Abstract
A disruption in any part of the vitamin D physiological pathway can result in vitamin D deficiency, which may lead to bone pain, muscle weakness, falls, low bone mass, and fractures. Recognizing the signs and symptoms helps physicians make a proper diagnosis and prescribe appropriate treatment. Physicians should suspect osteomalacia in patients who have prolonged vitamin D deficiency, a low serum calcium level, or a low serum phosphorus level. Patients with cystic fibrosis are at increased risk for deficiencies in fat-soluble vitamins, including vitamin D. Secondary hyperparathyroidism can develop in patients with chronic kidney disease as a result of low 25-hydroxyvitamin D levels or impaired conversion to 1,25-dihydroxyvitamin D. Patients may experience abnormal vitamin D metabolism as a result of taking anticonvulsants and other medications.
Persons obtain vitamin D through ultraviolet light-induced skin synthesis and ingestion of vitamin D–rich foods.1 The liver subsequently hydroxylates vitamin D to the storage form, 25-hydroxyvitamin D (25[OH]D), which undergoes another hydroxylation step in the kidney to become the bioactive form, 1,25-dihydroxyvitamin D (1,25[OH]2D). Regulated by parathyroid hormone (PTH), 1,25(OH)2D increases intestinal calcium absorption and is thought to affect bone metabolism and muscle function. A disruption in any part of the physiological pathway may result in hypovitaminosis D, or vitamin D deficiency.
Vitamin D status influences musculoskeletal health.1 Low vitamin D levels may lead to clinical manifestations, including bone pain, muscle weakness, falls, low bone mass, and fractures, with subsequent diagnoses of osteomalacia, osteoporosis, and myopathy.
Physicians, especially rheumatologists, often encounter patients who have signs and symptoms of vitamin D deficiency, and recognizing them helps in making a proper diagnosis and prescribing appropriate treatment. In this article, we describe 5 patients with hypovitaminosis D who demonstrate various signs and symptoms to highlight diagnosis and treatment challenges.
BACKGROUND
The National Center for Health Statistics conducts annual surveys to evaluate the health and nutritional status of the American civilian population. In the National Health and Nutrition Examination Survey conducted from 2001 through 2004, about 76% of 11,009 Americans 20 years or older had vitamin D insufficiency, defined as a serum 25(OH)D level lower than 30 ng/mL.2 Vitamin D deficiency, defined as a 25(OH)D level lower than 15 ng/mL, was present in 10% of adult men and 17% of adult women.2 Risk factors for hypovitaminosis D include decreased sun exposure, increased age, increased skin pigment, malabsorption, and conditions that contribute to abnormal vitamin D metabolism (eg, chronic kidney disease and anticonvulsant therapy).1
THE CASES
Case 1: Spontaneous fracture
A 41-year-old white woman reported that she had been walking across a room when she noted a “snap” and sudden pain in her right groin. She also reported having had left upper arm pain for 2 weeks. Her past medical history included anorexia nervosa, amenorrhea since her teenage years, polysubstance abuse, and a seizure disorder that was managed with phenytoin.
The woman weighed 75 lb and her height was 65.8 inches, with a body mass index (BMI) of 12.2. On examination, we observed painful but full right hip range of motion and elicited pain with palpation of her upper left humerus. There was normal shoulder range of motion.
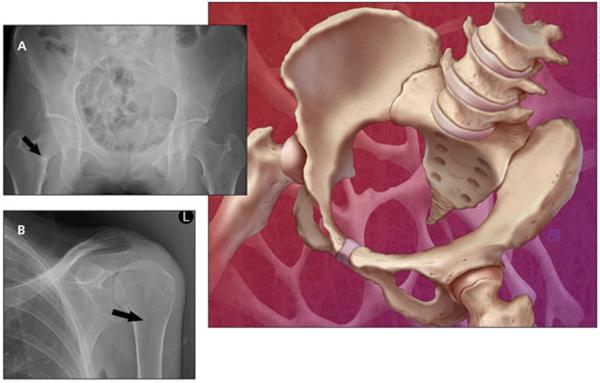
X-ray films of the patient's humerus and hip (Figure) revealed Looser zones (radiolucent areas occurring at right angles to the bone cortex that represent incomplete fractures resulting from osteomalacia). Laboratory testing revealed low serum albumin (3.2 g/dL), normal serum calcium (9 mg/dL), normal serum phosphorus (3.0 mg/dL), high PTH (82 pg/mL), low serum 25(OH)D (6 ng/mL), high alkaline phosphatase (ALP) (180 U/L), and low 24-hour urine calcium (80 mg) levels. Normal ranges are provided in Table 1. A bone mineral density (BMD) study performed 7 months earlier revealed a lumbar spine T-score of 3.4 and a left total hip T-score of 3.5.
Figure.
Pelvis (A) and humerus (B) x-ray films obtained for a patient with osteomalacia show Looser zones (arrows), which are radiolucent areas that occur at right angles to the bone cortex. They represent incomplete fractures resulting from osteomalacia.
Table 1.
Normative values for laboratory tests for vitamin D deficiency
| Test | Normal rangea |
|---|---|
| Serum 25(OH)D level | 30 – 8O ng/mL |
| Serum albumin level | 3.3 – 4.7 g/dL |
| Serum alkaline phosphatase level | 35 – 130 U/L |
| Serum calcium level | 8.5 – 10.2 mg/dL |
| Serum creatinine level | 0.6 – 1.3 mg/dL |
| Serum parathyroid hormone level | 14 – 72 pg/mL |
| Serum phosphorus level | 2.5 – 4.5 mg/dL |
| 24-hour urine calcium level | 100 – 300 mg |
| Estimated glomerular filtration ratsb | 60 – 120 mL/min/1.73 m2 |
25(OH)D, 25-hydroxyvitamin D.
Valuse reported for adults only.
Multiply result by 1.21 for African Americans.
The patient's medical history, laboratory values, and x-ray film results indicated osteomalacia, a disease of low BMD typically caused by vitamin D deficiency.3 In this patient, the deficiency resulted from malnutrition and long-term anticonvulsant therapy.
Comment
The many signs and symptoms of osteomalacia include muscle pain or weakness, sometimes leading to a waddling gait, and joint pain or bone pain, most often affecting the shoulders, pelvis, ribs, and spine.3 After several years of osteomalacia, there may be bone deformities, including kyphosis, scoliosis, protrusio acetabuli, and leg bowing. In addition, osteomalacia may result in subchondral microfractures, also called Looser zones.
Patients who have osteomalacia may present with abnormal laboratory findings. In 2 series, between 25% and 95% of patients had high serum ALP, 29% had low serum 25(OH)D, 50% had low serum calcium or phosphorus, 50% had high PTH, and 20% had low urine calcium levels.3,4 All 17 patients with a diagnosis of osteomalacia, made at the Mayo Clinic, had 2 or more of the following characteristics: low serum calcium, low serum phosphorus, or high serum ALP level or a radiographic finding (eg, Looser zones).3
Physicians should suspect osteomalacia in patients who have prolonged vitamin D deficiency, a low serum calcium level, or a low serum phosphorus level, any of which may lead to unmineralized osteoid.5 Prolonged vitamin D deficiency may result from limited sun exposure related to the patient's latitude of residence; increased skin pigmentation; or lifestyle choices, including the use of sun protection products.1 Deficiency also may result from conditions that cause malabsorption or malnutrition, including inflammatory bowel disease, celiac sprue, and GI surgery. In addition, abnormal vitamin D metabolism may result from renal disease, end-stage liver disease, or the use of anticonvulsants or tuberculosis medications.1,6
In patients with osteomalacia caused by vitamin D deficiency, we recommend that physicians monitor the success of therapy by noting clinical improvements and documenting normalization of initially abnormal laboratory values and increases in BMD. After management of vitamin D deficiency osteomalacia, one group reported dramatic short-term increases in BMD.6 Researchers investigated 26 patients with osteomalacia, including 23 who had vitamin D deficiency.5 Patients were treated with vitamin D3, 60,000 IU weekly; calcium (1 to 2 g/d); and casual sun exposure. Adjustments in therapy were made to normalize the symptoms and signs of disease. Over a follow-up period of 1.5 to 36 months, lumbar spine BMD increased between 25% and 51%.5 The investigators also observed similar dramatic increments in hip BMD that resulted from mineralization of unmineralized osteoid.3
Case 2: Cystic fibrosis
A 45-year-old white man with cystic fibrosis underwent a bone density study as part of his evaluation for eligibility to undergo lung transplantation; his femoral neck Z-score was 4. Thoracic x-ray films showed 4 compression fractures. Laboratory studies revealed a low serum 25(OH)D level (lower than 6 ng/mL), with normal serum calcium, phosphorus, intact PTH, ALP, and creatinine levels.
To manage the patient's vitamin D deficiency, we prescribed vitamin D2, 50,000 IU/d, for 14 days, then twice monthly. Two months later, his serum 25(OH)D level was 7 ng/mL. We then prescribed 50,000 IU of vitamin D2 daily for 30 days, followed by 50,000 IU once weekly. Two months later, the patient's serum 25(OH)D level remained low at 12 ng/mL.
We increased the patient's vitamin D dosage to 100,000 IU/d for 30 days, then 50,000 IU/d. Two months later, his serum 25(OH)D level had increased to 25 ng/mL.
After that, we advised the patient to increase his sun exposure and continue to take 100,000 IU/d of vitamin D2. Two months later, his 25(OH)D level was 31 ng/mL. Over the subsequent 3 years, 100,000 IU/d of vitamin D2 and casual sun exposure have maintained his serum 25(OH)D level just above 30 ng/mL.
Comment
Patients who have cystic fibrosis are at increased risk for deficiencies in fat-soluble vitamins, including vitamin D, because of pancreatic insufficiency.7 In one study, they absorbed significantly less vitamin D than did healthy controls, leading to a 76% and 23% prevalence of vitamin D insufficiency and deficiency, respectively.8
Because of the high prevalence, the Cystic Fibrosis Foundation advises physicians to check serum 25(OH)D levels in patients with cystic fibrosis at least yearly and prescribe at least 400 to 800 IU/d of vitamin D in addition to sun exposure.7 Higher vitamin D dosages may be necessary for some persons,7–9 as demonstrated by this patient. UV therapy can increase 25(OH)D levels in patients with cystic fibrosis via increased skin synthesis of vitamin D precursors. Researchers compared the ability of vitamin D2, vitamin D3, and UV therapy to increase serum 25(OH)D levels among 30 patients with cystic fibrosis.10 Serum 25(OH)D levels increased significantly in those receiving vitamin D2 or D3 therapy but not in those randomized to UV therapy. However, only 55% of the UV group adhered to therapy. Those adherent to UV therapy experienced increases in 25(OH)D levels similar to those receiving vitamin D2.
Case 3: Muscle weakness
A 41-year-old white woman presented to the clinic in winter reporting muscle weakness and falls. Her initial serum 25(OH)D level was 7 ng/mL in November. We instructed her to take vitamin D2, 50,000 IU/d, for 30 days. In January, her serum 25(OH)D level remained low at 10 ng/mL, prompting our prescription of vitamin D2, 100,000 IU/d, for 30 days. However, her 25(OH)D level remained low, at 9 ng/mL, in May. Although the patient stopped her vitamin D therapy, her 25(OH)D level was higher in July (19 ng/mL), corresponding with routine outdoor sun exposure. The following January, her 25(OH)D level again decreased to 6 ng/mL and the patient continued to experience muscle weakness and falls.
A 24-hour stool specimen revealed fat malabsorption. The patient subsequently received a diagnosis of chronic pancreatitis. However, the patient had a pork allergy that made pancreatic enzyme replacement therapy ineffective. In addition, intramuscular vitamin D therapy was no longer commercially available in the United States. Therefore, to elevate her low 25(OH)D level, we prescribed dermatology-directed broadband UV-B therapy twice weekly; the patient's 25(OH)D level increased to higher than 30 ng/mL. Although the long-term safety of UV-B therapy for achieving vitamin D repletion is currently uncertain,11 administration of UV-B by dermatologists permits surveillance for skin cancer in patients requiring this therapy.
Comment
Hypovitaminosis D is a known cause of sarcopenia, muscle weakness, and falls.1 Muscle biopsies in patients with these symptoms demonstrate type II fiber atrophy correlating with objective decreases in muscle strength and increased body sway.12–14 Taking vitamin D (800 IU/d) with calcium increases muscle strength and decreases body sway and fall frequency.13 Vitamin D metabolites bind to myocyte receptors and increase the numbers of myocytes and type II muscle fibers.13,14
Most studies document increased physical function after therapy with vitamin D and calcium, but studies of vitamin D used without calcium show no functional benefits.11 The apparent requisite administration of calcium with vitamin D suggests that ionized serum calcium or reversal of secondary hyperparathyroidism mediates the beneficial effects of vitamin D on skeletal muscle. More research is needed to clarify the appropriate vitamin D dosage and 25(OH)D level needed to achieve optimal muscle strength and function in older adults.
Case 4: Abnormal metabolism in chronic kidney disease (CKD)
A 92-year-old white man reported that he had turned over in bed and experienced severe back pain resulting from 2 compression fractures. Laboratory studies revealed a low 25(OH)D level (22 ng/mL), a high PTH level (98 pg/mL), and normal serum creatinine and calcium levels (1.2 mg/dL and 9.8 mg/dL, respectively). For osteoporosis and secondary hyperparathyroidism, we prescribed an oral bisphosphonate and vitamin D2, 50,000 IU/d, for 14 days, then once monthly thereafter.
One month later, the patient's 25(OH)D level was 45 ng/mL, with a serum calcium level of 9.6 mg/dL. Three months later, his serum 25(OH)D level was 32 ng/mL and his serum calcium level was 9.5 mg/dL, but his PTH level was higher at 102 pg/mL. Although he had a “normal” creatinine level of 1.2 mg/dL, his estimated glomerular filtration rate was 40 mL/min, indicating stage 3 CKD. With subsequent calcitriol therapy, the patient's PTH level normalized.
Comment
Secondary hyperparathyroidism can develop in patients with CKD as a result of low 25(OH)D levels or inadequate conversion of 25(OH)D to 1,25(OH)2D.15 Secondary hyperparathyroidism occurs in about 38% of patients with stage 3 and 68% of patients with stages 4 and 5 CKD.15,16 As renal function declines, patients experience reduced 1--hydroxylase enzyme activity, which limits or prevents conversion of 25(OH)D to 1,25(OH)2D.1 Subsequently, secondary hyperparathyroidism may develop in spite of adequate 25(OH)D stores.
According to the Kidney Disease Outcomes Quality Initiative, frequency of measurement and target serum PTH, calcium, and phosphorus levels vary with the stage of CKD (Table 2).17 For CKD stages 3 and 4, if the PTH level is high and the 25(OH)D level is lower than 30 ng/mL, experts recommend vitamin D2 to correct hypovitaminosis D. If the PTH level remains high in spite of vitamin D repletion (25[OH]D level higher than 30 ng/mL), the use of oral active vitamin D (eg, calcitriol) is advised to correct secondary hyperparathyroidism and minimize bone loss. 1
Table 2.
Guidelines for detecting and managing HTP in chronic kidney disease
| Chronic kidney disease stage | GFR range (mL/min/1.73 m2) | PTH measurement frequency | Measurement frequency for calcium, phosphorus levels | Target PTH (pg/mL) |
|---|---|---|---|---|
| 3 | 30 – 59 | Every 12 months | Every 12 months | 35 – 70 |
| 4 | 15 – 29 | Every 3 months | Every 3 months | 70 – 110 |
| 5 | < 15 or dialysis | Every 3 months | Every months | 150 – 300 |
HPT, hyperparathyroidism; GFR, glomerular filtration rate; PTH, parathyroid hormone.
Adapted from National Kidney Foundation. http://www.kidney.org/Professionals/kdogi/guidelines_bone/index.htm. 2009.17
Case 5: Abnormal metabolism resulting from anticonvulsant use
We evaluated a 45-year-old white man in the osteoporosis clinic who was taking phenytoin for several years to treat intractable seizures. The patient reported bilateral hip fractures and 5 compression fractures. A bone density study revealed low BMD. His serum 25(OH)D level was low (12 ng/mL), and he had a mildly elevated serum ALP level with normal serum phosphorus and PTH levels and renal function.
Comment
Patients may experience abnormal vitamin D metabolism as a result of taking anticonvulsants and other medications. The anticonvulsants phenytoin and carbamazepine and tuberculosis medications isoniazid and rifampin induce hepatic cytochrome P-450 enzyme activity, increasing the breakdown of 25(OH)D to inactive metabolites.6,18 Physicians may help patients overcome increased 25(OH)D metabolism by giving them additional vitamin D, but dosages of 400 to 4000 IU/d might be necessary.19,20 In a small prospective study, about 80% of patients needed vitamin D dosages of 2400 IU/d or higher to overcome hypovitaminosis D resulting from anticonvulsant therapy.20
To our knowledge, there are no official guidelines on how often to monitor 25(OH)D levels in patients receiving anticonvulsants known to alter vitamin D metabolism. In our opinion, such patients should undergo serum 25(OH)D measurement once yearly, preferably during wintertime, when 25(OH)D levels reach a nadir.
TREATMENT
Patients with hypovitaminosis D may present to a medical clinic with low bone mass, low-trauma fracture, bone or muscle pain, or muscle weakness. They also may present with a risk factor for vitamin D deficiency (eg, decreased sun exposure, increasing age, increasing skin pigment). Physicians should consider measuring vitamin D levels in these patients.
Physicians may use several methods to treat patients with vitamin D deficiency (Table 3). A loading dosage of vitamin D can quickly bring serum 25(OH)D levels into the normal range. One frequently cited loading dose involves administering 50,000 IU of vitamin D once weekly for 8 weeks.1 However vitamin D deficiency may recur unless physicians recommend treatment to maintain vitamin D repletion. Maintenance regimens include 400 to 1000 IU/d of vitamin D or 50,000 IU of vitamin D every 2 to 4 weeks.1 Patients with fat malabsorption or abnormal vitamin D metabolism often need higher doses of vitamin D, or UV-B therapy, to correct the deficiency and associated signs and symptoms.
Table 3.
Treatment options for patients with vitamin D deficiency
| Name | Formulation | Description |
|---|---|---|
| Ergocalciferol | Vitamin D2 | A plant source of vitamin D available only by prescription at 50,000 IU per capsule |
| Cholecalciferol | Vitamin D3 | Represents the vitamin D found in dairy products, fatty fish, and over-the-counter supplements in doses of 400 – 1000 IU per tablet |
| Calcitriol | 1.25(OH)2D | Represents the biologically active form of vitamin D; used in patients with chronic kidney disease who are unable to convert 25(OH)D to 1.25(OH)2D |
| Suntanning bed | UV-B | Induces skin synthesis of vitamin D precursor; long-term saftey with risk of skin cancer is unkonwn |
25(OH)D, 25-hydroxyvitamin D; 1.25(OH)2D, 1.25-dihydroxyvitamin D.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared to 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reginato AJ, Coquia JA. Musculoskeletal manifestations of osteomalacia and rickets. Best Pract Res Clin Rheumatol. 2003;17:1063–1080. doi: 10.1016/j.berh.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95:519–523. doi: 10.1016/0002-9343(93)90335-m. [DOI] [PubMed] [Google Scholar]
- 5.Bhambri R, Naik V, Malhotra N, et al. Changes in bone mineral density following treatment of osteomalacia. J Clin Densitom. 2006;9:120–127. doi: 10.1016/j.jocd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Brodie MJ, Boobis AR, Hillyard CJ, et al. Effect of rifampicin and isoniazid on vitamin D metabolism. Clin Pharmacol Ther. 1982;32:525–530. doi: 10.1038/clpt.1982.197. [DOI] [PubMed] [Google Scholar]
- 7.Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1 suppl):S1–S39. doi: 10.1378/chest.125.1_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 8.Wolfenden LL, Judd SE, Shah R, et al. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008;69:374–381. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rovner AJ, Stallings VA, Schall JI, et al. Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Am J Clin Nutr. 2007;86:1694–1699. doi: 10.1093/ajcn/86.5.1694. [DOI] [PubMed] [Google Scholar]
- 10.Khazai NB, Judd SE, Jeng L, et al. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab. 2009;94:2037–2043. doi: 10.1210/jc.2008-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cranney A, Horsley T, O'Donnell S, et al. AHRQ Evidence Report No. 158. Agency for Healthcare Research and Quality; Rockville, MD: 2007. [Accessed September 8, 2009]. Effectiveness and Safety of Vitamin D in Relation to Bone Health. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1b.chapter.73328. [Google Scholar]
- 12.Sørensen OH, Lund B, Saltin B, et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond) 1979;56:157–161. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]
- 13.Skaria J, Katiyar BC, Srivastava TP, Dube B. Myopathy and neuropathy associated with osteomalacia. Acta Neurol Scand. 1975;51:37–58. doi: 10.1111/j.1600-0404.1975.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 14.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882–8891. [PubMed] [Google Scholar]
- 15.Andress DL, Coyne DW, Kalantar-Zadeh K, et al. Management of secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Endocr Pract. 2008;14:18–27. doi: 10.4158/EP.14.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Craver L, Marco MP, Martínez I, et al. Mineral metabolism parameters throughout chronic kidney disease stages 1-5achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–1176. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- 17.KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Vol 42. National Kidney Foundation; New York: 2003. [Accessed August 17, 2009]. p. 43. http://www.kidney.org/Professionals/kdoqi/guidelines_bone/index.htm. [PubMed] [Google Scholar]
- 18.Pack AM, Gidal B, Vazquez B. Bone disease associated with antiepileptic drugs. Cleve Clin J Med. 2004;71(suppl 2):S42–S48. doi: 10.3949/ccjm.71.suppl_2.s42. [DOI] [PubMed] [Google Scholar]
- 19.Drezner MK. Treatment of anticonvulsant drug-induced bone disease. Epilepsy Behav. 2004;5(suppl 2):S41–S47. doi: 10.1016/j.yebeh.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Collins N, Maher J, Cole M, et al. A prospective study to evaluate the dose of vitamin D required to correct low 25-hydroxyvitamin D levels, calcium, and alkaline phosphatase in patients at risk of developing antiepileptic drug-induced osteomalacia. Q J Med. 1991;78:113–122. [PubMed] [Google Scholar]