Abstract
Green tea's health benefits have been attributed to its major polyphenols, the catechins: (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), and epicatechin (EC). Catechins (especially EGCG) modulate a wide range of biologically important molecules, including many membrane proteins. Yet, little is known about their mechanism(s) of action. We tested the catechins' bilayer-modifying potency using gramicidin A (gA) channels as molecular force probes. All the catechins alter gA channel function and modify bilayer properties, with a 500-fold range in potency (EGCG > ECG >> EGC > EC). Additionally, the gallate group causes current block, as evident by brief downward current transitions (flickers).
Keywords: polyphenols, epigallocatechin gallate, EGCG, membrane effect
Introduction
Green tea (Camellia sinensis) and green tea extracts have been reported to have a wide range of health benefits, being remedial for diverse diseases including cancer, cardiovascular disease, Alzheimer's and obesity [1]. These benefits have been attributed to green tea's major polyphenols, the catechins: EGCG, ECG, EGC, and EC (Fig. 1)—all of which are strong anti-oxidants. The most bioactive (and abundant) catechin is EGCG, followed closely by ECG, and then to a lesser extent EGC and EC; for reviews on the green tea catechins see [1–7].
Figure 1.
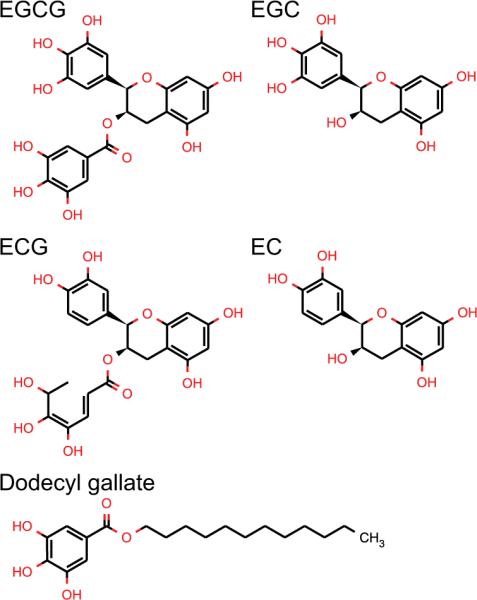
Structures of the green tea's catechins: (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), and epicatechin (EC) and of dodecyl gallate.
The green tea catechins, especially EGCG (the most studied), modulate numerous structurally dissimilar proteins at similar concentrations. Many of the affected proteins are membrane proteins e.g.: mitochondrial proton F0F1-ATPase [8], Kv1.5 potassium channels [9], hERG potassium channels [10], vascular endothelial growth factor receptor (VEGFR) [11], epidermal growth factor receptor (EGFR) [12]; for additional examples see Table 1 in [7]. The catechins localize to, and interact with, lipid bilayers [7,13–16]. They have been reported to interact with membrane lipid rafts [7,12], and their bilayer partitioning correlates with their biological effects [13]. The results suggest that the catechins may exert some of their effects through a common, bilayer-mediated mode of action—consistent with their effects on gA channels [12].
Table 1.
gA sequencesa
| [Ala1]gA | f-Ala Gly Ala Leu Ala Val Val Val Trp Leu Trp Leu Trp Leu Trp-e |
| [D-Ala1]gA− | f-Ala Gly Ala Leu Ala Val Val Val Trp Leu Trp Leu Trp-e |
| [MeTrp9,11,13,15]gA | f-Val Gly Ala Leu Ala Val Val Val MeW Leu MeW Leu MeW-e |
| [D-Val1-MeTrp9]gA | f-Val Gly Ala Leu Ala Val Val Val MeW Leu Trp Leu Trp Leu Trp-e |
| [Ser3]gA | f-Val Gly Ser Leu Ala Val Val Val Trp Leu Trp Leu Trp Leu Trp-e |
| des-(Val1-Gly2)gA | f-Ala Leu Ala Val Val Val Trp Leu Trp Leu Trp-e |
Altered residues, as compared to wild-type gA, are marked in bold. Underlined residues represent D-amino acids. The non-standard acronyms are: f, formyl; e, ethanolamide; and MeW, methylated tryptophan.
Membrane proteins are solvated by the lipid bilayer, and the proteins and their host bilayer are energetically coupled through hydrophobic interactions [17]. When membrane proteins undergo conformational transitions that involve their transmembrane domains, the bilayer adapts accordingly. This bilayer adaptation incurs an energetic cost, which varies with changes in the bilayer physical properties (e.g. thickness, intrinsic curvature, and the associated elastic and bending moduli), effectively coupling changes in bilayer properties to changes in membrane protein function. The bilayer properties are modified by amphiphiles that adsorb at the bilayer/solution interface e.g. [17], which might provide a mechanism by which the amphipathic catechins alter the function of diverse membrane proteins. To further explore the bilayer-modifying potency of the green tea catechins, we used gramicidin A (gA) channels as molecular force probes [17–19].
Materials and Methods
Lipid bilayers were formed using 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) from Avanti Polar Lipids (Alabaster, AL), which was used without further purification. The bilayer-forming solution was 2 % (w/v) DOPC in 99.9 % pure n-decane from ChemSampCo (Trenton, NJ). The catechins were from Sigma Chemical Co. (St. Louis, MO) at the highest available purity and diluted in DMSO (a few EGCG and EC experiments were done with catechins kindly provided by Dr. Yukihiko Hara, Mitsui Norin Co., Shizuoka, Japan). The gA analogues, Table 1, were prepared and purified as described previously [20]. Most experiments were done with the [Ala1]gA, which forms channels with average lifetimes ~175 ms, which is well suited for experiments in which the amphiphile may increase the channel appearance and lifetime.
Single-channel experiments were done using the bilayer punch method [21] at 25 ± 1 °C using a Dagan 3900A Integrating patch clamp (Minneapolis, MN), with 100 or 200 mV applied potential. The electrolyte solutions were 1 M CsCl or NaCl, 10 mM HEPES, pH 7.0. The current signal was low-pass Bessel filtered at 2–5 kHz, digitized at 20 kHz, and digitally filtered at 200–1000 Hz, as indicated. Single-channel current transition amplitudes and lifetimes were determined, and the respective histograms constructed, as described previously [21,22], e.g. supplementary material Fig. S1. Average channel lifetimes (τ) were determined by fitting a single exponential distribution N(t)/N(0) = exp{−t/τ}, where N(t) is the number of channels with lifetime longer than time t to the lifetime histograms [22]. The gA analogues and the catechins were added symmetrically, to the aqueous phases on both sides of the bilayer.
Results
To quantify the green tea catechins' effects on lipid bilayer properties, as sensed by a bilayer-spanning channel, we measured their effects on gA channels in planar bilayers. gA single-channel events were recorded in the absence and in the presence of increasing concentrations of the catechins. Single-channel current transition amplitudes were monitored using current transition amplitude histograms and the single-channel lifetime was determined by fitting single exponentials to normalized survivor histograms (see Fig. S1 for analysis with EC). Fig. 2 shows the increase in the normalized gA single-channel lifetime (τ) as a function of increasing [catechin]; EC, EGC, ECG, EGCG. All the catechins increase gA channel lifetime, with a 500-fold difference in potency; EGCG being the most potent and EC the least. Some catechins have additional effects, as illustrated in Fig. 3., which shows [Ala1]gA single-channel recordings without and with a selected concentration of the different catechins. EC and EGC do not alter the gA single-channel current steps, but ECG and EGCG cause qualitative changes in the channel appearance; namely downward flickers (current transitions) from the fully conducting state. Increasing the ECG or EGCG concentration increases the frequency of these downward flickers—indicating that EGCG and ECG produce intermittent interruptions of the ion flow through the channel. Occasionally the flickery current transitions were down to the non-conducting current level, but usually the transitions were too brief to be fully resolved; in some cases they would reach an ill-defined intermediate current level. At higher [EGCG], subconductance states are apparent (supplementary material Fig. S2), but the current amplitude of the fully conducting state is not changed.
Figure 2.
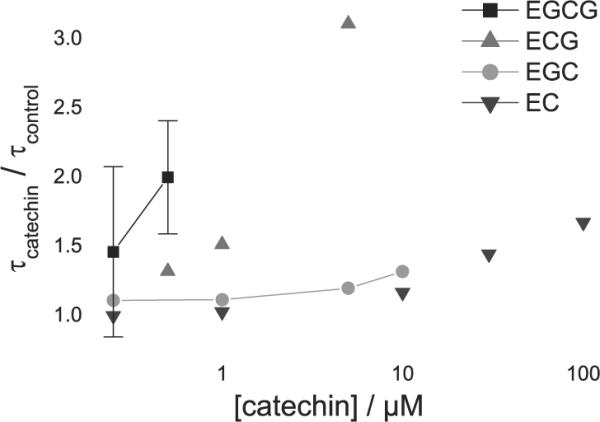
gA lifetime (τ) changes as a function of [catechin]. [Ala1]gA single-channel lifetimes, normalized relative to lifetimes determined before addition of the catechin; error bars show range for n = 2 and SE for n = 3–4. The large uncertainty in the EGCG results is due to the difficulties in determining the lifetimes of flickery channels, and the existence of long-lived subconductance states furthermore limited the concentration range that could be studied. 1 M NaCl or CsCl, 200 mV, filtered at 200–500 Hz.
Figure 3.
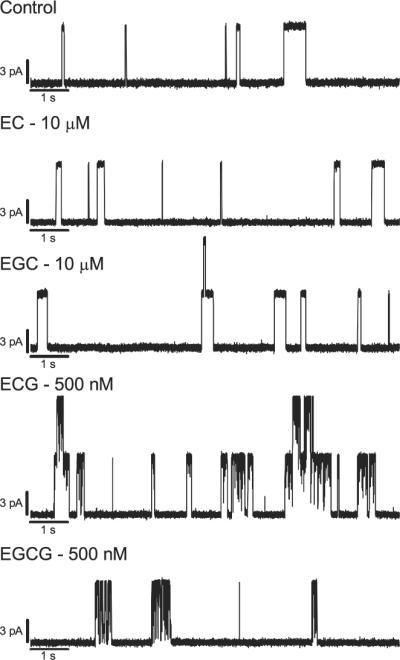
Single-channel current traces recorded in the presence of different catechins. [Ala1]gA current traces with: control, 10 μM EC, 10 μM EGC, 500 nM ECG, and 500 nM EGCG. 1 M CsCl, 200 mV, filtered at 1000 Hz.
To investigate the current flickers further, and to explore whether there could be direct binding to the gA channel, we measured the effect of EGCG on different gA analogues (Fig. 4). EGCG induces flickers in channels formed by gA analogues of opposite chirality (right- and left-handed helix sense) [Ala1]gA and [d-Ala1]gA− and variable sequence: [MeTrp9,11,13,15]gA, [d-Val1-MeTrp9]gA, [Ser3]gA, and des-(Val1-Gly2)gA (see Table 1 for amino acid sequence of the gA analogues). All these analogue channels show similar behavior with EGCG (Fig. 4). In [MeTrp9,11,13,15]gA, the four tryptophan indole NHs have been methylated, suggesting that EGCG-tryptophan interactions are unlikely to cause the flickers. [Ser3]gA, [d-Val1-MeTrp9]gA and des-(Val1-Gly2)gA have altered amino acid sequences at or close to the subunit interface, suggesting that the interface region is not important; des-(Val1-Gly2)gA channels also have a different channel length. No change was observed with a different permeant ion (Na+ instead of Cs+), and the flickers were observed also when EGCG or ECG was added asymmetrically to only one side of the bilayer and results with negative or positive potentials compared. The flickery behavior furthermore is not due to permanent chemical changes or damage to the channels, as the gA channel appearance rate and flicker frequency do not vary with time after catechin addition—and experiments with gA that was pre-incubated for 24 h with 25 μM EGCG caused no flickers (the final [EGCG] in the experimental chamber was ~25 nM, and we added the same amount of gA as in other experiments).
Figure 4.
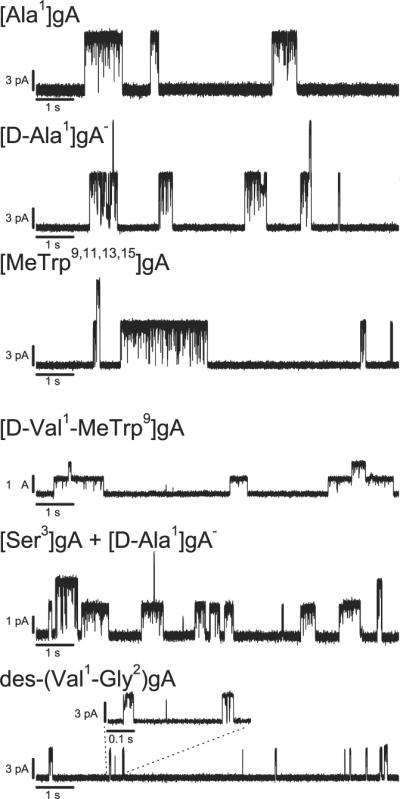
Effect of EGCG on channels formed by different gA analogues. In control current traces obtained with each of the gA analogues, no conductance flickers were observed. The traces are recorded with 0.5–1 μM EGCG, 1 M CsCl, 200 mV, filtered at 1000 Hz; except for the [D-Val1-MeTrp9]gA trace, which due to low conductance, is filtered at 200 Hz, and the [Ser3]gA + [D-Ala1]gA- (lower, higher conductance, respectively) trace is 1 M NaCl, filtered at 500 Hz.
Comparing the catechins that induce flickery behavior (EGCG and ECG) with those that do not (EGC and EC), the gallate group becomes the likely candidate for inducing the flickers (Figs. 1 and 3). To test this possibility, we used dodecyl gallate (a gallate group linked to a twelve carbon chain, which facilitates incorporation at the bilayer/solution interface), which was found to induce flickery gA channels at high nM concentrations (Fig. 5). In contrast to the green tea catechins, dodecyl gallate causes a reduction in gA channel lifetime: the normalized lifetime change (τdodecyl gallate/τcontrol) was 0.91 ± 0.04 at 0.5 μM and 0.77 ± 0.05 at 1 μM (mean ± SE).
Figure 5.
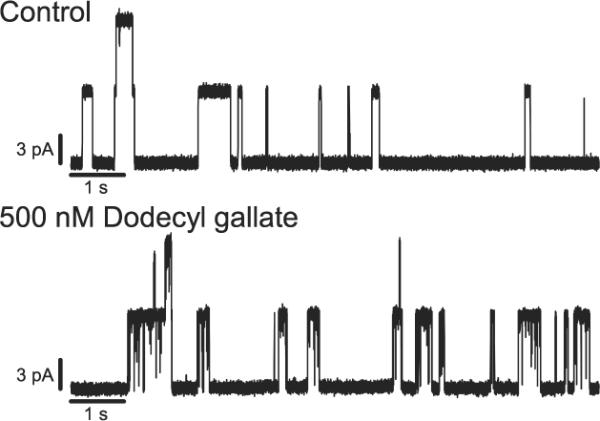
Effect of dodecyl gallate on [Ala1]gA single-channel behavior. The traces are recorded with 200 mV applied potential in 1 M CsCl and filtered at 500 Hz.
Discussion
The green tea catechins modify lipid bilayer properties, as sensed by gA channels. Incorporating the catechins into lipid bilayers increases the gA channel lifetime, meaning that the catechins reduce the disjoining force imposed by the bilayer on the channel [17]. EGCG is the most potent catechin, with detectable bilayer effects in the high nM range, followed closely by ECG. EGC and EC are far less potent, showing no effects at low μM concentrations and being only weak modifiers at higher concentrations (Fig. 2). The rank order of the green tea catechins' bilayer-modifying potency, EGCG > ECG >> EGC > EC, is similar to that reported for their biological effects, as summarized in [5,13,23,24]. Notably, the gallate-containing catechins are the most potent, in terms of both bilayer modification and their biological effects.
The catechins alter lipid bilayer properties at concentrations similar to those where they alter the function of many membrane proteins [7–12]—suggesting that they may exert their effects on membrane protein function by a common (e.g., bilayer mediated) mechanism, rather than by direct catechin binding to each of the diverse proteins. Because the green tea catechins adsorb at the bilayer/solution interface and modify bilayer properties, they will effectively alter the function of any membrane protein. The extent of the functional changes will vary among different proteins, depending on the extent of bilayer adjustment required for particular conformational changes (i.e., each protein's sensitivity to bilayer properties) [17]. Similar effects on bilayer properties, reported using gA channels as molecular force probes, have been observed with other phytochemicals; e.g. genistein [25], capsaicin [26] and curcumin [27].
The effects of EGCG and ECG on gA channels are complex, nevertheless, as these compounds in addition to altering bilayer properties (increasing the single-channel lifetime), also cause the appearance of current flickers or subconductance states (Figs. 3 and S2). The current flickers occurred only with the gallate-containing catechins and, because these two catechins have the greatest biological effect, we cannot exclude that the catechins biological effects somehow may be related to chemical interactions similar to those underlying gA channel block (albeit with very different targets).
To explore whether the gallate group caused the flickers, dodecyl gallate was tested and found to produce similar behavior (Fig. 5). In addition to causing flickery gA channels, dodecyl gallate also reduced the gA channel lifetime. The lifetime changes are opposite to those produced by the catechins, but consistent with the expected changes in bilayer properties caused by the insertion of a long saturated carbon chain (based on results with long-chain alcohols [28]). That the gallate group produces flickery gA channel behavior suggests direct interactions with the gA channel. The flicker frequency increased gradually with increasing concentration of gallate-containing molecules (Figs. 3 and S2). The flickers are not resolved at our highest filter frequency > 5000 Hz—indicating that the dissociation rate constants are high. To further explore the interaction of the gallate group with gA channels, we tested a number of gA analogues (Table 1). The general pattern did not depend on helix sense or the identity of residues close to the bilayer/solution interface or the subunit interface (Fig. 4). All of the tested analogue channels showed similar behavior, suggesting that the gallate-containing compounds disrupt ion flow by blocking the pore entrance rather than by binding at the channel/bilayer boundary. (The gallate group is not a very potent channel blocker, however; assuming there is one gallate group in a hemisphere of radius 1.5 nm centered at the channel entrance, the local gallate concentration in the vicinity of the gA channel would be ~250 mM.)
The biological effects of green tea catechins likely are due to multiple molecular interactions. Because the catechins accumulate at the bilayer/solution interface and (at sub-μM concentrations) modify bilayer properties that can influence membrane protein function, it becomes important to distinguish between indirect effects on protein function, due to the bilayer-protein hydrophobic coupling, and direct effects due to binding to one or more target proteins.
Supplementary Material
Acknowledgment
We thank Yasir Tarabichi for program development to facilitate analysis of flickery gA channels and Michael J. Bruno, Ruchi Kapoor, Kevin Lum, Sushmita Mukherjee, Radda Rusinova, and R. Lea Sanford for stimulating discussions. This work was supported by grants from the National Institutes of Health (R01GM021342 and ARRA supplement GM021342-35S1 to O.S.A.).
Abbreviations
- (DOPC)
1,2-dioleoyl-sn-glycero-3-phosphocholine
- (EGCG)
(−)-epigallocatechin gallate
- (ECG)
(−)-epicatechin gallate
- (EGC)
(−)-epigallocatechin
- (EC)
epicatechin
- (gA)
gramicidin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chin. Med. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- [3].Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- [4].Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–1855. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ravindranath MH, Saravanan TS, Monteclaro CC, Presser N, Ye X, Selvan SR, Brosman S. Epicatechins Purified from Green Tea (Camellia sinensis) Differentially Suppress Growth of Gender-Dependent Human Cancer Cell Lines. Evid. Based Complement. Alternat. Med. 2006;3:237–247. doi: 10.1093/ecam/nel003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol. Carcinog. 2006;45:431–435. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- [7].Patra SK, Rizzi F, Silva A, Rugina DO, Bettuzzi S. Molecular targets of (−)-epigallocatechin-3-gallate (EGCG): specificity and interaction with membrane lipid rafts. J. Physiol. Pharmacol. 2008;59(Suppl 9):217–235. [PubMed] [Google Scholar]
- [8].Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Choi BH, Choi JS, Min DS, Yoon SH, Rhie DJ, Jo YH, Kim MS, Hahn SJ. Effects of (−)-epigallocatechin-3-gallate, the main component of green tea, on the cloned rat brain Kv1.5 potassium channels. Biochem. Pharmacol. 2001;62:527–535. doi: 10.1016/s0006-2952(01)00678-5. [DOI] [PubMed] [Google Scholar]
- [10].Kelemen K, et al. Green tea flavonoid epigallocatechin-3-gallate (EGCG) inhibits cardiac hERG potassium channels. Biochem. Biophys. Res. Commun. 2007;364:429–435. doi: 10.1016/j.bbrc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [11].Lamy S, Gingras D, Beliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–385. [PubMed] [Google Scholar]
- [12].Adachi S, Nagao T, Ingólfsson HI, Maxfield FR, Andersen OS, Kopelovich L, Weinstein IB. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- [13].Nakayama T, Hashimoto T, Kajiya K, Kumazawa S. Affinity of polyphenols for lipid bilayers. BioFactors. 2000;13:147–151. doi: 10.1002/biof.5520130124. [DOI] [PubMed] [Google Scholar]
- [14].Uekusa Y, Kamihira M, Nakayama T. Dynamic Behavior of Tea Catechins Interacting with Lipid Membranes As Determined by NMR Spectroscopy. J. Agric. Food Chem. 2007;55:9986–9992. doi: 10.1021/jf0712402. [DOI] [PubMed] [Google Scholar]
- [15].Tamba Y, Ohba S, Kubota M, Yoshioka H, Yoshioka H, Yamazaki M. Single GUV Method Reveals Interaction of Tea Catechin (−)-Epigallocatechin Gallate with Lipid Membranes. Biophys. J. 2007;92:3178–3194. doi: 10.1529/biophysj.106.097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun Y, Hung WC, Chen FY, Lee CC, Huang HW. Interaction of tea catechin (−)-epigallocatechin gallate with lipid bilayers. Biophys. J. 2009;96:1026–1035. doi: 10.1016/j.bpj.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lundbæk JA, Collingwood SA, Ingólfsson HI, Kapoor R, Andersen OS. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J. R. Soc. Interface. 2010;7:373–395. doi: 10.1098/rsif.2009.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andersen OS, Nielsen C, Maer AM, Lundbæk JA, Goulian M, Koeppe RE., II Ion channels as tools to monitor lipid bilayer-membrane protein interactions: gramicidin channels as molecular force transducers. Methods Enzymol. 1999;294:208–224. doi: 10.1016/s0076-6879(99)94013-2. [DOI] [PubMed] [Google Scholar]
- [19].Andersen OS, Koeppe RE., II Bilayer thickness and membrane protein function: An energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- [20].Greathouse DV, Koeppe RE, II, Providence LL, Shobana S, Andersen OS. Design and characterization of gramicidin channels. Methods. Enzymol. 1999;294:525–550. doi: 10.1016/s0076-6879(99)94031-4. [DOI] [PubMed] [Google Scholar]
- [21].Andersen OS. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys. J. 1983;41:119–133. doi: 10.1016/S0006-3495(83)84414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Durkin JT, Koeppe RE, II, Andersen OS. Energetics of gramicidin hybrid channel formation as a test for structural equivalence. Side-chain substitutions in the native sequence. J. Mol. Biol. 1990;211:221–234. doi: 10.1016/0022-2836(90)90022-E. [DOI] [PubMed] [Google Scholar]
- [23].Ikeda I, Imasato Y, Sasaki E, Nakayama M, Nagao H, Takeo T, Yayabe F, Sugano M. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim. Biophys. Acta. 1992;1127:141–146. doi: 10.1016/0005-2760(92)90269-2. [DOI] [PubMed] [Google Scholar]
- [24].Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–8121. [PubMed] [Google Scholar]
- [25].Hwang TC, Koeppe RE, II, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- [26].Lundbæk JA, et al. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol. Pharmacol. 2005;68:680–689. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- [27].Ingólfsson HI, Koeppe RE, II, Andersen OS. Curcumin is a modulator of bilayer material properties. Biochemistry. 2007;46:10384–10391. doi: 10.1021/bi701013n. [DOI] [PubMed] [Google Scholar]
- [28].Ingólfsson HI, Andersen OS. Alcohol Effects on Lipid Bilayer Properties. Biophys. J. 2011;101:847–855. doi: 10.1016/j.bpj.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


