Abstract
Previous research has indicated that d-cycloserine [DCS; a N-methyl-d-aspartate (NMDA) partial agonist] facilitates the extinction of conditioned fear as well as the extinction of cocaine conditioned place preference. Sprague Dawley rats were first trained to self-administer cocaine and then we compared their extinction behavior (lever pressing) following treatment with vehicle; 15 mg/kg DCS; or 30 mg/kg DCS. We showed that 30 mg/kg DCS, but not 15 mg/kg significantly accelerated extinction of cocaine self-administration behavior when compared with saline by almost half (4 days vs. 9 days). At 2 weeks when all animals had extinguished, there were no longer differences between the groups. The present findings support of the potential of NMDA partial agonists as prospectively valuable in facilitating the extinction of cocaine-seeking behavior. More specifically, we demonstrate that 30 mg/kg DCS was effective at significantly accelerating the extinction of cocaine self-administration behavior in rats. These results provide further support for the potential of DCS as a treatment strategy for addiction.
Keywords: learning, glutamate, withdrawal, abstinence, drug abuse, NMDA, addiction, substance abuse
INTRODUCTION
It is estimated that over 6 million individual in the United States, aged 12 years and older have been exposed to cocaine (SAMHSA, 2007). Cocaine increases dopamine (DA) in the nucleus accumbens by blocking DA transporters, which is considered the mechanism by which it induces rewarding effects that can result in addiction (Ritz et al., 1987). DA is involved with reward and prediction of reward and with conditioned learning. DA modulation of the amygdala and the prefrontal cortex predominantly through D1 receptors are implicated in conditioning (Beninger and Gerdjikov, 2004) through neuroplastic adaptations in glutamatergic neurotransmission (Crombag et al., 2002; Kalivas and Volkow, 2005).
Specifically, changes in expression of α-amino-3-hydroxy-5-methyl-isoxazole propionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors, which contribute to the neuroplastic processes linked with learning and memory including long-term potentiation are involved in conditioning (Rao and Finkbeiner, 2007). Both NMDA and AMPA receptors are implicated in cocaine-seeking behavior controlled by drug-associated cues (Di Ciano and Everitt, 2001). Acute and chronic cocaine potentiate synaptic strength in the ventral tegmental area (VTA) through changes in AMPA receptors, which is blocked by NMDA receptor antagonists (Borgland et al., 2004).
The involvement of NMDA and AMPA receptors in cocaine addiction has raised interest in the therapeutic potential of medications that target NMDA and/or AMPA receptors. d-cycloserine (DCS), a partial NMDA receptor agonist (Klodzinska and Chojnacka-Wojcik, 2000), has been shown to facilitate extinction of previously conditioned fear and anxiety, both in preclinical and clinical models (Ressler et al., 2004; Faraone et al., 2005; Parnas, 2005; Davis et al., 2006; Guastella et al., 2007; Vervliet, 2008); furthermore, recent findings showed that DCS facilitated extinction of cocaine conditioned place preference (CPP) in rats (Botreau et al., 2006). The facilitated extinction was interpreted to reflect DCS’s enhancement of memory consolidation during the extinction conditions, via its effects on NMDA receptors (Botreau et al., 2006; Vervliet, 2008).
We recently showed a similar effect of DCS in extinction to CPP for cocaine in mice that was dose related and persisted for up to 2 weeks (Thanos et al., 2009). Specifically, extinction to cocaine CPP was significantly faster with DCS than with vehicle treatment (three sessions, vs. six sessions, respectively). After extinction was achieved; mice were retested for CPP 1 and 2 weeks later. All animals maintained extinction to CPP 1 week later but at 2 weeks, whereas the vehicle and the 15 mg/kg DCS treated animals maintained the extinction the 30 mg/kg DCS treated mice had renewed CPP and also showed inhibited locomotor activity. Though these results corroborated in mice the previously reported acceleration of extinction to cocaine induced CPP by DCS in rats we also show that the higher DCS doses had the opposite effect; that is it facilitated CPP reestablishment after extinction. Thus, while DCS could be beneficial in accelerating the extinction to conditioned responses in addiction, at higher doses, it could increase the risk of relapse. This highlights the importance of evaluating not only the short term beneficial therapeutic effects of DCS but also of evaluating the potential of longer lasting undesirable effects.
The purpose of this study was to further investigate the possible psychotherapeutic applications of DCS on the extinction of a rat model of cocaine self-administration. The self-administration model provides qualitative and quantitative measure of voluntary cocaine consumption analogous to the clinical situation and provides insight into a different aspect of drug abuse and addiction (motivational drive to exert the behavior) (Schuster and Johanson, 1988; Dykstra et al., 1997; Solinas et al., 2006) that distinct from CPP (conditioned behavior).
MATERIALS AND METHODS
Animals
Thirty six, 6-week-old, male Sprague-Dawley rats (Taconic Farms) were allowed 7 days to habituate to experimental conditions: temperature (72 ± 2 F); controlled humidity (40–60%); a 12 h reverse light cycle (lights off at 07:00 h). Rats were individually housed and kept on an ad libitum diet. For the cocaine self-administration experiments, only rat chow was restricted (18 g/day) to maintain a stable body weight, which was recorded daily throughout the experiment. All experiments were conducted in an accredited animal husbandry facility that was approved by the Association for Assessment of Laboratory Animal Care, and the Institutional Animal Care and Use Committee of Brookhaven National Laboratory.
Drugs
Cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) was prepared by dissolving it in 0.9% saline for doses of 0.750 mg/kg and 0.375 mg/kg for intravenous (i.v.) infusion. All cocaine solutions were infused intravenously at a volume of 100 μl. DCS (Sigma-Aldrich, St. Louis, MO) was used in a 15 mg/kg dose [1.5 M; 10 ml/kg intraperitoneally (i.p.)] and a 30 mg/kg dose (3 M; 10 ml/kg i.p.) during extinction. Saline (0.9% NaCl) was used (10 ml/kg i.p.) as the vehicle solution. Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) given i.p. for surgery under aseptic conditions.
Apparatus
The self-administration apparatus (Habitest—Coulbourn Instruments; Allentown, PA) was placed inside a rigid foam sound attenuated cubicle equipped with a 28 V exhaust fan. Each operant chamber contained a horizontal grid floor with metal side walls and clear front and back walls. One side wall contained two levers and a food receptacle in the center. The left lever was designated as the active lever, whereas the right lever was the inactive lever. Both levers were situated directly under their respective cue lights. The back wall was equipped with an infrared activity monitor that collected locomotor behavior. Attached to a swivel arm, the infusion line entered the chamber from the center of the ceiling to be connected to the catheter on the rat for drug delivery. The cocaine was injected i.v. through the infusion line with an infusion pump at a fixed rate of 0.025 ml/s for duration of 4 s. All experimental variables were programmed and controlled using Graphic State Version 3.02 software that allowed for behavioral data collection.
Tests and procedures
Food training
Rats were trained to respond to an operant lever response task for a food pellet before catheterization. Training sessions were conducted in the dark cycle from 8:00 h to 15:00 h and lasted for 4 days in 90 min daily sessions. A fixed-ratio 1 (FR1) reinforcement schedule with a 30 s timeout period was used. Pressing the active lever once released one (45 mg) food pellet into the food receptacle as the cue light was illuminated for a 30 s timeout period. During the timeout period, food was not released but the response recorded. Pressing the inactive lever had no programmed consequence.
Successful lever discrimination was achieved when rats met previously described criterion of an active/inactive lever press ratio ≥2:1 (Larson and Carroll, 2005). When rats exhibited lever discrimination they underwent surgery for catheterization. After surgery and recovery, rats went through one additional session of food retraining to ensure conditioning met aforementioned criterion to be able to move onto the cocaine self-administration phase of the experiment.
Jugular vein catheterization
Indwelling catheters were implanted in the right jugular vein of the rats for i.v. cocaine self-administration. Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) given i.p. for surgery under aseptic conditions. The silastic tubing end of the catheter was introduced into the right jugular vein and secured with sutures. On the other end of the catheter, a 22G cannula guide (Plastics One, Roanoke, VA) was routed subcutaneously along the right upper dorsum to the midscapular region. After surgery, rats were given a 3-day recovery period. On every day after the surgery, the rats were given an i.v. injection through the catheter of cefazolin and glycerol/heparin solution to prevent occlusion and/or infection. The catheter patency was also tested daily by administering a mixture of ketamine (5 mg/kg, i.v.) and midazolam (0.75 mg/kg, i.v.). The catheter was determined to be patent only if the rat lost righting reflex within 3 s of the injection. If the catheter was not patent, the rat was immediately taken out of the study.
Cocaine self-administration
Cocaine self-administration sessions (90 min/day) lasted for 15 days. All drug sessions were conducted in the dark cycle from 8:00 h to 15:00 h. A FR1 schedule was used with a 30 s timeout period. Immediately before and after the session, catheters were injected with saline to prevent occlusion. At the start of every session, rats received one priming infusion of cocaine. A single press of the active lever resulted in an immediate delivery of cocaine (0.75 mg/kg/infusion, i.v.) and a 30 s timeout period. During the timeout period, the cue light above the active lever was illuminated and the drug was not available. Lever presses were recorded during the timeout period. Inactive lever pressing during the session did not have a programmed consequence, but presses were recorded. During the first 7 days of cocaine self-administration, rats received an i.v. dose of 0.75 mg/kg/infusion cocaine in a volume of 0.1 ml with a FR1 schedule. For the last 8 days, the i.v. dose was halved to 0.375 mg/kg/infusion of cocaine under the FR1 schedule to look at the sensitivity in the dose response rate.
Cocaine extinction
The extinction procedures mimicked the self-administration procedures. Rats were placed in the operant chambers for extinction sessions (90 min/day) for 15 days. All extinction sessions were conducted in the dark cycle from 8:00 h to 15:00 h. During the extinction session, a single press of the left lever (active lever) resulted in an immediate delivery of saline. All other parameters from the self-administration sessions remained the same.
Groups
At the end of the self-administration phase, and before the start of the extinction phase; the rats were randomly assigned into three groups: vehicle (control), low dose DCS (15 mg/kg i.p. DCS) or high dose DCS (30 mg/kg i.p. DCS). Immediately after the end of each extinction session, the rats were injected with the vehicle solution, 15 mg/kg i.p. DCS, or 30 mg/kg i.p. DCS.
Statistics
Two-way analysis of variance (ANOVA, followed by pair-wise comparisons using the Holm-Sidak method) was utilized in the analysis of the self-administration, extinction, and locomotor activity data for both treatment and session as the variables. All statistical comparisons were performed using the SigmaStat 3.1 statistical software.
RESULTS
Food training
Food training (Fig. 1) allowed for the rats to acquire the ability to differentiate between the active lever and the inactive lever. A two-way ANOVA revealed a significantly greater number of active versus inactive lever presses [F(1, 3) = 12.04; P < 0.001; Figs. 2 and 3]. Pair-wise multiple comparisons (Holm-Sidak method) indicated that this difference was significant beginning at day 3 (t = 3.28; P < 0.001; Figs. 2 and 3).
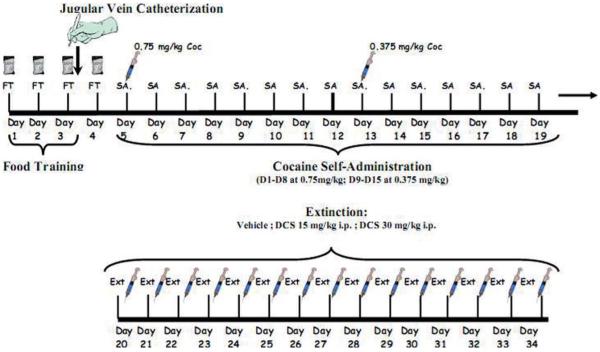
Fig. 1.
Study timeline: cocaine self-administration and extinction. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fig. 2.
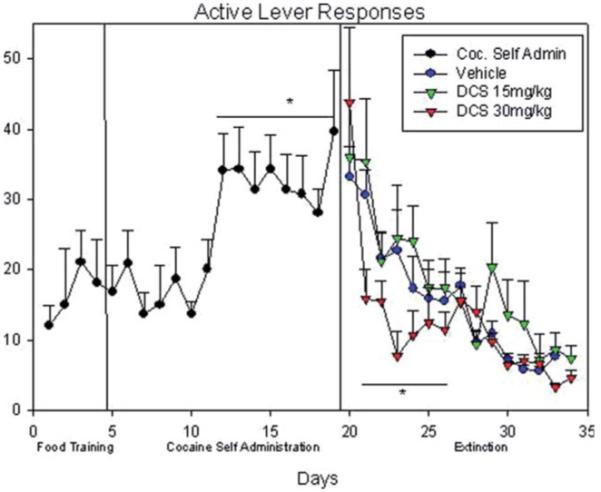
Active lever responses: food training, cocaine self-administration, and vehicle/15 mg/kg i.p. DCS/30 mg/kg i.p. DCS paired extinction. (*) indicates significant difference during the cocaine self-administrations sections; (**) indicates significant difference for 30 mg/kg i.p. DCS treatment group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fig. 3.
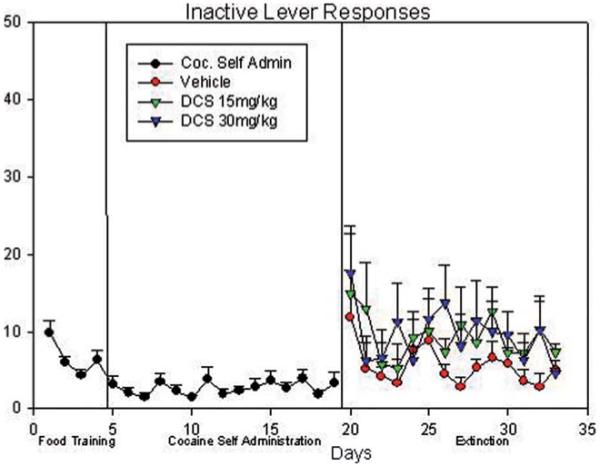
Inactive responses: food training, cocaine self-administration, and vehicle/15 mg/kg i.p.DCS/30 mg/kg i.p. DCS paired extinction. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cocaine self-administration
Rats as expected showed significantly greater number of cocaine (active) lever presses compared with the inactive lever which was maintained throughout the 15 days [two-way ANOVA; F(1, 23) = 300.53; P < 0.001; Figs. 2 and 3]. During the first week (days 5–11; Fig. 1), rats lever pressed for 0.75 mg/kg i.v. cocaine/ infusion (compared with the inactive lever). Similarly, during the second week, (days 12–19), rats again preferred and showed more lever presses for 0.35 mg/kg i.v. cocaine/ infusion (Fig. 2). A two-way ANOVA showed a significantly greater number of [F(1, 23) = 31.34; P < 0.001; Fig. 2] lever presses on days 12–19 (at 0.0375 mg/kg/infusion) as compared with the previous week (at 0.75 mg/kg/infusion). Furthermore, a two-way ANOVA showed as expected a significantly greater [F(1, 23) = 300.53; P < 0.001] number of active lever presses during cocaine self-administration compared with the inactive lever presses.
Pair-wise multiple comparisons indicated that there was a significantly greater number of active lever presses at both doses (t = 9.10; P < 0.001; 0.75 mg/kg cocaine during days 5–11 and at 0.375 mg/kg cocaine during days 12–19; t = 15.53; P < 0.001 Figs. 1-3).
Extinction: active lever
A two-way ANOVA used to examine active lever pressing during the extinction period (Fig. 1; days 20–34) yielded a significant difference in the number of active lever presses between the three treatment (low- and high-dose DCS, and vehicle) groups [F(1, 23) = 7.56; P < 0.001; Figs. 2 and 3]; as well as a significant difference between the number of days needed to achieve extinction [F(1, 23) = 3.89; P < 0.001; Fig. 2].
Pair-wise multiple comparisons (Holm-Sidak method) confirmed significant differences between the 15 mg/kg and 30 mg/kg i.p. DCS treatments (t = 2.85; P < 0.01), and between the vehicle and 30 mg/kg i.p. DCS treatments (t = 3.56; P < 0.001) favoring the higher DCS dose treatment of 30 mg/kg (Figs. 2 and 3). However, no statistical difference was obtained from the comparison between the vehicle and the 15 mg/kg i.p. DCS treatments (t = 0.65; P > 0.5). Furthermore, pair-wise comparisons revealed significant differences (P < 0.05; Fig. 2) in the 30 mg/kg treated rats and vehicle on extinction days 21–26 [day 21 (t = 6.04); day 22 (t = 3.16); day 23 (t = 4.02); day 24 (t = 3.12); day 25 (t = 2.01); day 26 (t = 2.16)]. Thus, 30 mg/kg i.p. DCS treated rats showed a significantly faster rate of extinguishing active lever responses.
Extinction: inactive lever
During the extinction period a two-way ANOVA was used to examine inactive lever pressing (Fig. 1; days 20–34). The results indicated a statistical significance in the number of inactive lever presses between all three treatment (low- and high-dose DCS, and vehicle) groups [F(1, 23) = 5.54; P < 0.01]. However, there was no significant difference found in the comparison of data among the extinction days [F(1, 23) = 0.94; P > 0.05].
Pair-wise multiple comparisons (Holm-Sidak method) confirmed significant differences between the 15 mg/kg i.p. DCS and vehicle treatments (t = 2.84; P < 0.01), and between the 30 mg/kg i.p. DCS and vehicle treatments (t = 2.52; P < 0.01). However, no statistical difference was obtained from the comparison between the 15 mg/kg i.p. and 30 mg/kg i.p. DCS treatments (t = 0.24; P > 0.5).
Locomotor
As expected, there was a significant increase in locomotor activity during the self-administration phase of the experiment compared with food training [F(1, 23) = 2.81; P < 0.01; Figs. 1 and 4]. During the extinction phase, a two-way ANOVA yielded no significant difference in locomotor activity among the three treatment groups [F(1, 23) = 0.74; P > 0.05; Fig. 4].
Fig. 4.
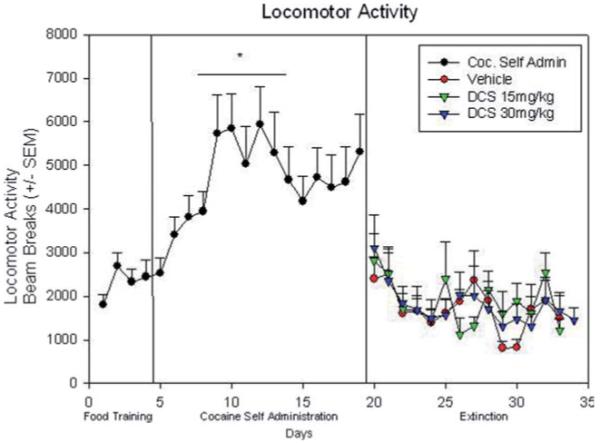
Locomotor activity during food training, cocaine self-administration, and vehicle/15 mg/kg i.p.DCS / 30 mg/kg i.p. DCS paired extinction: (*) indicates significant difference in locomotor activity during the cocaine self-administration section. [Color figure can be viewed in the online issue, which is available at wileyonline library.com.]
DISCUSSION
Previous studies have examined the behavioral and cognitive effects (Land and Riccio, 1999; Myers and Davis, 2002; Ho et al., 2005) of DCS and have reported that DCS may be involved in the facilitation of new memory formation that oppresses or overrides previously conditioned memories. In addition, it has been reported that as an NMDA partial agonist, DCS plays an important role in the consolidation of memories (Richardson, 2004), long-term potentiation or LTP (Yaka et al., 2007), and cocaine potentiated synaptic strength in the VTA (Borgland et al., 2004).
Evidence of the therapeutic effects of DCS, on extinction, has been widely described with respect to the extinction of conditioned fear and anxiety (Richardson, 2004; Hofmann et al., 2006; Norberg et al., 2008). Most recently, DCS has been examined for its properties of extinction of drug-seeking behavior after repeated drug exposures. The present findings, along with those of recent studies (Botreau et al., 2006; Paolone et al. 2009; Thanos et al., 2009), further support the notion that DCS may have therapeutic value for the extinction of cocaine seeking and abuse. Progressing from the original study on DCS-facilitated cocaine CPP extinction in rats (Botreau et al., 2006) and mice (Thanos et al., 2009); this study, utilizing a self-administration paradigm and two doses (15 mg/kg or 30 mg/kg i.p. DCS), revealed the dose effect and efficacy of DCS on the extinction of cocaine self-administration in rats.
Rats displayed similar cocaine self-administration behavior during the maintenance portion of the study as previously reported (Mark et al., 1999). When the cocaine dose was decreased in week 2 of self-administration, rats showed a corresponding increase in active lever responding for cocaine. Extinction of cocaine self-administration behavior in the rats indicated a progressive, daily, decrease in active lever responses; and slight increases in inactive lever responses. There were no significant differences in extinction between the vehicle (control) and the 15 mg/kg DCS rats. These results challenged those previously found in the extinction of a cocaine CPP (Botreau et al., 2006); study in which 15 mg/kg DCS were found effective in the facilitation of a cocaine CPP extinction. This is likely to reflect the differences in the neuronal processes that are involved in CPP, which does not require behavioral output other than choice of space verus those involved in drug self-administration, which require lever pressing to get the expected cocaine. This could be interpreted to indicate that whereas the lower DCS (15 mg/kg) dose may be sufficient to inhibit the place conditioning association it is insufficient to inhibit the motivational drive to lever press for cocaine.
The results from this study also indicated that there were differences in the rate at which the extinction took place between the vehicle (control) and the 30 mg/kg DCS treated rats. This higher DCS dose (30 mg/kg) showed a sharper decline in active lever responding and produced this effect rapidly (within the first treatment sessions). In addition, this effect was maintained throughout the first week extinction period (days 21–26). This facilitated cocaine extinction observed in the rats treated with 30 mg/kg DCS is consistent with findings for this dose of DCS on the extinction of fear and anxiety (Anthony and Nevins, 1993) and with findings of extinction on cocaine self-administration (Nic Dhonnchadha et al. 2010).
However, analysis of the inactive lever presses for all three treatment groups; during the extinction session indicate a somewhat erratic behavior. Although an increased number of inactive lever presses during the first few days of extinction is expected due to the initial cocaine-seeking response that rats show; this response typically lasts a few sessions. Indeed, the vehicle treated rats in this study within two extinction sessions showed a similar number of inactive lever responses in the extinction phase as in the cocaine self-administration phase. However, the DCS treated rats did show a greater number of inactive lever responses compared with the vehicle treated rats overall during extinction; although no significant difference was found on a day to day analysis. This increase in inactive lever responses during extinction of cocaine self-administration following DCS treatment has not been previously examined and further research is needed into its significance and mechanism. Thus, although DCS seems to facilitate the extinction of lever responding for cocaine; probably by enhancing learning of new contextual relationships during extinction sessions; this new learning of contextual relationships needs to be further examined in terms of responses to other cues.
Finally, there was no significant effect of DCS on locomotor activity throughout the extinction phase. This was in part consistent with the locomotor activity during CPP observed with 15 mg/kg DCS (Thanos et al. 2009). In addition, although previous data in CPP had shown inhibition of locomotor activity at 30 mg/kg, this was not observed here during the self-administration extinction paradigm. This may be due to the differences in the methods used (40 min per session, eight sessions of DCS treatment in the CPP study (Thanos et al., 2009); 90 min per session, 15 sessions of DCS treatment in this study. Although rats treated with DCS compared with control rats did not show significant locomotor side effects, further studies need to examine long-term effects following DCS treatment beyond 2 weeks.
CONCLUSION
This study demonstrated that DCS (30 mg/kg) can facilitate the extinction of the cocaine self-administration behavior in rats. These results when combined with data (from our lab and others) of facilitating cocaine CPP further supports the hypothesis that DCS may be useful in the treatment of drug abuse and addiction. Further investigations are required to evaluate the effects of DCS, it’s dosing, and long-term effects on the extinction of drug abuse behaviors as well reinstatement testing. Future research will examine whether these effects of DCS are selective for drug-seeking behavior or they are generalized to natural reinforcers such as sucrose or fatty foods.
Although DCS is approved in humans as an antibiotic for the treatment of tuberculosis, the clinical significance of our results in cocaine-dependent patients is limited since it remains unclear whether DCS exhibits generalized extinction (i.e., to other appetitive cues).
ACKNOWLEDGMENTS
The authors thank Jessica Steier for help with animal care.
Contract grant sponsor: The Intramural Research Program of NIAAA at the National Institute of Health (NIH); Contract grant sponsor: The NIDA summer research program to C.B.
REFERENCES
- Anthony EW, Nevins ME. Anxiolytic-like effects of N-methyl-D-aspartate-associated glycine receptor ligands in the rat potentiated startle test. Eur J Pharmacol. 1993;250:317–324. doi: 10.1016/0014-2999(93)90397-z. [DOI] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Gerdjikov T. The role of signaling molecules in reward-related incentive learning. Neurotox Res. 2004;6:91–104. doi: 10.1007/BF03033301. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: Electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. D-cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Preston KL, Bigelow GE. Discriminative stimulus and subjective effects of opioids with mu and kappa activity: Data from laboratory animals and human subjects. Psychopharmacology (Berl) 1997;130:14–27. doi: 10.1007/s002130050208. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Ho YJ, Hsu LS, Wang CF, Hsu WY, Lai TJ, Hsu CC, Tsai YF. Behavioral effects of d-cycloserine in rats: the role of anxiety level. Brain Res. 2005;1043:179–185. doi: 10.1016/j.brainres.2005.02.057. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Pollack MH, Otto MW. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev. 2006;12:208–217. doi: 10.1111/j.1527-3458.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Chojnacka-Wojcik E. Anticonflict effect of the glycineB receptor partial agonist. D-cycloserine, in rats. Pharmacological analysis. Psychopharmacology (Berl) 2000;152:224–228. doi: 10.1007/s002130000547. [DOI] [PubMed] [Google Scholar]
- Land C, Riccio DC. D-cycloserine: Effects on long-term retention of a conditioned response and on memory for contextual attributes. Neurobiol Learn Mem. 1999;72:158–168. doi: 10.1006/nlme.1998.3897. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Wheel running as a predictor of cocaine self-administration and reinstatement in female rats. Pharmacol Biochem Behav. 2005;82:590–600. doi: 10.1016/j.pbb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquistion of cocaine self-administration by augmenting extinction learning. Neuropsychopharm. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to d-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: Old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by d-cycloserine theoretical and clinical implications. Learn Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . National Survey on Drug Use and Health. 2007. [Google Scholar]
- Schuster CR, Johanson CE. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser. 1988;4:161–75. doi: 10.1007/978-3-642-73223-2_13. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Bermeo C, Wang GJ, Volkow ND. d-cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav Brain Res. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: effects of D-cycloserine. Acta Psychol (Amst) 2008;127:601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Yaka R, Biegon A, Grigoriadis N, Simeonidou C, Grigoriadis S, Alexandrovich AG, Matzner H, Schumann J, Trembovler V, Tsenter J, Shohami E. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. FASEB J. 2007;21:2033–2041. doi: 10.1096/fj.06-7856com. [DOI] [PubMed] [Google Scholar]