Abstract
It is known from primates that alliance partners may support each other’s interests in competition with others, for example, through repeated agonistic attacks against a particular individual. We examined serial aggressive interactions between greylag goose families and other flock members. We found that repeated attacks towards the same individual were common and that up to five serial attacks by family members followed an initial attack. Family size did not affect the frequency of such serial attacks. Juvenile geese evidently benefited most from active social support through serial attacks. About 60% of the juveniles’ lost primary interactions were subsequently reversed by another family member. This may be one of the reasons why juveniles rank higher in the social hierarchy than would be expected from their age and size alone. Losses in serial attacks predominantly occurred against other, presumably higher-ranking, family geese and ganders. We propose three major functions/consequences of serial attacks. Analogous to primates, serial attacks in greylag geese may serve to reinforce a losing experience of an opponent defeated in a preceding attack. On the side of the winning family, serial attacks may reinforce the experience of winning. Both winning and losing experiences are linked with physiological consequences in higher vertebrates, affecting the future social performance of winners or losers. Finally, serial attacks may signal the agonistic potential of a family to other flock members. This is supported by heart rate data, which indicate that greylags are competent to interpret third-party relationships.
Keywords: Anser anser, greylag goose, repeated agonistic interactions, social support, social physiology, status signalling
Aggressive conflicts between two individuals may concern and involve other group members (Das 2000), because individuals in social species live in social webs (Kummer 1971). Therefore, intra- and interdyadic conflicts need to be studied in the context of relationships with other group members (Das 2000). This is particularly important in cases in which individuals repeatedly support each other in agonistic conflicts, that is, form alliances (de Waal & Harcourt 1992), which, in turn, may influence the outcome of future agonistic interactions.
Aggression backed by allies is thought to be a key feature in the evolution of social organization in primates (reviewed in Silk et al. 2004). When ecological conditions favour collective defence of resources, selection is expected to favour investment in social relations with those who are likely to provide support. Females and offspring form alliances on behalf of maternal kin more often than on behalf of other individuals (reviewed in Gouzoules & Gouzoules 1987; Silk 1987, 2002; Pereira 1988; Chapais 2001; Silk et al. 2004), for example in cercopithecine female networks.
Several kinds of social interactions following aggressive conflict have been described in primates. These include ‘reconciliation’, that is, affiliative contacts between former opponents, ‘consolation’, that is, reaffirmative contacts between the victim of aggression and a bystander (de Waal & van Roosmalen 1979) and ‘redirection’, that is, aggression by the victim against an uninvolved individual (Aureli & van Schaik 1991). Another frequent behavioural response in primates, most pertinent for this study, is ‘further aggression’ (Watts et al. 2000, page 285), that is, the occurrence of repeated aggressive acts by a primary aggressor’s kin against the target immediately after an initial aggressive act has ended (long-tailed macaques, Macaca fascicularis: Aureli & van Schaik 1991; vervet monkeys, Cercopithecus aethiops: Cheney & Seyfarth 1986). Further aggression may be advantageous for alliance partners, because it will reassure kin and/or other group members that social bonds are still strong. In addition, eavesdroppers (McGregor et al. 1997) may be deterred from attacking the aggressors. This was shown, for example in great tits, Parus major (Peake et al. 2001, 2002) and Siamese fighting fish, Betta splendens (Oliveira et al. 1998), where males used the information they obtained on relative fighting ability in subsequent aggressive interactions with the males they had observed.
To our knowledge, no study has investigated whether birds repeatedly attack individuals with the help of social allies. In this study, we examined whether family members of greylag geese also take advantage of the social support described for primates, namely the repeated agonistic interactions against a previous opponent. We refer to these attacks as ‘serial attacks by social allies’, which conforms to the definition for primates (Watts et al. 2000, Table 14.1, page 285), except that in geese the initiator of the follow-up attack may either be the primary aggressor itself or one of its immediate kin rather than an unrelated individual (‘third party’; for a precise definition of serial aggression see Methods below).
Their social organization makes geese a candidate avian model for studying serial attacks. Similar to primates, a typical, hierarchically structured goose flock consists of individuals of various ages and sexes (e.g. Lamprecht 1986a, b, 1991; Lorenz 1991; Kotrschal et al. 1993). In goose flocks, families dominate pairs in aggressive encounters and pairs tend to win against single individuals (e.g. Black & Owen 1986; Lamprecht 1986a, b, 1991; Kotrschal et al. 1993). The dominance rank of a family is determined by the gander (male), whose agonistic motivation seems to be related to the number of offspring (Lamprecht 1986a, b). Furthermore, dominance rank is affected by the social support that pair partners or family members may provide for each other (Weiß et al. 2008). Long-term social bonds are formed between males and females (Lorenz 1991), males and males (Kotrschal et al. 2006), within families, and between female siblings, resulting in a loosely female-bonded clan structure (Frigerio et al. 2001; Weiß et al. 2008). Long-term relationships are maintained within, but not between families and clans.
Geese express their affiliation and social bonds through close proximity (Lorenz 1991; Frigerio et al. 2001) as well as with certain behaviours, such as ‘greeting’, the ‘triumph ceremony’ (Fischer 1965; Lorenz et al. 1978), or postcopulatory and vocal displays. Analogous to the expression of emotions via facial expression of primates, geese make use of specific neck and body postures including certain feather erection patterns (Fischer 1965) to express their emotional states. Geese are known to provide certain forms of active social support in agonistic encounters for each other (Scheiber et al. 2005a). This active support is often a supportive calling display (‘cheering on’) by the rest of the family, with each individual stretching the neck forward towards the opponent (Lorenz 1991). Passive support, defined by stress reduction (i.e. decreased glucocorticoid levels) resulting from the presence of a social ally, has also been shown (Frigerio et al. 2003; Scheiber et al. 2005a, 2009). In addition, social support may enhance not only success in agonistic encounters, but also access to food sources (Weiß & Kotrschal 2004). In geese, such interactions within dyads, families and clans may serve to reaffirm family bonds and to signal to nonkin outsiders both high status and the agonistic potential of one’s own family. Hence, as in primates, allies in geese not only support each other’s ecological interests, but also seem to affect each other’s psychophysiological states (DeVries et al. 2003).
Our aim in the present study was to investigate serial agonistic interactions in families of greylag geese. Since social support patterns found in greylag geese parallel those of many primates, we hypothesized that they will take advantage of serial agonistic attacks in a similar way as primates. We predicted that success in serial aggression will be enhanced, rendering it particularly beneficial after lost interactions. Furthermore, we predicted that serial attacks are more likely to occur after interactions in which low-ranking family members, that is, juvenile offspring, lost against unrelated opponents, as help by family members would be most beneficial in such situations. Similarly, serial attacks should be more likely to be performed by high-ranking family members, that is, parents, as these are more likely to displace the opponent successfully. In addition, we expected that the sex and social status of opponents would influence the likelihood of serial attacks. It may be particularly important to reverse a losing experience against paired males, one of the highest-ranking opponent categories in the flock (Lamprecht 1986a, b). This is especially crucial when juveniles are involved in serial attacks as paired males are likely to dominate juveniles outside the family unit. Therefore, we predicted that fathers would respond to losses of their own offspring by repeated attacks specifically against paired males.
METHODS
Study Area and Population
A nonmigratory flock of greylag geese was introduced into the valley of the River Alm in 1973 by Konrad Lorenz and coworkers (Lorenz 1991; Hemetsberger 2001; Kotrschal et al. 2006). Individuals are unrestrained and roam the valley between the Konrad Lorenz Forschungsstelle (KLF) and a lake approximately 10 km to the south, where they roost at night. At the time of data collection, the flock consisted of around 170 geese, which were all individually marked with coloured leg bands. As in other populations, natural predation, mainly by red foxes, Vulpes vulpes, is common and may account for losing up to 10% of the flock per year (Hemetsberger 2001).
The flock is supplemented with pellets and grain twice daily on the meadows around the research station, with small quantities from spring to autumn, and greater amounts during winter. Individual life history data and social backgrounds of all individuals have been monitored continuously since 1973. Geese breed in the valley, either at natural nest sites or in relatively predator-safe nestboxes provided by the KLF. Every few years a few geese are carefully hand-raised to keep the flock accessible to humans. Both hand-raised and goose-raised flock members are habituated to the close presence of humans. Geese do not show avoidance behaviour if approached up to 1 m distance, nor do they excrete elevated levels of immunoreactive corticosterone metabolites (Scheiber et al. 2005b) or show significant heart rate changes when familiar humans approach, as could be determined from 25 geese implanted with heart rate transmitters (C. Wascher, unpublished data). This indicates that human observers do not cause measurable arousal and are unlikely to have a negative effect on agonistic motivation even in the goose-raised geese.
Ten focal families were studied in 2003–2004, all raising at least one offspring (range 1–6 juveniles, juveniles) to fledging. For a detailed description, see Scheiber et al. (2005a). In total, we collected data from 53 individuals: 10 adult males, 10 adult females and 33 juveniles.
Data Collection
We collected behavioural data between August 2003 when the flock re-established itself after moult and February 2004 when the flock disintegrated into pairs and parent–offspring bonds loosened owing to the pairs preparing for breeding. One of us (I.S.) observed all agonistic interactions of the members of one focal family per day during morning feedings. A family was observed for the entire time it spent in the feeding area, or for a maximum of 1 h, if it did not leave until then. An agonistic interaction was defined as an encounter between two geese, in which one of them adopted a threat posture and caused the opponent to retreat. Retreating is defined as ceasing to participate in the interaction through withdrawal. The retreating goose was considered the loser of the interaction and the goose that elicited the retreat was considered the winner. In our flock, the initiator of an agonistic interaction is generally also the winner, and in the majority of cases is the higher-ranking of the two opponents (Weiß & Kotrschal 2004), which resembles patterns found in long-tailed macaques (de Waal et al. 1976; Netto & van Hooff 1986).
During each observation we recorded which focal individual was involved in an agonistic interaction, whether the interaction was won or lost and the social category (family, paired, single) and sex of the opponent (Scheiber et al. 2005a). We distinguished eight categories of opponents: (1) family males (i.e. paired males that during data collection had at least one fledged offspring); (2) paired males (i.e. males that were paired to either a goose or a gander); (3) single males; (4) family females; (5) paired females; (6) single females; (7) juveniles (i.e. related juveniles or offspring of other families; however, only one follow-up attack occurred against a related juvenile, see below); and lastly (8) the previous opponent (i.e. the individual involved in the preceding interaction). Category (8) thus comprises the opponents in serial attacks, which can be individuals of all social categories and of both sexes.
During our behavioural observations we noted whether the family member involved in an agonistic interaction or any of its kin interacted with the opponent sequentially, that is, within 5 s of the initial aggressive act ending (see social category 8 above). We define the original aggressive act as the ‘primary attack’, irrespective of whether the family member involved won or lost the interaction, and we denote the other combatant from outside the family as the opponent, regardless of the outcome of the agonistic interaction. Any agonistic interaction that follows a primary attack is defined as a ‘follow-up attack’. We define ‘stand-alone attacks’ as all other agonistic interactions with individuals of the flock, which, in essence, are primary attacks not followed by another attack against the same opponent.
Overall, we observed 4474 agonistic interactions in 22.5 h of observation time. Of these, 3577 were stand-alone attacks, 415 were primary attacks and 482 were follow-up attacks to these primary attacks. Follow-up attacks against the same opponent thus occurred in 10.4% of all observed events. We observed series of up to five follow-up attacks (Fig. 1). Agonistic interactions within a family unit were rare: only 23 of 3577 (0.64%) stand-alone attacks and only one of 415 serial attacks (0.24%) occurred within the family. To avoid pseudoreplication, we used only the first follow-up attack for all analyses (N = 415), no matter how long the series. Another source of pseudoreplication may be the occurrence of the same attacker–opponent dyads in different serial attacks. We cannot specify which of the serial attacks involved the same attacker and recipient for this data set, because we only recorded the opponent’s social category but not its identity. However, 16 years of data on the dominance hierarchy (B.M. Weiß, unpublished data) of the flock suggest that attacks against the same opponent occur approximately 15–20% of the time, dependent upon season. These values include both serial attacks as defined in this paper and subsequent attacks at a later time (which is what is of interest in the question of pseudoreplication); the number of sample units occurring repeatedly will thus be even lower. However, to avoid the problem of pseudoreplication altogether we analysed our data with generalized linear mixed models (see below) and used the focal identity as a random factor to account for possible multiple measurements of the same focal individuals (and thus the same attacker–opponent dyads).
Figure 1.
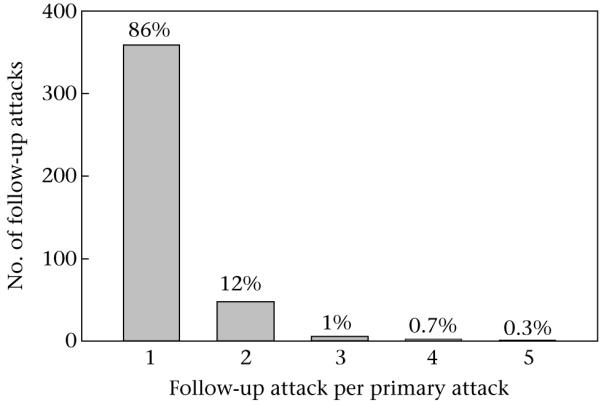
Occurrence of series of follow-up attacks (1–5) to a primary attack. Percentages are given above the bars.
Data Analyses
We conducted three generalized linear mixed models (GLMMs, see below). We applied the restricted maximum likelihood (REML) procedure for repeated sampling with an unbalanced design using the GenStat 10.1 statistical package (GenStat 2007). We present Wald statistics for REML, because the change in deviance when dropping a term from the model approximates a chi-square distribution (Foerster & Kempenaers 2005). We deleted sequentially fixed terms in order of decreasing significance; only terms, with P < 0.1 remained in the final model. Excluded terms were re-entered one by one into the final model to confirm that they did not explain a significant part of the variation (Poesel et al. 2006). In all three models we included individual identity as a random factor to account for repeated measures within individuals. A second random term, ‘family identity’ was included initially. However, ‘family identity’ was aliased with ‘individual, identity’, that is, the effects of the random term ‘family identity’ were strongly linked to the random term ‘individual identity’, and we therefore dropped ‘family identity’ from all analyses.
In the first GLMM we compared stand-alone and primary attacks to determine what might cause the continuation of an agonistic attack in the form of follow-up attacks. We constructed this GLMM with the binomial variable ‘stand-alone attack or primary attack’ as the response variable, ‘individual identity’ as a random term, and ‘sex of focal individual’, ‘age class of focal individual (adult–juvenile)’, ‘opponent category’, ‘number of offspring per family’ and ‘outcome (won–lost) from the focal individual’s perspective’ as fixed terms.
The second GLMM was constructed within serial attacks only, and compared primary and the first follow-up attacks. It was assembled with the binomial variable ‘primary attack or follow-up attack’ as the response variable, ‘individual identity’ as a random term, and ‘sex of focal individual’, ‘age class of focal individual (adult–juvenile)’ and ‘outcome (won–lost) from the focal individual’s perspective’ as fixed terms. By definition, the same opponent participated in both the primary and its resulting follow-up attack. Therefore, the fixed term ‘opponent category’ was excluded in this model.
Finally, the third GLMM investigated the factors that may influence the outcome of follow-up attacks. Here, the binomial response variable was ‘outcome of follow-up attack (won–lost)’, with ‘individual identity’ as a random term, and ‘sex of focal individual in primary attacks’, ‘sex of focal individual in follow-up attacks’, ‘age class of focal individual (adult–juvenile) in primary attacks’, ‘age class of focal individual (adult–juveniles) in follow-up attacks’, ‘opponent category’, ‘number of offspring per family’ and ‘outcome of primary attacks (won–lost) from the focal individual’s perspective’ as fixed terms.
RESULTS
Lost interactions were more frequent in primary (29% of 415 interactions) than in stand-alone attacks (17% of 3577 interactions), that is, lost interactions resulted in follow-up attacks more often than won interactions. Also, primary and, therefore, follow-up attacks were more likely to occur than stand-alone attacks if juveniles were involved in agonistic encounters (N = 3992 agonistic interactions in 53 individuals; Table 1). Overall, males won 95% and females won 85% of their stand-alone encounters, whereas juveniles only won 70% of their stand-alone agonistic interactions.
Table 1.
Statistical results of the three generalized linear mixed models (GLMMs)
| Fixed term | Full fixed model |
Final model |
|||
|---|---|---|---|---|---|
| Wald statistic | df | P | Wald statistic | P | |
| Stand alone versus primary attacks | |||||
| Age class | 3.42 | 1 | 0.070 | 6.42 | 0.014 |
| Opponent category | 7.75 | 7 | 0.459 | ||
| Sex | 0.43 | 1 | 0.515 | ||
| N offspring/family | 0.74 | 1 | 0.396 | ||
| Outcome | 22.71 | 1 | <0.001 | 24.46 | <0.001 |
| Primary versus follow-up attack | |||||
| Age class | 0.06 | 1 | 0.806 | ||
| Sex | 0.07 | 1 | 0.787 | ||
| Outcome | 15.3 | 1 | <0.001 | 20.18 | <0.001 |
| Outcome follow-up attack: won versus lost | |||||
| Opponent category | 62.54 | 7 | <0.001 | 67.87 | <0.001 |
| Age class follow-up attack | 13.35 | 1 | <0.001 | 14.38 | <0.001 |
| Age class primary attack | 3.43 | 1 | 0.065 | 3.69 | 0.056 |
| Sex follow-up attack | 13.92 | 1 | <0.001 | 14.82 | <0.001 |
| Sex primary attack | 0.03 | 1 | 0.852 | ||
| N offspring/family | 0.45 | 1 | 0.507 | ||
| Outcome primary attack | 11.90 | 1 | <0.001 | 11.97 | <0.001 |
For each GLMM, results of both the full fixed model, including all tested fixed terms, and the final model, including the significant fixed terms only, are given.
In the second analysis we compared primary and follow-up attacks, which showed that the latter were won more often than the preceding primary attacks (71% of 415 primary attacks, 83% of 415 follow-up attacks; Table 1; N = 830 agonistic interactions in 53 individuals).
The outcome of follow-up attacks was influenced by various factors (Table 1; N = 830 agonistic interactions in 53 individuals). Most importantly, the social class of the opponent influenced the outcome of a follow-up attack: against other family males and females only about half of the follow-up attacks were won, whereas most of the encounters with all other social categories were won (Fig. 2). Although sex of the individual involved in a primary attack did not determine the outcome of follow-up attacks, females lost follow-up attacks more often than males (females: 27% of 184 follow-up attacks; males: 7% of 222 follow-up attacks; nine follow-up attacks (six won, three lost) were by two juveniles, whose sex could not be determined before they disappeared; Table 1). Another factor that influenced the outcome of a follow-up attack was the outcome of the primary attack: if primary attacks were won, 94% of 293 follow-up attacks were also won. If primary attacks were lost, however, follow-up attacks were won in only 61% of 122 cases. Also, age class of the individual involved in the primary attack affected the outcome of the follow-up attack: if juveniles, rather than adults, performed the primary attacks, follow-up attacks were more likely to be lost (juveniles: 23% of 208 follow-up attacks; adults: 9% of 207 follow-up attacks). Similarly, if adults, rather than juveniles, performed the follow-up attacks, wins occurred more often (juveniles: 73% of 181 follow-up attacks; adults: 92% of 234 follow-up attacks).
Figure 2.
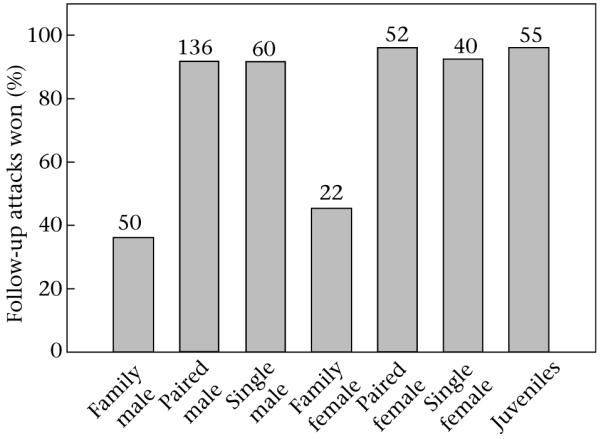
Percentage of won follow-up attacks against different opponents. The total number of agonistic interactions against any specific opponent category is given above the bars.
DISCUSSION
In our greylag geese, more than 10% of all observed agonistic interactions involved the opponent of the preceding interaction(s). This supports our hypothesis that, like primates, greylag geese may take advantage of serial aggression. Greylag goose family members were likely to win agonistic interactions, in both stand-alone and serial attacks. As predicted, our results indicate that serial attacks were more likely to occur when juveniles lost agonistic interactions. By attacking the previous opponent once more, the family member involved in the original attack and/or its kin could reverse their previous losses in about 60% of cases. Follow-up attacks were most often won if the family member involved in that attack was the male parent. About one-third of the follow-up attacks occurred and were won against paired males. This opponent category is among the highest ranking in the flock, and is, therefore, a potentially prestigious opponent, likely to dominate juveniles if these were not in the family unit. In contrast, lost follow-up interactions occurred primarily against other, presumably higher-ranking, family ganders and geese. Repeated aggressive acts against previous opponents of agonistic conflicts by kin and nonkin have been reported in several mammalian species (Cheney & Seyfarth 1986; York & Rowell 1988; Aureli & van Schaik 1991; Engh et al. 2005), but to our knowledge we are the first to show serial aggression against the same opponent in any avian species.
Although we showed previously that families with a larger number of offspring were involved in fewer agonistic interactions per capita and that glucocorticoid reduction in greylag families correlates positively with the number of young (Scheiber et al. 2005a), we found no relationship between the occurrence of serial attacks and number of offspring. In dark-bellied brent geese, Branta bernicla bernicla, the presence of offspring, rather than family size per se, seemed to be most pertinent for acquiring a high dominance status (Poisbleau et al. 2006). Lamprecht (1986a) proposed the ‘cooperative hypothesis’, which predicts that males are willing to fight harder in the presence of mates, and Gregoire & Ankney (1990) suggested that the presence of offspring stimulated parent snow geese, Anser caerulescens, to be more aggressive than pairs without young, making them also more likely to win agonistic encounters. By the same token, the support of parents in serial agonistic encounters may stimulate greylag goose juveniles, and allow them to be more successful in winning attacks against opponents to whom they had lost previously, and, therefore, provide a head start in the flock hierarchy.
Attacking the same opponent repeatedly may serve several functions. As outlined above, serial attacks reverse previous losing experiences about 60% of the time, particularly for juveniles. These reversals may aid juveniles in their struggle to establish themselves in the dominance structure of the flock, and may discourage future attacks by their opponents. Another possible function is that serial attacks may affect the emotional state and, hence, physiological stress responses of both opponents and even bystanders (Wascher et al. 2008). For the opponent, serial attacks may reinforce the previous losing experience (Scott & Fredericson 1951; Aureli & van Schaik 1991; Kazem & Aureli 2005). Winners of the follow-up attacks either reinforce a positive experience, or can reverse the negative associations of a previous loss. The former idea is supported by the fact that geese engage in serial attacks even if primary attacks were won. Attacking an opponent repeatedly would not only reinforce the existing dominance relationship in the flock, similar to what was shown for Japanese macaques, Macaca fuscata (Prud’homme & Chapais 1996; Chapais 2001), but may also signal to third parties postconflict condition and motivation (Kazem & Aureli 2005) as well as the strong bond of a group of allies (van Schaik & Aureli 2000). As a consequence, parental geese and even their offspring rank higher than any other social class in the flock, instead of rank being determined solely by age, pairing status and size (Black & Owen 1986; Lamprecht 1986a, b).
In conclusion, repeated attacks against the same opponents are a relatively common form of active social support in greylag geese, and may serve to reverse previous losses, reinforce a losing experience on the side of the opponent or signal the agonistic potential of a family to other flock members. Serial attacks are thus another example in which greylag geese parallel the social complexity and, in particular, forms of social support in primates.
Acknowledgments
We gratefully acknowledge financial support by the FWF-Projects 15766-B03 and 18601-B17, the ‘Verein der Förderer der Konrad Lorenz Forschungsstelle’ and the ‘Herzog von Cumberland Stiftung’. T. Bugnyar, D. Frigerio, J. Hemetsberger, K. Hirschenhauser, I. Nedelcu, C. Schloegl, R. Swoboda and C. Wascher promoted discussions on the topic, and three anonymous referees commented constructively on the manuscript. We are grateful to E. Price for correcting the English.
References
- Aureli F, van Schaik CP. Post-conflict behaviour in long-tailed macaques (Macaca fascicularis): II. Coping with the uncertainty. Ethology. 1991;89:101–114. [Google Scholar]
- Black JM, Owen M. Determinants of social rank in goose flocks: acquisition of social rank in young geese. Behaviour. 1986;102:129–146. [Google Scholar]
- Chapais B. Primate nepotism: what is the explanatory value of kin selection? International Journal of Primatology. 2001;22:203–299. [Google Scholar]
- Cheney D, Seyfarth R. The recognition of social alliances by vervet monkeys. Animal Behaviour. 1986;34:1722–1731. [Google Scholar]
- Das M. Conflict management via third parties. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. University of California Press; Berkeley: 2000. pp. 263–280. [Google Scholar]
- DeVries AC, Glasper EF, Detillion CE. Social modulation of stress responses. Physiology and Behavior. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Engh AL, Siebert ER, Greenberg DA, Holekamp KE. Patterns of alliance formation and postconflict aggression indicate spotted hyaenas recognize third party relationships. Animal Behaviour. 2005;69:209–217. [Google Scholar]
- Fischer H. Das Triumphgescheri der Graugans (Anser anser) Zeitschrift für Tierpsychologie. 1965;22:247–304. [PubMed] [Google Scholar]
- Foerster K, Kempenaers B. Effects of testosterone on male-male competition and male–female interactions in blue tits. Behavioral Ecology and Sociobiology. 2005;57:215–223. [Google Scholar]
- Frigerio D, Weiß BM, Kotrschal K. Spatial proximity among adult siblings in greylag geese (Anser anser): evidence for female bonding? Acta Ethologica. 2001;3:121–125. [Google Scholar]
- Frigerio D, Weiß BM, Dittami J, Kotrschal K. Social allies modulate corticosterone excretion and increase success in agonistic interactions in juvenile hand-raised greylag geese (Anser anser) Canadian Journal of Zoology. 2003;81:1746–1754. [Google Scholar]
- GenStat R. GenStat, Release 10.1. Lawes Agricultural Trust; Rothamsted: 2007. [Google Scholar]
- Gouzoules S, Gouzoules H. Kinship. In: Smuts B, Cheney D, Seyfarth R, Wrangham RW, Struhsacker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 299–305. [Google Scholar]
- Gregoire PE, Ankney CD. Agonistic behavior and dominance relationships among lesser snow geese during winter and spring migration. Auk. 1990;107:550–560. [Google Scholar]
- Hemetsberger J. Die Entwicklung der Grünauer Graugansschar seit 1973. In: Kotrschal K, Müller G, Winkler H, editors. Konrad Lorenz und Seine Verhaltensbiologischen Konzepte aus Heutiger Sicht. Filander Verlag; Fürth: 2001. pp. 249–260. [Google Scholar]
- Kazem AJN, Aureli F. Redirection of aggression: multiparty signalling within a network? In: McGregor PK, editor. Animal Communication Networks. Cambridge University Press; Cambridge: 2005. pp. 191–218. [Google Scholar]
- Kotrschal K, Hemetsberger J, Dittami J. Food exploitation by a winter flock of greylag geese: behavioral dynamics, competition and social status. Behavioral Ecology and Sociobiology. 1993;33:289–295. [Google Scholar]
- Kotrschal K, Hemetsberger J, Weiß BM. Making the best of a bad situation: homosociality in male greylag geese. In: Vasey P, Sommer V, editors. Homosexual Behaviour in Animals: an Evolutionary Perspective. Cambridge University Press; Cambridge: 2006. pp. 45–76. [Google Scholar]
- Kummer H. Primate Societies: Group Techniques of Ecological Adaptation. Aldine Atherton; Chicago: 1971. [Google Scholar]
- Lamprecht J. Structure and causation of the dominance hierarchy in a flock of bar-headed geese (Anser indicus) Behaviour. 1986a;96:28–48. [Google Scholar]
- Lamprecht J. Social dominance and reproductive success in a goose flock (Anser indicus) Behaviour. 1986b;97:50–65. [Google Scholar]
- Lamprecht J. Factors influencing leadership: a study of goose families (Anser indicus) Ethology. 1991;89:265–274. [Google Scholar]
- Lorenz K. Here I Am: Where Are You? Harcourt Brace Jovanovich; New York: 1991. [Google Scholar]
- Lorenz K, Kalas S, Kalas K. Das Jahr der Graugans. Piper Verlag; München: 1978. [Google Scholar]
- McGregor PK, Dabelsteen T, Holland J. Eavesdropping in a territorial songbird communication network: preliminary results. Bioacoustics. 1997;8:253–254. [Google Scholar]
- Netto WJ, van Hooff JARAM. Conflict interference and the development of dominance relationships in immature Macaca fascicularis. In: Else JG, Lee PC, editors. Primate Ontogeny, Cognition and Social Behaviour. Cambridge University Press; New York: 1986. pp. 291–300. [Google Scholar]
- Oliveira RF, McGregor PK, Latruffe C. Know thine enemy: fighting fish gather information from observing conspecific interactions. Proceedings of the Royal Society of London, Series B. 1998;265:1045–1049. [Google Scholar]
- Peake TM, Terry AMR, McGregor PK, Dabelsteen T. Male great tits eavesdrop on simulated male-to-male vocal interactions. Proceedings of the Royal Society of London, Series B. 2001;268:1183–1187. doi: 10.1098/rspb.2001.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake TM, Terry AMR, McGregor PK, Dabelsteen T. Do great tits assess rivals by combining direct experience with information gathered by eavesdropping? Proceedings of the Royal Society of London, Series B. 2002;269:1925–1929. doi: 10.1098/rspb.2002.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira ME. Agonistic interactions of juvenile savannah baboons. I. Fundamental features. Ethology. 1988;79:195–217. [Google Scholar]
- Poesel A, Kunc HP, Foerster K, Kempenaers B. Early birds are sexy: male age, dawn song and extrapair paternity in blue tits, Cyanistes (formerly Parus) caeruleus. Animal Behaviour. 2006;72:531–538. [Google Scholar]
- Poisbleau M, Fritz H, Valeix M, Perroi P-Y, Dalloyau S, Lambrechts MM. Social dominance correlates and family status in wintering dark-bellied brent geese, Branta bernicla bernicla. Animal Behaviour. 2006;71:1351–1358. [Google Scholar]
- Prud’homme J, Chapais B. Development of intervention behaviour in Japanese macaques: testing the targeting hypothesis. International Journal of Primatology. 1996;17:429–443. [Google Scholar]
- van Schaik CP, Aureli F. The natural history of valuable relationships in primates. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. University of California Press; Berkeley: 2000. pp. 307–333. [Google Scholar]
- Scheiber IBR, Weiß BM, Frigerio D, Kotrschal K. Active and passive social support in families of greylag geese (Anser anser) Behaviour. 2005a;142:1535–1557. doi: 10.1163/156853905774831873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiber IBR, Kralj S, Kotrschal K. Sampling effort/frequency necessary to infer individual acute stress responses from fecal analysis in greylag geese (Anser anser) Annals of the New York Academy of Sciences. 2005b;1046:154–167. doi: 10.1196/annals.1343.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiber IBR, Kotrschal K, Weiß BM. Benefits of family reunions: social support in secondary greylag goose families. Hormones and Behavior. 2009;55:133–138. doi: 10.1016/j.yhbeh.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JP, Fredericson E. The causes of fighting in mice and rats. Physiological Zoology. 1951;24:273–309. [Google Scholar]
- Silk JB. Social behavior in evolutionary perspective. In: Smuts B, Cheney D, Seyfarth R, Wrangham RW, Struhsacker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 318–329. [Google Scholar]
- Silk JB. Kin selection in primate groups. International Journal of Primatology. 2002;23:849–875. [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Animal Behaviour. 2004;67:573–582. [Google Scholar]
- de Waal FBM, Harcourt A. Coalitions and alliances: a history of ethological research. In: Harcourt A, de Waal FBM, editors. Coalitions and Alliances in Humans and Other Animals. Oxford University Press; Oxford: 1992. pp. 1–19. [Google Scholar]
- de Waal FBM, van Roosmalen D. Reconciliation and consolation among chimpanzees. Behavioral Ecology and Sociobiology. 1979;5:55–66. [Google Scholar]
- de Waal FBM, van Hooff JARAM, Netto WJ. An ethological analysis of types of agonistic interactions in a captive group of Java monkeys (Macaca fascicularis) Primates. 1976;17:257–290. [Google Scholar]
- Wascher CA, Scheiber IBR, Kotrschal K. Heart rate modulation in bystanding geese watching social and non-social events. Proceedings of the Royal Society of London, Series B. 2008;275:1653–1659. doi: 10.1098/rspb.2008.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DP, Colmenares F, Arnold K. Redirection, consolation, and policing. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. University of California Press; Berkeley: 2000. pp. 281–301. [Google Scholar]
- Weiß BM, Kotrschal K. Effects of passive social support in juvenile greylag geese (Anser anser): a study from fledging to adulthood. Ethology. 2004;110:429–444. [Google Scholar]
- Weiß BM, Kotrschal K, Frigerio D, Hemetsberger J, Scheiber IBR. Birds of a feather stay together: extended family bonds and social support in greylag geese (Anser anser) In: Ramirez RN, editor. Family Relations: Issues and Challenges. Nova Science Publishers; New York: 2008. pp. 69–88. [Google Scholar]
- York AD, Rowell TE. Reconciliation following aggression in patas monkeys, Erythrocebus patas. Animal Behaviour. 1988;36:502–509. [Google Scholar]


