Abstract
Rationale
While the personality dimensions of novelty seeking and sensation seeking are associated with drug abuse vulnerability, the mechanisms associated with this vulnerability remain obscure.
Objective
This study examined the behavioral effects of d-amphetamine in healthy volunteers scoring in the upper and lower quartiles based on age- and gender-adjusted population norms on the impulsive Sensation-Seeking Scale (SSS) of the Zuckerman–Kuhlman personality questionnaire (ZKPQ).
Method
Participants completed 7-day outpatient studies examining the subjective, performance, and cardiovascular effects of d-amphetamine (0, 7.5, and 15 mg/70 kg, p.o.) under double-blind conditions according to a randomized block design. Performance tasks included behavioral measures of impulsivity, including attention, inhibition, and risk-taking behavior.
Results
No differences in baseline performance or d-amphetamine effects on measures of attention, inhibition, and risk-taking behavior were observed. High impulsive sensation seekers reported greater increases on several subjective report measures associated with drug abuse potential, including visual analog scales feel drug, like drug, and high.
Conclusions
Healthy adults scoring in the top quartile on the population of the impulsive SSS of the ZKPQ may be vulnerable to the abuse potential of d-amphetamine.
Keywords: d-amphetamine, Sensation-seeking status, Vulnerability, Subjective effects, Performance effects, Abuse potential
Introduction
The personality dimension of novelty or sensation seeking is associated with the initiation of drug use and the frequency and amount of drug use among adolescents and young adults (Brennan et al. 1986; White et al. 2005; Wills et al. 1994, 1995). Furthermore, individuals with these personality traits are at increased risk for the development of drug abuse and dependence (Hawkins et al. 1992; Huba et al. 1981). One purpose of the present study was to examine potential pharmacological and behavioral factors associated with enhanced drug abuse vulnerability among high sensation seekers.
Sensation seeking was conceptualized as a neurobiologically based preference for novel, complex, ambiguous, and/or emotionally intense sensations and experiences and willingness to take risks for such experiences (Zuckerman 1994). The trait was associated with impulsivity and reward sensitivity and is moderately correlated with each of the two orthogonal basic dimensions of personality, extraversion, and constraint (Depue and Collins 1999; Zuckerman et al. 1993). Individual differences in sensation-seeking status were attributed to both genetic and environmental influences, as determined by twin and survey studies (e.g., Fulker et al. 1980; Stacy et al. 1991).
Preclinical studies have consistently found that animals emitting high levels of novelty-seeking behavior exhibit increased sensitivity to the effects of stimulant drugs (e.g., Bardo et al. 1996). Several recent clinical studies suggest that high sensation seekers are also more sensitive to the behavioral effects of stimulants (Hutchison et al. 1999; Perkins et al. 2000; Sax and Strakowski 1998; White et al. 2005). However, not all studies have found a positive association between sensation seeking and sensitivity to stimulant drugs (e.g., Alessi et al. 2003; Carrol et al. 1982; Chait 1993; Corr and Kumari 2000; de Wit et al. 1986). Across studies, novelty or sensation-seeking status, or associated traits were determined using a variety of questionnaires, including the Sensation-Seeking Scale-Form V (SSS-Form V), the Tridimensional Personality Questionnaire, and the Multidimensional Personality Questionnaire-Brief Form (Cloninger 1987; Patrick et al. 2002; Zuckerman et al. 1978). The association between sensation seeking and drug sensitivity was evaluated by examining correlations between scores on questionnaires with magnitude of drug response or by comparing drug response in subgroups of the study volunteers that vary in sensation-seeking status. Under these conditions, the association between sensation seeking and drug sensitivity can be influenced by multiple factors, including sample size, the relative distribution of sensation-seeking scores, and the relative contribution of influences on sensation-seeking status (i.e., genetic vs environmental) among the subjects in a given study (e.g., White et al. 2005).
A recent study by our group examined the effects of d-amphetamine in young adults who had been consistently in the top third of their age-based cohorts on the SSS-Form V across four consecutive annual assessments as adolescents (Kelly et al., personal communication). High sensation seekers were more sensitive to the performance, subjective, and cardiovascular effects of d-amphetamine than their low sensation-seeking classmates. However, groups differed on several factors in addition to sensation-seeking status (e.g., alcohol use, gender, extraversion, and measures of behavioral undercontrol), making it difficult to ascribe group differences to any specific factor. A primary objective of this study was to determine whether d-amphetamine effects would vary among groups of young adults recruited to reflect extremes on the impulsive SSS when controlling for gender, ethnicity, prior drug use, and associated personality components. To characterize the pharmacological effects of the drug in these groups, multiple doses were tested on two occasions to establish and replicate dose–response effects. While neither sensitization nor tolerance is typically observed during repeated testing with d-amphetamine in clinical studies with healthy volunteers (e.g., Kelly et al. 1991; Wachtel and de Wit 1999), the testing of doses on two occasions also permitted us to examine the possibility of group differences in sensitivity or tolerance associated with repeated testing.
Zuckerman and colleagues have attempted to identify basic dimensions of personality by factor analyzing scores on a variety of personality and temperament scales with known or suspected biological determinants (Zuckerman et al. 1988, 1991). One of the five basic dimensions that emerged through this analysis was impulsive sensation seeking. A new questionnaire (Zuckerman–Kuhlman Personality Questionnaire, ZKPQ) designed to measure these five basic dimensions has high content and construct validity (e.g., Goma-i-Freixanet et al. 2005; Zuckerman et al. 1993). The impulsive SSS of this questionnaire, which purportedly reflects a biologically based personality dimension, generates scores that are correlated with Form V of the SSS (Zuckerman et al. 1993). A second purpose of the present study was to determine whether the association between d-amphetamine and sensation-seeking status would also be observed if sensation-seeking status was determined using the biologically based impulsive SSS of the ZKPQ (Zuckerman et al. 1993).
Impulsive behavior was associated with individual differences in vulnerability to drug abuse (e.g., Bickel and Marsch 2001; de Wit and Richards 1997; Semple et al. 2005). Scores on the impulsive SSS of the ZKPQ are highly correlated with personality scales designed to assess impulsivity (e.g., Zuckerman et al. 1993; Goma-i-Freixanet et al. 2005). While associations between performance and personality measures of impulsivity have yet to be established (e.g., Evenden 1999; Holt et al. 2003; Zermatten et al. 2005), it is possible that increased risk for drug abuse among high impulsive sensation seekers may be associated with the impulsive behavior that is characteristic of these individuals. A final objective of the current study was to examine performance of high and low impulsive sensation seekers on behavioral tasks associated with impulsivity, including attention (rapid information processing task), inhibition (stop-signal task), and risk-taking behavior (gambling task) under control and active d-amphetamine conditions to examine the associations among drug effects, performance, and questionnaire assessments of impulsivity and sensation-seeking status.
Materials and methods
Participants
Healthy adult volunteers were recruited through advertisements placed on the University of Kentucky campus and in the local community. All volunteers completed a brief telephone interview or an internet-based questionnaire addressing general medical and legal status and completed the impulsive SSS from the ZKPQ (Zuckerman et al. 1988, 1993). Those reporting significant medical or legal problems or daily or regular heavy drug use (e.g., hallucinogens, alcohol, marijuana, stimulants, or sedatives) were not considered. Those reporting good health and occasional caffeine use with impulsive SSS scores falling in the upper (i.e., men >14 and women >13) and lower (i.e., men <7 and women <6) quartile of scores from college students (Zuckerman, personal communication) were contacted by telephone and invited to participate in the study.
During an orientation and medical screening day, volunteers completed a battery of medical and psychological questionnaires, including the Zuckerman SSS (Form V; Zuckerman et al. 1978), blood chemistry, liver function, and urinalysis tests. Volunteers were excluded if they had a history of medical illness (e.g., cardiovascular disease, neurological or psychiatric disorder) or if there were any indication of elevated medical risk associated with taking d-amphetamine. During a separate training session, participants practiced the study tasks until performance was consistent and accurate across consecutive trials. Forty-six volunteers (23 men: 1 African-American, 1 Asian, and 21 Caucasian; 23 women: 2 African-American and 21 Caucasian) were invited to complete the medical evaluation. Three chose not to participate, ten were excluded for medical reasons, eight withdrew after successfully completing the medical evaluation before beginning the study, and two withdrew after the training session.
Twenty-two out of 23 participants, all Caucasians, completed the study, which consisted of seven 4.5-h test days, Monday through Friday, each separated by a minimum of 48 h. One subject dropped out after the first session. Participants were instructed to abstain from medication and alcohol for 24 h before all scheduled test days, and to abstain from eating for 4 h before the start of each test day. At the start of a test day, which occurred at the same time each day, participants answered open-ended questions regarding sleep, medication use, eating behavior, and health status during the preceding 24 h, and completed field-sobriety, breath (Alco-Sensor III, Intoximeters; piCO Carbon Monoxide Monitor, Bedfont Scientific), and urine tests (cocaine, benzodiazepine, barbiturate, marijuana, amphetamine, and opiate OnTrak TesTstik, Varian; Clear-view HCG II, Unipath) to assess drug use and pregnancy. Participants then consumed a low-fat snack and behavioral assessments were completed before (i.e., time 0) and at hourly intervals for 3 h after dose administration. Each assessment was approximately 35 min in duration.
Upon successful completion of the 7-day protocol, participants received approximately *$350, including per diem and task earnings, and a bonus (*$140) for completing all scheduled test days and abstaining from drug use for the duration of the study. There were no group differences in earnings. The study was reviewed and approved by the University of Kentucky Medical Institutional Review Board.
Data from two female high sensation seekers were excluded from analysis due to tobacco and drug use during study participation, resulting in a final sample size of ten high (five women) and ten low (five women) impulsive sensation seekers. Low sensation seekers were significantly lower on the total score (p<0.001) and on the Thrill and Adventure Seeking (p=0.05), Experience Seeking (p<0.0001), and Boredom Susceptibility (p<0.0005) sub-scales of the SSS (Form V). Scores on the Disinhibition subscale were not different (p<0.2). Groups were not significantly different (two-sample t tests) in age (21.6 vs 21.7 years for low and high groups, respectively), height, weight, years of education (14.6 vs 13.9), or drug use. Alcohol (4.9±2.3 vs 5.9±3.1 drinks per week) and caffeine (34 vs 59 mg/day) use was modest for all participants. Four participants (one low sensation seeker) reported intermittent (i.e., less than daily) tobacco use, and two (one low) reported marijuana use on two or fewer occasions during the month preceding the study. No other drug use was reported (e.g., amphetamines and cocaine). Groups did not differ on any of the questionnaires examining symptoms of behavioral undercontrol (e.g., ADHD or conduct disorder), extraversion, mood disorders, or psychiatric symptoms, such as alcohol abuse or depression.
Design
A double-blind, placebo-controlled, randomized block design was used to examine the effects of one between-subject variable (high vs low sensation-seeking status) and three within-subject variables [d-amphetamine dose (0.0, 7.5, and 15.0 mg/70 kg), time (0, 60, 120, and 180 min postdose), and replication (two levels)].
Drug
Doses were prepared based on body weight by the University of Kentucky Investigational Pharmacy in size 00 opaque capsules with lactose filler. Placebo (0.0 mg/70 kg) was always administered during the first test day when data were recorded but not analyzed. Each dose (0.0, 7.5, and 15.0 mg/70 kg) was tested on two occasions in random order, and each dose was tested on one occasion before any dose was repeated.
Behavioral and subjective state assessment
During each assessment, subjects completed measures associated with drug abuse potential, including self-report (e.g., Foltin and Fischman 1991) and psychomotor and cognitive performance (e.g., Roache 1991) measures, in the following order:
-
–
Visual analog scales (VAS): Participants rated items (I feel stimulated, stressed, sedated, hungry, anxious, light-headed, thirsty, sleepy, sick to my stomach, down, high, and a drug effect, and I like the drug effect) presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not at all” and “Extremely.”
-
–
Profile of mood states (POMS): Participants completed an experimental version of the POMS consisting of 72 adjectives rated along a five-point scale, from “Not at all” to “Extremely,” which yielded scores on ten clusters: Anxiety, Depression, Anger, Vigor, Fatigue, Confusion, Friendliness, Elation, Arousal and Total Positive (McNair et al. 1971).
-
–
Addiction Research Center Inventory (ARCI): The 49-item short form of the true–false inventory yielded information on five dimensions: lysergic acid diethylamide (LSD) scale; amphetamine (A) scale; benzedrine group (BG) scale; morphine–benzedrine group (MBG) scale; and the pentobarbital, chlorpromazine, alcohol group (PCAG) scale (Martin et al. 1971).
-
–
Digit symbol substitution task (DSST): Participants completed a 1.5-min computerized version of the DSST adopted from (McLeod et al. 1982). Trial completion rate and accuracy was monitored on this psychomotor task.
-
–
Rapid information processing task (RIP): Participants completed a 5-min computerized version of the RIP (Fillmore et al. 2005). Information processing capacity (working memory) was determined based on the rate of digit presentation maintained and signal detection accuracy (proportion of hits) during this self-paced task.
-
–
Stop-signal task: Participants completed a 7.75-min computerized version of the stop-signal task (Fillmore et al. 2005). Inhibitory control was determined by examining reaction time and duration of the delay between go and stop signals that could effectively inhibit cued responding.
-
–
Risk-taking task: The risk-taking task consisted of 50 discrete two-choice trials in which participants selected between risky (each trial resulted in earning or losing between 25 and 75 cents) and nonrisky (each trial resulted in earning 1 cent) options (adopted from Lane et al. 2005a). Risk-taking behavior was determined based on the relative number of risky choices. Task duration was approximately 12 min.
-
–
Cardiovascular assessment: Oscillometric heart rate and systolic and diastolic blood pressure measures were obtained (Sentry II, NBS Medical).
Data analysis
Data were analyzed using a sensation-seeking status (between-group factor, two levels) × dose (within-group factor, three levels) × time (within-group factor, four levels) × replication (within-group factor, two levels) ANOVA. If the results of the ANOVA indicated a significant main effect of group, baseline (i.e., predrug) session values were added to the model as a covariate (i.e., ANCOVA) to control for possible group difference in baseline performance. Type I error was controlled by examining significant group × dose and group × dose × time interactions using simple-effects models, rather than conducting multiple pairwise comparisons. Results were considered significant at p<0.05. There were minimal interactions between replication and either sensation-seeking status (i.e., no evidence of group differences associated with repeated testing) or dose (i.e., no evidence of sensitization or tolerance associated with repeat testing), thus the effects of the replication factor are not reported. Data reported in the manuscript are the averages of the two replications.
Results
Table 1 summarizes the result of the ANOVA and presents d-amphetamine’s effects pooled across time as a function of dose for low and high impulsive sensation seekers. Significant stimulant-like drug effects were observed on cardiovascular measures (i.e., increased heart rate and systolic and diastolic blood pressure), task performance (e.g., enhanced DSST and RIP performance), and self-report measures (increases on VAS stimulated, feel drug, like drug, and high; ARCI A; BG; MBG; POMS vigor, elation, and arousal; and total positive scales; and decreases on VAS sleepy scale, ARCI, PCAG, and POMS fatigue and confusion scales).
Table 1.
Effects of d-amphetamine on study outcome measures (mean±SEM, pooled across time) for low and high sensation seekers, along with results of the ANOVA with sensation-seeking status (s: p<0.05; S: p<0.01), dose (d: p<0.05; D: p<0.01) and time (t: p<0.05; T: p<0.01)
| Low sensation seekers |
High sensation seekers |
|||||
|---|---|---|---|---|---|---|
| Dose (mg/70 kg) | 0 | 7.5 | 15 | 0 | 7.5 | 15 |
| DSST | ||||||
| Total TrialsT,D×T | 69.35 (1.03)* | 69.95 (0.96) | 69.78 (1.11) | 74.04 (0.80) | 75.14 (0.68) | 75.71 (0.69) |
| Proportion CorrectT | 0.93 (0.01) | 0.94 (0.01) | 0.93 (0.01) | 0.94 (0.00) | 0.94 (0.00) | 0.94 (0.00) |
| Stop Signal Task | ||||||
| Reaction TimeT | 531.7 (15.9) | 516.9 (15.2) | 530.4 (15.3) | 514.6 (13.3) | 514.5 (13.1) | 512.3 (13.2) |
| Proportion Correct | 0.93 (0.01) | 0.94 (0.01) | 0.94 (0.01) | 0.89 (0.02) | 0.89 (0.02) | 0.89 (0.02) |
| Proportion Stopst | 0.57 (0.03) | 0.56 (0.03) | 0.60 (0.02) | 0.49 (0.04) | 0.50 (0.04) | 0.50 (0.04) |
| RIP Task | ||||||
| Characters/min D,×t | 100.4 (2.78) | 104.3 (2.62) | 103.8 (2.42) | 102.6 (2.82) | 106.2 (2.82) | 105.8 (2.95) |
| Proportion HitsD,D×T | 0.51 (0.01) | 0.51 (0.02) | 0.54 (0.01) | 0.53 (0.01) | 0.53 (0.01) | 0.54 (0.01) |
| Risk-Taking Task | ||||||
| Risk Choices (N/50)^ | 17.40 (1.57) | 17.49 (1.43) | 16.65 (1.39) | 16.66 (2.03) | 14.40 (1.88) | 12.88 (1.90) |
| Cardiovascular | ||||||
| Heart RateD,t,D×T | 68.34 (1.21) | 71.78 (1.04) | 73.36 (1.00) | 62.75 (1.49) | 66.03 (1.34) | 66.64 (1.28) |
| SystolicD,T,D×T | 125.1 (1.81) | 129.9 (1.64) | 131.3 (1.95) | 124.8 (0.88) | 128.3 (1.24) | 133.8 (1.18) |
| DiastolicD,T,d×t | 69.30 (1.15) | 71.23 (1.15) | 72.05 (1.71) | 71.41 (0.77) | 74.68 (1.04) | 76.51 (1.08) |
| ARCI | ||||||
| PCAGs,D,T,D×T | 5.66 (0.43) | 4.73 (0.44) | 4.03 (0.38) | 4.24 (0.31) | 2.95 (0.21) | 2.68 (0.24) |
| BGD,T,D×T | 5.74 (0.36) | 5.75 (0.33) | 6.66 (0.35) | 5.14 (0.20) | 6.29 (0.22) | 6.90 (0.28) |
| LSD | 3.35 (0.24) | 3.26 (0.23) | 3.38 (0.23) | 3.09 (0.14) | 2.74 (0.15) | 2.83 (0.14) |
| MBGD,T,D×T | 2.79 (0.48) | 3.20 (0.49) | 4.36 (0.55) | 3.19 (0.33) | 4.98 (0.43) | 6.05 (0.52) |
| AD,T,D×t | 2.81 (0.33) | 2.86 (0.30) | 3.95 (0.35) | 1.53 (0.19) | 2.63 (0.28) | 3.30 (0.35) |
| POMS | ||||||
| Anxiety | 0.51 (0.04) | 0.49 (0.05) | 0.46 (0.04) | 0.36 (0.03) | 0.31 (0.03) | 0.32 (0.02) |
| Depressiont | 0.08 (0.02) | 0.08 (0.02) | 0.07 (0.02) | 0.07 (0.03) | 0.01 (0.00) | 0.01 (0.01) |
| Anger | 0.14 (0.02) | 0.15 (0.03) | 0.12 (0.02) | 0.05 (0.02) | 0.02 (0.00) | 0.02 (0.00) |
| VigorD,T,D×T | 0.75 (0.08) | 0.93 (0.08) | 1.10 (0.09) | 0.93 (0.09) | 1.14 (0.09) | 1.29 (0.11) |
| FatigueT,D×T,s×d×t | 0.47 (0.06) | 0.41 (0.07) | 0.28 (0.05) | 0.39 (0.07) | 0.20 (0.05) | 0.21 (0.05) |
| Confusiond,t,d×t | 0.61 (0.04) | 0.58 (0.04) | 0.50 (0.03) | 0.46 (0.03) | 0.38 (0.03) | 0.39 (0.03) |
| FriendlyD,t,d×t | 1.20 (0.09) | 1.25 (0.09) | 1.39 (0.09) | 1.46 (0.09) | 1.67 (0.08) | 1.73 (0.10) |
| ElationD,T,D×T | 0.88 (0.09) | 1.00 (0.09) | 1.09 (0.09) | 1.04 (0.08) | 1.15 (0.08) | 1.33 (0.10) |
| ArousalD,T,D×T | 0.17 (0.12) | 0.43 (0.12) | 0.78 (0.11) | 0.44 (0.14) | 0.87 (0.10) | 1.01 (0.13) |
| Total PositiveD,T,D×T | 0.79 (0.09) | 0.92 (0.09) | 1.02 (0.10) | 0.96 (0.09) | 1.14 (0.08) | 1.32 (0.10) |
| VAS | ||||||
| StimulatedD,T,s×t,D×T | 10.70 (1.84) | 14.18 (1.94) | 15.74 (2.32) | 10.04 (1.27) | 19.16 (2.16) | 26.29 (3.07) |
| Sedatedt | 11.81 (2.26) | 8.55 (1.63) | 7.08 (1.48) | 14.28 (2.80) | 6.64 (0.99) | 7.88 (1.36) |
| HungryT | 13.39 (2.28) | 12.25 (2.03) | 8.79 (1.48) | 14.04 (2.03) | 13.25 (1.85) | 14.58 (2.14) |
| Anxious | 14.20 (2.64) | 13.58 (2.61) | 14.49 (2.95) | 5.81 (1.16) | 5.26 (0.92) | 6.68 (1.21) |
| Light-HeadedT,s×t,d×t | 9.89 (1.76) | 5.30 (1.11) | 5.73 (1.36) | 6.20 (1.28) | 5.30 (1.11) | 6.10 (1.11) |
| Thirstys×d | 13.79 (2.28) | 14.69 (2.38) | 11.70 (1.82) | 16.35 (1.92) | 18.44 (1.91) | 24.96 (2.54) |
| SleepyT,d×t | 23.13 (2.87) | 18.36 (2.53) | 13.43 (2.03) | 23.38 (2.83) | 17.23 (2.05) | 15.06 (2.15) |
| Sick-to-Stomach | 4.88 (0.90) | 3.60 (0.77) | 4.61 (0.99) | 5.69 (0.98) | 5.73 (0.94) | 5.08 (0.88) |
| Down | 6.41 (1.10) | 6.34 (1.38) | 4.34 (1.09) | 10.38 (2.29) | 4.46 (0.76) | 5.70 (1.31) |
| Highd,T,s×d,D×T | 5.93 (1.36) | 4.96 (1.01) | 5.73 (1.44) | 5.35 (0.94) | 12.84 (1.80) | 17.94 (2.81) |
| Feel drug s,D,T,S×D,s×t,D×T,S×D×T | 5.88 (1.19) | 5.34 (1.27) | 5.83 (1.36) | 7.41 (1.48) | 15.11 (2.51) | 25.80 (3.37) |
| Like drugs,D,T,s×d,S×T,D×T,S×D×T | 2.49 (0.45) | 5.53 (1.48) | 4.24 (1.39) | 8.20 (1.67) | 16.15 (2.87) | 24.15 (3.44) |
Mean (SEM)
Number of risk choices out of 50 trials
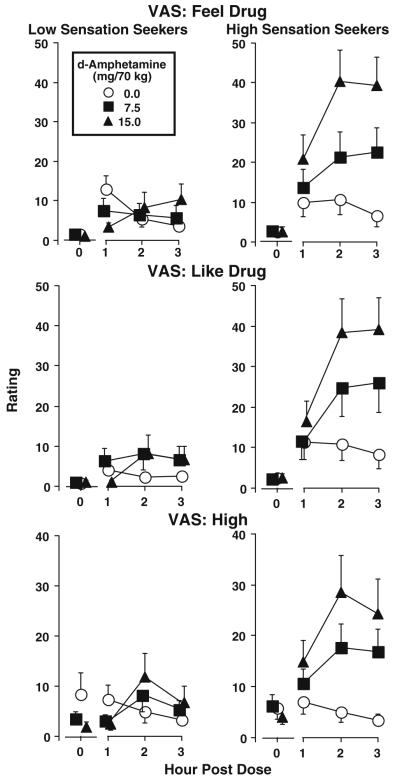
Significant differences in d-amphetamine effects were also observed among low and high sensation seekers on multiple self-report measures. Figure 1 presents the effects of d-amphetamine on VAS ratings of feel drug (top panels), like drug (middle panels), and high (bottom panels). Significant sensation-seeking group × dose [feel drug: F (2,36)=7.97, p<0.005; like drug: F(2,36)=6.52, p<0.05; high: F(2,36)=4.21, p<0.05] and sensation-seeking group × dose × time [feel drug: F(6,108)=3.34, p<0.005; like drug: F(6,108)=3.03, p<0.01] interactions were observed. A significant main effect of sensation-seeking group was observed on VAS feel drug ratings [F(1,18)=6.27, p<0.05], which raised the concern that group differences in drug effect were potentially related to group differences in baseline ratings. However, a supplemental ANCOVA controlling for group differences in baseline feel drug ratings confirmed the sensation-seeking group × dose interaction [F(2,36)=8.26, p<0.001].
Fig. 1.
Time-course effects of d-amphetamine (0.0, 7.5, and 15.0 mg/70 kg) on the VAS feel drug (top row), like drug (middle row), and high (bottom row) for low (left column) and high (right column) impulsive sensation seekers. Error bars represent ±1 SEM
Simple-effects analyses of the two-way group × dose interactions on VAS ratings of feel drug, like drug, and high indicated significant dose effects only among high sensation seekers [feel drug: F(2,36)=15.94, p<0.001; like drug: F(2,36)=10.39, p<0.001; high: F(1,36)=7.97, p<0.005] and greater d-amphetamine effects occurring among high sensation seekers on all three measures at the 7.5 mg/70 kg [feel drug: F(1,36)=8.98, p<0.01; like drug: F(1,36)=9.22, p<0.005; high: F(1,36)=6.17, p<0.05] and 15 mg/70 kg [feel drug: F(1,36)=37.28, p<0.001; like drug: F(1,36)=32.39, p<0.001; high: F(1,36)=14.83, p<0.001] doses. Simple-effects analyses of the three-way group × dose × time interactions indicated that the enhanced d-amphetamine effects among high sensation seekers were observed 1 [like drug: F(5,108)=4.79, p<0.001], 2 [feel drug: F(5,108)=20.22, p<0.001; like drug: F(5,108)=27.22, p<0.001], and 3 [feel drug: F(5,108)=17.46, p<0.001;like drug: F(5,108)=31.58, p<0.001] hours postdose.
Similar differences in d-amphetamine effects among low and high sensation seekers were obtained on the VAS Thirsty [group × time: F(2,36)=4.67, p<0.05] and POMS Fatigue [group × dose × time: F(6,108)=2.27, p<0.05] scales (Table 1). High sensation seekers reported greater increases on VAS Thirsty than low sensation seekers only at the 15.0 mg/70 kg dose [F(1,36)=23.90, p<0.001]. Significant dose × time interactions were observed for both low and high sensation seekers on the POMS Fatigue Scale [low sensation seekers: F(11,108)=5.03, p<0.001; high sensation seekers: F(11,108)=3.83, p<0.001], although decreases in Fatigue ratings across time were greater for high sensation seekers at the 15.0 mg/70 kg dose [F(7,108)=5.04, p<0.001].
The magnitude of d-amphetamine effects were also greater among high sensation seekers on several additional measures (e.g., VAS stimulated; ARCI A, BG, and MBG scales; see Table 1), although these differences did not reach statistical significance. Effect sizes for the group × drug and group × drug × time interactions on these measures ranged from 0.3 to 2.5 (i.e., small to large effect sizes).
In contrast to the self-report measures, drug effects did not vary as a function of sensation-seeking status on any task performance or cardiovascular measure.
Discussion
The main finding of this study is that impulsive sensation seeking young adults without extensive histories of drug use or prior exposure to d-amphetamine reported greater stimulant-like effects of d-amphetamine than low impulsive sensation seekers on self-report measures that were associated with the reinforcing effects of drugs, including VAS ratings of feel drug, like drug, and high (e.g., Foltin and Fischman 1991), and other measures of stimulant drug effect (i.e., decreases in POMS fatigue). While group differences in d-amphetamine effects on other self-report measures did not reach significance, small to large effect sizes were observed for group by drug and group by drug by time interactions (e.g., VAS stimulated, ARCI A, BG, and MBG scales), suggesting that significant group differences could have emerged with a larger sample size. Furthermore, by not including volunteers who were regular or heavy drug users for study participation who were also likely to be high impulsive sensation seekers, the current study adopted a conservative approach to examine group differences in sensitivity to drug effects that could have reduced the overall effect size. Many factors can influence the reinforcing effects of d-amphetamine, and verbal report measures are not always consistent with drug-taking behavior (e.g., Chait 1993; Johanson et al. 1983; Johanson and Uhlenhuth 1980), so additional studies incorporating direct measures of drug-taking behavior should be undertaken to examine more carefully the relative reinforcing effects of d-amphetamine among impulsive sensation seekers.
These results, obtained using the impulsive SSS of the ZKPQ (Zuckerman et al. 1993), are consistent with those of several previous studies using a variety of different questionnaires to examine the association between sensation-seeking dimensions of personality and the subjective effects of d-amphetamine, thereby extending and validating the association (Hutchison et al. 1999; Kelly et al., submitted for publication; Sax and Strakowski 1998; White et al. 2005). It is unlikely that group differences in d-amphetamine effects were associated with pharmacokinetic factors, as comparable drug effects were observed on the majority of measures, including cardiovascular and performance measures. Furthermore, no evidence of sensitization or tolerance was apparent, which is consistent with previous studies examining the effects of repeated d-amphetamine administration (e.g., Kelly et al. 1991; Wachtel and de Wit 1999). Unanticipated group differences were also observed on VAS ratings of Thirsty, although the effects were consistent with those on other self-report measures in reflecting greater sensitivity to drug effect among high sensation seekers.
It is important to note, however, that an association between sensation-seeking status and sensitivity to stimulant drug effects was not obtained in all studies (e.g., Alessi et al. 2003; Carrol et al. 1982; Chait 1993; Corr and Kumari 2000; de Wit et al. 1986). Several factors could contribute to inconsistent outcomes across studies, including sample size and the distribution of sensation-seeking scores in the study populations (for discussion, see White et al. 2005). Individual differences in sensation-seeking status were attributed to both genetic and environmental influences (e.g., Fulker et al. 1980; Stacy et al. 1991). Another factor that could contribute to inconsistent outcomes across studies is the relative contribution of genetic and environmental influences on the expression of the trait in the study population. In the current study, biological influence was enhanced by recruiting participants who scored at the extremes of the population on the biologically based impulsive SSS of the ZKPQ. Future studies will be needed to determine whether the association between sensation-seeking status and sensitivity to stimulant drug effects is limited to those individuals who are in the extremes of the population distributions and/or exhibiting relatively high levels of biological influence on trait expression.
The present study also examined performance on tasks involving attention (RIP), inhibition (stop signal task), and risk-taking behavior (gambling) among low and high impulsive sensation seekers under placebo and active dose conditions. d-amphetamine engendered stimulant effects on RIP and stop signal task performance (see Fillmore et al. 2005 for a detailed report of these data). No d-amphetamine effects were apparent on risk-taking behavior. Performance on the risk-taking task was altered by both sedatives and THC, suggesting that the absence of d-amphetamine effects is not a consequence of task insensitivity (Lane et al. 2004, 2005a,b). It is important to note that no impulsive sensation seeking group differences were observed on attention, inhibition, or risk-taking behavior under placebo or active d-amphetamine doses. These data suggest that the association between impulsive sensation seeking and d-amphetamine sensitivity is not likely mediated through changes in impulsive behavior.
The apparent lack of association between personality and performance measures of impulsivity in the present study does not suggest a problem of measurement. There is ample precedence for the limited convergence among personality and performance measures of impulsivity (e.g., Holt et al. 2003; Petry 2001). The discordance is associated with the multifactorial nature of impulsivity, the quantitative distinction between personality, which reflects behavioral tendency, and performance, which is an instance of behavior that contributes to an overall tendency (e.g., Evenden 1999; Miller and Lynam 2003). As such, given the relatively small sample size in the current study, the absence of an association between personality and performance measures of impulsivity might not be interpreted as a problem with measurement, but rather one of low statistical power.
The main finding of this study is that high sensation seekers, matched to low sensation seekers in age, gender, education, other personality dimensions (e.g., extraversion), mood, psychiatric symptoms, measures of behavioral undercontrol, and drug use, reported greater sensitivity to the stimulant-like effects of d-amphetamine on self-report measures that were associated with the reinforcing effects of drugs, including VAS ratings of feel drug, like drug, and high (e.g., Foltin and Fischman 1991). However, the enhanced sensitivity was specific to self-report measures; d-amphetamine effects on cardiovascular and performance measures were not different between groups. High novelty or sensation-seeking status is associated with an increased frequency and amount of drug use (Brennan et al. 1986; Wills et al. 1994, 1995) and increased risk for the development of drug abuse and dependence (Hawkins et al. 1992; Huba et al. 1981). The results of this study suggest that escalating patterns of drug use and the development of problems associated with that use among high impulsive high sensation seekers might be due, in part, to increased sensitivity to the reinforcing effects of drugs.
Acknowledgements
This research project was supported by grants P50 05312 and R01 DA 15392 from the National Institute on Drug Abuse. The authors wish to thank Stephanie Douglas, Cleeve Emurian, Beth Eaves, Oriaku Akuma-Kalu Njoku, and Susan Holiday for help in executing the study.
Contributor Information
Thomas H. Kelly, Departments of Behavioral Science, Psychiatry and Psychology, College of Medicine and College of Arts and Sciences, University of Kentucky, Lexington, KY 40536-0086, USA
Glenn Robbins, Department of Behavioral Science, College of Medicine, University of Kentucky, Lexington, KY 40536-0086, USA.
Catherine A. Martin, Departments of Psychiatry and Behavioral Science, College of Medicine, University of Kentucky, Lexington, KY 40536-0086, USA
Mark T. Fillmore, Departments of Psychology and Behavioral Science, College of Arts and Sciences and College of Medicine, University of Kentucky, Lexington, KY 40536-0086, USA
Scott D. Lane, University of Texas Health Science Center, Houston, TX, USA
Nancy G. Harrington, Department of Communication, College of Communications and Information Sciences, University of Kentucky, Lexington, KY 40536-0086, USA
Craig R. Rush, Departments of Behavioral Science, Psychiatry and Psychology, College of Medicine and College of Arts and Sciences, University of Kentucky, Lexington, KY 40536-0086, USA
References
- Alessi SM, Greenwald M, Johanson CE. The prediction of individual differences in response to d-amphetamine in healthy adults. Behav Pharmacol. 2003;14:19–32. doi: 10.1097/00008877-200302000-00002. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Brennan AF, Walfish S, AuBuchon P. Alcohol use and abuse in college students. I. A review of individual and personality correlates. Int J Addict. 1986;21:449–474. doi: 10.3109/10826088609083536. [DOI] [PubMed] [Google Scholar]
- Carrol EN, Zuckerman M, Vogel WH. A test of the optimal level of arousal theory of sensation seeking. J Pers Soc Psychol. 1982;42:572–575. doi: 10.1037//0022-3514.42.3.572. [DOI] [PubMed] [Google Scholar]
- Chait LD. Factors influencing the reinforcing and subjective effects of d-amphetamine in humans. Behav Pharmacol. 1993;4:191–199. [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Corr PJ, Kumari V. Individual differences in mood reactions to d-amphetamine: a test of three personality factors. J Psychopharmacol. 2000;14:371–377. doi: 10.1177/026988110001400406. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: Reward and impulsivity. Nebr Symp Motiv. 1997;45:19–55. [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Kelly TH, Martin CA. Effects of d-amphetamine in human models of information processing and inhibitory control. Drug Alcohol Depend. 2005;77:151–159. doi: 10.1016/j.drugalcdep.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Methods for the assessment of abuse liability of psychomotor stimulants and anorectic agents in humans. Br J Addict. 1991;86:1633–1640. doi: 10.1111/j.1360-0443.1991.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Fulker DW, Eysenck SB, Zuckerman M. A genetic and environmental analysis of sensation seeking. J Res Pers. 1980;14:261–281. [Google Scholar]
- Goma-i-Freixanet M, Wismeijer AA, Valero S. Consensual validity parameters of the Zuckerman–Kuhlman personality questionnaire: evidence from self-reports and spouse reports. J Pers Assess. 2005;84:279–286. doi: 10.1207/s15327752jpa8403_07. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J. Is discounting impulsive? Evidence from temporal and probability discounting in gambling and non-gambling college students. Behav Processes. 2003;64:355–367. doi: 10.1016/s0376-6357(03)00141-4. [DOI] [PubMed] [Google Scholar]
- Huba GJ, Newcomb MD, Bentler PM. Comparison of canonical correlation and interbattery factor analysis on sensation seeking and drug use domains. Appl Psychol Meas. 1981;5:291–306. [Google Scholar]
- Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp Clin Psychopharmacol. 1999;7:493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacology. 1980;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Kilgore K, Uhlenhuth EH. Assessment of dependence potential of drugs in humans using multiple indices. Psychopharmacology. 1983;81:144–149. doi: 10.1007/BF00429009. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Fischman MW. The effects of repeated amphetamine exposure on multiple measures of human behavior. Pharmacol Biochem Behav. 1991;38:417–426. doi: 10.1016/0091-3057(91)90301-h. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, Tcheremissine OV. Alcohol effects on human risk taking. Psychopharmacology. 2004;172:68–77. doi: 10.1007/s00213-003-1628-2. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005a;30:800–809. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- Lane SD, Tcheremissine OV, Lieving LM, Nouvion S, Cherek DR. Acute effects of alprazolam on risky decision making in humans. Psychopharmacology. 2005b;181:364–373. doi: 10.1007/s00213-005-2265-8. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum Comput. 1982;14:463–466. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of mood states (manual) Educational and Industrial Testing Services; San Diego, CA: 1971. [Google Scholar]
- Miller JD, Lynam DR. Psychopathy and the five-factor model of personality: a replication and extension. J Pers Assess. 2003;81:168–178. doi: 10.1207/S15327752JPA8102_08. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the multidimensional personality questionnaire. Psychol Assess. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Wilson A. Greater sensitivity to subjective effects of nicotine in non-smokers high in sensation seeking. Exp Clin Psychopharmacol. 2000;8:462–471. doi: 10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Roache JD. Performance and physiological measures in abuse liability evaluation. Br J Addict. 1991;86:1595–1600. doi: 10.1111/j.1360-0443.1991.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM. Enhanced behavioral response to repeated d-amphetamine and personality traits in humans. Biol Psychiatry. 1998;44:1192–1195. doi: 10.1016/s0006-3223(98)00168-1. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Zians J, Grant I, Patterson TL. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29:85–93. doi: 10.1016/j.jsat.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Newcomb MD, Bentler PM. Social psychological influences on sensation seeking from adolescence to adulthood. Pers Soc Psychol Bull. 1991;17:701–708. [Google Scholar]
- Wachtel SR, de Wit H. Subjective and behavioral effects of repeated d-amphetamine in humans. Behav Pharmacol. 1999;10:271–281. doi: 10.1097/00008877-199905000-00004. [DOI] [PubMed] [Google Scholar]
- White TL, Lott DC, de Wit H. Personality and the subjective effects of acute amphetamine in healthy volunteers. Neuropsychopharmacology. 2005;31(5):1064–1074. doi: 10.1038/sj.npp.1300939. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J Subst Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Wills TA, DuHamel K, Vaccaro D. Activity and mood temperament as predictors of adolescent substance use: test of a self-regulation mediation model. J Pers Soc Psychol. 1995;68:901–916. doi: 10.1037//0022-3514.68.5.901. [DOI] [PubMed] [Google Scholar]
- Zermatten A, Van der Linden M, d’Acremont M, Jermann F, Bechara A. Impulsivity and decision making. J Nerv Ment Dis. 2005;193:647–650. doi: 10.1097/01.nmd.0000180777.41295.65. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge University Press; MA: 1994. [Google Scholar]
- Zuckerman M, Eysenck SB, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, Camac C. What lies beyond E and N? Factor analyses of scales believed to measure basic dimensions of personality. J Pers Soc Psychol. 1988;54:96–107. [Google Scholar]
- Zuckerman M, Kuhlman DM, Thornquist M, Kiers H. Five (or three) robust questionnaire scale factors of personality without culture. Pers Individ Differ. 1991;12:929–941. [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models of personality: the big three, the big five, and the alternative five. J Pers Soc Psychol. 1993;65:757–768. [Google Scholar]