Abstract
One pathway by which infant mammals gain information about their environment is through ingestion of milk. We assessed the relationship between stress-induced cortisol concentrations in milk, maternal and offspring plasma, and offspring temperament in rhesus monkeys. Milk was collected from mothers after a brief separation from their infants at 3–4 months postpartum, and blood was drawn at this time for both mothers and infants. Offspring temperament was measured at the end of a 25-hour assessment. Cortisol concentrations in milk were in a range comparable to those found in saliva, and were positively correlated with maternal plasma levels. Mothers of males had higher cortisol concentrations in milk than did mothers of females, and cortisol concentrations in maternal milk were related to a Confident temperament factor in sons, but not daughters. This study provides the first evidence that naturally occurring variation in endogenous glucocorticoid concentrations in milk are associated with infant temperament.
Keywords: infant development, personality, lactation, maternal programming, Macaca mulatta
Introduction
During lactation, mammalian mothers provide extensive behavioral care to and physiological investment in their offspring, influencing infant developmental trajectories. This maternal care provides information about the world into which the infant is born; milk that mothers supply to their infants is one mechanism of information transfer (German, Dillard, & Ward, 2002; Bernt & Walker, 1999). It is well established across mammals that the energetic value of milk is positively associated with infant growth and development (reviewed in Hinde et al. 2009). Whether or not the composition of maternal milk directly influences infant behavior is less understood (Hinde & Capitanio, 2010). Milk contains numerous non-nutritive bioactive components, including hormones (Akers, 2002; Donovan & Odle, 1994; Rodriguez-Palmero, Koletzko, Kunz, & Jensen, 1999; Schwalm & Tucker, 1978), that may have important effects on the behavioral development of infants. One class of biologically active components in milk is glucocorticoids (corticosterone in rodents; cortisol in humans and nonhuman primates), which have the potential to influence infant behavior through calibration of the hypothalamic-pituitary-adrenal (HPA) axis of infants. Glucocorticoids (GCs) in milk are reflective of serum blood GC concentrations in response to physical, pharmacological, and psychological challenges (Bremel & Gangwer, 1978; Groer, Humenick, & Hill, 1994; Pearlman, 1983), with glucocorticoid concentrations equilibrating rapidly between plasma and milk (Fox, Butler, Everett, & Natzke, 1981; Angelucci, Patacchioli, Scaccianoce, Di Sciullo, Cardillo, & Maccari, 1985; Termeulen, Butler, & Natzke, 1981). In this way, milk may serve as a salient biochemical signal of environmental conditions from mother to infant, entraining infant physiological functioning and patterns of responding.
Glucocorticoid concentrations in milk may affect behavior through effects on broad dispositional characteristics. Temperament is an individual’s consistent pattern of responsiveness that is thought to be partially heritable, biologically based, present early in life, and stable over time (Allport, 1937). The HPA axis has long been identified as an important physiological underpinning of temperament, with both regulation and reactivity of the system providing important information about the relationship between physiological and psychological arousal (Levine, Haltmeyer, Karas, & Denenberg, 1967; Mcewen, 2001; Stansbury & Gunnar, 1994; Suomi, 1991; Mendoza & Mason, 1989; Capitanio, Mendoza, & Bentson, 2004). For example, in comparison to socially inhibited children, uninhibited children have lower morning basal cortisol concentrations (Kagan, Reznick, & Snidman, 1988) and greater lability in HPA axis responsivity to social stress (Gunnar, Tout, De Haan, Pierce, & Stansbury, 1997). Characteristic dispositions to respond show consistency from childhood into adulthood (Gest, 1997) and glucocorticoids contribute to these lasting patterns by strengthening or weakening neural pathways that become canalized over time (Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002; Korte, 2001; Casolini, Cigliana, Alema, Ruggieri, Angelucci, & Catalani, 1997). These neural pathways influence the likelihood of future behavioral responses resulting in individual differences in temperament (Gunnar & Quevedo, 2006). Neural changes are influenced by maternal indicators of environmental conditions, such as the quality of maternal behavioral care (Fish, Shahrokh, Bagot, Caldji, Bredy, Szyf, & Meaney, 2004), but may also be influenced through physiological signaling during lactation. To date, however, little is known about how maternal physiological investment during lactation shapes infant behavior and temperament (Hinde & Capitanio, 2010).
Glucocorticoids have been indentified in the milk of several species, including humans (for review, see Alexandrova & Macho, 1983; humans, Kulski & Hartmann, 1981; Patacchioli, Cigliana, Cilumbriello, Perrone, Capri, Alema, Zichella, & Angelucci, 1992; dairy cattle, Tucker & Schwalm, 1977; rodents, Angelucci, Patacchioli, Chierichetti, & Laureti, 1983). Infant mammals have corticosteroid (mineralocorticoid and glucocorticoid) receptors in their intestinal tract which provide a potential pathway for maternal GCs to influence infant physiology following milk ingestion (for review see Pacha, 2000). Glucocorticoid receptors (GRs) are found at higher densities in the intestines during infancy, but intestinal GRs decrease to adult levels after weaning (Henning & Kretchmer, 1973). This is especially striking because GR density increases in other body tissues during development (Henning, Ballard, & Kretchmer, 1975).
Bioactive maternal glucocorticoids are transmitted to mammalian infants via milk ingestion and the effects show sex biases. Angelucci and colleagues delivered radiolabeled corticosterone to adult female rats by oral administration in drinking water, which resulted in increased corticosterone concentrations in both maternal plasma and milk, but within the range of naturally occurring stress-induced cortisol concentrations (Angelucci et al., 1983). After infants ingested the milk of these mothers, labeled corticosterone was found in their gastric contents, plasma, and brains. Infants also displayed higher circulating morning plasma concentrations of corticosterone (Angelucci et al., 1983). Ingesting elevated cortisol concentrations in infancy caused lasting changes to offspring: adult females, whose mothers were administered exogenous corticosterone, had elevated corticosterone concentrations in comparison to female controls, whereas adult males had lower plasma corticosterone concentrations in comparison to male controls (Angelucci et al., 1983).
Glucocorticoids in milk are also implicated in infant behavioral outcomes in both rats and humans. Offspring whose mothers were administered exogenous corticosterone, described above, displayed fewer behavioral indicators of anxiety in response to stress than did controls. This effect was present during infancy, as well as after weaning into adulthood (Angelucci et al., 1985; Catalani, Casolini, Scaccianoce, Patacchioli, Spinozzi, & Angelucci, 2000; Catalani, Casolini, Cigliana, Scaccianoce, Consoli, Cinque, Zuena, & Angelucci, 2002). In humans, infant fear behavior is positively correlated with mother’s plasma cortisol concentrations but only in infants that were breast-fed (Glynn, Davis, Schetter, Chicz-Demet, Hobel, & Sandman, 2007) suggesting that maternal physiology, rather than behavior, is responsible for this effect. To date, however, no studies have directly investigated cortisol concentrations in milk and infant temperament in human or non-human primates.
To better understand the relationship between cortisol in milk and infant temperament we investigated these measures in a non-human primate: the rhesus monkey (Macaca mulatta). Rhesus macaques display substantial individual variation in behavior and temperament (Suomi & Ripp, 1983; Capitanio, Mason, Mendoza, Del Rosso, & Roberts, 2006). Additionally the regulation of their HPA axis has been well characterized (Levine & Wiener, 1988; Clarke, 1993; Gorman, Mathew, & Coplan, 2002) and HPA regulation and responsivity to stress have been found to be affected by early experience (Capitanio, Mendoza, Mason, & Maninger, 2005). The aims of the present study were to determine the correlation between cortisol concentrations in maternal plasma and milk, and the association between milk cortisol concentrations and infant temperament, as assessed in response to a stressful experience.
Methods
Subjects
Subjects were 44 adult female rhesus monkeys (Macaca mulatta) and their infants. Twenty mothers had male offspring (6 primiparous, 14 multiparous) and 24 had female offspring (14 primiparous, 10 multiparous). Infants were between 91–124 days old (mean age=110 days) at the time of the assessment (see below). Dominance rank did not differ between mothers of male and female offspring (Mann-Whitney U = 211.5, p = 0.468) or between primiparous and multiparous mothers (Mann-Whitney U = 223.0, p = 0.665). Prior to assessment, mothers and infants were housed outdoors in half-acre enclosures consisting of 100–150 animals of mixed age- and sex-classes of close kin, distant kin, and non-kin at the California National Primate Research Center (CNPRC). A commercial diet (Outdoor Monkey Lab Diet, PMI Nutrition Int’l, Brentwood, Missouri) was provided twice daily, fruit and vegetable supplements were provided twice weekly, and water was available ad libitum.
Subject Relocation
Mothers and their offspring were captured from their homecage, separated from one another, and relocated to separate novel environments (between 0800–0900h) for a 25-hour period. Infants were weighed immediately after separation, and were tested in cohorts of five to eight animals. Housing for mothers and for infants was in standard laboratory cages (60cm X 65cm X 79cm, Lab Products, Inc., Maywood, NJ), with all members of a given cohort housed in the same room throughout the 25-hr period. Mothers and infants were housed in separate rooms with no visual, auditory, or olfactory contact possible. At the conclusion of the 25-hour testing period, infants and mothers were reunited in the mother’s holding cage and were housed together for one hour before being returned to their outdoor enclosures.
Infant Biobehavioral Assessment
All 44 infant subjects were part of an ongoing biobehavioral assessment program at the CNPRC described in detail elsewhere (Capitanio et al., 2005; Golub, Hogrefe, Widaman, & Capitanio, 2009; Hinde & Capitanio, 2010). Over the 25-hr. testing period, behavioral data were collected in a variety of standardized testing situations, including behavioral observations in the novel holding cage, subjects’ recognition memory, and subjects’ responsivity to social stimuli, graded conditions of challenge, and novel objects. Blood samples (1.0 ml) were also collected from unanesthetized infants 2 hours after separation and relocation via femoral venipuncture.
Temperament Ratings
Infant temperament ratings were conducted in order to collect an overall “thumbnail” portrait of the animal’s functioning during the entire Infant Biobehavioral Assessment period. At the conclusion of the 25-hr. testing period, observers who performed the testing rated each infant on 16 trait adjectives describing characteristics of temperament (see Table 3 in Golub et al., 2009; Hinde & Capitanio, 2010). Ratings were made using a seven point Likert scale, with a score of 1 reflecting a total absence of the observation of behaviors related to the characteristic and a score of 7 indicating the observation of an extremely large amount of the behaviors reflecting that characteristic. Agreement between independent observers (a difference of no more than 1 scale point) for each trait was significantly greater than chance using chi-square (p<0.000001). Ratings on each adjective were z-scored across all subjects within a given birth year. Exploratory and confirmatory factor analyses of the data from the full sample between 2001 and 2005 (n=1284, described in Golub et al., 2009) revealed four factors (named for the trait adjective with the highest positive loading): Confident (active, bold, confident, curious, playful), Gentle (calm, curious, flexible, gentle), Vigilant (vigilant, not depressed, not tense, not timid), and Nervous (fearful, nervous, timid, not calm, not confident). The trait adjectives preceded by the word “not” reflect a negative loading in the factor analysis. A composite score for each factor was created by adding the z-scores of each trait rating adjective (or the reverse-coded z-score for negative loadings). These scores were then standardized within each factor scale and z-scores were created. Cronbach’s alpha (scale reliability) values, based upon the full sample of 347 animals assessed this year, were 0.91 (Confident), 0.78 (Gentle), 0.87 (Vigilant), and 0.61 (Nervous).
Sample Collection
After 3.5–4 hours of milk accumulation, mothers were sedated with ketamine hydrochloride intramuscularly (5–10 mg/kg). Blood was collected on a randomly selected subset of subjects (n=16: 6 with female offspring, 10 with male offspring) via femoral venipuncture into pre-chilled EDTA tubes which were immediately placed in ice. Subjects were then administered exogenous oxytocin (2 IU/kg) intramuscularly for myoepithelial cell contraction and milk let down. Milk was collected by gentle hand stripping of the nipple. To minimize sampling bias (Oftedal, 1984) the mammary was fully evacuated which occurred within 10–15 minutes for all subjects as described elsewhere (Hinde, Power, & Oftedal, 2009).
Cortisol Assays
Blood samples were centrifuged at 3000 RPM for 10 min, and the plasma fraction was removed and frozen at −80°C prior to being assayed for cortisol concentration by coated-tube RIA (Diagnostic Products Corp., Los Angeles, CA). The interassay coefficient of variation (CV) was 7.3% and intra-assay CV was 5.1%.
Milk was briefly vortexed, aliquoted, and frozen at −80°C until thawed for analysis. Once thawed, it was centrifuged at 3000 RPM for 10 min., consistent with methods used in other species (Butler & Des Bordes, 1980; Agrimonti, Frairia, Fornaro, Torta, Borretta, Trapani, Bertino, & Angeli, 1982; Groer, Davis, Casey, Short, Smith, & Groer, 2005; Spencer, Boyd, Cabrera, & Allee, 2003). Centrifugation resulted in the separation of aqueous component from suspended particles in milk and a floating lipid layer. Concentrations of cortisol in the aqueous component were estimated in duplicate using commercial RIA kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA). During assay development we determined that the partial removal of lipids and suspended particles through centrifugation resulted in reduced cortisol concentrations that were 81% of whole milk values, consistent with results in cows (Schwalm & Tucker, 1978), and that cortisol concentrations in milk were approximately 10% of plasma values. Assay procedures were modified as follows: 1) standards were diluted to concentrations ranging from 2.76 to 345 nmol/L; 2) sample volume was increased to 200 μl, and 3) incubation times were extended to 3 h. Serial dilution of samples indicated that the modified assay displays a linearity of .98 and a least detectable dose of 1.3854 nmol/L. All samples were run in one assay with an intra-assay CV of 2.54%.
Milk Fat and Protein Assays
Composition analyses of proximate milk constituents were conducted in the Nutrition Laboratory at the Smithsonian National Zoological Park in Washington DC using standard methods described elsewhere (Hinde et al., 2009; Oftedal & Iverson, 1995; Milligan, Gibson, Williams, & Power, 2008; Power, Verona, Ruiz-Miranda, & Oftedal, 2008). To determine fat, total lipids were measured by sequential extractions with ethanol, diethyl ether and petroleum ether by a micro modification of the Rose-Gottleib procedure. The amount of nitrogen in each sample was determined using a CHN elemental gas analyzer (Model 2400, PerkinElmer, Norwalk, CT) using a 2-s oxygen burst to promote complete combustion. Crude protein was estimated as 6.38*nitrogen. These methods have been validated at the SNZP Nutrition Laboratory using both fresh cow milk and powdered cow milk from the National Institute of Standards and Technology (Hinde et al., 2009).
Data analysis
Bivariate correlations were conducted to compare cortisol concentrations in maternal milk to cortisol concentrations in maternal plasma, maternal milk components, offspring plasma, and offspring temperament factor scores. A Bonferroni correction for multiple comparisons was applied to the correlations between cortisol concentrations in milk and offspring temperament factor scores in order minimize the probability of Type I error, α′ = 0.00625 = α/k, where k is the number of tests (8: 4 each for males and females). The significance of the difference between correlations was computed as described in Blalock (1972). Partial correlations were also conducted to assess the unique contribution of cortisol in maternal milk with infant temperament, controlling for other components in milk. A t-test was performed to compare the cortisol concentrations in the milk and plasma of mothers of sons and daughters. There were no differences based on maternal parity in maternal cortisol concentrations in milk or infant temperament factor scores, therefore groups were combined in the final analyses.
Results
Individual Differences in Cortisol Concentrations in Milk
Cortisol concentrations in the milk of rhesus monkeys were variable across individuals and in the range of salivary values (74.94 – 757.01 nmol/L, M = 231.40 nmol/L, SD = 159.54). Cortisol concentrations in milk were significantly correlated with cortisol concentrations in mothers’ plasma (r = 0.586, p = 0.017). Cortisol concentrations in milk were also associated with the nutritional value of milk; milk protein and cortisol concentrations were positively correlated (r = 0.441, p = 0.003), as were cortisol and fat concentrations in milk (r = 0.398, p = 0.007).
Cortisol Concentrations and Offspring Characteristics
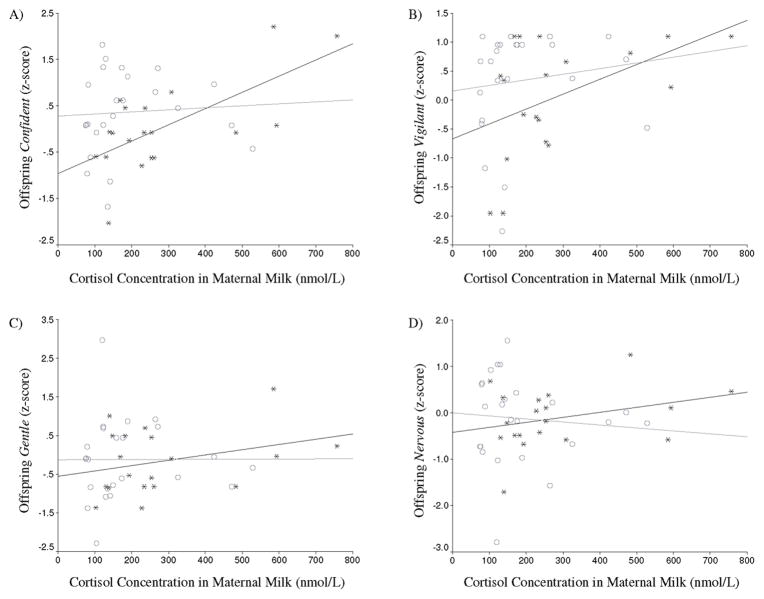
Cortisol concentrations in milk were found to be higher for mothers of males than for mothers of females (t(42) = 2.085, p = 0.043), but concentrations in maternal plasma did not differ for mothers of males and females (t(14) = 0.298, p = 0.77). Cortisol concentrations in maternal milk were not correlated with concentrations in the plasma of offspring (r = 0.086: males: r = 0.259; females r = 0.149, all n.s); however, they were correlated with scores on one infant temperament factor in sons, but not daughters. After the Bonferroni correction for multiple comparisons, maternal cortisol in milk was correlated with their son’s z-scores on the temperament factor Confident (males: r = 0.669, p = 0.002; females: r = 0.064, p = 0.767), but not for the temperament factors Vigilant (males: r = 0 .475, p = 0.04; females: r = −0.139, p = 0.517), Gentle (males: r = 0.306, p = 0.203; females: r = 0.003, p = 0.988) or Nervous (males: r = 0.311, p = 0.194; females: r = −0.088, p = 0.683). The correlation coefficients were found to be significantly different between male and female infants for the Confident factor (z = 2.28, p = 0.022). This relationship also remained after controlling for nutritional components in milk; when controlling for protein and fat content partial correlations reveal that maternal cortisol concentrations in milk continue to be correlated with Confident factor scores for male infants (r = 0.5167, p = 0.034), but not female infants (r = −0.0298, p = 0.895).
Discussion
Stress-induced cortisol concentrations in milk were correlated with maternal plasma cortisol concentrations and were in a range comparable to those found in saliva, indicating that cortisol found in milk is likely free rather than bound to CBG or albumin (Umeda, Hiramatsu, Iwaoka, Shimada, Miura, & Sato, 1981). Additionally, naturally occurring variation of endogenous glucocorticoid concentrations in maternal milk in response to stress was associated with one dimension of offspring temperament, and showed sex-specific effects. This suggests that the transmission of biochemical markers from mother to infant through the consumption of milk may not only influence infant physiology (Angelucci et al., 1983), but may further influence characteristics of infant temperament with important, and differential, consequences for sons and daughters.
For sons, Confident factor scores were significantly and positively correlated with maternal cortisol concentrations in milk, whereas there were no correlations between daughters’ temperament factor scores and cortisol in maternal milk. High scores on the Confident factor reflect an infant that has a bold, active, curious and playful approach during a stressful experience. In rodents there are sex-dependent effects on HPA axis regulation in infants exposed to increased maternal cortisol concentrations through milk (Catalani et al., 2000; Catalani et al., 2002), which may underlie the differences in behavioral responsivity observed here. Males may be more sensitive than females to experimentally induced variability of their mother’s HPA axis (Parker, Buckmaster, Sundlass, Schatzberg, & Lyons, 2006). Males exposed to pharmacologically elevated GC concentrations ingested through milk show an increase in both mineralocorticoid (MR) and glucocorticoid receptors (GR) in the hippocampus in comparison to controls (Catalani et al., 2000; Casolini, Domenici, Cinque, Alema, Chiodi, Galluzzo, Musumeci, Mairesse, Zuena, Matteucci, Marano, Maccari, Nicoletti, & Catalani, 2007; Casolini et al., 1997), but no differences in hippocampal MR or GR densities have been observed in females exposed to increased cortisol through milk (Catalani et al., 2002). In this way exposure to cortisol concentrations in milk may lead to sex-dependent effects that contribute to lasting changes in physiological functioning by entraining neuroendocrine regulation in males but not females. This post-natal vulnerability to maternal influence may provide an opportunity for mothers to fine-tune the quality of sons during development.
Previous research has demonstrated associations between early life exposure to elevated glucocorticoid concentrations and characteristics of confidence, consistent with the results presented here. Infant monkeys exposed to brief intermittent separation stress, and the resultant periodic activation of the HPA axis, are more curious and exploratory in novel situations later in life (Parker, Buckmaster, Schatzberg, & Lyons, 2004). Similarly, rodent pups exposed to elevated GC concentrations in milk, increased pharmacologically to stress-induced levels, exhibit fewer behavioral indicators of anxiety in response to novelty from infancy to adulthood than those that were not exposed to increased GCs (Catalani et al., 2002; Catalani et al., 2000). Our results suggest that individual differences in maternal milk GC concentrations in response to stress are associated with individual differences in sons’ behavioral responsivity to a novel situation, with greater GC concentrations in milk corresponding to a son that is more confident. Maternal indicators of environmental condition may play an important role in this trait specifically due to its significance in the life-history of males. Male rhesus macaques emigrate from their natal group (Pusey & Packer, 1986) and attain rank in a new social group based partially on personality characteristics, whereas female rhesus monkeys remain in their natal group and inherit rank from their mother (Walters & Seyfarth, 1986). Indeed, adolescent boldness and confidence positively predict adult rank attainment in male vervet monkeys (Fairbanks, Jorgensen, Huff, Karin, Yung-Yu, & Mann, 2004). For this reason the Confident temperament factor may be particularly sensitive to maternal hormones in a way that the other temperament factors - Vigilant, Gentle, and Nervous –are not.
In conjunction with the sex-biased effects of maternal cortisol on aspects of infant temperament, mothers of males had higher cortisol concentrations in milk than did mothers of females, but determining causal explanations of this difference remains difficult. Among polygynous mammals, mothers in better condition are predicted to bias investment toward sons because of their greater potential reproductive output compared to daughters (Trivers & Willard, 1973; reviewed in rhesus macaques in Bercovitch, Widdig, & Nürnberg, 2000; Hinde, 2009). Maternal condition has been assessed through the examination of glucocorticoid concentrations (Love, Chin, Wynne-Edwards, & Williams, 2005) as glucocorticoids play a prominent role in energy balance (Dallman, Strack, Akana, Bradbury, Hanson, Scribner, & Smith, 1993; Munck & Naray-Fejes-Toth, 1994). In our population of rhesus macaques, however, mothers of sons and daughters do not differ in their condition and presumably pay the same energetic costs to rear offspring; they are equally likely to get pregnant and produce a surviving infant in the subsequent birth season (Hinde, 2009). Further, mothers of sons and daughters in the present study did not differ in plasma cortisol concentrations, suggesting that sex-biased differences in cortisol concentrations in milk are not due to differences in maternal condition and may be specific to mammary function or offspring development. We also found that cortisol in milk was positively correlated with energetic components in milk, specifically protein and fat concentrations, which are higher in the milk produced for sons (Hinde, 2007). Energetic aspects of milk have been shown to predict infant behavior (Hinde & Capitanio, 2010); when controlling for these factors, however, the association between cortisol concentration in maternal milk and Confident factor scores in sons remained. Evidence from several species indicates that male infants assimilate and metabolize maternal milk energy differently than do female infants (see Hinde, 2009 for review) and cortisol may be an important factor in calibrating metabolic processes (German et al., 2002; Rodriguez-Palmero et al., 1999).
This study was part of a larger project, and consequently, there are several limitations in experimental design and control. The measures of cortisol reported here (for both mothers and infants) were collected at a single time point and do not reflect basal levels or baseline concentrations; these measures reflect cortisol concentrations after activation of the HPA axis in response to separation and relocation stress. However, the HPA axis shows trait-like consistency (Stansbury & Gunnar, 1994); therefore maternal cortisol concentrations in milk are also likely to be trait-like and show consistency across a variety of physiological and psychological stressors. Due to testing parameters, samples were collected at different times for infants and mothers (two and four hours post-separation, respectively); the lack of relationship between maternal cortisol concentrations in milk and offspring plasma cortisol concentrations may be due, in part, to this. Moreover, mothers and infants were separated at the time of testing, so transmission of milk from mother to offspring was not possible. Finally, correlations between cortisol in milk and dimensions of infant temperament may reflect underlying similarities between mothers and sons in behavioral and physiological reactivity, perhaps as a result of sons’ direct inheritance of x-linked genes associated with HPA axis functioning from their mother (e.g. Monoamine Oxidase A gene (Jabbi, Korf, Kema, Hartman, Van Der Pompe, Minderaa, Ormel, & Den Boer, 2007)), rather than the direct transmission of maternal signals to offspring through milk from their mother. This may be addressed through longitudinal and cross-fostering studies that are better able to tease apart the influence of genetics and the environment.
The data presented here represent an important first step in understanding the role of maternal glucocorticoids in milk and the potential consequences for one dimension of infant temperament. Our results suggest that confidence in males may be more strongly associated with their mother’s physiological functioning than is the confidence of daughters. This relationship provides a potential physiological mechanism through which mothers may fine-tune their sons’ development during early life. Future studies should further investigate individual differences in concentrations of cortisol in milk over lactation, its relationship to regulation of the HPA axis in offspring, and the consequences for offspring temperament.
Figure 1.
Scatterplot of cortisol concentration in maternal milk and offspring temperament factor scores by offspring sex (males: *; females: ○ ). (A) Confident factor scores, (B) Vigilant factor scores, (C) Gentle factor scores, (D) Nervous factor scores.
Acknowledgments
We thank L. DelRosso, L. Calonder, C. Stanko, L. Laughlin, N. Maninger, for contributions to this project. We would also like to thank two anonymous reviewers for their insightful comments that improved the quality of the paper. This research was supported by NSF DDIG #0525025, the American Society of Primatologists, and NIH #RR019970, RR000169. All procedures were approved by the Institutional Animal Care and Use Committee at UC Davis and conducted in accordance with the laws of the United States of America.
Contributor Information
Katie Hinde, Email: kjhinde@ucdavis.edu.
Sally P. Mendoza, Email: spmendoza@ucdavis.edu.
John P. Capitanio, Email: jpcapitanio@ucdavis.edu.
References
- Agrimonti F, Frairia R, Fornaro D, Torta M, Borretta G, Trapani G, et al. Circadian and circaseptan rhythmicities in corticosteroid-binding globulin (CBG) binding activity of human milk. Chronobiologia. 1982;9(3):281–290. [PubMed] [Google Scholar]
- Akers RM. Lactation and the mammary gland. Ames: Iowa State Press; 2002. [Google Scholar]
- Alexandrova M, Macho L. Glucocorticoids in human, cow and rat milk. Endocrinol Exp. 1983;17(3–4):183–189. [PubMed] [Google Scholar]
- Allport G. Personality: A psychological interpretation. New York: Henry Holt; 1937. [Google Scholar]
- Angelucci L, Patacchioli FR, Chierichetti C, Laureti S. Perinatal mother-offspring pituitary-adrenal interrelationship in rats: corticosterone in milk may affect adult life. Endocrinol Exp. 1983;17(3–4):191–205. [PubMed] [Google Scholar]
- Angelucci L, Patacchioli FR, Scaccianoce S, Di Sciullo A, Cardillo A, Maccari S. A model for later-life effects of perinatal drug exposure: maternal hormone mediation. Neurobehav Toxicol Teratol. 1985;7(5):511–517. [PubMed] [Google Scholar]
- Bercovitch FB, Widdig A, Nürnberg P. Maternal investment in rhesus macaques (Macaca mulatta ): reproductive costs and consequences of raising sons. Behavioral Ecology and Sociobiology. 2000;48(1):1–11. [Google Scholar]
- Bernt KM, Walker WA. Human milk as a carrier of biochemical messages. Acta Paediatr Suppl. 1999;88(430):27–41. doi: 10.1111/j.1651-2227.1999.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Blalock HM. Social Statistics. New York: McGraw-Hill; 1972. [Google Scholar]
- Bremel RD, Gangwer MI. Effect of adrenocorticotropin injection and stress on milk cortisol content. J Dairy Sci. 1978;61(8):1103–1108. doi: 10.3168/jds.S0022-0302(78)83693-5. [DOI] [PubMed] [Google Scholar]
- Butler WR, Des Bordes CK. Radioimmunoassay technique for measuring cortisol in milk. J Dairy Sci. 1980;63(3):474–477. doi: 10.3168/jds.S0022-0302(80)82956-0. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, Del Rosso LA, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery rearing of nonhuman primates in the 21st century. New York: Springer; 2006. pp. 191–213. [Google Scholar]
- Capitanio JP, Mendoza SP, Bentson KL. Personality characteristics and basal cortisol concentrations in adult male rhesus macaques (Macaca mulatta) Psychoneuroendocrinology. 2004;29(10):1300–1308. doi: 10.1016/j.psyneuen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Casolini P, Cigliana G, Alema GS, Ruggieri V, Angelucci L, Catalani A. Effect of increased maternal corticosterone during lactation on hippocampal corticosteroid receptors, stress response and learning in offspring in the early stages of life. Neuroscience. 1997;79(4):1005–1012. doi: 10.1016/s0306-4522(96)00668-9. [DOI] [PubMed] [Google Scholar]
- Casolini P, Domenici MR, Cinque C, Alema GS, Chiodi V, Galluzzo M, et al. Maternal exposure to low levels of corticosterone during lactation protects the adult offspring against ischemic brain damage. J Neurosci. 2007;27(26):7041–7046. doi: 10.1523/JNEUROSCI.1074-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Cigliana G, Scaccianoce S, Consoli C, Cinque C, et al. Maternal corticosterone influences behavior, stress response and corticosteroid receptors in the female rat. Pharmacol Biochem Behav. 2002;73(1):105–114. doi: 10.1016/s0091-3057(02)00755-4. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100(2):319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, et al. Feast and Famine: Critical Role of Glucocorticoids with Insulin in Daily Energy Flow. Frontiers in Neuroendocrinology. 1993;14(4):303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Donovan SM, Odle J. Growth factors in milk as mediators of infant development. Annu Rev Nutr. 1994;14:147–167. doi: 10.1146/annurev.nu.14.070194.001051. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Huff A, Karin B, Yung-Yu H, Mann JJ. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. 2004;64:1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, et al. Epigenetic Programming of Stress Responses through Variations in Maternal Care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Fox L, Butler WR, Everett RW, Natzke RP. Effect of adrenocorticotropin on milk and plasma cortisol and prolactin concentrations. J Dairy Sci. 1981;64(9):1794–1803. doi: 10.3168/jds.S0022-0302(81)82768-3. [DOI] [PubMed] [Google Scholar]
- German JB, Dillard CJ, Ward RE. Bioactive components in milk. Curr Opin Clin Nutr Metab Care. 2002;5(6):653–658. doi: 10.1097/00075197-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Gest SD. Behavioral inhibition: stability and associations with adaptation from childhood to early adulthood. J Pers Soc Psychol. 1997;72(2):467–475. doi: 10.1037//0022-3514.72.2.467. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Schetter CD, Chicz-Demet A, Hobel CJ, Sandman CA. Postnatal maternal cortisol levels predict temperament in healthy breastfed infants. Early Hum Dev. 2007;83(10):675–681. doi: 10.1016/j.earlhumdev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Mathew S, Coplan J. Neurobiology of early life stress: nonhuman primate models. Semin Clin Neuropsychiatry. 2002;7(2):96–103. doi: 10.1053/scnp.2002.31784. [DOI] [PubMed] [Google Scholar]
- Groer M, Davis M, Casey K, Short B, Smith K, Groer S. Neuroendocrine and immune relationships in postpartum fatigue. MCN Am J Matern Child Nurs. 2005;30(2):133–138. doi: 10.1097/00005721-200503000-00012. [DOI] [PubMed] [Google Scholar]
- Groer MW, Humenick S, Hill PD. Characterizations and psychoneuroimmunologic implications of secretory immunoglobulin A and cortisol in preterm and term breast milk. J Perinat Neonatal Nurs. 1994;7(4):42–51. doi: 10.1097/00005237-199403000-00005. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The Neurobiology of Stress and Development. Annu Rev Psychol. 2006 doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Dev Psychobiol. 1997;31(1):65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Henning SJ, Ballard PL, Kretchmer N. A study of the cytoplasmic receptors for glucocorticoids in intestine of pre- and postweanling rats. J Biol Chem. 1975;250(6):2073–2079. [PubMed] [Google Scholar]
- Henning SJ, Kretchmer N. Development of intestinal function in mammals. Enzyme. 1973;15(1):3–23. [PubMed] [Google Scholar]
- Hinde K. First-time macaque mothers bias milk composition in favor of sons. Curr Biol. 2007;17(22):R958–959. doi: 10.1016/j.cub.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Hinde K. Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. Am J Hum Biol. 2009;21(4):512–519. doi: 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- Hinde K, Capitanio JP. Lactational programming? Mother’s milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta) American Journal of Primatology. 2010 doi: 10.1002/ajp.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K, Power ML, Oftedal OT. Rhesus macaque milk: magnitude, sources, and consequences of individual variation over lactation. Am J Phys Anthropol. 2009;138(2):148–157. doi: 10.1002/ajpa.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol Psychiatry. 2007;12(5):483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240(4849):167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25(2):117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Kulski JK, Hartmann PE. Changes in the concentration of cortisol in milk during different stages of human lactation. Aust J Exp Biol Med Sci. 1981;59(Pt 6):769–778. doi: 10.1038/icb.1981.66. [DOI] [PubMed] [Google Scholar]
- Levine S, Haltmeyer GC, Karas GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiology & Behavior. 1967;2(1):55–59. [Google Scholar]
- Levine S, Wiener SG. Psychoendocrine aspects of mother-infant relationships in nonhuman primates. Psychoneuroendocrinology. 1988;13(1–2):143–154. doi: 10.1016/0306-4530(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, Williams TD. Stress Hormones: A Link between Maternal Condition and Sex-Biased Reproductive Investment. 2005;166:751–766. doi: 10.1086/497440. [DOI] [PubMed] [Google Scholar]
- McEwen BS. From molecules to mind: Stress, individual differences, and the social environment. Ann NY Acad Sciences. 2001;935:42–49. [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Primate relationships: social dispositions and physiological responses. In: Seth PK, Seth S, editors. Perspectives in primate biology. Vol. 2. New Delhi: Today and tomorrow’s printers and publishers; 1989. pp. 129–143. [Google Scholar]
- Milligan LA, Gibson SV, Williams LE, Power ML. The composition of milk from Bolivian squirrel monkeys (Saimiri boliviensis boliviensis) Am J Primatol. 2008;70(1):35–43. doi: 10.1002/ajp.20453. [DOI] [PubMed] [Google Scholar]
- Munck A, Naray-Fejes-Toth A. Glucocorticoids and Stress: Permissive and Suppressive Actions. 1994;746:115–130. doi: 10.1111/j.1749-6632.1994.tb39221.x. [DOI] [PubMed] [Google Scholar]
- Oftedal OT. Milk composition, milk yield and energy output at peak lactation: a comparative review. Zool Soc Lond. 1984;51:33–85. [Google Scholar]
- Oftedal OT, Iverson SJ. Comparative analysis of nonhuman milks: a phylogenetic variation in the gross composition of milks. In: Jensen RG, editor. Handbook of Milk Composition. San Diego: Academic Press; 1995. pp. 749–789. [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61(9):933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci U S A. 2006;103(8):3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patacchioli FR, Cigliana G, Cilumbriello A, Perrone G, Capri O, Alema GS, et al. Maternal plasma and milk free cortisol during the first 3 days of breast-feeding following spontaneous delivery or elective cesarean section. Gynecol Obstet Invest. 1992;34(3):159–163. doi: 10.1159/000292751. [DOI] [PubMed] [Google Scholar]
- Pearlman WH. Glucocorticoids in milk: a review. Endocrinol Exp. 1983;17(3–4):165–174. [PubMed] [Google Scholar]
- Power ML, Verona CE, Ruiz-Miranda C, Oftedal OT. The composition of milk from free-living common marmosets (Callithrix jacchus) in Brazil. Am J Primatol. 2008;70(1):78–83. doi: 10.1002/ajp.20459. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Packer C. Dispersal and Philoparty. In: Smuts B, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: The University of Chicago Press; 1986. pp. 250–266. [Google Scholar]
- Rodriguez-Palmero M, Koletzko B, Kunz C, Jensen R. Nutritional and biochemical properties of human milk: II. Lipids, micronutrients, and bioactive factors. Clin Perinatol. 1999;26(2):335–359. [PubMed] [Google Scholar]
- Schwalm JW, Tucker HA. Glucocorticoids in mammary secretions and blood serum during reproduction and lactation and distributions of glucocorticoids, progesterone, and estrogens in fractions of milk. J Dairy Sci. 1978;61(5):550–560. doi: 10.3168/jds.s0022-0302(78)94409-0. [DOI] [PubMed] [Google Scholar]
- Spencer JD, Boyd RD, Cabrera R, Allee GL. Early weaning to reduce tissue mobilization in lactating sows and milk supplementation to enhance pig weaning weight during extreme heat stress. J Anim Sci. 2003;81(8):2041–2052. doi: 10.2527/2003.8182041x. [DOI] [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monogr Soc Res Child Dev. 1994;59(2–3):108–134. [PubMed] [Google Scholar]
- Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Found Symp. 1991;156:171–183. doi: 10.1002/9780470514047.ch11. discussion 183–178. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Ripp C. A history of motherless mother monkey mothering at the University of Wisconsin Primate Laboratory. In: Reite M, Caine N, editors. Child Abuse: The Nonhuman Primate Data. New York: Liss; 1983. pp. 49–78. [Google Scholar]
- Termeulen SB, Butler WR, Natzke RP. Rapidity of cortisol transfer between blood and milk following adrenocorticotropin injection. J Dairy Sci. 1981;64(11):2197–2200. doi: 10.3168/jds.S0022-0302(81)82829-9. [DOI] [PubMed] [Google Scholar]
- Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179(68):90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Tucker HA, Schwalm JW. Glucocorticoids in mammary tissue and milk. J Anim Sci. 1977;45(3):627–634. doi: 10.2527/jas1977.453627x. [DOI] [PubMed] [Google Scholar]
- Umeda T, Hiramatsu R, Iwaoka T, Shimada T, Miura F, Sato T. Use of saliva for monitoring unbound free cortisol levels in serum. Clin Chim Acta. 1981;110(2–3):245–253. doi: 10.1016/0009-8981(81)90353-3. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Seyfarth RM. Conflict and Cooperation. In: Smuts B, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: The University of Chicago Press; 1986. pp. 306–317. [Google Scholar]