Abstract
We analyzed the association between the evolution of the V3–V5 regions of env and disease progression in our SIV/SHIV macaque model of morphine dependence and AIDS. Previous studies revealed two distinct disease patterns- fast progression and normal progression. To determine the effect of the two distinct patterns of disease in the evolution of SIV/17E-Fr envelope, we analyzed env sequences from three morphine-dependent macaques that developed accelerated AIDS and three morphine-dependent macaques that developed AIDS at a slower rate and compared them to control macaques. Morphine-dependent animals exhibited a higher percentage of diversity in both compartments within V4 when compared to controls. Divergence from the inoculum was significantly greater in the morphine group as compared to controls in CSF but not in plasma. We also found a direct correlation in V4 evolution and rapid disease progression. These results indicate that morphine dependence plays a role in the pathogenesis of SIV/SHIV infection and env evolution.
Keywords: SIV, SHIV, Envelope evolution, Macaque, Morphine, Variable region
INTRODUCTION
Injection drug use (IDU) continues to be an important cause of human immunodeficiency virus (HIV) infection in the United States and other countries (CDC, 2003; Chu & Levy, 2005; Kerr, 2005; Qian et al., 2006; Cohn, 2002). The relationship between IDU and the differences in the virology and immunopathogenesis of HIV and its effect in antiretroviral treatments remains to be elucidated. Human studies on the influence of IDU in disease progression and survival rates although meaningful, provide insufficient information because of the many factors present among injection drug users unrelated to HIV. However there are ample indirect proofs that illicit drug abuse may adversely affect HIV/AIDS. These include (i) morphine-mediated increase in HIV-1 replication in tissue culture (Ho et al., 2003; Li et al., 2003; Guo et al., 2002; Schweitzer et al., 1991); (ii) AIDS as major cause of death in IDU cohort (Tyndall et al., 2001; CDC, 2001); and, (iii) morphine mediated exaggeration of bacterial infection, including direct correlation between bacterial infections like tuberculosis and AIDS (Cohn, 2002).
In order to circumvent major gaps in HIV human studies, various animal models of HIV/AIDS have been established, allowing for a better control of parameters (Bonyhadi & Kaneshima, 1997; Esparza, 1990; Fultz, 1993; Gardner, 1990; Kinman et al., 2004; Persidsky et al., 2005; Persidsky et al., 1995; van Maanen & Sutton, 2003; Vodros & Fenyo, 2004). These animal models have provided a way to test pre-clinical effectiveness of drugs, develop vaccines, examine virulence, and determine the role of antiviral immune responses, among others. One such model, the non-human primate, has proven to be particularly valuable for the study of viral pathogenesis and disease progression because of the similarities between simian immunodeficiency virus (SIV) AIDS-like illness and HIV-AIDS (Haigwood, 2004; Hu, 2005; Levy, 1996; Vodros & Fenyo, 2004).
In view of the fact that both survival advantage and disadvantage have been reported in the setting of drug abuse and that results from animal studies are inconclusive, we developed a non-human primate model of drug abuse and AIDS in Indian rhesus macaques. In our model we use a mixture of three viruses that had been previously shown to cause massive CD4+ cell loss and neurological disorders in animals (Kumar et al., 2004; Kumar et al., 2006). Using this model, we have previously shown that morphine-dependent macaques showed significantly higher virus replication. We have also demonstrated that 50% of the morphine-dependent and infected animals develop SHIV/SIV-induced disease within 20 weeks after infection whereas other morphine-dependent and control animals survived for much longer.
In our attempt to establish a reason for accelerated disease progression in half of the morphine-dependent macaques, we sought to determine whether there was a correlation between virus evolution and disease progression. Earlier studies from this laboratory have indicated an inverse correlation between SIVtat evolution both in plasma as well as cerebrospinal fluid (CSF) compartments (Noel, Jr. et al., 2006; Noel, Jr. & Kumar, 2006). This observation was later extended to our study on SIV envelope (env) wherein we found similar correlation within variable regions 1 and 2 (Tirado & Kumar, 2006). In this study we sought to characterize the changes within env V3-V5 regions to determine whether there is an indirect correlation as was seen previously, or if evolution of some variable regions of env is directly related to disease progression in morphine-dependent macaques. The data presented represent a comprehensive analysis of envelope genetic variation in plasma and CSF from rapid and normal progressor morphine-dependent and control macaques. Our results indicate a direct correlation between env V4 evolution and rapid disease progression in morphine-dependent macaques.
RESULTS
We have sequenced and analyzed a total of 125 plasma and 123 CSF SIV envelope (env) clones, covering the variable regions V3–V5 from six morphine-dependent (1/04L, 1/28Q, 1/42N, 1/02N, 1/52N and 1/56L) and three control rhesus macaques (2/02P, 2/31P, and 2/AC42) to assess compartmentalization of viral evolution. Plasma and CSF were collected at regular intervals from each macaque after experimental inoculation with SIV/SHIV. An earlier study showed that morphine dependence lead to higher viral load in SIV/SHIV-infected macaques (Kumar, Torres et al., 2004). Among morphine-dependent macaques, half progressed rapidly, maintained high viral loads and low CD4+ T-cell counts (1/04L, 1/28Q, and 1/42N). These animals, termed rapid progressors, died on or before the 20th week post-infection. The other three animals within the morphine-dependent group, termed normal progressors, showed intermediate viral loads and CD4+ T-cell counts (1/02N, 1/52N, and 1/56L). The control animals showed the least severe disease as compared to the rapid progressors and moderately better than the normal progressors (Noel, Jr. & Kumar, 2006; Noel, Jr. et al., 2006; Kumar, Torres et al., 2004; Kumar et al., 2006). Retroviral infections are characterized by different levels of viral genetic variation within the host. This variability may be contributed by viral factors such as replication, mutation, and recombination and by host factors such as immune response and target cell range (van Marle & Power, 2005; Negroni & Buc, 2001; Nowak, 1992). In this study we examined the evolution of SIV env from the morphine-dependent macaques and compared it to control group to elucidate whether morphine exerts any effect over viral evolution. We also analyzed the rapid progressors and normal progressors from the morphine-dependent group and compared them to control macaques to determine any correlation between virus evolution and disease progression. We have found that SIV env evolution is to some extent independent of selective immune pressure as evidenced by the changes seen in the rapid progressors which we had previously shown to lack effective humoral and cellular immune responses (Kumar et al., 2006).
Contrasting env diversity in plasma and CSF
Sequence data was obtained from clones derived from collection at weeks 12 and 18 post-infection (wpi). Viral RNA was extracted and amplified by RT-PCR prior to cloning and sequencing. The sequenced clones represented a ~905 base pair fragment encompassing the V3 to V5 regions of SIV env.
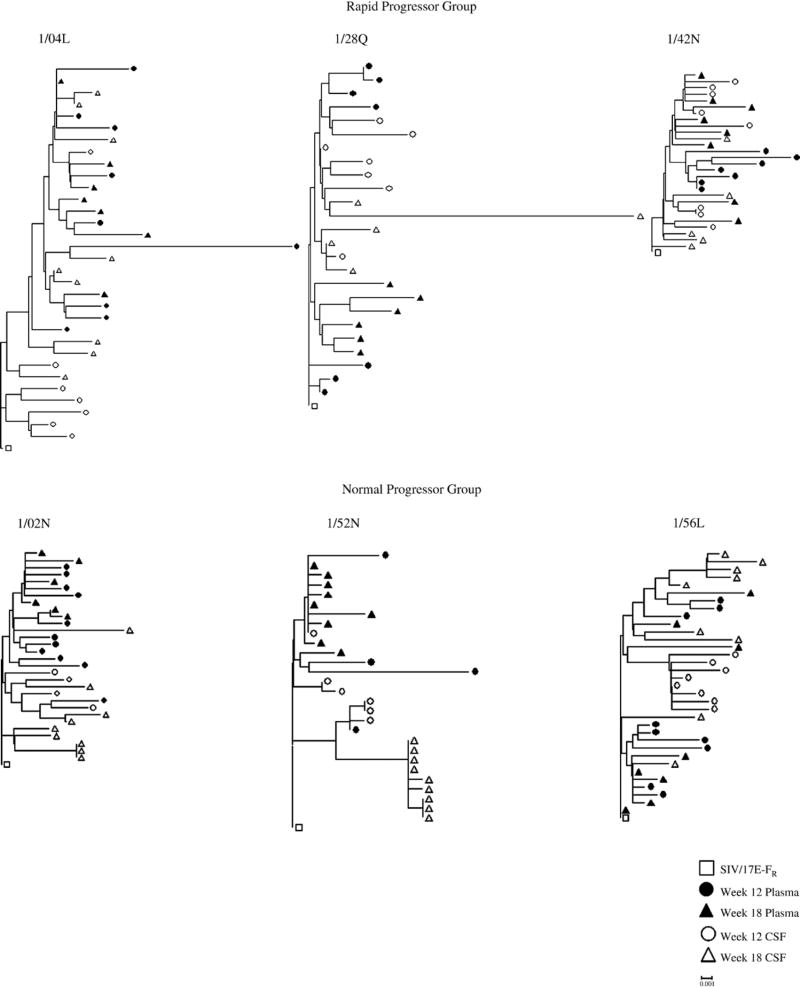
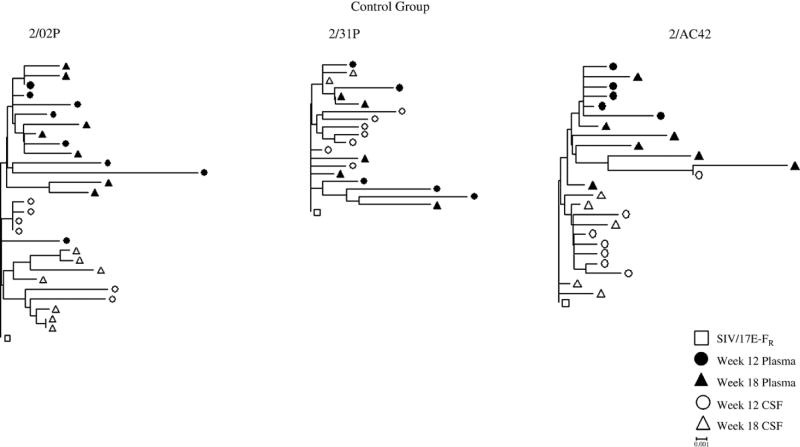
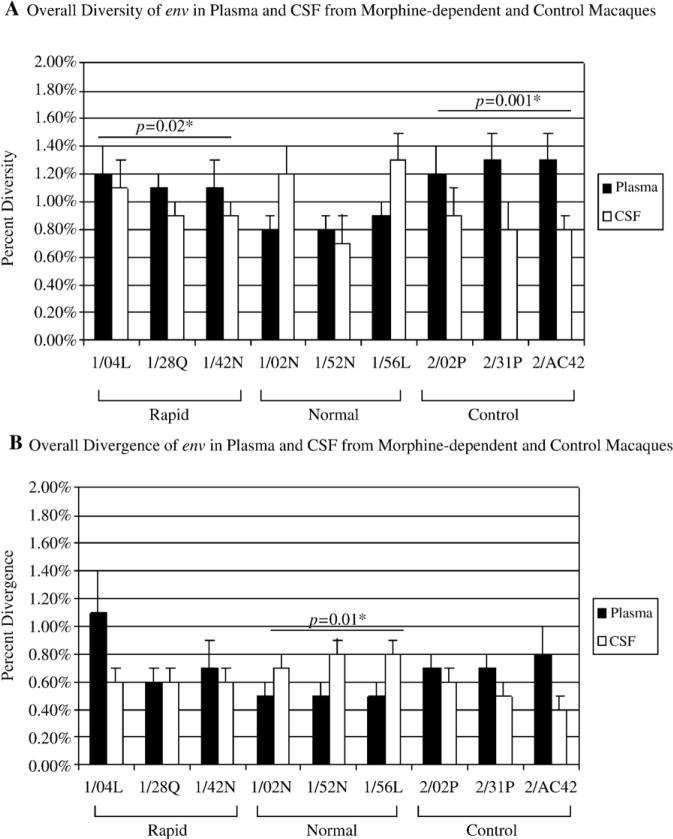
Phylogenetic analysis of plasma and CSF-derived clones within 18 wpi revealed differences in the evolution of the viral quasispecies within the two compartments. Neighbor-joining trees indicated that there is better compartmentalization in the normal progressors and control macaques than in the rapid progressors (Figure 1). We then examined the sequences in each animal independent of time to determine the overall diversity and the nucleotide distance from the SIV/17E-Fr inoculum (divergence). Analysis of the plasma-derived clones revealed a significant difference (p= 0.005) in the overall sequence diversity of the clones from morphine-dependent macaques (0.98%) when compared to control macaques (1.27%) with the control macaques exhibiting a higher percentage (Figure 2A). There is no statistically significant difference in divergence for the morphine group when compared to control group. Analysis of the two morphine sub-groups revealed no significant difference when comparing the diversity of the rapid progressors to control macaques. However, a significant difference (p= 0.003) was detected when comparing the diversity in the normal progressors (0.83%) to control macaques (1.27%) (Figure 2A). A statistically significant (p= 0.010) difference was also detected for the divergence from the SIV/17E-Fr inoculum for the normal progressors (0.50%) but not for the rapid progressors (0.80%) when compared to control macaques (0.73%) (Figure 2B).
Figure 1. Phylogenetic reconstruction of SIV env of plasma and CSF sequences from morphine-dependent and control macaques infected with SHIVKU-1B, SHIV 89.6P, and SIV/17 E-Fr.
DNA sequences were derived from RT-PCR of viral RNA extractions from plasma (solid symbols) and CSF (open symbols) at weeks 12 (circles) and 18 (triangles) post-infection. The phylogenetic trees were generated by the neighbor-joining method using the MEGA 3.1 program. The trees were rooted using the corresponding sequence from SIV/17E-Fr env (□). All trees are drawn to the same scale. The distance scale is indicated in the lower right of the figure.
Figure 2. Diversity and Divergence of env in plasma and CSF from morphine-dependent and control macaques.
The env sequences in plasma (solid bars) and CSF (open bars) from morphine-dependent and control macaques were aligned and used to calculate diversity (A) and divergence (B) using MEGA 3.1. The mean percent diversity and divergence are plotted for each monkey. Error bars indicate mean of standard error. *P value of the comparison between plasma and CSF.
In CSF, as seen in plasma, there was a significant difference (p= 0.05) in the overall sequence diversity of the clones from morphine-dependent macaques when compared to control macaques. However, in contrast to plasma, the morphine-dependent macaques showed greater diversity than controls (1.02% and 0.83%, respectively) (Figure 2A). Analysis of the divergence from the SIV/17E-Fr inoculum revealed that there was a significant difference between the two groups (p= 0.03), with the morphine-dependent macaques exhibiting a higher average percentage of divergence (0.68%) as compared to the control macaques (0.50%) (Figure 2B). Analysis of the morphine sub-groups revealed a significant difference (p= 0.03) in diversity between the rapid progressors (0.97%) and control macaques (0.83%) but no significant difference between normal progressors and control macaques. Divergence from the SIV/17E-Fr inoculum was only significantly different (p= 0.05) between the normal progressors (0.77%) and the control macaques (0.50%) (Figure 2B).
Analysis of the diversity and divergence of the clones between plasma and CSF compartments revealed significant differences in diversity in rapid progressors and control macaques with the plasma-derived clones exhibiting higher diversity, whereas the normal progressors exhibited a higher divergence in the CSF compartment as compared to plasma (Figure 2).
Evolution within individual variable regions shows conflicting pattern of responses to morphine, compartment and rate of progression
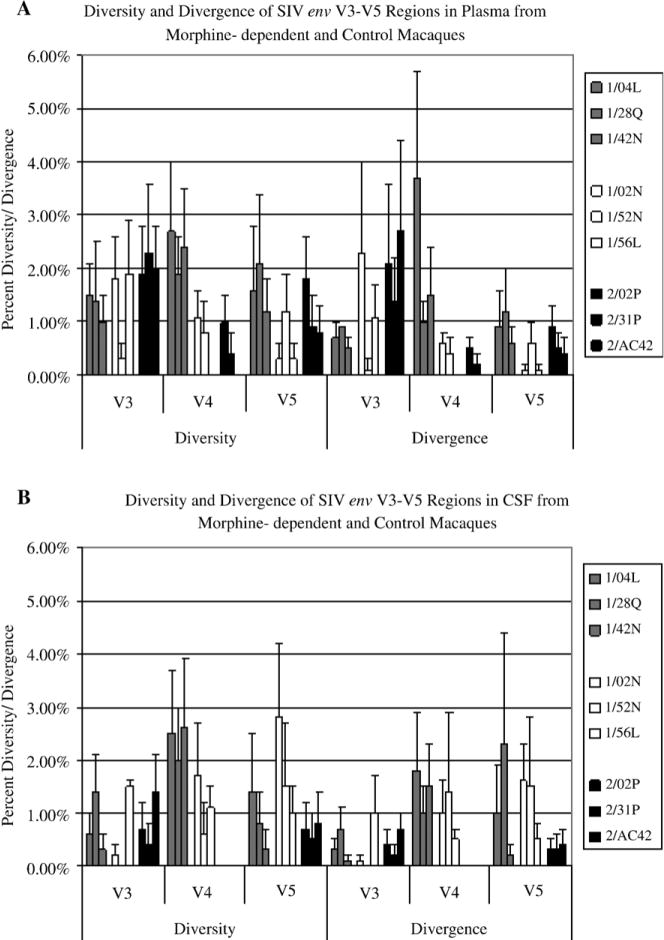
Overall analysis of diversity and divergence within the envelope variable regions in plasma-derived clones revealed an inverse correlation in SIV env evolution between morphine-dependent and control macaques within variable region 3. The morphine-dependent macaques exhibited a 1.31% in diversity and 0.94% in divergence whereas the control macaques showed significantly greater (p= 0.04) diversity (2.07%) and divergence (2.07%) (Figure 3A). No significant differences where detected within variable region 3 from CSF-derived clones.
Figure 3. Diversity and Divergence of env variable regions 3 to 5 in plasma and CSF from morphine-dependent and control macaques.
The env sequences in plasma (A) and CSF (B) from morphine-dependent and control macaques were aligned and used to calculate diversity and divergence using MEGA 3.1. The mean percent diversity and divergence are plotted for each monkey. A: Overall diversity and divergence of env in plasma. B: Overall diversity and divergence of env in CSF. Error bars indicate mean of standard error.
In contrast, within variable region 4 a direct correlation in diversity was detected for both plasma and CSF, in morphine-dependent macaques when compared to controls. Morphine-dependent plasma-derived clones exhibited a 1.48% diversity versus a 0.47% diversity in control macaques (p= 0.04) (Figure 3A). Morphine-dependent CSF-derived clones exhibited a 1.75% diversity versus 0% diversity in control macaques (p= 0.004) (Figure 3B). Although the percentage of diversity in CSF was higher than in plasma, statistical analysis revealed no significant difference in diversity between the two compartments. In both plasma and CSF the rapid progressors exhibited a significantly higher percentage in diversity when compared to control macaques (Figure 3).
Analysis of the overall plasma sequence diversity and divergence of env within variable region 5 revealed no significant differences in morphine-dependent animals when compared to control macaques. However, a significant difference (p=0.02) was detected in the divergence from the SIV/17 E-Fr inoculum of the morphine-dependent CSF-derived clones when compared to control macaques (1.19% and 0.33%, respectively) (Figure 3B). No significant differences in diversity and divergence within V5 were detected when comparing the two morphine sub-groups to control macaques from both compartments.
Analysis of env variable region evolution at each time point
Plasma shows significant changes within V3 and V4
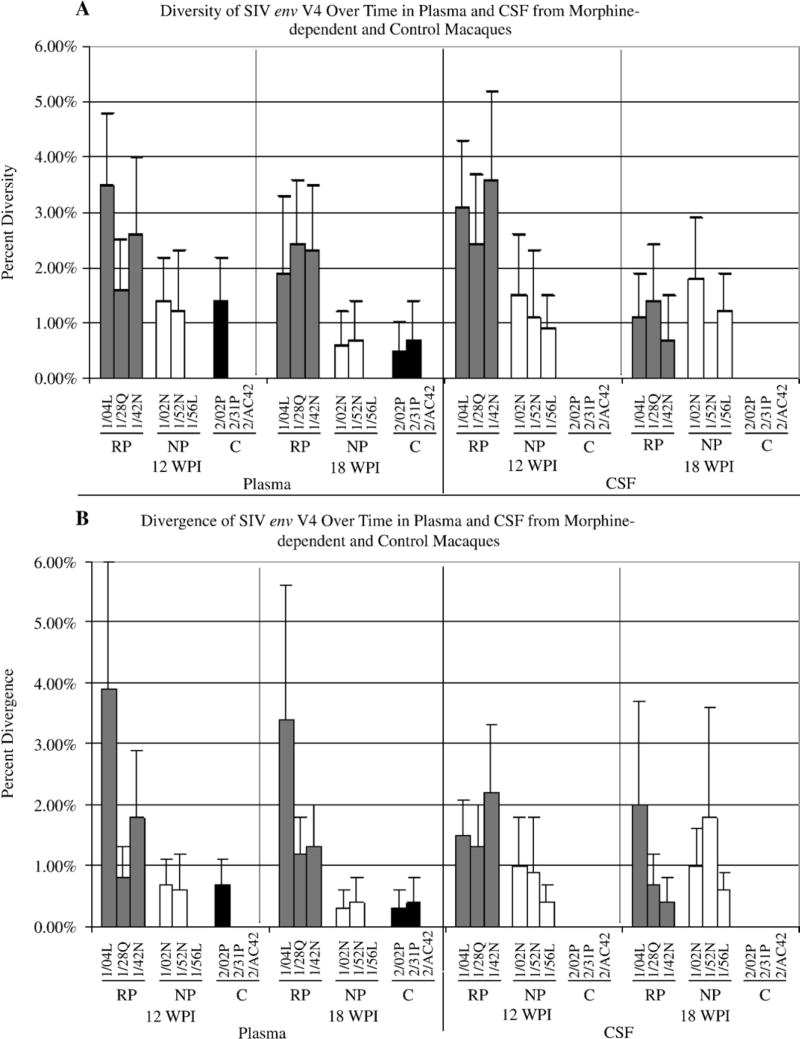
We also analyzed the nucleotide distance within and between groups in the variable regions over time. As shown in Figure 4, plasma-derived clones from the morphine group exhibited a higher percentage of diversity within V4 although this difference was only significant at 18 wpi (morphine: 1.32%, control: 0.6%; p= 0.045). Comparison of the plasma-derived morphine subgroups revealed a significant difference when comparing the rapid progressors to control but not when comparing the normal progressors to control. The rapid progressors exhibited a 2.57% in diversity at 12 wpi (p= 0.009) and 2.2% at 18 wpi (p= 0.010) whereas the control group exhibited 0.47% and 0.6% in diversity at 12 and 18 wpi, respectively. Analysis of the divergence from the SIV/17E-Fr revealed a significant difference only when comparing the rapid progressors to control group at 12 wpi (rapid progressors: 2.17%, control: 0.23%; p= 0.05).
Figure 4. Diversity and divergence of env variable 4 in plasma and CSF over time from morphine-dependent and control macaques.
The env V4 sequences in plasma and CSF from morphine-dependent and control macaques at 12 and 18 wpi were aligned and used to calculate diversity and divergence using MEGA 3.1. The mean percent diversity and divergence are plotted for each monkey. A: Over time diversity, B: Over time divergence. Error bars indicate mean of standard error.
No significant differences were detected in diversity over time for V3 and V5. Divergence, however, within V3 was significantly lower at 12 wpi in the morphine group (0.87%) than in controls (1.93%, p= 0.009). Among morphine-dependent macaques, the rapid progressors were significantly less divergent from the inoculum than the controls (0.75% vs. 1.93%, p= 0.031) while the morphine-dependent normal progressors were less divergent (1.23%) but not at a statistically significant level. No significant differences were detected for the divergence over time within V5 from plasma-derived clones (data not shown).
CSF shows contrasting pattern with most significant changes in V4
Analysis of the diversity of CSF-derived clones within variable regions revealed that the morphine group exhibited a higher percentage of diversity (12 wpi: 2.1%, p= 0.003; 18 wpi: 6.2%, p=0.005) and divergence (12 wpi: 1.2%, p= 0.002; 18 wpi: 1.1%) within variable region 4 at 12 and 18 weeks post-infection when compared to control macaques (diversity and divergence 12 and 18 wpi: 0%) (Figure 4). Comparison of the CSF-derived morphine sub-groups revealed a significant difference in diversity when comparing the rapid progressors to control at 12 and 18 wpi (rapid progressors: 12 wpi: 3.03%, p= 0.006; 18 wpi: 1.07%, p= 0.017; control: 12 and 18 wpi: 0%). Comparison of the normal progressors to control also revealed a significant difference but only at 12 wpi (normal progressors: 1.17%, control: 0%; p= 0.011). Between the morphine and control groups, no significant differences were detected over time for the diversity and divergence within variable region 3 (data not shown). Within variable region 5 there is only a significant difference in diversity between the normal progressors (1.4%) and control group (0.73%, p= 0.015) at 18 wpi. Analysis of the divergence from the SIV/17 E-Fr inoculum revealed that there is a significant difference between morphine and control group at 18 wpi (morphine: 1.23%, control: 0.37%; p= 0.033) but not at 12 wpi. In both compartments, the rapid progressors exhibited higher diversity in V4 when compared to control macaques.
Significant differences in amino acid changes within V4 are detectable earlier in infection
Based on the deduced amino acid sequences, the majority of amino acid changes occurred outside of the variable regions (data not shown). Most of the amino acid changes within variable regions 3 to 5 represent similar substitutions, indicating a single evolutionary event. Analysis of the overall frequency of amino acid mutations in plasma-derived clones from morphine-dependent macaques revealed a higher frequency of mutations in the morphine group within V4 as compared to control macaques (0.619 and 0.99, respectively; p= 0.04). This difference is significant at 12 wpi but not at 18 wpi (Table 1) indicating that mutations accumulate in V4 early after infection. Comparison of the morphine sub-groups with control macaques revealed a significant difference in the rapid progressors within V4 (p= 0.05) and V5 (p= 0.008) at 12 wpi but not at 18 wpi (Table 1). No statistically significant frequency of amino acid mutations was detected either in V3 for the rapid progressors and in V3 to V5 for the normal progressors.
Table 1.
Mean Frequency of SIV Envelope Mutations in Plasma and CSF by Variable Region from Morphine-dependent and Control Macaques
| Group | Monkey Number | Sampling Time (week) | Mean frequency of mutations by variable regiona |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | CSF | |||||||||
| Morphine | No. of clones | V3 | V4 | V5 | No. of clones | V3 | V4 | V5 | ||
| Rapid | 1/04L | 12 | 9 | 0.333 | 1.889 | 0.667 | 7 | 0.143 | 0.571 | 0.429 |
| 18 | 7 | 0.286 | 1.857 | 0.286 | 9 | 0.111 | 1.110 | 0.444 | ||
| 1/28Q | 12 | 7 | 0 | 0.429 | 0.857 | 7 | 0.142 | 0.714 | 1.143 | |
| 18 | 6 | 0 | 0.500 | 0.167 | 5 | 0 | 0.400 | 0.800 | ||
| 1/42N | 12 | 7 | 0.143 | 1.000 | 0.429 | 8 | 0 | 1.250 | 0.125 | |
| 18 | 8 | 0 | 0.625 | 0.125 | 5 | 0.200 | 0.200 | 0 | ||
| Normal | 1/02N | 12 | 10 | 0.200 | 0.400 | 0.100 | 7 | 0 | 0.571 | 0.857 |
| 18 | 6 | 0.500 | 0.167 | 0 | 9 | 0 | 0.556 | 0.556 | ||
| 1/52N | 12 | 3 | 0 | 0.333 | 0.667 | 6 | 0 | 0.375 | 0 | |
| 18 | 9 | 0 | 0.222 | 0 | 9 | 0 | 1.000 | 0.111 | ||
| 1/56L | 12 | 8 | 0.125 | 0 | 0 | 8 | 0 | 0.250 | 0.125 | |
| 18 | 8 | 0.125 | 0 | 0.125 | 9 | 0 | 0.333 | 0.333 | ||
| Control | ||||||||||
| 2/02P | 12 | 8 | 0 | 0.250 | 0.375 | 6 | 0.167 | 0 | 0 | |
| 18 | 7 | 0.571 | 0.143 | 0.286 | 7 | 0.286 | 0 | 0 | ||
| 2/31P | 12 | 5 | 0 | 0 | 0.400 | 7 | 0.143 | 0 | 0.143 | |
| 18 | 5 | 0 | 0.200 | 0 | 2 | 0 | 0 | 0 | ||
| 2/AC42 | 12 | 5 | 0.250 | 0 | 0 | 7 | 0 | 0 | 0.143 | |
| 18 | 7 | 0.143 | 0 | 0.286 | 5 | 0 | 0 | 0 | ||
Mean frequency of mutation by variable region was region in a monkey at a given time point divided
As with plasma-derived clones, analysis of the overall frequency of amino acid mutations in CSF-derived clones from morphine and control groups revealed a higher frequency of mutation in the morphine group within V4 (morphine: 0.611; control: 0.260; p= 0.003). However, over time this difference was significant at 12 and 18 wpi (Table 1). In contrast to plasma-derived clones, significant differences were also detected for V3 and V5. Morphine-dependent CSF-derived clones exhibited a higher frequency of amino acid mutations within V5 (morphine: 0.410, control: 0.152, p= 0.02) and this difference was significant at 18 wpi but not at 12 wpi. Whereas, the control group exhibited a higher frequency of amino acid mutations within V3 (morphine: 0.050, control: 0.078; p= 0.03) and this difference was significant at 12 wpi but not at 18 wpi (Table 1). Comparison of the morphine sub-groups with control macaques revealed no significant differences between rapid progressors and control macaques within V3 and significant differences within V4 (p= 0.004) and V5 (p= 0.023) with the rapid progressors exhibiting higher frequency of amino acid changes, but only significant at 12wpi. The normal progressors exhibited a significantly higher frequency of amino acid mutations within V4 (0.514, p= 0.003), and V5 (0.330, p= 0.044) when compared to control macaques (V4: 0 and V5: 0.048), whereas as the control macaques exhibited a higher frequency of mutation within V3 (0.147 vs. 0, p= 0.019).
DISCUSSION
Injection drug use continues to be one of the major cofactors among HIV positive individuals as more than 25% of persons living with AIDS have been reported to belong to this category in United States of America (2003). Several studies with this cohort of HIV-infected individuals have failed to provide a direct correlation between drug abuse and the impact on HIV infection and disease progression because of the many factors unrelated to HIV (Alcabes & Friedland, 1995).
In HIV, evolution of env during the course of infection allows for infection of a broader range of target cells and this shift influences viral pathogenesis and disease course. These changes may be a combinatorial result of viral replication/recombination and the host’s selective pressure. Regardless of how env is modulated, the changes generated may affect distinct domains of env, which have discrete functions in viral pathogenesis. Among the regions of env, the V1–V2 region and the V3 loop have been implicated as determinants in cell tropism and co-receptor utilization, with the V3 playing a major role in this function (Cho et al., 1998; Harrowe & Cheng-Mayer, 1995; Hoffman et al., 1998). Studies within the V4 region have revealed that it can tolerate substantial changes in length, amino acid composition, and glycosylation without compromising function (Ren et al., 2005). Thus, suggesting that there is a minimal contribution of the V4 region to HIV-1 envelope glycoprotein function. However, because of its exposure on the assembled envelope glycoprotein trimer and its hypervariability, V4 may play a pivotal role in immune evasion. In contrast, other studies have revealed a more active role of the V4–V5 region in co-receptor utilization (Cho et al., 1998; Hu et al., 2000; Smyth et al., 1998).
The SIV/SHIV morphine-dependent macaque model of AIDS provides an ideal system in which to investigate the molecular basis of antigenic variation because of the similarities to human AIDS, and the capacity to control the inoculum, timing, and drug exposure. In our model system we have seen that morphine accelerates the onset of disease. Morphine-dependent macaques exhibited a more prominent loss of CD4+ T cells, higher viral set point and more pronounced viral replication in the cerebral compartment. Thus, indicating positive correlation between morphine and levels of viral replication (Kumar et al., 2004; 2006). We wanted to determine whether morphine could potentiate viral evolution in the same way as disease progression. However, studies on SIV tat evolution, both in plasma and CSF compartments, revealed an inverse correlation with disease progression (Noel, Jr. & Kumar, 2006; Noel, Jr. et al., 2006). Morphine-dependent macaques exhibited a lower tat sequence diversity and divergence in plasma (Noel, Jr. & Kumar, 2006) and CSF (Noel, Jr. et al., 2006) when compared to control group. Within the morphine sub-groups, the rapid progressors sustained the lowest diversity and divergence, whereas there were no significant differences between the normal progressors and the controls. A similar pattern of evolution was also found within the 5′ end of SIV env encompassing V1 and V2 (Tirado & Kumar, 2006). In the present study we examined the effect of morphine in the evolution of the 3′ end of SIV env within plasma and cerebrospinal fluid (CSF) to establish a correlation with disease progression. We wanted to determine whether changes in V3 to V5 regions associated with disease progression.
Analysis of env evolution within the entire region encompassing V3–V5 revealed that the virus in plasma showed generally the same trend, inverse correlation with disease progression, as that seen in previous studies with tat and env V1–V2 region. However, in CSF there was a direct correlation with disease progression, with the morphine-dependent macaques exhibiting greater diversity and divergence that the control group. Analysis of the individual regions also revealed differences in evolution between the two compartments, with plasma showing significant differences within V3 and V4 and CSF mainly within V4. A consistent pattern does emerge between the two compartments in the V4 region. In both plasma and CSF, the rapid progressors exhibited higher diversity when compared to control macaques. We have also found a direct correlation with disease progression in the frequency of amino acid mutations within V4. The rapid progressors showed higher mutation frequency as compared to control macaques and this difference was significant at the earlier time point (12 weeks). A possible explanation for greater diversity within V4 in rapid progressors is that this group always maintained a very high viral load in both plasma and CSF as compared to normal progressors and control macaques (Kumar et al., 2006). In addition, two of the three rapid progressors showed progressive neurological signs including jerky movements, increased aggressiveness, ataxia and drooling. These results suggest that changes within V4 may be related to disease progression, perhaps through modification of co-receptor use as has been suggested by others (Cho et al., 1998; Hu et al., 2000; Smyth et al., 1998).
This study provides evidence that there are remarkable differences between morphine-dependent and control macaques indicating a contribution of morphine in both the pathogenesis of SIV/SHIV infection and env evolution. Further analysis of envelope and other genes in various compartments may provide a better understanding of the accelerated form of the disease in morphine-dependent macaques.
METHODS
Animal Model
Establishment of morphine addiction has been previously described (Kumar et al., 2004; 2006; Noel, Jr. & Kumar, 2006). Briefly, morphine dependence was established by injecting increasing doses of morphine (1 to 5 mg/kg of body weight over a two-week period) by the intramuscular route at 8-h intervals. The animals were maintained at three daily doses of morphine (5 mg/kg) for an additional 18 weeks. All macaques were infected by the intravenous route with a 2-ml inoculum containing 104 50% tissue culture infective doses each of simian-human immunodeficiency virus SHIVKU-1B (Singh et al., 2002), SHIV89.6P (Reimann et al., 1996a; Reimann et al., 1996b) and SIV/17E-Fr (Flaherty et al., 1997). The animals were monitored for a period of 20 weeks. Blood was collected into EDTA vacutainer tubes. The cerebrospinal fluid was collected by inserting a 23-gauge needle at the junction of the spinal cord and brain. The clear liquid was spun down at 3,000 rpm for 10 minutes. The supernatant was collected and used for RNA extraction. The experimental protocol was approved by the Institutional Animal Care and Use Committee, and the research was conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
Amplification, cloning and sequencing of V3-V5 regions of env
Viral RNA was isolated from plasma and CSF samples at 12 and 18 weeks using the QIAmp UltraSens Viral RNA Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacture’s recommendations. A 905-bp fragment of the env region of SIV/17E-Fr was amplified in a reverse transcription polymerase chain reaction (RT-PCR) using the One-Step RT-PCR kit (Qiagen, Inc., Valencia, CA). The oligonucleotide primers used in the RT-PCR were 5′-TTGTGCACCTCCAGGTTATG-3′ (nucleotides 7299-7318) and 5′-TTTGTGCTAGGGTTCTTGGG-3′ (nucleotides 8204-8185). The RT-PCR reaction included initial reverse transcription at 50 °C for 30 min and denaturation at 95 °C for 15 min followed by 40 cycles of amplification (95 °C for 30 sec, 52 °C for 45 sec and 72 °C for 3 min) as well as a final extension at 72 °C for 10 min. The 905-bp fragment was confirmed by agarose electrophoresis prior to cloning into pCR2.1 using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA). The insert was confirmed by performing a PCR using the DNA from transformed Escherichia coli and the same primer set as described above. Clones were sequenced using the M13 forward site on pCR2.1 using Big-Dye terminator chemistry on the Applied Byosystems 3100 Genetic Analyzer, by the DNA Sequencing Facility of Florida State University, Department of Biological Sciences.
Sequence analysis and statistics
All sequence files were first manually verified and edited as necessary using the software ChromasLite 2.0 (Technelysium Pty Ltd, Australia). Edited sequences were aligned using BioEdit version 7.0.5.2 (Hall TA,) and Clustal W (Thompson et al., 1994). The Clustal W program (runs within BioEdit) was set to perform multiple sequence alignments using the default penalties. Aligned sequences were used to calculate pairwise DNA distances and to generate phylogenetic trees using the program MEGA version 3.1 (Kumar et al., 2004). All phylogenetic analyses used the SIV/17E-Fr inoculum sequence as a reference. Statistical comparisons were done using a one-tailed T-test. The statistical cut-off for significance in these analyses was p=0.05. Sequences in this report are available from GenBank with accession numbers from DQ784853 to DQ785100.
Acknowledgments
We thank Dr. Martin Hill, Department of Physiology and Pharmacology, Ponce School of Medicine for insightful discussions. We acknowledge the support of the Molecular Biology Core and the RCMI Publications Office (Grant # 2G12RR003050-21). This work was supported by National Institute on Drug Abuse (DA015013) and National Institute on Alcohol and Alcoholism (AA015045).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- CDC. HIV and AIDS--United States, 1981–2000. MMWR Morb Mortal Wkly Rep. 2001;50:430–434. [PubMed] [Google Scholar]
- CDC. HIV diagnoses among injection-drug users in states with HIV surveillance--25 states, 1994–2000. MMWR Morb Mortal Wkly Rep. 2003;52:634–636. [PubMed] [Google Scholar]
- Alcabes P, Friedland G. Injection drug use and human immunodeficiency virus infection. Clin Infect Dis. 1995;20:1467–1479. doi: 10.1093/clinids/20.6.1467. [DOI] [PubMed] [Google Scholar]
- Bonyhadi ML, Kaneshima H. The SCID-hu mouse: an in vivo model for HIV-1 infection in humans. Mol Med Today. 1997;3:246–253. doi: 10.1016/s1357-4310(97)01046-0. [DOI] [PubMed] [Google Scholar]
- Cho MW, Lee MK, Carney MC, Berson JF, Doms RW, Martin MA. Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4. J Virol. 1998;72:2509–2515. doi: 10.1128/jvi.72.3.2509-2515.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu TX, Levy JA. Injection drug use and HIV/AIDS transmission in China. Cell Res. 2005;15:865–869. doi: 10.1038/sj.cr.7290360. [DOI] [PubMed] [Google Scholar]
- Cohn JA. HIV-1 infection in injection drug users. Infect Dis Clin North Am. 2002;16:745–770. doi: 10.1016/s0891-5520(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Esparza J. Animal models for the evaluation of drugs and vaccines for HIV infection and AIDS: report of a WHO Working Group. Dev Biol Stand. 1990;72:367–372. [PubMed] [Google Scholar]
- Flaherty MT, Hauer DA, Mankowski JL, Zink MC, Clements JE. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J Virol. 1997;71:5790–5798. doi: 10.1128/jvi.71.8.5790-5798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz PN. Nonhuman primate models for AIDS. Clin Infect Dis 17 Suppl. 1993;1:S230–S235. doi: 10.1093/clinids/17.supplement_1.s230. [DOI] [PubMed] [Google Scholar]
- Gardner MB. Animal models for development of an AIDS vaccine. Int Rev Immunol. 1990;7:31–49. doi: 10.3109/08830189009061763. [DOI] [PubMed] [Google Scholar]
- Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50:435–442. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigwood NL. Predictive value of primate models for AIDS. AIDS Rev. 2004;6:187–198. [PubMed] [Google Scholar]
- Hall TA. BioEdit: A user friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl Acids Symp Ser. 41:95–98. [Google Scholar]
- Harrowe G, Cheng-Mayer C. Amino acid substitutions in the V3 loop are responsible for adaptation to growth in transformed T-cell lines of a primary human immunodeficiency virus type 1. Virology. 1995;210:490–494. doi: 10.1006/viro.1995.1367. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Guo CJ, Yuan CS, Douglas SD, Moss J. Methylnaltrexone antagonizes opioid-mediated enhancement of HIV infection of human blood mononuclear phagocytes. J Pharmacol Exp Ther. 2003;307:1158–1162. doi: 10.1124/jpet.103.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman TL, Stephens EB, Narayan O, Doms RW. HIV type I envelope determinants for use of the CCR2b, CCR3, STRL33, and APJ coreceptors. Proc Natl Acad Sci U S A. 1998;95:11360–11365. doi: 10.1073/pnas.95.19.11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QX, Barry AP, Wang ZX, Connolly SM, Peiper SC, Greenberg ML. Evolution of the human immunodeficiency virus type 1 envelope during infection reveals molecular corollaries of specificity for coreceptor utilization and AIDS pathogenesis. J Virol. 2000;74:11858–11872. doi: 10.1128/jvi.74.24.11858-11872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SL. Non-human primate models for AIDS vaccine research. Curr Drug Targets Infect Disord. 2005;5:193–201. doi: 10.2174/1568005054201508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C. Injection drug use fuels HIV/AIDS epidemic across Eurasia. Lancet Infect Dis. 2005;5:539. doi: 10.1016/s1473-3099(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Kinman LM, Worlein JM, Leigh J, Bielefeldt-Ohmann H, Anderson DM, Hu SL, Morton WR, Anderson BD, Ho RJ. HIV in central nervous system and behavioral development: an HIV-2287 macaque model of AIDS. AIDS. 2004;18:1363–1370. doi: 10.1097/01.aids.0000131307.62828.a1. [DOI] [PubMed] [Google Scholar]
- Kumar R, Orsoni S, Norman L, Verma AS, Tirado G, Giavedoni LD, Staprans S, Miller GM, Buch SJ, Kumar A. Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques. Virology. 2006 doi: 10.1016/j.virol.2006.06.020. on line publication. [DOI] [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78:11425–11428. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Levy JA. The value of primate models for studying human immunodeficiency virus pathogenesis. J Med Primatol. 1996;25:163–174. doi: 10.1111/j.1600-0684.1996.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Merrill JD, Mooney K, Song L, Wang X, Guo CJ, Savani RC, Metzger DS, Douglas SD, Ho WZ. Morphine enhances HIV infection of neonatal macrophages. Pediatr Res. 2003;54:282–288. doi: 10.1203/01.PDR.0000074973.83826.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negroni M, Buc H. Mechanisms of retroviral recombination. Annu Rev Genet. 2001;35:275–302. doi: 10.1146/annurev.genet.35.102401.090551. [DOI] [PubMed] [Google Scholar]
- Noel RJ, Jr, Kumar A. Virus replication and disease progression inversely correlate with SIV tat evolution in morphine-dependent and SIV/SHIV-infected Indian rhesus macaques. Virology. 2006;346:127–138. doi: 10.1016/j.virol.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Noel RJ, Jr, Marrero-Otero Z, Kumar R, Chompre-Gonzalez GS, Verma AS, Kumar A. Correlation between SIV Tat evolution and AIDS progression in cerebrospinal fluid of morphine-dependent and control macaques infected with SIV and SHIV. Virology. 2006;349:440–452. doi: 10.1016/j.virol.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Nowak MA. Variability of HIV infections. J Theor Biol. 1992;155:1–20. doi: 10.1016/s0022-5193(05)80545-4. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Nottet HS, Sasseville VG, Epstein LG, Gendelman HE. The development of animal model systems for HIV-1 encephalitis and its associated dementia. J Neurovirol. 1995;1:229–243. doi: 10.3109/13550289509114019. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Potula R, Haorah J. Rodent model systems for studies of HIV-1 associated dementia. Neurotox Res. 2005;8:91–106. doi: 10.1007/BF03033822. [DOI] [PubMed] [Google Scholar]
- Qian HZ, Schumacher JE, Chen HT, Ruan YH. Injection drug use and HIV/AIDS in China: Review of current situation, prevention and policy implications. Harm Reduct J. 2006;3:4. doi: 10.1186/1477-7517-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, Sodroski J, Letvin NL. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996a;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann KA, Li JT, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori DC, Lee-Parritz DE, Lu Y, Collman RG, Sodroski J, Letvin NL. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996b;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Sodroski J, Yang X. An unrelated monoclonal antibody neutralizes human immunodeficiency virus type 1 by binding to an artificial epitope engineered in a functionally neutral region of the viral envelope glycoproteins. J Virol. 2005;79:5616–5624. doi: 10.1128/JVI.79.9.5616-5624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer C, Keller F, Schmitt MP, Jaeck D, Adloff M, Schmitt C, Royer C, Kirn A, Aubertin AM. Morphine stimulates HIV replication in primary cultures of human Kupffer cells. Res Virol. 1991;142:189–195. doi: 10.1016/0923-2516(91)90056-9. [DOI] [PubMed] [Google Scholar]
- Singh DK, McCormick C, Pacyniak E, Griffin D, Pinson DM, Sun F, Berman NE, Stephens EB. Pathogenic and nef-interrupted simian-human immunodeficiency viruses traffic to the macaque CNS and cause astrocytosis early after inoculation. Virology. 2002;296:39–51. doi: 10.1006/viro.2002.1364. [DOI] [PubMed] [Google Scholar]
- Smyth RJ, Yi Y, Singh A, Collman RG. Determinants of entry cofactor utilization and tropism in a dualtropic human immunodeficiency virus type 1 primary isolate. J Virol. 1998;72:4478–4484. doi: 10.1128/jvi.72.5.4478-4484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado G, Kumar A. Evolution of SIV envelope in morphine-dependent rhesus macaques with rapid disease progression. AIDS Res Hum Retroviruses. 2006;22:114–119. doi: 10.1089/aid.2006.22.114. [DOI] [PubMed] [Google Scholar]
- Tyndall MW, Craib KJ, Currie S, Li K, O’Shaughnessy MV, Schechter MT. Impact of HIV infection on mortality in a cohort of injection drug users. J Acquir Immune Defic Syndr. 2001;28:351–357. doi: 10.1097/00126334-200112010-00008. [DOI] [PubMed] [Google Scholar]
- van Maanen M, Sutton RE. Rodent models for HIV-1 infection and disease. Curr HIV Res. 2003;1:121–130. doi: 10.2174/1570162033352075. [DOI] [PubMed] [Google Scholar]
- van Marle G, Power C. Human immunodeficiency virus type 1 genetic diversity in the nervous system: evolutionary epiphenomenon or disease determinant? J Neurovirol. 2005;11:107–128. doi: 10.1080/13550280590922838. [DOI] [PubMed] [Google Scholar]
- Vodros D, Fenyo EM. Primate models for human immunodeficiency virus infection. Evolution of receptor use during pathogenesis. Acta Microbiol Immunol Hung. 2004;51:1–29. doi: 10.1556/AMicr.51.2004.1-2.1. [DOI] [PubMed] [Google Scholar]