIBO, mast cell and neutrophil infiltration is reduced and expression of innate immune markers is more normal (16). Also, when treated with laxative, CF mice have normal numbers of small intestinal bacteria and, again, the innate immune changes are ameliorated (34). Although there are many changes in innate immune markers in the CF small intestine, our previous microarray study did not show changes in IL-1β or TNFα (33). Therefore, it is uncertain that the changes observed in IAP expression are related to the mild immune response in the CF mouse intestine.
An alternative possibility is that decreased IAP in the CF intestine is a result of malnutrition. It has been shown in rodents that prolonged fasting or starvation strongly decrease IAP expression (48; 49). Also, early weaning in pigs, which involves nutritional stress, results in decreased IAP levels (50). CF mice on the liquid diet are about 70% the weight of age and diet matched WT mice (16). However, the mechanism linking nutrition to IAP expression is currently unknown and, although CF mice grow poorly, it not clear if this degree of malnutrition is sufficient to result in the strong decrease in IAP in CF.
In addition to its ability to detoxify LPS, IAP has been shown to have other important physiological functions (46). One is that IAP dephosphorylates luminal ATP, thereby contributing to a feedback loop controlling P2Y1 purinergic signaling and influencing bicarbonate secretion in the intestine (51). Thus, a consequence of decreased IAP in the intestine would be to increase bicarbonate secretion. However, bicarbonate secretion in the intestine is CFTR-dependent, so a decrease in IAP in CF is not productive in this respect. An additional consideration is that ATP in high enough concentrations is considered an ‘endogenous danger signal’ that has pro-inflammatory effects (52). So, a decrease in IAP activity in the CF intestine may contribute to an inflammatory process.
Another role of IAP is in dietary lipid assimilation and IAP deficient (Akp3 null) mice show enhanced lipid uptake (53). In CF, malnourishment is a significant problem and particularly fat maldigestion and malabsorption are common (9). Poor fat assimilation is also true in CF mice (10). A decrease in IAP in the CF intestine may be an adaptive change in an attempt to increase dietary lipid assimilation. How IAP participates in fat assimilation is not well understood, and whether a deficiency in dietary fat assimilation is important in regulating Akp3 gene expression is unknown.
The other aspect of the mucosal barrier we investigated in the CF mouse was permeability of the gut to macromolecules. We observed increased amounts of serum albumin in the lumen of the small intestine, and greater passage of fluorescent dextran from the lumen to the blood in CF mice as compared to WT. After antibiotic treatment of CF mice, passage of the fluorescent dextran from the intestine into the blood was reduced by about one half. A similar degree of reduced permeability occurred in laxative treated CF mice. An even greater decrease in permeability was observed in CF mice given exogenous IAP, and these mice also had a greater than 80% reduction in small intestinal bacterial overgrowth. Impaired intestinal permeability appears to be a ubiquitous feature of intestinal dysfunction and it occurs even with mild inflammation (54). Additionally, increased permeability is a common feature of microbial dysbiosis. In patients with SIBO with colonic type bacteria there is increased intestinal permeability (55), and CF mice also have SIBO with predominantly colonic type bacteria (16; 17). Since the interventions we used dramatically decrease bacterial load in CF mice (16; 34) it is suggested that signals from the microbial dysbiosis in CF affect the epithelium to make it more permeable.
Although IAP affects the gut microbiota (45), how it does so is unknown. A possible mechanism of the effects of exogenous IAP in the CF intestine could be involve a decrease in bacterial LPS which in turn will reduce stimulation of immune responses. Previous work showed that eradication of bacterial overgrowth in the CF mouse reduced mucus accumulation (31; 34), and bacteria colonize this mucus. Thus, it is possible that bacterial LPS increases mucus production which in turn enhances the niche for bacterial growth. More work is needed to investigate the effects of exogenous IAP on the CF intestinal phenotype.
An interesting question is the extent to which IAP levels and epithelial permeability are causally related. While IAP is considered part of the mucosal defenses of the intestine because of its LPS detoxifying activity, there is also data suggesting that IAP is more directly involved in the physical barrier of the epithelium. After ischemia-reperfusion, IAP-deficient mice exhibit significantly increased bacterial translocation across the gut wall to mesenteric lymph nodes (49). It has not been reported whether epithelial permeability to tracer macromolecules is altered in the IAP deficient mouse. To address this we used the IAP inhibitor L-Phe, added to the liquid diet. Previous work showed that oral L-Phe increased passage of gavaged LPS into blood in rats (56) and increased the severity of experimental colitis induced with dextran sodium sulfate in mice (39). When we treated mice with oral LPhe, intestinal permeability was not affected in either WT or CF mice. Therefore, it appears that IAP is not required for normal low permeability and that its inhibition alone is not sufficient to increase permeability. However, there was more than a 5-fold increase in postweaning deaths of L-Phe treated CF mice as compared to CF controls. This was observed in a small sample size, but might indicate the importance of the residual IAP activity in the CF intestine where increased permeability is also present. Further work is needed to address this issue.
In summary, CF mice, like human CF patients, have significantly decreased IAP levels and increased intestinal permeability. Interventions that improve other aspects of the CF intestinal phenotype also increase IAP levels and decrease intestinal permeability. In the untreated CF mouse small intestine there is SIBO with Gram negative bacteria, decreased IAP activity, and increased permeability. These conditions are expected to allow greater biologically active LPS to become systemic in CF which may have a significant impact on distant sites like the airways. Another important novel finding was that exogenous IAP reduced permeability in the CF intestine to WT levels and also reduced bacterial overgrowth more than 80%. Thus, exogenous IAP may be a new therapy for CF intestinal disease. Because systemic exposure to gut bacterial products like LPS can affect distant organs, our work suggests that therapies that improve the mucosal barrier function of the intestine could have broader beneficial effects such as lessening airway inflammation in CF.
Acknowledgments
This work was supported by National Institutes of Health grant R21 AI083479 and a pilot project as part of P20 RR024214, COBRE on Molecular Regulation of Cell Development and Differentiation from the National Center for Research Resources.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Barraclough M, Taylor CJ. Twenty-four hour ambulatory gastric and duodenal pH profiles in cystic fibrosis: effect of duodenal hyperacidity on pancreatic enzyme function and fat absorption. J Pediatr Gastroenterol Nutr. 1996;23:45–50. doi: 10.1097/00005176-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 3.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 4.Houwen RH, van der Doef HP, Sermet I, et al. Defining DIOS and constipation in cystic fibrosis with a multicentre study on the incidence, characteristics, and treatment of DIOS. J Pediatr Gastroenterol Nutr. 2010;50:38–42. doi: 10.1097/MPG.0b013e3181a6e01d. [DOI] [PubMed] [Google Scholar]
- 5.Courtney JM, Bradley J, McCaughan J, et al. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol. 2007;42:525–532. doi: 10.1002/ppul.20619. [DOI] [PubMed] [Google Scholar]
- 6.Milla CE. Association of nutritional status and pulmonary function in children with cystic fibrosis. Curr Opin Pulm Med. 2004;10:505–509. doi: 10.1097/01.mcp.0000138995.08494.69. [DOI] [PubMed] [Google Scholar]
- 7.Stallings VA, Stark LJ, Robinson KA, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Baker SS, Borowitz D, Duffy L, et al. Pancreatic enzyme therapy and clinical outcomes in patients with cystic fibrosis. J Pediatr. 2005;146:189–193. doi: 10.1016/j.jpeds.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Borowitz D, Durie PR, Clarke LL, et al. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2005;41:273–285. doi: 10.1097/01.mpg.0000178439.64675.8d. [DOI] [PubMed] [Google Scholar]
- 10.Bijvelds MJ, Bronsveld I, Havinga R, et al. Fat absorption in cystic fibrosis mice is impeded by defective lipolysis and post-lipolytic events. Am J Physiol Gastrointest Liver Physiol. 2005;288:G646–G653. doi: 10.1152/ajpgi.00295.2004. [DOI] [PubMed] [Google Scholar]
- 11.De Lisle RC, Isom KS, Ziemer D, et al. Changes in the exocrine pancreas secondary to altered small intestinal function in the CF mouse. Am J Physiol Gastrointest Liver Physiol. 2001;281:G899–G906. doi: 10.1152/ajpgi.2001.281.4.G899. [DOI] [PubMed] [Google Scholar]
- 12.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 13.Werlin SL, uri-Silbiger I, Kerem E, et al. Evidence of Intestinal Inflammation in Patients With Cystic Fibrosis. J Pediatr Gastroenterol Nutr. 2010;51:304–308. doi: 10.1097/MPG.0b013e3181d1b013. [DOI] [PubMed] [Google Scholar]
- 14.Raia V, Maiuri L, De Ritis G, et al. Evidence of chronic inflammation in morphologically normal small intestine of cystic fibrosis patients. Pediatr Res. 2000;47:344–350. doi: 10.1203/00006450-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Hallberg K, Grzegorczyk A, Larson G, et al. Intestinal permeability in cystic fibrosis in relation to genotype. J Pediatr Gastroenterol Nutr. 1997;25:290–295. doi: 10.1097/00005176-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun. 2004;72:6040–6049. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canale-Zambrano JC, Auger ML, Haston CK. Toll-like Receptor-4 Genotype Influences the Survival of Cystic Fibrosis Mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G381–G390. doi: 10.1152/ajpgi.00003.2010. [DOI] [PubMed] [Google Scholar]
- 18.Clarke LL, Gawenis LR, Bradford EM, et al. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1050–G1058. doi: 10.1152/ajpgi.00393.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lisowska A, Wojtowicz J, Walkowiak J. Small intestine bacterial overgrowth is frequent in cystic fibrosis: combined hydrogen and methane measurements are required for its detection. Acta Biochim Pol. 2009;56:631–634. [PubMed] [Google Scholar]
- 20.Fridge JL, Conrad C, Gerson L, et al. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2007;44:212–218. doi: 10.1097/MPG.0b013e31802c0ceb. [DOI] [PubMed] [Google Scholar]
- 21.Lewindon PJ, Robb TA, Moore DJ, et al. Bowel dysfunction in cystic fibrosis: importance of breath testing. J Paediatr Child Health. 1998;34:79–82. doi: 10.1046/j.1440-1754.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien S, Mulcahy H, Fenlon H, et al. Intestinal bile acid malabsorption in cystic fibrosis. Gut. 1993;34:1137–1141. doi: 10.1136/gut.34.8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reims A, Strandvik B, Sjovall H. Epithelial electrical resistance as a measure of permeability changes in pediatric duodenal biopsies. J Pediatr Gastroenterol Nutr. 2006;43:619–623. doi: 10.1097/01.mpg.0000232573.33526.f5. [DOI] [PubMed] [Google Scholar]
- 24.Dalzell AM, Freestone NS, Billington D, et al. Small intestinal permeability and orocaecal transit time in cystic fibrosis. Arch Dis Child. 1990;65:585–588. doi: 10.1136/adc.65.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escobar H, Perdomo M, Vasconez F, et al. Intestinal permeability to 51Cr-EDTA and orocecal transit time in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1992;14:204–207. doi: 10.1097/00005176-199202000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Hendriks HJ, van Kreel B, Forget PP. Effects of therapy with lansoprazole on intestinal permeability and inflammation in young cystic fibrosis patients. J Pediatr Gastroenterol Nutr. 2001;33:260–265. doi: 10.1097/00005176-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Purohit V, Bode JC, Bode C, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poelstra K, Bakker WW, Klok PA, et al. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am J Pathol. 1997;151:1163–1169. [PMC free article] [PubMed] [Google Scholar]
- 29.Van Biervliet S, Eggermont E, Carchon H, et al. Small intestinal brush border enzymes in cystic fibrosis. Acta Gastroenterol Belg. 1999;62:267–271. [PubMed] [Google Scholar]
- 30.Van Biervliet S, Eggermont E, Marien P, et al. Combined impact of mucosal damage and of cystic fibrosis on the small intestinal brush border enzyme activities. Acta Clin Belg. 2003;58:220–224. doi: 10.1179/acb.2003.58.4.002. [DOI] [PubMed] [Google Scholar]
- 31.De Lisle RC, Roach EA, Norkina O. Eradication of small intestinal bacterial overgrowth in the cystic fibrosis mouse reduces mucus accumulation. J Pediatr Gastroenterol Nutr. 2006;42:46–52. doi: 10.1097/01.mpg.0000189322.34582.3e. [DOI] [PubMed] [Google Scholar]
- 32.Jeffrey I, Durrans D, Wells M, et al. The pathology of meconium ileus equivalent. J Clin Pathol. 1983;36:1292–1297. doi: 10.1136/jcp.36.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norkina O, Kaur S, Ziemer D, et al. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1032–G1041. doi: 10.1152/ajpgi.00473.2003. [DOI] [PubMed] [Google Scholar]
- 34.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G577–G584. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- 35.Bol-Schoenmakers M, Fiechter D, Raaben W, et al. Intestinal alkaline phosphatase contributes to the reduction of severe intestinal epithelial damage. Eur J Pharmacol. 2010;633:71–77. doi: 10.1016/j.ejphar.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Lukas M, Drastich P, Konecny M, et al. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm Bowel Dis. 2010;16:1180–1186. doi: 10.1002/ibd.21161. [DOI] [PubMed] [Google Scholar]
- 37.Ramasamy S, Nguyen DD, Eston MA, et al. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21377. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehouse JS, Riggle KM, Purpi DP, et al. The Protective Role of Intestinal Alkaline Phosphatase in Necrotizing Enterocolitis. J Surg Res. 2010;163:79–85. doi: 10.1016/j.jss.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Campbell EL, Macmanus CF, Kominsky DJ, et al. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke LL, Gawenis LR, Franklin CL, et al. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46:612–618. [PubMed] [Google Scholar]
- 41.Bowers GN, Jr., McComb RB. A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin Chem. 1966;12:70–89. [PubMed] [Google Scholar]
- 42.Cesarone CF, Bolognesi C, Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979;100:188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- 43.Narisawa S, Hoylaerts MF, Doctor KS, et al. A novel phosphatase upregulated in Akp3 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1068–G1077. doi: 10.1152/ajpgi.00073.2007. [DOI] [PubMed] [Google Scholar]
- 44.Shephard MD, Peake MJ, Walmsley RN. Quantitative method for determining serum alkaline phosphatase isoenzyme activity II. Development and clinical application of method for measuring four serum alkaline phosphatase isoenzymes. J Clin Pathol. 1986;39:1031–1038. doi: 10.1136/jcp.39.9.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malo MS, Alam SN, Mostafa G, et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010;59:1476–1484. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- 46.Lalles JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010;68:323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 47.Malo MS, Biswas S, Abedrapo MA, et al. The pro-inflammatory cytokines, IL-1beta and TNF-alpha, inhibit intestinal alkaline phosphatase gene expression. DNA Cell Biol. 2006;25:684–695. doi: 10.1089/dna.2006.25.684. [DOI] [PubMed] [Google Scholar]
- 48.Hodin RA, Chamberlain SM, Meng S. Pattern of rat intestinal brush-border enzyme gene expression changes with epithelial growth state. Am J Physiol. 1995;269:C385–C391. doi: 10.1152/ajpcell.1995.269.2.C385. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg RF, Austen WG, Jr., Zhang X, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lackeyram D, Yang C, Archbold T, et al. Early weaning reduces small intestinal alkaline phosphatase expression in pigs. J Nutr. 2010;140:461–468. doi: 10.3945/jn.109.117267. [DOI] [PubMed] [Google Scholar]
- 51.Akiba Y, Mizumori M, Guth PH, et al. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1223–G1233. doi: 10.1152/ajpgi.00313.2007. [DOI] [PubMed] [Google Scholar]
- 52.Ishii KJ, Akira S. Potential link between the immune system and metabolism of nucleic acids. Curr Opin Immunol. 2008;20:524–529. doi: 10.1016/j.coi.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Nakano T, Inoue I, Koyama I, et al. Disruption of the murine intestinal alkaline phosphatase gene Akp3 impairs lipid transcytosis and induces visceral fat accumulation and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1439–G1449. doi: 10.1152/ajpgi.00331.2006. [DOI] [PubMed] [Google Scholar]
- 54.Bjarnason I, Takeuchi K, Bjarnason A, et al. The G.U.T. of gut. Scand J Gastroenterol. 2004;39:807–815. doi: 10.1080/00365520410003326. [DOI] [PubMed] [Google Scholar]
- 55.Riordan SM, McIver CJ, Thomas DH, et al. Luminal bacteria and small-intestinal permeability. Scand J Gastroenterol. 1997;32:556–563. doi: 10.3109/00365529709025099. [DOI] [PubMed] [Google Scholar]
- 56.Koyama I, Matsunaga T, Harada T, et al. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin Biochem. 2002;35:455–461. doi: 10.1016/s0009-9120(02)00330-2. [DOI] [PubMed] [Google Scholar]