Abstract
The final steps of oogenesis occur during oocyte maturation that generates fertilization-competent haploid eggs capable of supporting embryonic development. Cyclin-dependent kinase 1 (CDK1) drives oocyte maturation and its activity and actions on substrates are tightly regulated. CDC14 is a dual-specificity phosphatase that reduces CDK1 activity and reverses the actions of CDK1 during mitosis. In budding yeast, Cdc14 is essential for meiosis, but it is not known whether its mammalian homolog CDC14A is required for meiosis in females. Here, we report that CDC14A is concentrated in the nucleus of meiotically incompetent mouse oocytes but is dispersed throughout meiotically competent oocytes. During meiotic progression CDC14A has no specific sub-cellular localization except between metaphase of meiosis I (Met I) and metaphase of meiosis II (Met II) when it co-localizes with the central portion of the meiotic spindle. Overexpression of CDC14A generally delays meiotic progression after resumption of meiosis whereas microinjection of oocytes with an antibody against CDC14A specifically delays exit from Met I. Each of these perturbations generates eggs with chromosome alignment abnormalities and eggs that were injected with the CDC14A antibody had an elevated incidence of aneuploidy. Collectively, these data suggest that CDC14A regulates oocyte maturation and functions to promote the meiosis I-to-meiosis II transition as its homolog does in budding yeast.
Keywords: CDC14A, meiosis, oocyte maturation, protein phosphatase, CDK
Introduction
Haploid gametes, which are essential for sexual reproduction, are generated from diploid precursor cells via a two-part cell division called meiosis. In mammals, meiotic maturation in oocytes is a prolonged process characterized by several cell cycle arrests. The first arrest at prophase of meiosis I (MI) occurs after the completion of homologous recombination in the developing fetus. During this arrest, these meiotically incompetent oocytes undergo a growth period during which they increase in volume (>200 fold) and accumulate organelles and macromolecules that are necessary to support meiotic maturation, fertilization and early embryonic cell divisions.1 During oocyte growth oocytes sequentially acquire meiotic competence, i.e., the ability to resume and complete meiosis.2-4 Resumption of meiosis, which in vivo is triggered by a surge in luteinizing hormone, requires high levels of cyclin-dependent kinase (CDK1) activity.5 CDK1 activity must decrease between MI, when homologous chromosomes segregate, and arrest at metaphase of meiosis II (MII). CDK1 activity is high at metaphase II (Met II) arrest and must decrease again when sister chromatid separation is initiated by fertilization or egg activation (MII exit).
Fluctuations in CDK1 activity drive meiotic maturation in oocytes and yet little is known about how these fluctuations are achieved. CDC14 is a dual-specificity phosphatase that preferentially dephosphorylates CDK substrates.6 Because CDC14 reverses the action of CDKs, it is required for a wide variety of cellular processes. For example, in budding yeast, Cdc14 is essential for multiple steps in mitotic exit. Cdc14 mutants arrest in late anaphase and have elongated spindles and 2C DNA content.7 During mitotic exit Cdc14 downregulates CDK activity and regulates formation of the spindle midzone that drives spindle elongation and coordinates cytokinesis.8-10 In fission yeast the Cdc14 homolog Clp1/Flp1 is not essential for mitotic exit but it is required for proper cytokinesis because it promotes stabilization of the contractile ring.11 This role in promoting cytokinesis is conserved in multi-cellular organisms such as C. elegans and human tissue culture cells.12,13
In mammals, there are two CDC14 homologs, CDC14A and CDC14B. In human cell lines CDC14A co-localizes with interphase centrosomes and altered expression of CDC14A causes several defects.12,14 Cell lines that overexpress CDC14A undergo premature centrosome splitting and have multi-polar mitotic spindles, whereas cell lines in which CDC14A is depleted by siRNA have centrosome splitting and cytokinesis defects. Both depletion and overexpression of CDC14A causes defects in chromosome segregation that lead to genomic instability. It is not known if any of these functions of CDC14A are conserved in mouse somatic cells.
Cdc14 is essential for meiosis in budding yeast.15,16 It is not known, however, if either mammalian CDC14 homolog is required for meiotic maturation in oocytes. We report here a role for CDC14A during mammalian meiosis in mouse oocytes. Our data reveal that CDC14A localization in oocytes correlates with acquisition of meiotic competence because it localizes in the nucleus of meiotically incompetent oocytes and is more dispersed throughout the oocyte once oocytes are competent to resume meiosis. Moreover, similar to its role in yeast, our data obtained by experimentally increasing or decreasing CDC14A activity suggest that CDC14A is required for promoting a timely transition from MI to MII and maintaining proper chromosome number in mouse oocytes.
Results
CDC14A localizes in the nucleus of prophase I arrested oocytes
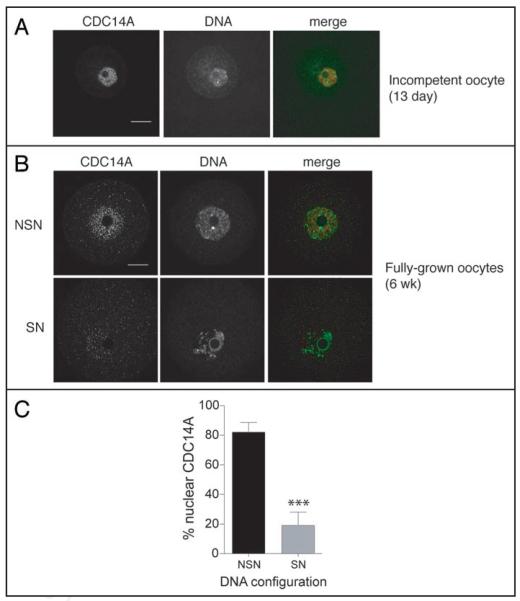
In human somatic cells, CDC14A has no distinct localization during prophase.12 In contrast, CDC14A localized inside the nucleus of meiotically incompetent oocytes (Fig. 1A). Following completion of the growth phase, fully-grown meiotically competent oocytes can be divided into two groups based on their chromatin configuration with respect to the nucleolus.17 In one group the chromatin is not condensed [referred to as non-surrounded nucleolus (NSN)] and in the other group the chromatin is condensed and forms a tight ring around the nucleolus [referred to as surrounded nucleolus (SN)]. A SN DNA configuration is associated with meiotic competence whereas a NSN DNA configuration is associated with meiotic incompetence.3,4 Interestingly, the nuclear staining for CDC14A appeared stronger in oocytes with a NSN DNA configuration when compared to oocytes with a SN configuration for which the staining was more dispersed throughout the entire oocyte (Fig. 1B and C). Thus, because of the observation that CDC14A is in the GV in incompetent oocytes and is dispersed in meiotically competent oocytes, there is a correlation between CDC14A localization and acquisition of meiotic competence.
Figure 1.
Immunocytochemical detection of CDC14A in prophase-arrested oocytes. (A) Meiotically incompetent and (B) meiotically competent oocytes were fixed in 3.7% pararformaldehyde and stained with an anti-CDC14A antibody. DNA was visualized with Sytox Green. In the merged images, CDC14A is red and DNA is green and the scale bars are 20 μm. These experiments were repeated 3 times and a total of 60 oocytes were visualized by confocal microscopy. (C) Images obtained in panel B were analyzed for DNA configuration and concentration of CDC14A in the nucleus. Concentration of nuclear CDC14A was assessed by using Image J to measure the intensity of CDC14A signal in the nucleus versus the signal intensity in the cytoplasm. Oocytes with a CDC14A signal ratio greater than 1 (with the nuclear signal being in the numerator) were considered to be oocytes with nuclear-concentrated CDC14A. The data are expressed as mean ± the S.E.M, and the data was analyzed by Student’s t-test. ***p < 0.0001; SN, surrounded nucleolus; NSN, non-surrounded nucleolus.
CDC14A localization is dynamic during oocyte maturation
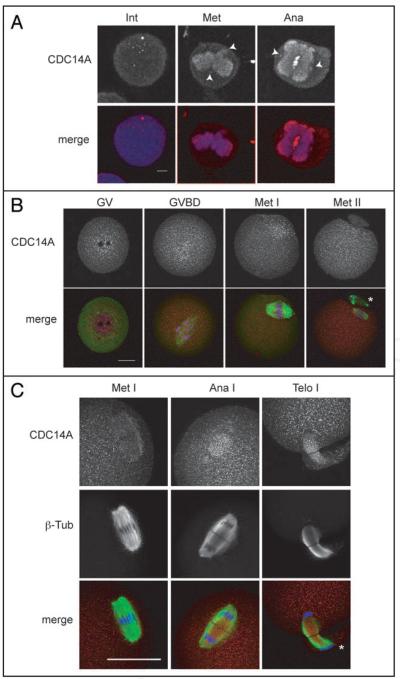
In human somatic cells CDC14A co-localizes with γ-tubulin at the centrosome during interphase and mitosis, and with the central spindle during anaphase.12,14 Similar to hCDC14A, we found that mCDC14A localizes at the centrosome and central spindle in somatic NIH 3T3 cells (Fig. 2A). To determine where CDC14A localized during mammalian meiosis, we collected prophasearrested [germinal vesicle (GV)] oocytes, cultured them in vitro and fixed them at various times during meiosis. After meiosis resumed [GV breakdown (GVBD)], CDC14A remained around the region of the condensed chromosomes, and was dispersed throughout the cytoplasm during both metaphases of MI (Met I) and MII (Met II) (Fig. 2B). Furthermore, CDC14A was more dispersed at Met II than it was at Met I because at Met I we still observed a significant portion of the protein around the region of the chromosomes. In contrast to somatic cells and the localization of CDC14B in oocytes,18 we never observed CDC14A co-localization with γ-tubulin at microtubule organizing centers (MTOCs; centrosome-like) in mouse oocytes.
Figure 2.
Immunocytochemical detection of CDC14A in NIH 3T3 cells and oocytes. (A) Mouse somatic 3T3 cells were fixed in cold methanol for 20 min prior to immunological detection. The arrows indicate localization at the mitotic centrosome during interphase (int), metaphase (met) and anaphase (ana) as determined by morphology and location with respect to the mitotic spindle. The scale bar is 5 μm. In the merged images CDC14A is red and DNA is blue. (B and C) GV-intact oocytes were collected and matured in vitro for 0 h (GV), 3 h (GVBD), 7 h (Met I), 9 h (Ana I, Telo I) and 16 h (Met II), prior to fixation in 3.7% paraformaldehyde. The scale bars are 20 μm and the asterisks indicate polar bodies. These experiments were conducted twice and a total of 15-20 oocytes per stage and 50 somatic cells were analyzed by confocal microscopy.
During meiosis in budding yeast, Cdc14 is sequestered in the nucleolus except at anaphase of MI (Ana I) when it is released onto the spindle where Cdc14 regulates the MI-to-MII transition.16 In mouse oocytes, CDC14A localized to the central region of the Ana I spindle (Fig. 2C) and persisted there through telophase of MI (Telo I) before it became dispersed again at Met II (Fig. 2B). The localization on the spindle between MI and MII suggests that CDC14A plays a role in regulating the MI-to-MII transition in female mammalian meiosis as it does in lower eukaryotes.
Overexpression of CDC14A prevents completion of meiotic maturation
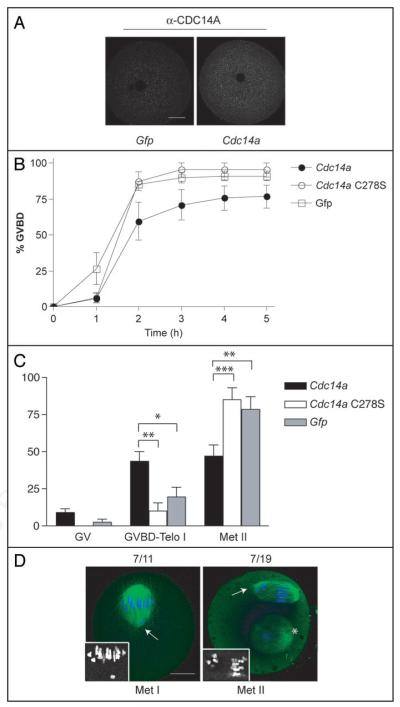
Overexpression of CDC14B prevents oocytes from resuming meiosis through activating the CDH1 regulatory subunit of the anaphase-promoting complex.18 We asked whether CDC14A similarly delays GVBD when overexpressed in mouse oocytes. To this end, we microinjected GV-intact oocytes with Cdc14a mRNA, allowed meiotic resumption to occur 4 h post-injection by washing the maturation inhibitor (milrinone) from the culture medium and monitored the disappearance of the GV every hour. As a control, we injected oocytes with catalytically inactive Cdc14a-C278S or Gfp mRNAs. Microinjection of Cdc14a expanded the endogenous pool by 1.5-fold when compared to Gfp mRNA-injected controls as assessed by immunocytochemistry (Fig. 3A). We found that CDC14A-overexpressing oocytes underwent GVBD with very similar kinetics as control oocytes (Fig. 3B). These data suggest that CDC14A does not play a role in preventing meiotic resumption.
Figure 3.
Overexpression of CDC14A delays meiotic progression after GVBD. (A) Oocytes were microinjected with the indicated mRNA and were held for 16 h in maturation medium containing milrinone prior to fixation and immunodetection of CDC14A. To estimate the level of overexpression the average intensities of the overexpressing cells (n = 15) were compared to the average intensities in Gfp-injected cells using Image J software. Scale bar is 20 μm. (B) Oocytes were injected with the indicated mRNAs and held in milrinone-containing medium for 4 h. The oocytes were washed out of the maturation inhibitor, and the absence of a GV (GVBD) was monitored every hour by light microscopy. These experiments were repeated 3 times, n = 60 for each group and the data are presented as the mean ± S.E.M. p = 0.0746 (2-way ANOVA) (C) Oocytes were injected with the indicated mRNAs, held in milrinone for 4 h and matured in the absence of milrinone for 16 h. Cells were fixed and stained with anti-β-tubulin (to visualize the spindle) and propidium iodide (to visualize DNA) to determine the meiotic stage. These experiments were conducted 3 times, n = 45 for each group. Two-way ANOVA was used to analyze the data and the data are presented as the mean ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.0001. (D) Representative images of oocytes overexpressing CDC14A where DNA is blue, and the spindle (β-tubulin) is green. The insets contain zoomed in black and white images of the chromosomes at Met I and Met II. The scale bar is 20 μm, the asterisk indicates a polar body and the arrows point to misaligned chromosomes.
Overexpression of CDC14A in human somatic cells leads to multi-polar spindles, aneuploidy and progressive cell death.12,14 We assessed the consequences of overexpressing CDC14A during oocyte maturation on chromosome and spindle dynamics by microinjecting mRNAs encoding Cdc14a into GV-intact oocytes that were then matured in vitro to Met II. Overexpression of CDC14A prevented completion of meiotic maturation because only 50% of the oocytes reached Met II after 16 h of maturation compared to ~90% that reach Met II in the controls (Fig. 3C). More than two-thirds of the oocytes that underwent GVBD but were not at Met II were blocked at pro Met I or Met I. The remaining one-third were exiting MI and were at either Ana I or Telo I (data not shown). Furthermore, this defect depended upon the catalytic activity of CDC14A because 85% of oocytes overexpressing the catalytically inactive C278S mutant completed meiosis by 16 h (Fig. 3C).
Although the slight delay in GVBD can account for ~20% of oocytes not reaching Met II within 16 h (Fig. 2B), it cannot account for the entire population (35%) that are unable to reach Met II within this time frame, suggesting that excess CDC14A delays meiotic events between meiotic resumption and MII arrest. After lengthy incubations (30 h) nearly all the Cdc14a-injected oocytes had extruded a polar body, suggesting that overexpression of CDC14A caused a delay and not a block in meiotic progression (data not shown). Furthermore, we observed severe chromosome alignment perturbations in 63% of oocytes overexpressing CDC14A that were at Met I and in 36% of oocytes that reached Met II after 16 h of maturation (Fig. 3D). Collectively, these data suggest that CDC14A is required to promote the transition from MI to MII.
Disruption of CDC14A function delays the MI-to-MII transition
The requirement for hCDC14A in regulating centrosome splitting and cytokinesis in somatic cells was determined by RNA interference (RNAi) depletion strategies.14 We attempted a similar strategy to study the requirement for mCDC14A in oocytes but found that even after long culture times following microinjection of long Cdc14a double-stranded RNA (dsRNA) (up to 72 h), there was no apparent decrease in the amount of CDC14A protein as determined by immunocytochemistry despite an efficient reduction (~90%) of Cdc14a message (data not shown).
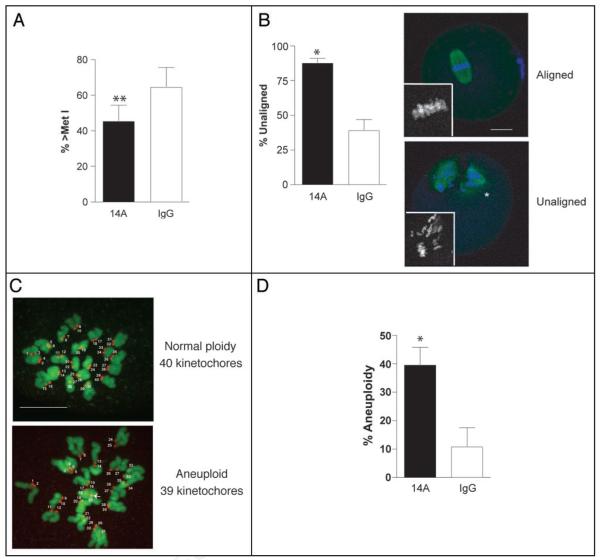
As an alternative approach, we microinjected GV-intact oocytes with a polyclonal antibody made against the C-terminal region of CDC14A and then matured these cells in vitro to Met II. Oocytes injected with the CDC14A antibody underwent GVBD and progressed to Met I at the same rate as control IgG-injected oocytes (data not shown). At 10 h following initiation of maturation, only 45% of oocytes injected with the CDC14A antibody had progressed past Met I, whereas nearly 70% of the control oocytes were past Met I and either in Ana I or Telo I (Fig. 4A). Although there is no published data demonstrating that this polyclonal anti-body specifically inhibits CDC14A activity, these data suggest that CDC14A is required for the timely progression from MI to MII and are consistent to the time in which Cdc14 functions in lower eukaryotes.16 Moreover, we observed no difference in the incidence of polar body extrusion of anti-CDC14A-injected oocytes when compared to control IgG-injected oocytes when matured in vitro to MII for 16 h (data not shown). Anti-CDC14A-injected eggs, however, had a two-fold higher incidence of misaligned chromosomes on the Met II plate (88% compared to 39%; Fig. 4B).
Figure 4.
CDC14A-disrupted oocytes are delayed in the MI-MII transition. Following microinjection of 10 mg/ml anti-CDC14A antibody or non-immune rabbit IgG, oocytes were either held in milrinone-containing CZB overnight and matured for 10 h the following day (A, repeated 6 times, n = 90) or matured in vitro for 16 h (B-D, repeated 3 times, n = 50) prior to fixation. (A and B) Spindles were stained with anti-β-tubulin (green) and DNA was visualized with propidium iodide (blue). Slides were scanned using confocal microscopy to observe clearly the meiotic cell cycle stage (A) or chromosome alignment at Met II (B). The lower panel of A contains representative images of eggs with aligned or unaligned chromosomes and the insets contain zoomed in black and white images of the chromosomes. The scale bar is 20 μm and the arrowheads indicate polar bodies. (C and D) After maturation, eggs were treated with monastrol for 1 h prior to fixation. Kinetochores were stained with an anti-CREST serum (red, numbered) and DNA was visualized with Sytox Green (green). Eggs containing more or less than 40 kinetochores were considered aneuploid. The numbers label each kinetochore, the arrow points to an unpaired kinetochore and the scale bar in this z-projection is 10 μm. The data were analyzed by a pairwise Student’s t-test and are expressed as mean ± S.E.M. *p < 0.05; **p < 0.01.
Next, we assessed whether chromosome misalignment correlates with altered chromosome numbers in anti-CDC14A-injected eggs. To this end, we treated in vitro matured eggs with monastrol, an Eg5 inhibitor that collapses the meiotic spindle and spreads the chromosomes inside the egg. This treatment permitted accurate counting of the number of kinetochores stained with CREST anti-serum in intact eggs (Fig. 4C); its advantages over standard chromosome-spreading techniques for ploidy assessment will be described elsewhere.19 We found that eggs that had been injected with the anti-CDC14A antibody had a four-fold higher incidence of aneuploidy as compared to IgG-injected controls (40% compared to 10%; Fig. 4D).
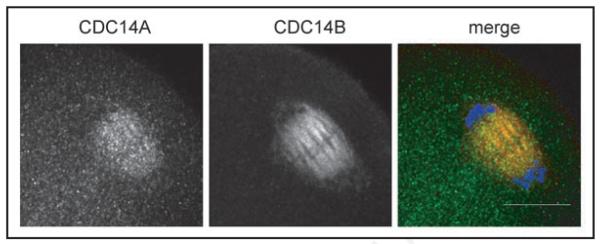
Although the Ana I spindle fails to disassemble in budding yeast strains that lack functional Cdc14, the cells attempt to segregate sister chromatids, which ultimately results in inviable dyads.16 In addition to CDC14A, mammals contain a second CDC14 homolog called CDC14B that co-localizes with the meiotic spindle in oocytes.18 We found that CDC14A and CDC14B co-localize on the central meiotic spindle during the MI-to-MII transition in mouse oocytes (Fig. 5), suggesting that these phosphatases play a redundant role in regulating this meiotic transition. Although all of CDC14B co-localizes with the meiotic spindle, CDC14A was still detected in the cytoplasm suggesting that only a portion of the protein co-localized with the spindle. These data suggest that CDC14B may play a more dominant role than CDC14A in regulating the meiotic spindle during the MI-to-MII transition. Importantly, this is the only time during meiotic maturation that we have observed co-localization of the 2 phosphatases (data not shown), indicating that they likely play different roles during meiosis and only overlap during MI exit.
Figure 5.
CDC14A and CDC14B co-localize on the central spindle during Ana I. GV-intact oocytes were matured in vitro for 9 h prior to fixation in MTSB-XF to preserve the meiotic spindle. The scale bar is 20 μm and in the merge CDC14A is green, CDC14B is red and DNA is blue. This experiment was conducted twice and a total of 20 oocytes were analyzed.
Discussion
We report here, for the first time, that CDC14A plays a role in meiotic maturation in mouse oocytes. During fetal development oocytes arrest in the dictyate stage prior to MI and then begin a prolonged growth phase. During this growth phase the oocyte acquires meiotic and developmental (the ability to support early embryonic development) and meiotic competence.2,20,21 Meiotic competence is fully acquired once an oocyte reaches ~80% of its full size, transcription is silenced and CDK1 protein accumulates.1-4,22-24 Furthermore, meiotically competent oocytes contain condensed chromatin that surrounds the nucleolus. Nuclear and cytoplasmic swap studies recently revealed that meiotic competence depends upon cytoplasmic factors whereas molecules required for developmental competence reside in the GV.25 We found that the nuclear localization of CDC14A highly correlated with chromatin configuration (Fig. 1B and C). The configuration of chromatin in meiotically incompetent oocytes is not condensed and CDC14A is concentrated in the GV. In contrast, in meiotically competent oocytes where chromatin is condensed, CDC14A is dispersed between the GV and cytoplasm. Meiotic competence is also associated with an increase in CDK1 protein that is localized in the GV.22-24 Therefore, there is an inverse correlation between the nuclear localization of CDK1 and CDC14A at the time of meiotic competence acquisition. In fission yeast Cdk1 phosphorylates and inactivates Cdc14 (Clp1/Flp1).26 It will be interesting to see if this negative feedback loop is conserved in mouse oocytes, and will likely be important to understanding how meiotic competence acquisition is regulated.
In budding yeast, Cdc14 mutants are unable to form viable spores due to defects in proper MI exit.16 We demonstrate that overexpression of CDC14A prevents completion of meiotic maturation (Fig. 3B), and that microinjection of a polyclonal antibody against CDC14A delays the transition between MI and MII and generates aneuploid eggs (Fig. 4). Although CDC14A was successfully depleted using siRNAs in human cells14 we find that in oocytes mCDC14A is surprisingly stable because 72 h after injection of long dsRNA CDC14A protein is not sufficiently reduced. Although there has not been a report demonstrating that the polyclonal antibody used in our studies neutralizes CDC14A activity, the delay in the timing of MI exit in our anti-CDC14A injected oocytes is consistent with what has been reported in lower eukaryotes. Moreover, CDC14A is dispersed at metaphase of MI and then localizes on the central meiotic spindle during the MI-to-MII transition, further suggesting that it plays a role in MI exit (Figs. 2C and 5).
In the mitotic cell cycle, DNA is replicated one time prior to separation of sister chromatids. DNA re-replication is prevented and DNA segregation is activated by CDKs.27,28 In yeast, Cdc14 antagonizes CDK action in late mitosis to promote DNA replication factor loading onto origins of replication and to promote mitotic exit.29 To generate gametes that contain half the number of chromosomes from diploid precursors in meiosis, two rounds of chromosome segregation must occur without an intervening round of DNA synthesis. This unique cell cycle situation demands that the mechanisms that regulate mitosis be altered so that correct chromosome segregation can occur during meiosis.
In budding yeast, one way this alteration is achieved is through uncoupling the spindle and chromosome cycles between MI and MII. During this transition, the spindle reduplicates while DNA replication is inhibited. Cdc14 is required for spindle duplication because Cdc14 mutants contain persistent MI spindles, but Cdc14 is not required to prevent DNA re-replication because Cdc14 mutants do not re-replicate their DNA.16 This meiosisspecific change in Cdc14 function occurs because yeast contain a CDK-related kinase called Ime2 that is only expressed during meiosis and whose consensus phosphorylation sites differs from that of CDKs and are not recognized by Cdc14.30 Although CDK and Ime2 phosphorylate different residues, their phosphorylation affects their substrates in similar ways. Therefore, during the MI-to-MII transition Ime2 phosphorylates DNA replication factors and Cdc14 does not recognize these phosphorylated residues. This mechanism prevents re-replication in a cell with low CDK activity. Mouse has several Ime2-like homologs (MAK, ICK and RAGE) and transcripts for all of these kinases are present in oocytes.31 It will be interesting to determine whether this regulatory mechanism is conserved in mammals.
In mammalian oocytes, CDK1 activity is partially reduced between MI and MII32 likely because cyclin B1 protein is only partially destroyed.33,34 To promote MI exit in mouse oocytes, separase binds to CDK1 and inhibits its activity.35 This mechanism contrasts to how MI exit in budding yeast is regulated because in yeast separase activates the release of Cdc14 from the nucleolus so that it can act upon its substrates. We found that injection of oocytes with a polyclonal antibody against CDC14A delays the MI-to-MII transition suggesting that it functions to promote MI exit in mammalian oocyte meiosis. As mentioned above, Cdk1 phosphorylates and inactivates Cdc14 (Clp1/Flp1) in fission yeast.26 During mitotic exit when Cdk1 activity decreases, Clp1/Flp1 is activated by auto-dephosphorylation in trans. Therefore, it is tempting to speculate that CDC14A is inactivated by CDK1 prior to Met I, and, because CDK1 activity declines during MI exit, CDC14A reactivates itself to promote Ana I by dephosphorylating CDK1 substrates. To address this model, a robust method that assesses changes in CDC14A activity during oocyte maturation must be developed.
As the spindle elongates and chromosomes segregate, a region called the central spindle, which is composed of anti-parallel, interdigitating microtubules, forms. In the center of this structure is an electron dense region called the spindle midzone that often appears as a dark band in immunofluorescent images because it is refractory to antibody staining. The central spindle and spindle midzone control cytokinesis and therefore the integrity of the central spindle is essential for cell division.36 CDC14A and CDC14B co-localize during this transition on the central meiotic spindle (Fig. 5) suggesting that they are functionally redundant during this phase of meiosis. A subset of proteins required to coordinate central spindle formation are phosphorylated and inhibited by CDK1 during mitotic metaphase.37-39 CDC14 promotes mitotic anaphase, in part, by reversing the CDK1 phosphorylation on these substrates and thereby promote their localization to the central spindle and spindle midzone.8,13 We find that although oocytes with depleted CDC14B extrude polar bodies, they contain abnormal spindles and have cytokinesis defects.18 Collectively, these data indicate that CDC14A and CDC14B control proper MI exit through reversing CDK1 action to control spindle elongation. The generation of a Cdc14a and Cdc14b double mutant mouse strain will be a more robust way to address the requirement of these phosphatases during this meiosis-specific transition.
Materials and Methods
Oocyte collection, culture and microinjection
Meiotically competent, germinal vesicle-intact oocytes from eCG-primed (44–48 h prior to collection), 6 wk-old female CF-1 mice (Harlan) were collected as previously described.40 Meiotic resumption was inhibited by adding milrinone (2.5 μM final concentration) to the collection, culture or injection medium. Meiotically incompetent oocytes were obtained from 13-day-old female CF-1 mice as described previously.41 Oocytes were cultured in CZB medium in an atmosphere of 5% CO2 in air at 37°C.42 Oocytes were microinjected in bicarbonate-free Whitten’s medium supplemented with 10 mM Hepes (pH 7.3) and 0.01% polyvinylalcohol.43 Oocytes were injected with 7 pl of mRNA at 1 μg/μl as previously described.44 The CDC14A (Zymed 34-8100) and IgG (Jackson Laboratory) antibodies were concentrated to 10 mg/mL in PBS using a Microcon YM-30 centrifugal filter device (Millipore) per the manufacturer’s instructions. The oocytes were held in CZB plus milrinone for 4 h post-microinjection except for oocytes shown in Figure 4A where they were held for 14 h. In the maturation experiments oocytes were washed and cultured in milrinone-free CZB medium. All animal experiments were approved by the Institutional Animal Use and Care Committee and were consistent with NIH guidelines.
Cloning
Full-length cDNA of Cdc14a (IMAGE: 30356962) was acquired from the IMAGE collection (Open Biosystems), cloned into the pIVT expression vector and verified by sequencing. Site-directed mutagenesis on the pIVT construct was performed to generate catalytically inactive mutant of Cdc14a (Quik Change, Stratagene). In this mutant, C278 was changed to an S (TGC to TCC).
In vitro synthesis of mRNA
Plasmids containing Cdc14a and EGfp sequences were linearized with NotI and then in vitro transcribed using a T7 mMessage mMachine kit (Ambion). The resulting RNA was purified using RNeasy Mini Kit (Qiagen) per manufacturer’s instructions.
Immunocytochemistry
Oocytes and eggs were fixed in 3.7% paraformaldehyde in PBS for 1 h at room temperature or in MTSB-XF buffer45 for 30 min at 37°C. Oocytes were permeabilized in 0.1% Triton X-100 plus 0.3% BSA in PBS for 15 min at room temperature, rinsed through 3 drops of blocking solution (0.3% BSA plus 0.01%Tween-20 in PBS) prior to blocking for 15 min. In a humidified chamber, oocytes were incubated in blocking solution containing primary antibody for 1 h. The following dilutions were used: CDC14A (Zymed 34-8100, 1:30), CDC14B (Abcam ab26194, 1:100), CREST (Immunovision HCT-0100, 1:40) and β-tubulin (Sigma T4026, 1:500). After washing, secondary antibodies were applied for 1 h. The secondary antibodies (Jackson) were Cy5-conjugated anti-chicken and anti-rabbit IgG, Alexa 488-conjugated anti-rabbit, Alexa 594-conjugated anti-human (Invitrogen) and FITC-conjugated anti-mouse IgG (Southern Biotech). DNA was detected by either mounting the cells in VectaShield containing 3 μg/ml propidium iodide or by a 15 min incubation in blocking buffer containing Sytox Green (1:5,000) (Invitrogen). Somatic NIH 3T3 cells were fixed for 20 min in ice-cold methanol and stained with the Cdc14A anti-body at 1:100. Fluorescence was detected on a Leica TCS SP laser-scanning confocal microscope with Leica Confocal Software (Leica Microsystems) and images were processed using Photoshop software (Adobe Systems, Inc.). Most images were viewed under a 40X oil immersion objective (NA 1.25). Images that focus on the meiotic spindle and those of NIH 3T3 cells were viewed under a 63X oil immersion objective (NA 1.32) and the kintochores were viewed under a 100X oil immersion objective (NA 1.4) using a Leica DMI4000B spinning disc confocal.
Ploidy assessment
In vitro matured eggs were treated with monastrol (Sigma, 100 μM) for 1 h in a humidified chamber prior to 20 min fixation in 2% paraformaldehyde in PBS. A 9 μm z-series was obtained by scanning in 0.4 μm increments and kinetochores were counted using Image J software (NIH).
Statistical analysis
Two-way ANOVA or Student t-test, as indicated in the figure legends, was used to evaluate the difference between groups using Prism software (Graph Pad Software, Inc., San Diego, CA). Differences of p < 0.05 were considered to be significant.
Acknowledgements
The authors thank Francesca Duncan for critical reading of this manuscript and assistance with developing the ploidy assessment protocol and imaging in Figure 4. The authors also thank Jun Ma for growing the NIH 3T3 cells used in Figure 2A and Michael Lampson for use of his confocal microscope. K.S. was supported by F32 HD055822 from the NIH. This work was supported by a grant from the NIH (HD22681) to R.M.S.
Abbreviations
- Ana
anaphase
- GV
germinal vesicle
- GVBD
germinal vesicle breakdown
- Int
interphase
- MI
meiosis I
- MII
meiosis II
- Met
metaphase
- NSN
non-surrounded nucleolus
- RNAi
RNA interference
- SN
surrounded nucleolus
- Telo
telophase
References
- 1.Schultz RM. Oogenesis and the control of meiotic maturation. Cambridge University Press; New York: 1986. [Google Scholar]
- 2.Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976;50:531–6. doi: 10.1016/0012-1606(76)90172-x. [DOI] [PubMed] [Google Scholar]
- 3.Moore GP. The RNA polymerase activity of the preimplantation mouse embryo. Journal of embryology and experimental morphology. 1975;34:291–8. [PubMed] [Google Scholar]
- 4.Moore GP, Lintern-Moore S. Transcription of the mouse oocyte genome. Biol Reprod. 1978;18:865–70. doi: 10.1095/biolreprod18.5.865. [DOI] [PubMed] [Google Scholar]
- 5.Masui Y, Clarke HJ. Oocyte maturation. Int Rev Cytol. 1979:57. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- 6.Gray CH, Good VM, Tonks NK, Barford D. The structure of the cell cycle protein Cdc14 reveals a proline-directed protein phosphatase. The EMBO journal. 2003;22:3524–35. doi: 10.1093/emboj/cdg348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pringle JRaH LH. The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1981. [Google Scholar]
- 8.Khmelinskii A, Lawrence C, Roostalu J, Schiebel E. Cdc14-regulated midzone assembly controls anaphase B. The Journal of cell biology. 2007;177:981–93. doi: 10.1083/jcb.200702145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaspersen SL, Charles JF, Tinker-Kulberg RL, Morgan DO. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Molecular biology of the cell. 1998;9:2803–17. doi: 10.1091/mbc.9.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, Amon A. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell. 1998;2:709–18. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 11.Trautmann S, Wolfe BA, Jorgensen P, Tyers M, Gould KL, McCollum D. Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr Biol. 2001;11:931–40. doi: 10.1016/s0960-9822(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser BK, Zimmerman ZA, Charbonneau H, Jackson PK. Disruption of centrosome structure, chromosome segregation and cytokinesis by misexpression of human Cdc14A phosphatase. Molecular biology of the cell. 2002;13:2289–300. doi: 10.1091/mbc.01-11-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruneberg U, Glotzer M, Gartner A, Nigg EA. The CeCDC-14 phosphatase is required for cytokinesis in the Caenorhabditis elegans embryo. The Journal of cell biology. 2002;158:901–14. doi: 10.1083/jcb.200202054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mailand N, Lukas C, Kaiser BK, Jackson PK, Bartek J, Lukas J. Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat Cell Biol. 2002;4:317–22. doi: 10.1038/ncb777. [DOI] [PubMed] [Google Scholar]
- 15.Buonomo SB, Rabitsch KP, Fuchs J, Gruber S, Sullivan M, Uhlmann F, et al. Division of the nucleolus and its release of CDC14 during anaphase of meiosis I depends on separase, SPO12 and SLK19. Dev Cell. 2003;4:727–39. doi: 10.1016/s1534-5807(03)00129-1. [DOI] [PubMed] [Google Scholar]
- 16.Marston AL, Lee BH, Amon A. The Cdc14 phosphatase and the FEAR network control meiotic spindle disassembly and chromosome segregation. Dev Cell. 2003;4:711–26. doi: 10.1016/s1534-5807(03)00130-8. [DOI] [PubMed] [Google Scholar]
- 17.De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;292:1–12. doi: 10.1016/j.ydbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Schindler K, Schultz RM. CDC14B acts through FZR1 (CDH1) to prevent meiotic maturation of mouse oocytes. Biol Reprod. doi: 10.1095/biolreprod.108.074906. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan FE, Chiang T, Lampson MA and Schultz RM. Unpublished data.
- 20.Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation and fertilization in vitro. Biol Reprod. 1989;41:268–76. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- 21.Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol. 1991;143:162–72. doi: 10.1016/0012-1606(91)90063-9. [DOI] [PubMed] [Google Scholar]
- 22.de Vantery C, Stutz A, Vassalli JD, Schorderet-Slatkine S. Acquisition of meiotic competence in growing mouse oocytes is controlled at both translational and posttranslational levels. Dev Biol. 1997;187:43–54. doi: 10.1006/dbio.1997.8599. [DOI] [PubMed] [Google Scholar]
- 23.Mitra J, Schultz RM. Regulation of the acquisition of meiotic competence in the mouse: changes in the subcellular localization of cdc2, cyclin B1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. J Cell Sci. 1996;109:2407–15. doi: 10.1242/jcs.109.9.2407. [DOI] [PubMed] [Google Scholar]
- 24.Chesnel F, Eppig JJ. Synthesis and accumulation of p34cdc2 and cyclin B in mouse oocytes during acquisition of competence to resume meiosis. Mol Reprod Dev. 1995;40:503–8. doi: 10.1002/mrd.1080400414. [DOI] [PubMed] [Google Scholar]
- 25.Inoue A, Nakajima R, Nagata M, Aoki F. Contribution of the oocyte nucleus and cytoplasm to the determination of meiotic and developmental competence in mice. Human reproduction (Oxford, England) 2008;23:1377–84. doi: 10.1093/humrep/den096. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe BA, McDonald WH, Yates JR, 3rd, Gould KL. Phospho-regulation of the Cdc14/Clp1 phosphatase delays late mitotic events in S. pombe. Dev Cell. 2006;11:423–30. doi: 10.1016/j.devcel.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–74. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 28.Blow JJ, Dutta A. Preventing re-replication of chromosomal DNA. Nature reviews. 2005;6:476–86. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Amours D, Amon A. At the interface between signaling and executing anaphase—Cdc14 and the FEAR network. Genes Dev. 2004;18:2581–95. doi: 10.1101/gad.1247304. [DOI] [PubMed] [Google Scholar]
- 30.Holt LJ, Hutti JE, Cantley LC, Morgan DO. Evolution of Ime2 phosphorylation sites on Cdk1 substrates provides a mechanism to limit the effects of the phosphatase Cdc14 in meiosis. Mol Cell. 2007;25:689–702. doi: 10.1016/j.molcel.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan H, O’Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev Biol. 2005;286:493–506. doi: 10.1016/j.ydbio.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Choi T, Aoki F, Mori M, Yamashita M, Nagahama Y, Kohmoto K. Activation of p34cdc2 protein kinase activity in meiotic and mitotic cell cycles in mouse oocytes and embryos. Development. 1991;113:789–95. doi: 10.1242/dev.113.3.789. [DOI] [PubMed] [Google Scholar]
- 33.Donahue RP. Maturation of the mouse oocyte in vitro I. Sequence and timing of nuclear progression. The Journal of experimental zoology. 1968;169:237–49. doi: 10.1002/jez.1401690210. [DOI] [PubMed] [Google Scholar]
- 34.Hampl A, Eppig JJ. Analysis of the mechanism(s) of metaphase I arrest in maturing mouse oocytes. Development. 1995;121:925–33. doi: 10.1242/dev.121.4.925. [DOI] [PubMed] [Google Scholar]
- 35.Gorr IH, Reis A, Boos D, Wuhr M, Madgwick S, Jones KT, et al. Essential CDK1-inhibitory role for separase during meiosis I in vertebrate oocytes. Nat Cell Biol. 2006;8:1035–7. doi: 10.1038/ncb1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCollum D. Cytokinesis: the central spindle takes center stage. Curr Biol. 2004;14:953–5. doi: 10.1016/j.cub.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 37.Fu C, Yan F, Wu F, Wu Q, Whittaker J, Hu H, et al. Mitotic phosphorylation of PRC1 at Thr470 is required for PRC1 oligomerization and proper central spindle organization. Cell Res. 2007;17:449–57. doi: 10.1038/cr.2007.32. [DOI] [PubMed] [Google Scholar]
- 38.Wheatley SP, Hinchcliffe EH, Glotzer M, Hyman AA, Sluder G, Wang Y. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. The Journal of cell biology. 1997;138:385–93. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishima M, Pavicic V, Gruneberg U, Nigg EA, Glotzer M. Cell cycle regulation of central spindle assembly. Nature. 2004;430:908–13. [Google Scholar]
- 40.Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–73. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- 41.Ihara M, Stein P, Schultz RM. UBE2I (UBC9), a SUMO-conjugating enzyme, localizes to nuclear speckles and stimulates transcription in mouse oocytes. Biol Reprod. 2008;79:906–13. doi: 10.1095/biolreprod.108.070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–88. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 43.Whitten W. Nutrient requirements for the culture of preimplantion mouse embryo in vitro. Adv Biosci. 1971;6:129–39. [Google Scholar]
- 44.Kurasawa S, Schultz RM, Kopf GS. Egg-induced modifications of the zona pellucida of mouse eggs: effects of microinjected inositol 1,4,5-trisphosphate. Dev Biol. 1989;133:295–304. doi: 10.1016/0012-1606(89)90320-5. [DOI] [PubMed] [Google Scholar]
- 45.Messinger SM, Albertini DF. Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100:289–98. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]