Abstract
Hepatocellular carcinoma (HCC) accounts for 80–90% of primary liver tumors and is one of the most common and devastating malignant diseases worldwide. The MAPK signaling pathway is activated in over 90% of HCCs, and RKIP has been identified as an inhibitor of the MAPK pathway. It has been observed that downregulation of RKIP expression in HCC tumors contributes to constitutive activation of the ERK/MAPK pathway and promotes proliferation and migration of HCC cells. More important, activation of IGF-I/ERK/MAPK pathways can be blocked by restoration of RKIP levels. The protein levels of RKIP are significantly reduced in HCC, whereas mRNA levels only decreased in 41% of HCC samples studied, suggesting that the downregulation of RKIP in HCC may be influenced through multiple mechanisms both at the mRNA and protein levels. In this context, mTOR inhibitor, insulin, and proteasome inhibitors were found to modulate RKIP expression in FOCUS HCC cells. A better understating of mechanisms by which RKIP expression is downregulated in HCC may be critical to develop a possible target for therapeutic intervention of HCC.
Keywords: hepatocellular carcinoma, Raf kinase inhibitor protein, post-transcriptional regulation
I. INTRODUCTION
The World Health Organization reported in 2009 that liver cancer is the fourth leading cause of cancer deaths worldwide, resulting in about 610,000 deaths per year. Primary hepatocellular carcinomas (HCCs) account for 80–90% of these liver tumors.1 Hepatocarcinogenesis is a multistep process, in the majority of cases slowly developing with a well-defined etiology of viral infection and chronic alcohol abuse, leading to chronic hepatitis and cirrhosis regarded as preneoplastic stages, and finally HCC.2 During the long preneoplastic stage, in which the liver is often the site of chronic hepatitis and/or cirrhosis, hepatocyte cycling is accelerated by upregulation of mitogenic pathways, in part through epigenetic mechanisms, and leads to produce aberrant and dysplastic hepatocytes. Development of dysplastic hepatocytes in foci and nodules and emergence of hepatocellular carcinoma are associated with the accumulation of irreversible structural alterations in genes and chromosomes. However, there are currently no consistent genetic sequences of events identified that lead to HCC formation, and HCCs exhibit the extensive heterogeneity of gene alterations, suggesting that multiple molecular signaling pathways may be involved in its development.3 A great number of growth factors, receptors, and downstream elements of their signaling cascade are known to be involved in HCC development. In addition, specialized pathways associated with Wnt and Hedgehog are described as one of those factors triggering the malignant outcomes owing to liver disorder.4–6
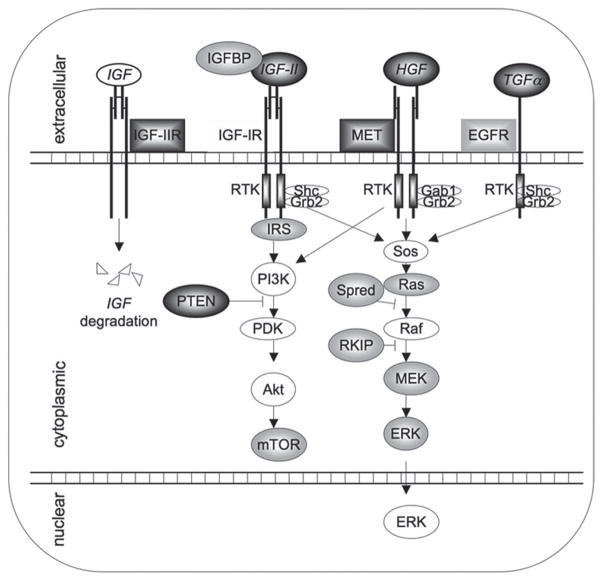
The MAPK signaling pathways are highly conserved and involved in cell growth, differentiation, survival, and invasion.7 Many different growth factors, including insulin and insulinlike growth factor (IGF), activate the ERK/MAPK pathway (Fig. 1).
FIGURE 1.
Schematic and simplified display of different growth factors and their downstream signaling pathways that are frequently involved in the development and progression of human hepatocellular carcinogenesis. Predominantly dysregulated signaling components are highlighted in dark gray. Molecules not expressed by tumor cells (HGF) and distinct protein family members dysregulated in HCCs (IRS) are presented in light gray. Gab1: GRB2-associated binding protein 1; Grb2: growth factor receptor-bound protein 2; IGFBP: IGF binding protein; IRS: insulin receptor substrate; MEK: mitogen-activated protein kinase kinase; mTOR: mammalian target of rapamycin; PI3K: phosphatidylinositol 3-kinase; PIP2: phosphatidylinositol bisphosphate; PKC: protein kinase C; PLC: phospholipase C; PTEN: phosphatase and tensin homologue; RKIP: Raf kinase inhibitor protein; RTK: receptor tyrosine kinase; Shc: (Src homology 2 domain containing) transforming protein; SOS: son of sevenless.
The IGF/ERK/MAPK signaling pathway has a major role in regulation of fetal development, proliferation, differentiation, cell growth, and apoptosis. Dysregulation of IGF signaling in HCC predominantly occurs at the level of IGF-II. IGF-II gene expression is increased in multiple malignancies, including HCC.8 IGF-II is overexpressed in 16–40% of human HCCs, and possibly even in some premalignant lesions,9–11 HCC cell lines,11,12 and several HCC animal models.13,14 More than 70% of IGF-II is bound to IGF-binding protein 3 (IGFBP3), the most abundant circulating binding protein for IGFs.15 Thus, downregulation of IGFBPs may contribute to elevated IGF function in tumor tissues. Indeed, expression levels of IGFBP-1, -3, and -4 have frequently been reduced in HCCs.16,17 Constitutive activation of components of this pathway due to overexpression of insulin receptor substrate 1 (IRS-1) has been observed in situations of unrestrained growth, including the majority of human HCCs.18,19 Conversely, inhibition of IGF/ERK/MAPK signaling by a dominant-negative IRS-1 protein has reversed the malignant phenotype of human HCC cells.20 This aberrant activation of the ERK/MAPK signaling cascade is associated with increased HCC tumor size.21,22 In addition, overexpression and phosphorylation of MAPK (ERK1/2) was detected in 91% and 69% of HCCs, respectively.23 Overexpression of Ras proteins is frequently observed in HCC.24 In HCV-associated HCC, the ERK/MAPK pathway is activated, having a positive role in HCC proliferation.25
The Raf kinase inhibitor protein (RKIP) was identified as an inhibitor of the MAPK signaling pathway.26 Prior studies have found an inverse relationship between RKIP expression and tumor metastasis in human breast cancer, ovarian cancer, colorectal cancer, and prostate cancer.27–33 Little is known about the role of RKIP in human hepatocarcinogenesis and how RKIP may be regulated in HCC cells. In this article, we summarize our studies on RKIP expression in human and mouse HCC, and functional consequences of RKIP expression in HCC cell lines, and discuss possible mechanisms by which RKIP protein is downregulated in HCC.
II. DOWNREGULATION OF RKIP PROTEIN EXPRESSION IN HCC
One investigation evaluated the expression level of RKIP protein by immunohistochemical staining in 17 pairs of human HCC tumors and corresponding adjacent peritumoral tissues.6 RKIP staining was detected in 82.3% (14 of 17) peritumal tissues, but only in 12% (2 of 17) of HCC tumor tissues (p < 0.001) (Figs. 2A and 2B). In addition, Western blot analysis using 8 of the 17 paired samples showed decreased RKIP protein levels in 88% (7 of 8) of HCC tumors compared to adjacent peritumoral tissues. Consistent with these results, an increase in ERK and MAPK phosphorylation was also found in these seven HCC tumor samples, demonstrating that the ERK/MAPK signaling cascade was activated in the down-regulation/loss of RKIP protein expression in human HCC. Interestingly, RKIP mRNA levels evaluated by real-time RT-PCR were only decreased in 41% of HCC tumor samples, increased in 47%, and revealed no change in 12% of the HCC samples. Overall, there was no significant difference in of RKIP mRNA between HCC tumors and corresponding peritumoral tissues (p > 0.5). Moreover, there was no correlation between expression levels of RKIP mRNA and protein in the same HCC samples. In this regard, reduction of RKIP protein expression in HCC may be through mainly post-transcriptional regulations including mRNA/protein stability and/or translation.
FIGURE 2.
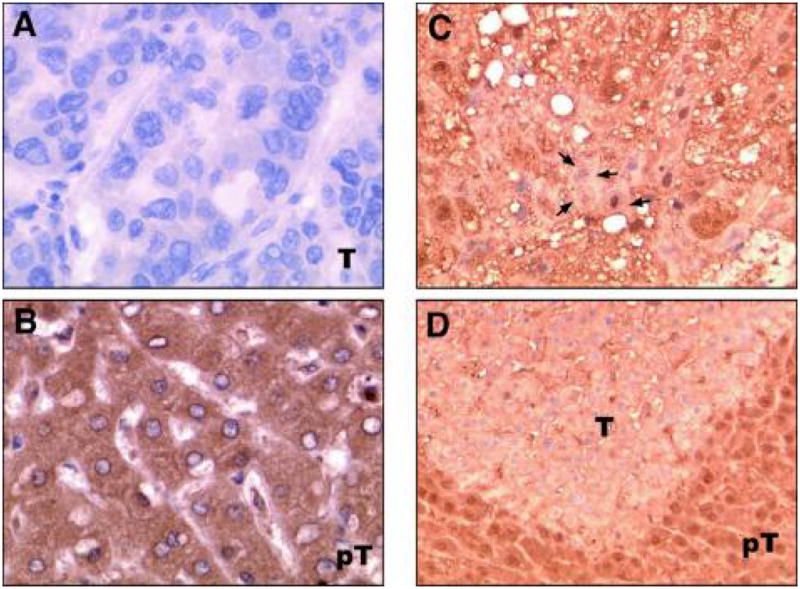
Downregulation of RKIP protein expression in HCC assessed by immunohistochemical staining. (Left panel) RKIP protein expression in human HCC tissue samples. Representative examples of HCC and peritumoral areas were immunostained with anti-RKIP antibody, and counterstained with hematoxylin. Magnification was 200x. (A) Negative (−) staining of a HCC tumor. (B) Strong staining of peritumoral tissues. (Right panel) RKIP protein expression in dysplastic hepatocyte and HCC of ATX+/IRS-1+ transgenic mice. (C) Loss of RKIP protein expression was found in dysplastic hepatocytes (arrows). (D) A small HCC (T) shows absence of RKIP expression, while a high level of RKIP expression in normal surrounding hepatocytes (pT) in the liver.
In corroboration with these results, RKIP protein expression was downregulated in dysplastic hepatocytes and HCC of ATX+/IRS-1+ transgenic mice. Loss of RKIP protein expression was found in dysplastic hepatocytes (Fig. 2C, arrows) and a small HCC (Fig. 2D, T) derived from the liver of an ATX+/IRS-1+ double transgenic animal, while a high level of RKIP expression was found in normal surrounding hepatocytes (Fig. 2D, pT) in the liver.
Taken together, downregulation of RKIP protein was found in both human and transgenic murine HCC. The loss of RKIP-mediated Raf kinase inhibition may lead to aberrant phosphorylation and activation of MEK by Raf, allowing MEK to then phosphorylate and activate ERK. The enhanced proliferative stimulus of HCC may be due, in part, to this loss of RKIP protein and the corresponding dysregulation of the ERK/MAPK cascade.
III. EFFECTS OF RKIP EXPRESSION ON HCC CELL PROLIFERATION AND MIGRATION
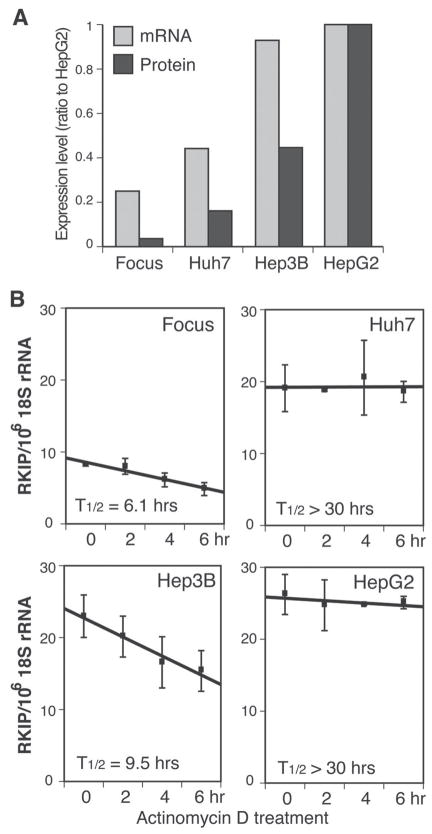
It was determined the levels of RKIP protein and mRNA in four human HCC cell lines, namely, FOCUS, Huh7, Hep3B, and HepG2. These cell lines have different degrees of differentiation (FOCUS < Huh7 < Hep3B < HepG2) according to characteristics such as morphology, growth rate, production of liver specific proteins such as albumin, α-anti-trypsin, and transferrin, as well as an anchorage-independent growth in soft agar and tumor formation in nude mice. Interestingly, the least differentiated FOCUS cells showed the lowest level of RKIP mRNA and protein levels, whereas the well-differentiated HepG2 cells expressed the highest level of RKIP protein as well as mRNA. These results suggest a correlation between RKIP expression and HCC cellular differentiation. The expression levels of RKIP mRNA were reasonably correlated with RKIP protein levels, with the exception of Hep3B cells, which had similar mRNA levels, while RKIP protein was less than 50% compared to HepG2 cells (Fig. 3A). These observations are consistent with the findings in human HCC tumor samples and underscore that regulation of RKIP expression in HCC may be significantly affected by post-transcriptional mechanisms.
FIGURE 3.
Levels of RKIP mRNA, protein, and mRNA stability of RKIP in four HCC cell lines. (A) Comparison of RKIP mRNA and protein expression levels in different HCC cell lines. RKIP protein levels were measured by Western blot analysis and normalized to actin. Levels of RKIP mRNA expression were quantified by quantitative real-time RT-PCR and normalized by copy number of 18S rRNA as an internal control. Expression levels were plotted as in relation to the level of RKIP in HepG2. (B) RKIP mRNA stability in four HCC cell lines. Various HCC cell lines (FOCUS, Huh7, Hep3B, HepG2) were seeded onto six-well plates with 105 cells per well, 48 h prior to treatment with or without 10 μg/ml of actinomycin D. Total RNA were purified using TRIzol reagent after 0, 2, 4, and 6 h of actinomycin D treatment. The RKIP mRNA levels were quantified using quantitative real-time RT-PCR. The results reported are the mean of four assays. The half-life (T1/2) of RKIP mRNA is also indicated.
Low RKIP levels correlated with activation of ERK/MAPK signaling. In FOCUS cells, which express low RKIP protein levels, high MEK and ERK phosphorylation were observed. In contrast, MEK and ERK phosphorylation were low in HepG2 cells, which express high levels of RKIP protein. Thus, it is possible that RKIP expression influences directly on this signaling pathway in HCC cell lines. As expected, on restoration of RKIP in FOCUS cells, which express low RKIP expression, IGF-1–induced phosphorylation of both MEK and ERK was diminished compared to control cells as measured by Western blot analysis. Knockdown of RKIP using siRNA in HepG2 cells exhibited the increased IGF-1–induced MEK and ERK phosphorylation.6 It is known that activated ERK translocates to the nucleus and modulates gene expression through the phosphorylation of target transcriptional factors. Therefore, the effect of RKIP on nuclear phospho-ERK accumulation was investigated. In this regard, IGF-1 stimulation of FOCUS cells showed an increase in nuclear phospho-ERK, which was abolished by restoration of RKIP as assessed by Western blot analysis as well as double-label immunofluorescent staining with anti-RKIP and anti–phospho-ERK antibodies. These results indicate that the level of RKIP is a key factor in the modulation of IGF-1–induced ERK/MAPK signaling in HCC cells.
Finally, functional analysis was accessed since activation of the ERK/MAPK pathway in HCC leads to cell proliferation, migration, and inhibition of apoptosis. In this regard, ectopic RKIP expression by stable transfection did not affect the basal cell proliferation rate. However, IGF-1–stimulated FOCUS cells showed an increased cell proliferation rate, whereas this increase was abolished in the RKIP transfected FOCUS cells. Moreover, overexpression of RKIP also significantly decreased IGF-1–mediated FOCUS HCC cell motility compared to controls as measured by the Transwell chamber cell motility assay and further confirmed by a wound-healing assay.6 These findings are consistent with the idea that RKIP overexpression antagonized IGF-1 activation of the ERK/MAPK signaling, resulting in the downstream biological consequences being reduced proliferation and migration of HCC cells.
IV. MOLECULAR MECHANISMS OF RKIP EXPRESSION IN HCC
Although studies exhibited downregulation of RKIP in human and mouse HCC,6,34 the mechanisms responsible for downregulation of RKIP expression in HCC still remain to be studied. Our observations indicate that downregulation of RKIP expression in tumors is likely to be complex and involve transcriptional and/or post-transcriptional regulation.
Hypermethylation of promoter regions of DNA is an important epigenetic mechanism for gene silencing, commonly used by cancer cells to inactivate tumor suppressor genes. However, it has been reported that hypermethylation of promoter regions is not the cause of RKIP downregulation in prostate cell lines and colorectal cancer.35,36 Recently, Arai et al. investigated DNA methylation profiles in human HCC as well as noncancerous liver tissue samples and reported at which genomic locations had hypo- or hypermethylation in HCC compared to noncancerous liver tissue.37 According to this report, regulation of RKIP by hypermethylation can be excluded. Snail, a mediator of the epithelial-mesenchymal transition, inhibits RKIP transcription and negatively correlates with RKIP levels in tumors, consistent for a role of RKIP in metastasis.35 However, there is no correlation between levels of Snail and RKIP expression in HCC cell lines tested (unpublished data). So far, there are no reported studies that demonstrate how RKIP expression is regulated at the transcriptional level in human HCC, and further investigations are required.
One of post-transcriptional regulatory mechanisms explored was the examination of mRNA stability in four different HCC cell lines. The half-life of RKIP mRNA was measured by quantitative real-time RT-PCR with 18s rRNA as an internal control after 0, 2, 4, and 6 h postactinomycin D treatment as shown in Fig. 3B. FOCUS cells were found to have the shortest RKIP mRNA half-life (6.1 h). The shorter half-life of Hep3B (9.5 h) in comparison to HepG2 (>30 h) could explain why the level of RKIP protein expression in Hep3B was lower than that for a HepG2 although the level of RKIP mRNA was similar in both cell lines. However, the half-life of RKIP mRNA does not correlate with the level of RKIP protein expression in HCC cell lines in general. Based on quantitation of RKIP transcript levels in the various metastatic cancer cell lines such as breast and prostate cancer cell lines by qPCR, the findings demonstrated that they correlated with the levels of the protein, suggesting that RKIP expression is downregulated at the RNA level via changes in mRNA stability or transcription initiation. Nevertheless, it is unlikely that downregulation of RKIP protein is related to mRNA levels in certain HCC cell lines. This suggests that mRNA stability may only be one aspect of a complex mechanism for the downregulation of RKIP protein.
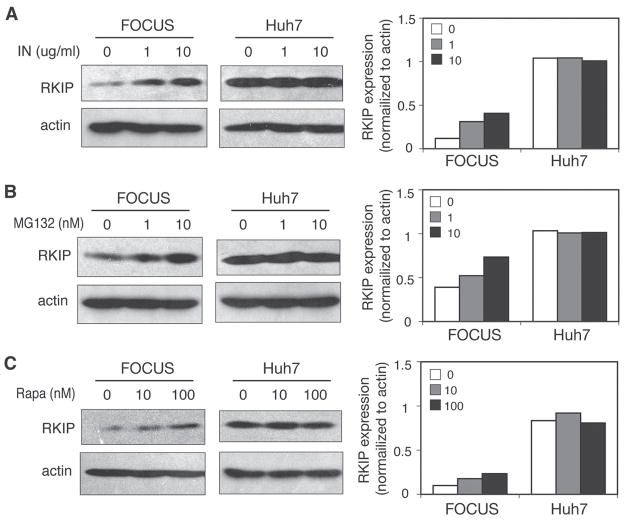
An attempt was made to examine various reagents by which RKIP protein might be restored in HCC cell lines. The effect of various growth factors on RKIP expression was tested at various time points on FOCUS and Huh7 HCC cell lines (data not shown). Among these growth factors including insulin, IGF-I, and IGF-II, it was found that insulin had the greatest effect on RKIP protein expression in a dose-dependent manner in FOCUS cells, not in Huh7 cells. As shown in Fig. 4A, insulin treatment of FOCUS cells increased RKIP protein expression in a dose-dependent manner, while there was no effect on RKIP expression in Huh7 HCC cells. A comprehensive understanding of the response to insulin in FOCUS cells will require further studies.
FIGURE 4.
Differential modulation of RKIP protein levels by insulin, MG132, and rapamycin in different HCC cell lines. FOCUS and Huh7 HCC cells were plated onto six-well plates with a confluence of 60–80%. Cells were then starved with a serum-free medium of DMEM for 24 h. After 24 h of inactivation, cells were treated with different reagents as indicated for 30 min to 2 h. Cells were harvested and subjected to Western blot analysis. Actin was used as a loading control. RKIP protein levels were modulated by insulin (A), MG132 (B), and rapamycin (C) in a dose-dependent manner in FOCUS cells, not in Huh7 cells.
To determine the role of proteosomal degradation in RKIP regulation, the proteosome inhibitor MG132 was added to FOCUS and Huh7 cells (Fig. 4B). RKIP protein levels, as measured by Western blotting, indicated that RKIP was stabilized in the cytoplasm of FOCUS cells by inhibiting degradation via the ubiquitination pathway. In contrast, Huh7 cells showed no change in RKIP protein stability after MG132 treatment.
The mammalian target of rapamycin (mTOR) is a key molecule in translation and may have an effect on RKIP expression through translational regulation. In this context, FOCUS (the least differentiated and lower levels of RKIP expression) and Huh7 HCC cells were examined for the effect of rapamycin on RKIP protein expression and downstream of mTOR signaling. Rapamycin treatment did increase RKIP protein expression as measured by Western blot as shown in Fig. 4C. Among downstream components of mTOR, there was no apparent change in p-eIF4E or p-4EBP1, but phosphorylation of P70S6 kinase and S6 were decreased by treatment with rapamycin. Without any upstream signal, inhibition of mTOR by rapamycin resulted in an increased level of RKIP protein in FOCUS HCC cells, suggesting that mTOR may be a negative regulator for RKIP expression. However, there was no effect on RKIP protein level in Huh7 HCC cells. This indicates that the regulating mechanisms of RKIP expression are likely different in different HCC cell lines.
V. CONCLUSION
What causes the differences in RKIP mRNA and protein expression in the HCC cell lines as well as human HCC is not yet defined. It has been shown that downregulation of RKIP protein expression is correlated with increased malignancy and a lack of differentiation. However, regulation of RKIP mRNA transcription is unlikely to completely account for the observed patterns of protein expression. This was especially apparent in some human HCC samples, where protein expression was attenuated while mRNA expression was apparently unaffected. This paradox seemed to indicate that RKIP regulation is most likely taking place at the post-transcriptional level as well as the transcriptional level. To our knowledge, there have been no studies to date to address these important regulatory phenomena. Our observations indicate that RKIP expression is regulated by multiple mechanisms that are dependent on mRNA stability, translational controls, and proteosomal degradation. Further studies to define mechanisms by which RKIP expression is regulated in HCC may be critical to develop possible targets for therapeutic intervention of HCC.
Acknowledgments
The authors acknowledge grants from the National Institutes of Health (Grants No. CA-35711, No. CA-123544 (JRW), and No. P20 RR015578) and a Developmental Research Award from Rhode Island Hospital (MK).
ABBREVIATIONS
- Gab1
GRB2-associated binding protein 1
- Grb2
growth factor receptor-bound protein 2
- HCC
hepatocellular carcinoma
- IGF
insulinlike growth factor
- IGFBP
IGF binding protein
- IRS-1
insulin receptor substrate 1
- MEK
mitogen-activated protein kinase kinase
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositol 3-kinase
- PIP2
phosphatidylinositol biphosphate
- PKC
protein kinase C
- PLC
phospholipase C
- PTEN
phosphatase and tensin homologue
- RKIP
Raf kinase inhibitor protein
- RTK
receptor tyrosine kinase
- SOS
son of sevenless
References
- 1.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Libbrecht L, Desmet V, Roskams T. Preneoplastic lesions in human hepatocarcinogenesis. Liver Int. 2005;25:16–27. doi: 10.1111/j.1478-3231.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- 3.Feitelson MA, Sun B, Satiroglu Tufan NL, Liu J, Pan J, Lian Z. Genetic mechanisms of hepatocarcinogenesis. Oncogene. 2002;21:2593–604. doi: 10.1038/sj.onc.1205434. [DOI] [PubMed] [Google Scholar]
- 4.Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25:212–25. doi: 10.1055/s-2005-871200. [DOI] [PubMed] [Google Scholar]
- 5.Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS, Diehl AM. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–57. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- 6.Lee HC, Tian B, Sedivy JM, Wands JR, Kim M. Loss of Raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology. 2006;131:1208–17. doi: 10.1053/j.gastro.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill E, Kolch W. Conferring specificity the ubiquitous Raf/MEK signalling pathway. Br J Cancer. 2004;90:283–8. doi: 10.1038/sj.bjc.6601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Q, Liu YF, Zhang JF, Zhang SX, Li DF, Yang JJ. Expression of insulin-like growth factor II in hepatitis B, cirrhosis and hepatocellular carcinoma: its relationship with hepatitis B virus antigen expression. Hepatology. 1994;20:788–99. doi: 10.1002/hep.1840200404. [DOI] [PubMed] [Google Scholar]
- 9.Cariani E, Lasserre C, Seurin D, Hamelin B, Kemeny F, Franco D, Czech MP, Ullrich A, Brechot C. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors, and liver cirrhosis. Cancer Res. 1988;48:6844–9. [PubMed] [Google Scholar]
- 10.Aihara T, Noguchi S, Sasaki Y, Nakano H, Monden M, Imaoka S. Clonal analysis of precancerous lesion of hepatocellular carcinoma. Gastroenterology. 1996;111:455–61. doi: 10.1053/gast.1996.v111.pm8690212. [DOI] [PubMed] [Google Scholar]
- 11.Breuhahn K, Vreden S, Haddad R, Beckebaum S, Stippel D, Flemming P, Nussbaum T, Caselmann WH, Haab BB, Schirmacher P. Molecular profiling of human hepatocellular carcinoma defines mutually exclusive interferon regulation and insulin-like growth factor II overexpression. Cancer Res. 2004;64:6058–64. doi: 10.1158/0008-5472.CAN-04-0292. [DOI] [PubMed] [Google Scholar]
- 12.Lund P, Schubert D, Niketeghad F, Schirmacher P. Autocrine inhibition of chemotherapy response in human liver tumor cells by insulin-like growth factor-II. Cancer Lett. 2004;206:85–96. doi: 10.1016/j.canlet.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Schirmacher P, Held WA, Yang D, Chisari FV, Rustum Y, Rogler CE. Reactivation of insulin-like growth factor II during hepatocarcinogenesis in transgenic mice suggests a role in malignant growth. Cancer Res. 1992;52:2549–56. [PubMed] [Google Scholar]
- 14.Harris TM, Rogler LE, Rogler CE. Reactivation of the maternally imprinted IGF2 allele in TGFalpha induced hepatocellular carcinomas in mice. Oncogene. 1998;16:203–9. doi: 10.1038/sj.onc.1201519. [DOI] [PubMed] [Google Scholar]
- 15.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 16.Gong Y, Cui L, Minuk GY. The expression of insulin-like growth factor binding proteins in human hepatocellular carcinoma. Mol Cell Biochem. 2000;207:101–4. doi: 10.1023/a:1007010818094. [DOI] [PubMed] [Google Scholar]
- 17.Huynh H, Chow PK, Ooi LL, Soo KC. A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation. Cell Growth Differ. 2002;13:115–22. [PubMed] [Google Scholar]
- 18.Khamzina L, Gruppuso PA, Wands JR. Insulin signaling through insulin receptor substrate 1 and 2 in normal liver development. Gastroenterology. 2003;125:572–85. doi: 10.1016/s0016-5085(03)00893-x. [DOI] [PubMed] [Google Scholar]
- 19.Wands J, Moradpour D. Hepatology: A textbook of liver disease. 5. Chapter 10 Philadelphia, PA: W.B. Saunders; 2006. Molecular pathogenesis of hepatocellular carcinoma. [Google Scholar]
- 20.Tanaka S, Wands JR. A carboxy-terminal truncated IRS-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J Clin Invest. 1996;98:2100–8. doi: 10.1172/JCI119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito T, Sasaki Y, Wands JR. Overexpression of human insulin receptor substrate 1 induces cellular transformation with activation of mitogen-activated protein kinases. Mol Cell Biol. 1996;16:943–51. doi: 10.1128/mcb.16.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka S, Mohr L, Schmidt EV, Sugimachi K, Wands JR. Biological effect of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology. 1997;26:598–604. doi: 10.1002/hep.510260310. [DOI] [PubMed] [Google Scholar]
- 23.Huynh H, Nguyen TT, Chow KH, Tan PH, Soo KC, Tran E. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol. 2003;3:19. doi: 10.1186/1471-230X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Zhao LJ, Wang L, Ren H, Cao J, Li L, Ke JS, Qi ZT. Hepatitis C virus E2 protein promotes human hepatoma cell proliferation through the MAPK/ERK signaling pathway via cellular receptors. Exp Cell Res. 2005;305:23–32. doi: 10.1016/j.yexcr.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Keller ET, Fu Z, Brennan M. The role of Raf kinase inhibitor protein (RKIP) in health and disease. Biochem Pharmacol. 2004;68:1049–53. doi: 10.1016/j.bcp.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Hagan S, Al-Mulla F, Mallon E, Oien K, Ferrier R, Gusterson B, Garcia JJ, Kolch W. Reduction of Raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin Cancer Res. 2005;11:7392–7. doi: 10.1158/1078-0432.CCR-05-0283. [DOI] [PubMed] [Google Scholar]
- 28.Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, Yao Z, Keller ET. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate. 2006;66:248–56. doi: 10.1002/pros.20319. [DOI] [PubMed] [Google Scholar]
- 29.Li HZ, Wang Y, Gao Y, Shao J, Zhao XL, Deng WM, Liu YX, Yang J, Yao Z. Effects of Raf kinase inhibitor protein expression on metastasis and progression of human epithelial ovarian cancer. Mol Cancer Res. 2008;6:917–28. doi: 10.1158/1541-7786.MCR-08-0093. [DOI] [PubMed] [Google Scholar]
- 30.Granovsky AE, Rosner MR. Raf kinase inhibitory protein: a signal transduction modulator and metastasis suppressor. Cell Res. 2008;18:452–7. doi: 10.1038/cr.2008.43. [DOI] [PubMed] [Google Scholar]
- 31.Zlobec I, Baker K, Minoo P, Jass JR, Terracciano L, Lugli A. Node-negative colorectal cancer at high risk of distant metastasis identified by combined analysis of lymph node status, vascular invasion, and Raf-1 kinase inhibitor protein expression. Clin Cancer Res. 2008;14:143–8. doi: 10.1158/1078-0432.CCR-07-1380. [DOI] [PubMed] [Google Scholar]
- 32.Li HZ, Gao Y, Zhao XL, Liu YX, Sun BC, Yang J, Yao Z. Effects of raf kinase inhibitor protein expression on metastasis and progression of human breast cancer. Mol Cancer Res. 2009;7:832–40. doi: 10.1158/1541-7786.MCR-08-0403. [DOI] [PubMed] [Google Scholar]
- 33.Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM, Minn AJ, Rosner MR. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–58. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuierer M, Bataille F, Weiss T, Hellerbrand C, Bosserhoff A. Raf kinase inhibitor protein is downregulated in hepatocellular carcinoma. Oncol Rep. 2006;16:451–6. [PubMed] [Google Scholar]
- 35.Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, Yeung KC. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008;27:2243–8. doi: 10.1038/sj.onc.1210860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minoo P, Zlobec I, Baker K, Tornillo L, Terracciano L, Jass JR, Lugli A. Loss of Raf-1 kinase inhibitor protein expression is associated with tumor progression and metastasis in colorectal cancer. Anatomic Pathol. 2007;127:820–7. doi: 10.1309/5D7MM22DAVGDT1R8. [DOI] [PubMed] [Google Scholar]
- 37.Arai E, Ushijima S, Gotoh M, Ojima H, Kosuge T, Hosoda F, Shibata T, Kondo T, Yokoi S, Imoto I, Inazawa J, Hirohashi S, Kanai Y. Genome-wide DNA methylation profiles in liver tissue at the precancerous stage and in hepatocellular carcinoma. Int J Cancer. 2009;125:2854–62. doi: 10.1002/ijc.24708. [DOI] [PubMed] [Google Scholar]