Abstract
Migration of keratinocytes to re-epithelialize wounds is a key step in dermal wound healing. In aged human skin, wound healing rates decrease and cellular damage by reactive oxygen species (ROS) accumulates. The relationship between age, ROS and human skin keratinocyte migration is not clearly understood. In this study, 4% and 21% oxygen tensions were used to modify levels of ROS produced by metabolism to model low and high oxidative stress conditions. When migration of keratinocytes from young and old primary skin was compared using an in vitro scratch assay, old keratinocytes migrated faster in high oxygen tension than did young keratinocytes, whereas young keratinocytes migrated faster in low oxygen tension. Although all young and old cells at the scratch margins showed intense increases in dihydroethidium oxidation immediately after scratching, the old keratinocytes grown at 21% oxygen demonstrated a greater decrease in the DHE oxidation following scratching and migrated the fastest. These results show that old and young keratinocytes respond to oxygen tension differently and support the hypothesis that keratinocyte migration is affected by the capacity to remove ROS.
Keywords: cell migration, keratinocyte, oxygen tension, ROS, skin
Introduction
Wound healing is a complex process composed of several phases and involving many types of cells. One of the most important events is re-epithelialization, the migration of keratinocytes from wound margins to resurface the wounded area. It occurs approximately 24 h after injury (1). Several factors including age, matrix formation, moisture and oxygen tension affect the amount and rate of re-epithelialization (1–4). To date, much of this research has been conducted in mouse skin or in culture with human neonatal foreskin cells or established cell lines. These models differ from primary adult human skin keratinocytes in proliferation and migration, often making comparisons to adult human skin wound healing difficult.
Many factors that interact with extracellular matrix proteins to increase or decrease keratinocyte migration affect wound closure and are altered in ageing skin. Especially in elderly patients, poor tissue oxygenation leading to hypoxia is a major problem for chronic skin wounds (5). Furthermore the amounts of such factors can vary according to environment. For example, laminin-5 and ezrin, which increase keratinocyte migration, and moesin, which decreases keratinocyte migration, are increased in low oxygen conditions (2), which also affects the steady-state levels of reactive oxygen species (ROS), such as superoxide. Thus, ROS altering proteins would be expected to modify wound healing as well. Matrix metalloproteinase 1 increases ROS and is increased in aged human dermis (4), suggesting a link between age, ROS and wound healing. In fact, therapeutic techniques that enhance wound healing include colloidal membranes, matrix applications to wounds and occlusive and semi-occlusive bandages (2). Wound occlusion lowers the oxygen tension in the wounds and increases wound healing and keratinocyte migration. It may be that occlusion decreases the amount of aerobic respiration of the cells and therefore decreases ROS in the wounds. Thus, one of the factors that affect keratinocyte migration is likely the presence of ROS in the wound.
Because the inception of the free radical theory of ageing by Harman in the 1950s, free radicals and ROS have been linked to age-related conditions, including inflammatory diseases, hypertension and cancer (6–8). Age-related ROS damage was shown to increase in parallel with decreased wound healing in rats (9). Because there is a relationship between wound healing and ROS, and a relationship between ageing and ROS, the delay observed in wound closure in ageing humans may be related to accumulation of ROS damage in ageing keratinocytes. The work presented here demonstrates a correlation between ageing, cell migration and ROS in primary human skin keratinocytes.
Materials and methods
Keratinocyte cultures
Human keratinocytes were isolated from normal skin specimens obtained from University of Iowa Hospitals and Clinics Surgical Pathology with IRB approval. Ages of specimens were recorded, but no other identifying information was obtained, except that the skin was not from patients with diabetes, smokers or patients with skin diseases. Samples used in this study were adults, aged 22–25 and 72–90. To obtain keratinocytes, pieces of skin were incubated in dispase II (Roche, Indianapolis, IN, USA) overnight at 4°C; then, the epidermis was mechanically separated from the dermis and placed in 0.25% trypsin (Invitrogen, Carlsbad, CA, USA) for 30 min at 37°C. Serum containing medium was added to inactivate the trypsin; then, agitation was used to dissociate individual basal keratinocytes. Isolated keratinocytes were cultured on collagen type IV–coated culture dishes in keratinocyte serum-free medium (KSFM; Invitrogen, Carlsbad, CA, USA) and 1.5% antibiotic/antimycotic (Invitrogen) at 37°C in a 5% CO2 incubator at either 4% or 21% O2.
Cell migration assays
Keratinocytes were grown in 4% or 21% O2 to near confluency on non-coated culture dishes; then, a scratch was made using a sterile micropipette tip. This created an open area into which the keratinocytes migrated (see Fig. 1). Two areas of the scratch margins were imaged daily with a Nikon TS100 phase microscope equipped with a Retiga 2000R digital camera until scratches were closed. The area of the scratch remaining was measured on the images at three points per field, with two fixed fields averaged. Measurements were stopped when cells no longer made positive progress towards the scratch origin. The scratch measurements were used to calculate rate of cell migration per day and to determine the per cent of the scratch remaining each day. Paired t-test was used to compare the treatment groups.
Figure 1.
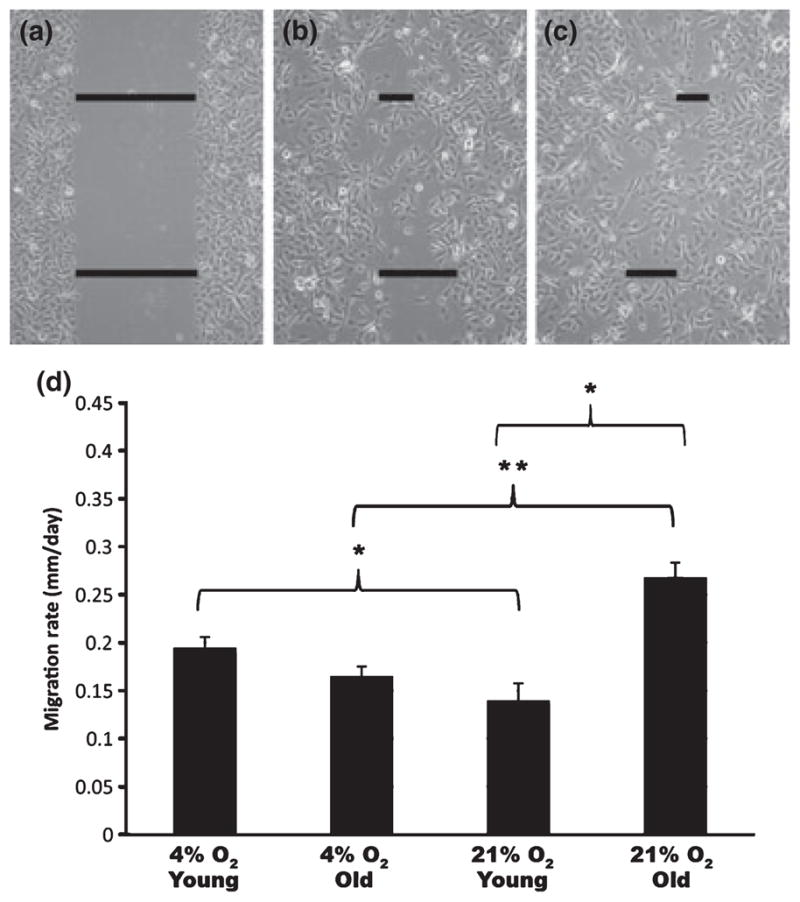
Oxygen tension differentially affects the rates of migration of keratinocytes isolated from young and old skin. (a–c) Representative samples of phase contrast images of scratch assays on day 0 (a), day 1 (b), and day 2 (c) after scratching to demonstrate how scratch widths were measured. (d) Graph depicting mean migration rates from keratinocytes isolated from old and young skin cultured in 4% O2 or 21% O2 conditions (n = 5 young skin samples and 5 old skin samples). Mean ± SEM (n = 30 cultures for each condition). *P < 0.001, ** P < 0.01.
Live cell imaging
Cell migration assays were performed in 21% oxygen conditions using the Large Scale Digital Cell Analysis System (LSDCAS) at the Holden Comprehensive Cancer Center. Scratch margins were imaged sequentially in 20 coordinates every 1 min over a 48- or 72-h time period. Images were compiled to form digital movies. Keratinocytes from five samples of young and three samples of old skin were imaged.
Cellular proliferation analysis
Cultures were prepared as described earlier. At 0, 6, 24 and 48 h after scratching, medium was replaced with 6 mg/ml of colcemid (Invitrogen) in KSFM (Invitrogen) and 1.5% antibiotic/antimycotic (Invitrogen). For each time point, a separate culture dish was treated with colcemid because colcemid destabilizes microtubules and would have significant effect on cell migration. Scratched cultures and unscratched controls were incubated with the colcemid at 37°C in a 5% CO2 incubator at either 4% or 21% O2 for 4 h, then fixed in cold 3:1 methanol: acetic acid, dried overnight and stained with 10% Giemsa in Gurrs buffer. This method allowed us to determine proliferating cells on the scratch margins as well as away from the margins in situ. Ten random fields were chosen from each unscratched control dish. The average number of cells per field was determined. The numbers of metaphase positive cells were counted in the 10 fields of unscratched control dishes and in 10 fields away from the scratch area in the experimental dishes. For the scratched dishes, the number of metaphase positive cells was counted along the full length of scratch margin. (see Fig. 2) For 0 and 6 h after scratching, the scratch margin was defined at three cells in thickness (Fig. 2b,c). For 24 and 48 h after scratching, all cells within the original scratch area as evidenced by residual cellular debris were counted (Fig. 2d). Metaphase positive cells were independently scored by two individuals in a blinded fashion. To confirm the validity of our metaphase count method, we also repeated the proliferation experiments using exponentially growing human skin keratinocyte cultures.
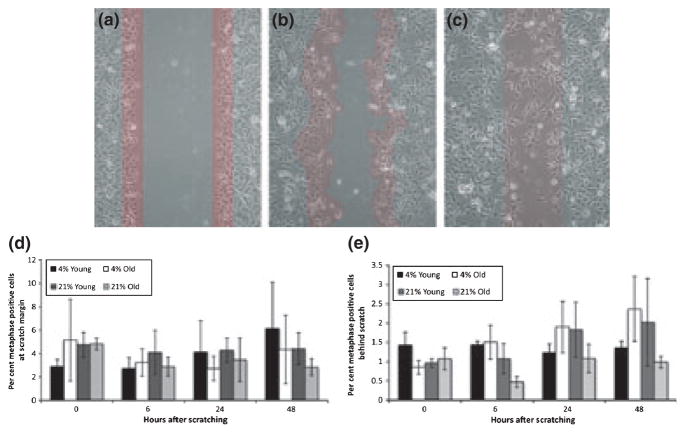
Figure 2.
Differences in migration rate among young and old keratinocytes do not result from differences in proliferation. (a–c) Phase contrast images with red overlay to illustrate the method for counting cells on the migratory edge. At 0 h (a) and 24 h (b), cells within a 3-cell width of the scratch margin were counted. At 48 h (c), cells that had moved into the centre of the scratch (as indicated by residual cellular scratch debris) were counted. (d) Bar graph showing the per cent of metaphase positive cells on the migratory edge at 0, 6, 24 and 48 h after scratching. (e) Bar graph showing the per cent of metaphase positive cells behind the migratory edge 0, 6, 24 and 48 h after scratching for young and old keratinocytes grown at 4% and 21% oxygen tension., Mean ± SEM (n = 4 young keratinocyte cultures grown at 4% and 21% O2, n = 5 old keratinocyte cultures grown at 4% and 21% O2). No significant differences were found.
For statistical analysis, we compared the proliferation between the four treatment groups within each time point for both the per cent metaphase positive cells behind the scratch and at the margin. For analysis of cells behind the scratch, we used the fold change of metaphase positive cells per field at each time point after scratching (0, 6, 24 and 48 h) over the number of metaphase positive cells per field in an unscratched control. For analysis of the proliferation of cells on the margin, we compared the average metaphase positive cells over the number of cells contained in the length of the scratch (constituting a three cell thickness). Means of counts were statistically analysed using Student’s t-test.
Measurement of intracellular ROS
Cultures were prepared as described previously. At 0, 6, 24 and 48 h after scratching, cells were rinsed with PBS containing 5 mM pyruvate (Invitrogen) and then incubated for 5 min. with 5 μM dihydroethidium (DHE) (Invitrogen) in KSFM. The fluorescence of two fields for each culture was imaged (excitation 488 nm, emission 585 nm) with a Nikon TS100 phase microscope equipped with a Retiga 2000R digital camera and Q Imaging system software. Metamorph imaging software (MDS Analytical Technologies, Toronto, ON, Canada) was used to determine the total intensity for cells on the scratch front of each field. Scratch fronts constitute three cells in width. Cells behind the scratch margin were also analysed to determine the total intensity for cells behind the scratch front. Because of the nature of DHE labelling assays, the fluorescent intensity numbers are arbitrary, and any measurements taken at different days cannot be compared unless normalized to one control. It was technically impossible to perform a DHE labelling in situ in all of the skin samples (young, old, 4%, 21%) on the same day. Therefore, we normalized the DHE fluorescence intensity numbers at the scratch margins for each sample (age, % O2) to that of its own unscratched culture dish that was labelled with DHE at the same time.
For statistical analysis, we compared fluorescence rate (fluorescence fold change over control) among the four groups (young cells at 4%, old cells at 4%, young cells at 21% and old cells at 21%) at each time point, using one-way ANOVA. To find the time effect on levels of ROS we fitted a linear model, within each of the four groups with the fluorescence rate (fold change over control) as the response variable and time as covariate variable.
Western blot analysis
For Western blot analysis of cytosolic and mitochondrial forms of superoxide dismutase (SOD1 and SOD2, respectively), protein extracts from unscratched young and old keratinocytes grown in 4% and 21% oxygen were harvested and pelleted on ice in Ripa buffer (Pierce Biotechnology Inc., Rockford, IL, USA) with protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). Protein concentrations were determined by Bradford assay, and 20 μg of each sample was resolved using a 12% poly-acrylamide gel and then transferred to a nitrocellulose membrane. The membrane was blocked for 2 h at 4°C in 5% non-fat milk and 1% Tween-20 in TBS and then incubated in the following concentrations of primary antibody overnight at 4°C: rabbit polyclonal directed against SOD1 (1:500, cat. no. 07-403; Millipore, Temecula, CA, USA), rabbit polyclonal directed against SOD2 (1:2000, cat. no. LF-PA0021; Affinity Bioreagents, Golden, CO, USA), rabbit polyclonal directed against actin (1:1000, cat. no. A2066; Sigma, St. Louis, MO, USA). After incubation the membrane was rinsed in TBS-T and then incubated with secondary infrared antibody (IRDye 800-conjugated affinity-purified IgG goat; Rockland Inc, Philadelphia, PA, USA) directed against rabbit (1:2000, cat. no. 611-132-122; Rockland Inc) in 2% non-fat milk and 1% Tween-20 in TBS for 45 min at room temperature. After rinsing, protein concentrations were detected with an Odyssey Infrared Imaging System (LI-COR Biotechnology, Lincoln, NE, USA). Metamorph imaging software (MDS Analytical Technologies) was used to perform densitometry analysis.
Results
Human keratinocytes isolated from young and old skin migrate at different rates in 4% O2 and 21% O2
To determine whether migration of keratinocytes from old skin differed from keratinocytes from young skin, we used a standard cell migration assay (10). Keratinocytes were grown to near confluency, scratched, and photographs taken every day until the scratches were closed (Fig. 1). We photographed two fields in each scratch and averaged three measurements in each field (Fig. 1a–c). In general, all keratinocytes covered the scratched areas in 72–96 h. However, a comparison of each age group revealed a differential migratory response of these cells when grown in low (4%) vs high (21%) oxygen tension (Fig. 1d). Young keratinocytes migrated faster in 4% oxygen when compared to young cells in 21% oxygen, whereas old keratinocytes migrated faster when grown in 21% oxygen when compared to old cells in 4% oxygen. The old keratinocytes cultured in 21% oxygen migrated the fastest of all the cells.
Differences in migration are not attributed to differences in proliferation
It was unclear whether the different closure rates of young and old keratinocytes grown at low and high oxygen tensions were attributed to changes in migration only or whether differences in proliferation contributed. To assess this, we determined the proportion of cells in metaphase using mitotic inhibition with colcemid, a microtubule depolymerizer. We paired the colcemid treatment with the scratch assays and determined the proliferation of keratinocytes on the migratory edge and behind the edge at various times during scratch closure. For each time point we used a separate culture dish owing to well-known inhibitory effects of colcemid on cell migration. From the initial time of scratching to 24 h, cells on the migratory edge (red area in Fig. 2a,b) and cells behind the edge were counted. By 48 h, the migratory edge was less distinct, so cells that had moved into the original scratch were counted by two individuals in a blinded fashion (red area in Fig. 2c). We found no significant difference between the percentage of mitotic cells on the scratch margin (Fig. 2d) or several fields away from the scratch margin (Fig. 2e). Additionally, the percentage of proliferating cells was not significantly different between young and old keratinocytes or between cells grown at 4% and 21% oxygen tension. Thus, oxygen tension affects cell migration, but not proliferation of the keratinocytes. Furthermore, reliability of our proliferation experiments was confirmed in exponentially growing human skin keratinocytes. The number of metaphase positive cells (40–50% of total population) was in agreement with cell count numbers in exponentially growing cell cultures (data not shown).
Old keratinocytes cultured in high oxygen tension display the ability to dissipate increased levels of ROS
Because previous work postulated that an increase in ROS leads to an ageing phenotype (6,7,11), we investigated the levels of ROS in young and old keratinocytes incubated in high and low oxygen tension using DHE oxidation. DHE is a redox-sensitive probe that has been widely used to detect intracellular superoxide. Higher oxygen tension should equate to higher ROS levels as increased oxidative stress results in greater metabolic products derived from the respiratory chain, such as superoxide (11). Using flow cytometry to assess the levels of DHE oxidation, we found the expected increase in DHE staining in young keratinocytes incubated at 21% oxygen when compared to those incubated at 4% oxygen. In contrast, for old keratinocytes, we found increased DHE staining in cells grown at 4% oxygen when compared to those incubated at 21% oxygen (data not shown). Because DHE staining presumably detects superoxide, and SOD 1 and SOD 2 are the enzymes responsible for dismuting superoxide, we expected to find differences in the levels of the SOD 1 (cytosolic) and SOD 2 (mitochondrial) enzymes in the cultures of keratinocytes from young and old skin. However, Western blot analysis revealed no differences in the immunoreactive protein of SOD1 (Fig. 3a,b) or SOD2 (Fig. 3a,c) in these cultures.
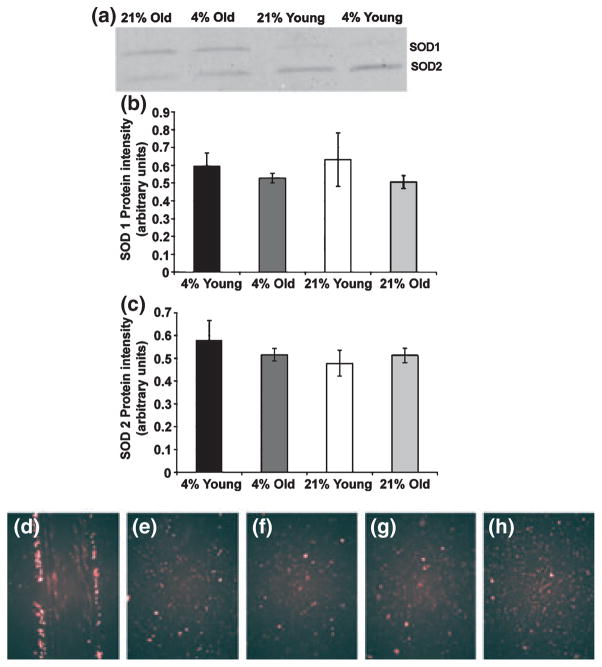
Figure 3.
Keratinocytes with faster cell migration decreased levels of reactive oxygen species more rapidly than keratinocytes with slower migration rates. (a) A representative western blot of superoxide dismutase (SOD) 1 and SOD 2 (b–c) Graphs of western blot analysis of SOD 1 (b) and SOD 2 (c) in keratinocytes isolated from young and old skin and grown at 4% and 21% O2 (n = 4). No significant differences in amount of protein were found. (d–h) Epiflourescent images showing that dihydroethidium oxidation increased immediately after scratching in cells at the edge of the scratch (d) and decreased progressively after scratching 6 h (e), 24 h (f), 48 h (g) to near-control fluorescence (h). Shown are representative young keratinocyte samples.
Although no differences in SOD 1 and SOD 2 were seen in the total culture isolates, previous work had shown an increase in ROS at mouse dermal wound margins (12), so we questioned whether the extent of ROS was evenly distributed among the keratinocytes in the scratched cultures or whether the presence of superoxide could be localized to the few cells along the scratch margins. To answer this question, we used DHE oxidation in scratched cultures in situ, thereby allowing us to examine ROS in cells directly along the scratch margin, as well as in cells away from the margin. Both young and old keratinocytes along the scratch margin showed an intense increase in DHE oxidation immediately after scratching (for an example see Fig. 3d) when compared to unscratched controls (Fig. 3h). This intense DHE fluorescence was greatly decreased by 6 h after scratching (Fig. 3e) and had returned to near-control levels by 24–48 h (Fig. 3f,g). Both young and old keratinocytes showed the same immediate intense DHE oxidation in cells at the scratch margins and minimal DHE oxidation in cells behind the scratch margin. Because the fluorescence intensity numbers obtained from the analysis software are in arbitrary units, the only way to compare data obtained from cultures labelled on different days is to normalize the data. When we normalized the fluorescence intensities at the scratch margins to the fluorescence intensities of unscratched cultures, the old keratinocytes had much higher DHE oxidation overall (approximately 400-fold) at the scratch margins when compared to young keratinocytes. The cells also differed in how much DHE oxidation was dissipated (Table 1). Old keratinocytes incubated at 21% O2 dissipated the most DHE oxidation of all groups (97.5 ± 1.2%, Table 1). These cells also had the fastest migration rate (Fig. 1b), suggesting that the capacity to decrease levels of ROS correlates with an increase in migration rate. Although not statistically significant, the results imply a strong relationship between these two events, as suggested by a Spearman correlation coefficient of 0.8.
Table 1.
The greatest per cent decrease in DHE fluorescence occurred in old keratinocytes incubated at 21% O2. The numbers represent the per cent decrease in DHE fluorescence at 6, 24 and 48 h after scratching
| Group | % decrease in DHE fluorescence
|
||
|---|---|---|---|
| Time after scratching
| |||
| 6 h | 24 h | 48 h | |
| 4% O2 – Young | 84.9 ± 3.8 | 93.1 ± 1.5 | 93.3 ± 0.5 |
| 4% O2 – Old | 83.7 ± 5.6 | 83.9 ± 8.5 | 83.1 ± 4.2 |
| 21% O2 – Young | 81.4 ± 3.5 | 88.4 ± 3.3 | 91.1 ± 1.5 |
| 21% O2 – Old | 79.4 ± 10.8 | 92.7 ± 4.3 | 97.5 ± 1.2* |
Mean ± SEM (n = 4 young 4%, all others n = 5).
P < 0.05 compared to ‘4% O2-Old group’.
DHE, dihydroethidium.
Discussion
It is estimated that by the year 2050, the number of US citizens over the age of 85 will have increased to 18.2 million. With this expected large increase in the elderly population, the focus on geriatric medicine and care is intensifying, including managing wound care (13). Keratinocyte migration is at the forefront of wound closure. Their migration from wound margins to the centre of the insult is essential to re-epithelialize the wounds. Earlier studies in the literature reported that the rate of wound closure decreases with age in humans (14,15) and cellular damage by ROS increases with age (8). Furthermore, any stress on skin keratinocytes (e.g. UV exposure) has been shown to generate ROS, which further leads to oxidative damage in cell membranes (16) as well as increases in stress inducible enzymes such as aldo-keto reductase 1C (17). These studies strongly suggest that keratinocyte migration can be affected by ROS directly or through its downstream effects. Therefore, in the current study, we investigated the relationship between migration and level of ROS in skin keratinocytes. To control the level of ROS in the cells, we selected 21% and 4% oxygen conditions to model ambient oxygen tension and subepidermal oxygen tension, respectively. In addition, 4% oxygen tension likely models occluded wound conditions. Based on previous observations of primary keratinocytes and the increase in cellular respiration that produces ROS at high oxygen tensions (18), we hypothesized that keratinocytes cultured at 21% oxygen tension would migrate slower than those cultured at 4%. Instead, we found an age-related difference. Young keratinocytes did exactly as we expected. However, old keratinocytes reacted the opposite with the cells at 21% oxygen migrating the fastest of all the experimental groups. We also found an age-related difference in the dissipation of increased ROS levels in keratinocytes. Although scratching greatly increased ROS levels in both old and young keratinocytes, the old keratinocytes decreased their ROS level to a greater extent than did the young keratinocytes. Thus, our data suggest a link between ROS and keratinocyte migration.
Studies examining wound closure in situ reported that ambient oxygen tension is required for complete wound healing, albeit that reduced oxygen might jump start wound healing (1). When we controlled the oxygen tension in keratinocyte cultures, we found that the age of the cells had a direct relation to how they reacted at 4% and 21% oxygen tension. Additionally, we demonstrated that the rates of migration were not related to proliferation, but instead to age and oxygen tension. Our finding of no change in proliferation in 4% oxygen differed from other studies that showed that cell proliferation varied with levels of ROS (19). In these studies, they found that BHK-21 fibroblasts were stimulated to proliferate in the presence of low levels of H2O2, while high levels not only depressed proliferation but lead to apoptosis and necrosis (19). However, these studies examined fibroblasts, and it may be that keratinocytes react differently to oxygen levels.
Consistent with previous experiments that showed intense DHE staining on mouse dermal wound edges (12,20), our adult human keratinocyte scratch assays showed intense DHE fluorescence in cells at the margins immediately after scratching, and this staining intensity was comparable among age and oxygen tension groups. The level of ROS is controlled in the cell by antioxidants, such as SOD that dismutes superoxide. When we investigated the levels of SOD1 and SOD2, we found that both young and old keratinocytes contained similar levels of immunoreactive protein for these two enzymes, suggesting that age itself may not cause a change in the SOD levels in human keratinocytes. Additionally, the extent that old keratinocytes decreased their ROS after scratching was greater than the young keratinocytes, suggesting that old keratinocytes may use an alternative mechanism to decrease rapidly increased ROS levels.
Initially, we suspected that the DHE staining might be a result of direct cellular damage produced when scratching destroyed the integrity of cells in the scratched area. However, using a specially designed large-scale real-time imager (21), we were able to see that the cells on the scratch margin not only were not dead but migrated within a few hours after scratching – even before the cells behind the margin (see Movie S1 in supplemental data). Furthermore, many of the cells at the margin subsequently divided, demonstrating that they remained alive. Thus, the increased DHE was a direct result of scratching, not cell death. Even though keratinocyte migration in re-epithelialization is only one part of a much larger response to wounding, it is intriguing that keratinocytes from young and old skin show a differential response in the scratch assay. Although what causes this response is still unknown, it is clear that it is occurring on a cellular level and may be related to steady-state levels of ROS.
Superoxide is short lived and rapidly dismuted in cells, resulting in the relatively stable hydrogen peroxide (22). Research in zebra-fish demonstrated that hydrogen peroxide can act as a leucocyte recruitment signal to initiate wound healing (23). These authors found that hydrogen peroxide produced by an NADPH oxidase acted to encourage wound healing by increasing directionality and motility of leucocytes to the wound site from substantial distances away from the injury. ROS in primary human keratinocytes may respond with a similar signalling mechanism. It is possible that the effects observed in our studies resulted from superoxide quickly dismuting to produce hydrogen peroxide, with the resulting hydrogen peroxide effecting a direction for keratinocytes to migrate (24).
In conclusion, occluding wounds may result in ROS being differentially present in old and young skin keratinocytes. While wound occlusion has been used for decades, our data suggest that the effects of wound occlusion on old patients when compared to young patients might be worth re-evaluation. Although the exact mechanism regulating the differential response to oxygen tension in young and old keratinocytes following scratching remains unclear and merits further investigation, this study illustrates that young and old keratinocytes have a complex relationship between oxygen tension, ROS and migration that was previously unknown. Adult human keratinocytes isolated from young and old skin respond to oxygen tension in opposite ways with regard to decreasing ROS levels and increasing migration. These findings may suggest that future studies regarding wound healing should not assume the same migratory effects or ROS regulation will be seen in human keratinocytes from young and old skin.
Supplementary Material
This movie, showing transmitted light time-lapse images of skin keratinocytes isolated from a 90-year-old individual, demonstrates that the cells on the scratch margin are not terminally damaged owing to scratching.
Acknowledgments
The authors thank the members of the Bickenbach lab and Dr. Thomas Waldschmidt for helpful discussions. We also thank Diqiong Xie, Department of Biostatistics, for her assistance in statistical analyses and Dr. Michael Mackey, Technical Director of the LSDCAS core facility for his expertise in live cell imaging. This research was funded in part by grants from the National Institutes of Health to JRB (R21AR053936 and R01AR053619) and by a grant from the McCord Research Foundation to NAB and JRB.
Abbreviations
- SOD
superoxide dismutase
- DHE
dihydroethidium
- ROS
reactive oxygen species
Footnotes
Conflict of interest
None.
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Rodriguez PG, Felix FN, Woodley DT, et al. Dermatol Surg. 2008;34:1159–1169. doi: 10.1111/j.1524-4725.2008.34254.x. [DOI] [PubMed] [Google Scholar]
- 2.O’Toole EA, Marinkovich MP, Peavey CL, et al. J Clin Invest. 1997;100:2881–2891. doi: 10.1172/JCI119837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Toole EA. Clin Exp Dermatol. 2001;26:525–530. doi: 10.1046/j.1365-2230.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisher GJ, Quan T, Purohit T, et al. Am J Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia YP, Zhao Y, Tyrone JW, et al. J Invest Dermatol. 2001;116:50–56. doi: 10.1046/j.1523-1747.2001.00209.x. [DOI] [PubMed] [Google Scholar]
- 6.Harman D. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 7.Harman D. Ann N Y Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 8.Muller FL, Lustgarten MS, Jang Y, et al. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Khodr B, Khalil Z. Free Radic Biol Med. 2001;30:1–8. doi: 10.1016/s0891-5849(00)00378-6. [DOI] [PubMed] [Google Scholar]
- 10.Woodley DT, Bachmann PM, O’Keefe EJ. J Cell Physiol. 1988;136:140–146. doi: 10.1002/jcp.1041360118. [DOI] [PubMed] [Google Scholar]
- 11.Birch-Machin MA. Clin Exp Dermatol. 2006;31:548–552. doi: 10.1111/j.1365-2230.2006.02161.x. [DOI] [PubMed] [Google Scholar]
- 12.Roy S, Khanna S, Nallu K, et al. Mol Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittman J. J Wound Ostomy Continence Nurs. 2007;34:412–415. doi: 10.1097/01.WON.0000281658.71072.e6. quiz 416–417. [DOI] [PubMed] [Google Scholar]
- 14.Wicke C, Bachinger A, Coerper S, et al. Wound Repair Regen. 2009;17:25–33. doi: 10.1111/j.1524-475X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- 15.Engeland CG, Bosch JA, Cacioppo JT, et al. Arch Surg. 2006;141:1193–1197. doi: 10.1001/archsurg.141.12.1193. discussion 1198. [DOI] [PubMed] [Google Scholar]
- 16.Bellei B, Mastrofrancesco A, Briganti S, et al. Exp Dermatol. 2008;17:115–124. doi: 10.1111/j.1600-0625.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- 17.Marin YE, Seiberg M, Lin CB. Exp Dermatol. 2009;18:611–618. doi: 10.1111/j.1600-0625.2008.00839.x. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni AC, Kuppusamy P, Parinandi N. Antioxid Redox Signal. 2007;9:1717–1730. doi: 10.1089/ars.2007.1724. [DOI] [PubMed] [Google Scholar]
- 19.Burdon RH, Gill V, Alliangana D. Free Radic Res. 1996;24:81–93. doi: 10.3109/10715769609088004. [DOI] [PubMed] [Google Scholar]
- 20.Graf J. Plast Reconstr Surg. 2010;125:378–383. doi: 10.1097/PRS.0b013e3181c2a571. [DOI] [PubMed] [Google Scholar]
- 21.Davis PJ, Kosmacek EA, Sun Y, et al. J Microsc. 2007;228:296–308. doi: 10.1111/j.1365-2818.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- 22.McCord JM, Fridovich I. J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 23.Zelko IN, Mariani TJ, Folz RJ. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 24.Groeger G, Quiney C, Cotter TG. Antioxid Redox Signal. 2009;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This movie, showing transmitted light time-lapse images of skin keratinocytes isolated from a 90-year-old individual, demonstrates that the cells on the scratch margin are not terminally damaged owing to scratching.