Abstract
Since its discovery approximately 200 years ago, chitosan, as a cationic natural polymer, has been widely used as a topical dressing in wound management owing to its hemostatic, stimulation of healing, antimicrobial, nontoxic, biocompatible and biodegradable properties. This article covers the antimicrobial and wound-healing effects of chitosan, as well as its derivatives and complexes, and its use as a vehicle to deliver biopharmaceuticals, antimicrobials and growth factors into tissue. Studies covering applications of chitosan in wounds and burns can be classified into in vitro, animal and clinical studies. Chitosan preparations are classified into native chitosan, chitosan formulations, complexes and derivatives with other substances. Chitosan can be used to prevent or treat wound and burn infections not only because of its intrinsic antimicrobial properties, but also by virtue of its ability to deliver extrinsic antimicrobial agents to wounds and burns. It can also be used as a slow-release drug-delivery vehicle for growth factors to improve wound healing. The large number of publications in this area suggests that chitosan will continue to be an important agent in the management of wounds and burns.
Keywords: antimicrobial activity, chitin, chitosan, drug delivery, wound dressing, wound healing, wound infection
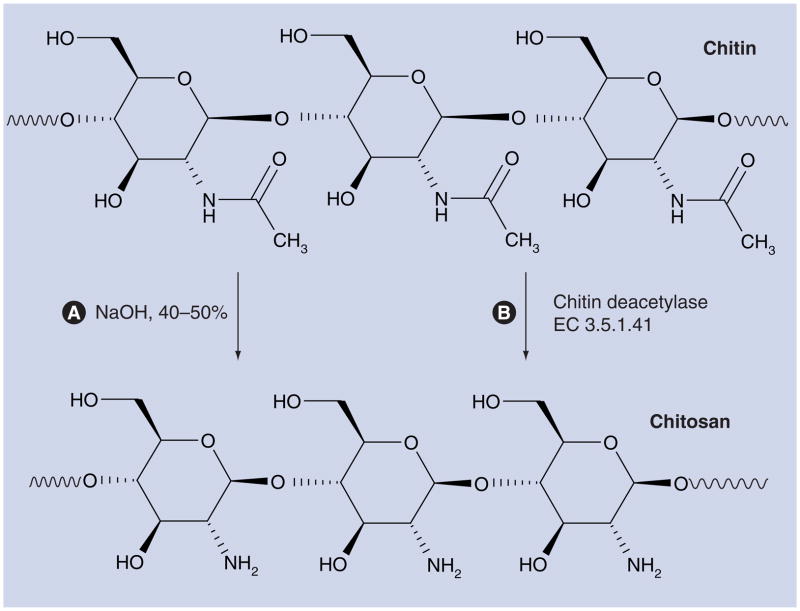
Chitosan is a β-1,4-linked polymer of glucosamine (2-amino-2-deoxy-β-D-glucose) and lesser amounts of N-acetylglucosamine. It is a derivative of chitin (poly-N-acetylglucosamine) (Figure 1), which is the second most abundant biopolymer after cellulose. Chitosan was first discovered in 1811 by Henri Braconnot [1], a French chemist and pharmacist. Bracannot observed that a certain substance (chitin) found in mushrooms did not dissolve in sulfuric acid. Later in the century, chitin was found in crustaceans (such as crabs, lobsters, shellfish and shrimp), the indigestible outer skeleton of insects and the material from which the cell walls of the mycelial fungi are made. It is also found in the radulas of mollusks, and the beaks of cephalopods (including squid and octopuses). Over the last 200 years, the study and application of chitosan has taken on many different forms. Researchers continue to build on the original finding of Bracannot, discovering new uses for chitin and chitosan as they find different forms of it in nature.
Figure 1. Preparation of chitosan from chitin.
(A) Preparation of chitosan from chitin by alkaline deacetylation. (B) Preparation of chitosan from chitin by enzymatic deacetylation.
Chitosan preparations of various molecular weights, degrees of deacetylation (DDA; defined as the molar fraction of glucosamine residues), and with further molecular derivatization patterns have attracted much attention because of their potentially beneficial biological properties. It can truly be said that the beneficial medical effects of chitosan can be applied from head to toe (Figure 2). Chitosan’s properties of binding with red blood cells allow it to rapidly clot blood, and it has recently gained regulatory approval in the USA for use in bandages and other hemostatic agents [2,3]. In addition, chitosan modulates the functions of inflammatory cells and subsequently promotes granulation and organization [4]. As a semipermeable biological dressing, it maintains a sterile wound exudate beneath a dry scab, preventing dehydration and contamination of the wound, to optimize conditions for healing. Furthermore, chitosan is a polymer with a number of basic amino groups and hence possesses an overall cationic charge, especially at acidic pH. This is due to the presence of primary amines on the molecule that bind protons according to the equation:
Figure 2.
Schematic depiction of the applications of chitosan in wound-healing and burn therapy, showing it can be usefully applied ‘from head to toe’.
In common with many cationic polymers, chitosan has pronounced antimicrobial effects due to destabilization of the outer membrane of Gram-negative bacteria [5,6] and permeabilization of the microbial plasma membrane [5,7]. In this article, we review the antimicrobial and wound-healing effects of chitosan as well as its derivatives and complexes. The use of chitosan preparations as drug-delivery vehicles and characterization of physical and biological properties of chitosan formulations are also briefly discussed. Topics have been categorized into three levels including in vitro, animal and clinical studies.
Antimicrobial effects of chitosan preparations
Wound infection is a manifestation of disturbed host–bacteria equilibrium in a traumatized tissue environment in favor of the bacteria. A wound infection not only has the possibility to elicit a systemic response (sepsis), but is highly likely to inhibit the multiple processes involved in the orchestrated progression of normal wound healing. Each process involved in healing is affected when bacteria proliferate in a wound [8]. Chitosan, as a cationic natural polymer, has been widely investigated as an antimicrobial agent for preventing and treating infections owing to its intrinsic antimicrobial properties, and also its ability to effectively deliver extrinsic antimicrobial compounds into the infected area.
Many factors present in the chitosan molecule or its environment can influence the antimicrobial properties, such as the molecular weight, DDA and the ionic strength and pH of the dissolving medium. Also, the physical state of the chitosan can present very different antimicrobial properties, such as whether the chitosan is present in the form of films, hydrogels, coatings, in solutions or in combinations with other materials.
The exact mechanisms of the antimicrobial actions of chitosan are still uncertain, but many new developments have been made in exploring this aspect. It has been proposed that interaction between positively charged chitosan molecules and negatively charged microbial cell membranes leads to the disruption of microbial membrane, and subsequently the leakage of proteinaceous and other intracellular constituents [5,6,9–11]. At a lower concentration (<0.2 mg/ml), the polycationic chitosan binds to the negatively charged bacterial surface to cause agglutination, while at higher concentrations, the larger number of positive charges have imparted a net positive charge to the bacterial surfaces to keep them in suspension [5].
It is also proposed that chitosan interacts with the membrane of the cell to alter cell permeability [5,7,11]. Studies using fluorescent probes, 1-N-phenylnaphthylamine, nile red and propidium iodide, and field emission scanning electron microscopy suggested that chitosan-arginine’s antibacterial activity is, at least in part due to its interaction with the cell membrane, in which it increases membrane permeability [7].
In vitro studies
Andres et al. investigated the interaction between chitin or chitosan powder and various kinds of pathogenic microorganisms [10]. First of all, physicochemical characterizations of chitin and chitosan powder were performed. The deacetylation yields were 35, 60 and 80% ± 10%. The experimental studies focused on the measurements of the mortality constant rate for various bacterial strains – Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis and Staphylococcus saprophyticus. An explanation of the antibacterial mechanisms was proposed involving the cell wall disruption due to free amino groups present in chitosan.
In another study, No et al. compared the antibacterial activities of chitosans and chitosan oligomers against both Gram-negative and Gram-positive bacteria [12]. Chitosans showed higher antibacterial activities than chitosan oligomers and markedly inhibited growth of most bacteria tested, although inhibitory effects differed with molecular weights of chitosan and the particular bacterium. Chitosan generally showed stronger bactericidal effects with Gram-positive bacteria than with Gram-negative bacteria in the presence of 0.1% chitosan. As a chitosan solvent, 1% acetic acid was effective in inhibiting the growth of most of the bacteria tested, except for lactic acid bacteria that were more effectively suppressed with 1% lactic or formic acids. Antibacterial activity of chitosan was inversely affected by pH, with higher activity at lower pH value.
Raafat et al. investigated the antimicrobial mode of action of chitosan using a combination of approaches [11]. It was found that chitosan exhibited a dose-dependent growth-inhibitory effect. A simultaneous permeabilization of the cell membrane to small cellular components, coupled to a significant membrane depolarization, was detected. A concomitant interference with cell wall biosynthesis was not observed. Chitosan treatment of 22 Staphylococcus simulans cells did not give rise to cell wall lysis; the cell membrane also remained intact. Analysis of transcriptional response data revealed that chitosan treatment leads to multiple changes in the expression profiles of Staphylococcus aureus SG511 genes involved in the regulation of stress and autolysis, as well as genes associated with energy metabolism. Finally, the investigators speculated that binding of chitosan to teichoic acids, coupled with a potential extraction of membrane lipids (predominantly lipoteichoic acid) results in a sequence of events ultimately leading to bacterial death.
Muzzarelli et al. tested the antimicrobial efficacy of N-carboxybutyl chitosan, which was prepared from crustacean chitosan (DDA = 73%), against 298 strains of Gram-positive and Gram-negative pathogens and Candida spp. [13]. It was found that N-carboxybutyl chitosan was particularly active against Candida and Gram-positive bacteria. When a thin pad obtained by pressing freeze-dried N-carboxybutyl chitosan between steel plates was used, growth of all strains was inhibited. All Candida and most staphylococci were killed, while no bactericidal activity was observed with streptococci and enterococci. Electron microscopy studies indicated that, in Staphylococci, the presence of N-carboxybutyl chitosan caused fraying and weakening of the outer part of the cell wall, which locally appeared thicker than in controls; duplication was also depressed. In Gram-negative organisms an abnormally expanded periplasmic space was observed in cells close to the N-carboxybutyl chitosan pad. The intracellular material in Gram-negative organisms appeared more tightly packed than it did in controls. Fragments of cell wall and bacterial ‘shadows’ lacking any intracellular organization were also detected. Candida albicans strains close to N-carboxybutyl chitosan showed cell damage to various extents. In general, their cell walls were still identifiable, but intracellular structures had either disappeared or changed their normal characteristics or distributions.
Seyfarth et al. studied the antifungal activities of water-soluble low- and high-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against the fungal species of C. albicans, Candida krusei and Candida glabrata [14]. In the study, the investigators used a microplate nephelometer to measure the fungal growth. The investigators observed a concentration-dependent antifungal activity of low- and high-molecular-weight chitosan hydrochloride against the fungal species in acid medium. In addition, the investigators found an influence of molecular weight on the antifungal activity: a low-molecular weight is associated with low antifungal activity. Another interesting detail was the low activity of carboxymethyl chitosan against the fungal species. The authors concluded that the polycationic character of chitosan is crucial for antifungal activity, because this functional group masks the cationic amino groups.
Kulkarni et al. reported the antibacterial activity of chitosan after conversion into thiazolidinone derivatives (TDCs) [15]. TDCs were prepared by converting chitosan into chitosan’s Schiff’s bases, followed by treatment with mercaptoacetic acid. Polymer samples (both original chitosan and chemically modified chitosan TDCs) of a concentration of 100 ppm were tested for antimicrobial activity against E. coli, Shigella dysentrae, P. aeruginosa and Bacillus subtilis using a disc diffusion method by measuring the zone of inhibition. It was observed that the antibacterial activity of chitosan is increased approximately tenfold in the corresponding TDC. The increased antibacterial activity of chemically modified chitosan was proposed to be due to the newly introduced groups and the increased interaction and polyelectrolyte complexes between the polymer and the bacterial cell wall. The diffusive permeability of a polymer was also an important parameter for antibacterial activity.
Tang et al. investigated the antibacterial activity of another chitosan derivative, arginine-functionalized chitosan, on the Gram-negative bacteria Pseudomonas fluorescens and E. coli [7]. The investigators observed that two different arginine-functionalized chitosans (6% arginine-substituted and 30% arginine-substituted) both strongly inhibited P. fluorescens and E. coli growth. At the concentration of 5000 mg/l, 6%- and 30%-substituted chitosan-arginine killed 2.7 logs and 4.5 logs of P. fluorescens, and 4.8 logs and 4.6 logs of E. coli in 4 h, respectively. At low concentrations (<500 mg/l), the 6%-substituted chitosan-arginine was more effective in inhibiting cell growth, even though the 30%-substituted chitosan-arginine appeared to be more effective in permeabilizing the cell membranes of both P. fluorescens and E. coli.
For the purpose of controlling the infections associated with medical implants, Li et al. reported chitosan hydrogel based on the modifications of chitosan by adding a hydrophobic alkyl side chain and cationic charge through quaternization of the amino group, hydrophilic poly(ethylene glycol) (PEG) with six ethylene glycol repeats (PEG6) and methacrylate functionality [6]. The investigators demonstrated that the chitosan hydrogel had the microbe membrane suction ability and, subsequently, excellent antimicrobial/antifungal activities against P. aeruginosa, E. coli, S. aureus and Fusarium solani.
Similar in vitro studies on the antimicrobial effects of chitosan as well as its derivatives and complex were also carried out by Tsai et al. [16], Altiok et al. [17], Rossi et al. [18] and Ong et al. [19]. Table 1 is a summary of the literature on in vitro studies.
Table 1.
Antimicrobial effects of chitosan preparations: a summary of in vitro studies.
| Chitosan preparations | Microorganisms | Major results/conclusions | Ref. |
|---|---|---|---|
| Chitin and chitosan powder | Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis and Staphylococcus saprophyticus | Effective antimicrobial effect | [10] |
| Chitosan solution | Staphylococcus aureus | Chitosan led to multiple changes in the bacterial gene expression; binding of chitosan to teichoic acid led to death of bacteria | [11] |
| Chitosan and chitosan oligomer | Gram-positive and Gram-negative bacteria | Chitosan generally showed stronger bactericidal effects with Gram-positive bacteria than Gram-negative bacteria | [12] |
| N-carboxybutyl chitosan | 298 strains Gram-positive bacteria, Gram-negative bacteria and Candida spp. | Particularly active against Candida and Gram-positive bacteria; no bactericidal activity was observed with streptococci and enterococci | [13] |
| Solutions of chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide, and N-acetyl-D-glucosamine | Candida albicans, Candida krusei and Candida glabrata | Antifungal activity decreases with declining molecular mass and increasing masking of the protonated amino groups with functional groups | [14] |
| Thiazolidinone derivatives of chitosan | E. coli, Shigella dysenteriae, P. aeruginosa and Bacillus subtilis | Better antimicrobial activity than chitosan’s Schiff’s bases | [15] |
| Chitosan hydrolysate | Bacillus cereus, E. coli, S. aureus, P. aeruginosa, Salmonella enterica serovar Typhi and Saccharomyces cerevisiae. | Strong activity at 100 ppm against many pathogens and yeast species | [16] |
| Chitosan film loaded with thyme oil | E. coli, Klebsiella pneumoniae, P. aeruginosa and S. aureus | Chitosan films with 1.2% (v/v) showed antimicrobial activity on all microorganisms tested | [17] |
| Chitosan hydrochloride, 5-methyl-pyrrolidinone chitosan | S. aureus, Staphylococcus epidermidis, P. aeruginosa, C. albicans and Aspergillus niger | Antimicrobial activity against bacteria and C. albicans is shown by the dressing | [18] |
| Chitosan incorporated with polyphosphate and silver | S. aureus and P. aeruginosa | Complete kill of P. aeruginosa and a >99.99% kill of S. aureus | [19] |
| Arginine-functionalized chitosan | Pseudomonas fluorescens and E. coli | At the concentration of 5000 mg/l, 6%- and 30%-substituted chitosan-arginine killed 2.7 logs and 4.5 logs of P. fluorescens, and 4.8 logs and 4.6 logs of E. coli in 4 h, respectively | [7] |
| Chitosan hydrogel | P. aeruginosa, E. coli, S. aureus and Fusarium solani | Excellent antimicrobial/antifungal activities | [6] |
Animal studies
Treatment of open-skin wound infections
Burkatovskaya et al. compared the antimicrobial ability of HemCom™ bandage, a chitosan acetate bandage, with alginate sponge bandage and silver sulfadiazine cream in mouse models of infected open wounds [20]. P. aeruginosa, Proteus mirabilis and S. aureus, which had all been stably transformed with the entire bacterial lux operon, were used to allow in vivo bioluminescence imaging of infection. An excisional wound in BALB/c mice was inoculated with 50–250 million bacterial cells followed after 30 min by application of HemCon™ bandage, alginate sponge bandage, silver sulfadiazine cream or no treatment. Animal survival was followed over 15 days with observations of bioluminescence emission and animal activity daily. Chitosan acetate-treated mice infected with P. aeruginosa and P. mirabilis all survived while those receiving no treatment, alginate and silver sulfadiazine demonstrated 25–100% mortality. Chitosan acetate was much more effective than other treatments in rapidly reducing bacterial luminescence, which was correlated to the bacterial colony forming units in the wounds. S. aureus formed only nonlethal localized infections after temporary immunosuppression of the mice, but HemCon™ was again more effective in reducing bacterial luminescence. The data suggest that chitosan acetate rapidly kills bacteria in the wound before systemic invasion can take place, and is superior to alginate bandage and silver sulfadiazine that may both encourage bacterial growth in the short term.
Ong et al. refined the chitosan dressing by incorporating polyphosphate and silver for improved hemostatic and antimicrobial effects [19]. Both in vitro and animal studies were carried out. It was found that the optimal chitosan-polyphosphate formulation (ChiPP) accelerated blood clotting, increased platelet adhesion, generated thrombin faster and absorbed more blood than chitosan. Silver-loaded ChiPP exhibited significantly greater bactericidal activity than ChiPP in vitro, consistently achieving a complete kill of P. aeruginosa and a >99.99% kill of S. aureus. In vivo animal test demonstrated that the silver dressing also significantly reduced mortality from 90% to 14.3% in a P. aeruginosa wound infection model in mice. Although the dressing exerted severe cytotoxicity against cultured fibroblasts, wound healing was not inhibited.
Treatment of burn infections
Using the same HemCon™ bandage reported by Burkatovskaya et al. [20], Dai et al. [21] investigated its efficacy for treating burn infections in mice. They applied the dressing to third degree burns in mice that had been infected with bioluminescent P. aeruginosa and P. mirabilis.
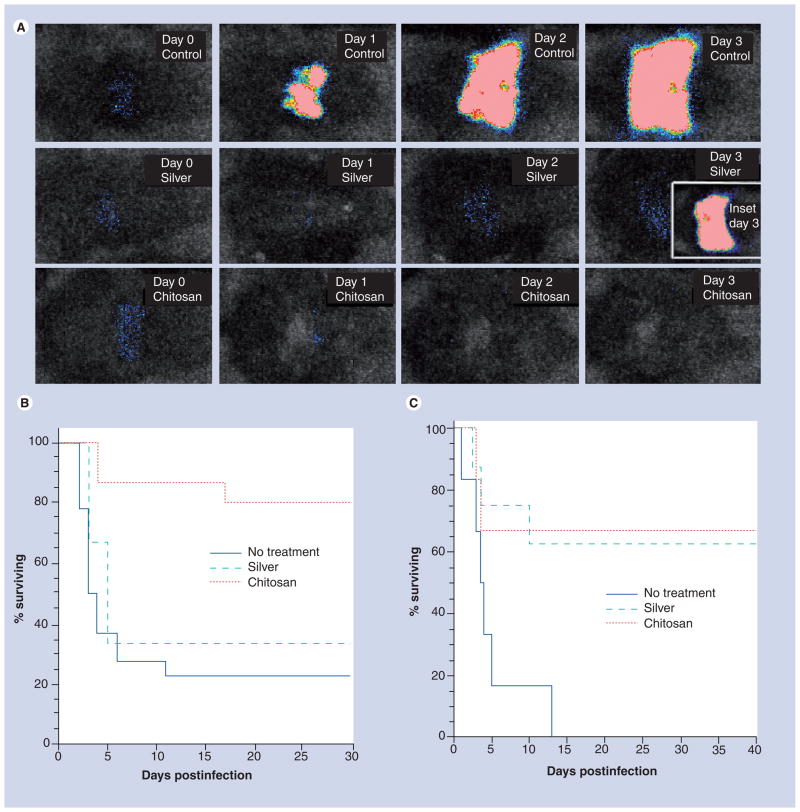
Figure 3a shows the successive bacterial luminescence images from day 0 to day 3 after P. aeruginosa infection of an untreated burn, a silver dressing-treated burn, and a chitosan acetate-treated burn, respectively. In the untreated burn the bacteria multiplied approximately 1000-fold from day 0 to day 3, while in both the silver dressing and chitosan acetate bandage-treated burns, a decrease in bacterial luminescence signal was seen at day 1 as the dressing immediately quenched the light. There was a detectable increase in signal from the silver-dressing burn at day 2 and 3 (compared with day 1) that was not seen in the chitosan-acetate burn. The inset showing a silver-treated burn at day 3 was taken after the silver dressing was removed from a dead mouse and shows a vigorous bacterial proliferation underneath the dressing.
Figure 3. Treatment of mouse burn infections using a chitosan acetate bandage.
(A) Representative successive bioluminescence images from days 0–3 of mice with Pseudomonas aeruginosa-infected burns with no treatment, treated with silver dressing and treated with chitosan acetate bandage. The inset at day 3 in silver dressing burn shows the bioluminescence signal after the dressing was removed from a dead mouse. (B) Survival curves of mice with P. aeruginosa-infected burns treated with chitosan acetate bandage (n = 15), silver dressing (n = 11) or no treatment (n = 15). (C) Survival curves of mice with Proteus mirabilis-infected burn treated with chitosan acetate bandage (n = 12), silver dressing (n = 8) or no treatment (n = 13).
Reprinted from [21] with permission.
In the case of P. aeruginosa infections (Figure 3b), the survival rate of mice treated with the chitosan acetate bandage was 73.3%, whereas the survival rate of mice treated with a nanocrystalline silver dressing was 27.3% (p = 0.0055), and that of untreated mice was 13.3% (p < 0.0002). For P. mirabilis infections (Figure 3C), the comparable survival rates were 66.7, 62.5 and 23.1%, respectively. Quantitative bacterial luminescence signals demonstrated that the chitosan acetate bandage effectively controlled the growth of bacteria in the burns and prevented the development of systemic sepsis, as shown by blood culture.
Treatment of surgical-site infections
Surgical-site infection is one of the complications in the use of mesh in hernia repair. Triclosan-embedded commercial absorbable suture materials are used to reduce the infection rate. To achieve a better therapeutic outcome, Cakmak et al. tested the use of meshes coated with triclosan-loaded chitosan gel to prevent and treat mesh infections in a rat model. Simultaneous and 24-h S. aureus inoculation was used to model mesh infection, and the rats were observed for 8 days by means of surgical-site infections [22]. It was reported that grafts coated with triclosan-loaded chitosan gel presented satisfactory preventive effects against graft infection.
Treatment of osteomyelitis
A chitosan bar loaded with gentamicin was investigated by Aimin et al. for the potential treatment of osteomyelitis [23]. The chitosan bar was prepared using combined crosslinking, solvent evaporation and a cylinder model cutting technique. Sustained diffusion of gentamicin to the surrounding medium was observed in vitro. The gentamicin released from the bar showed significant antibacterial activity. The bar implanted in the proximal portion of the rabbit tibia produced a low blood concentration of gentamicin, but a much higher concentration was produced in local bone and in the hematoma. In all bone tissue around the bar, the gentamicin concentration exceeded the MIC for the common causative organisms of osteomyelitis for approximately 8 weeks. No systemic side effects caused by the implant were observed. The investigators suggested that, based on the test results together with the chitosan characteristics of biodegradable, antibiotic and immunologic activity, the chitosan bar loaded with gentamicin seems to be a clinically useful method for the treatment of bone infection. This system has an advantage over other systems in that it avoids a second operation for removal of the carrier.
Treatment of oral mucositis
A thermally sensitive mucoadhesive gel based on chitosan derivatives was developed by Rossi et al. for the treatment of oral mucositis [24]. Trimethyl chitosan or methylpyrrolidinone chitosan was mixed with glycerophosphate (GP) according to different polymer/GP molar ratios and characterized for gelation properties by means of rheological analysis in comparison with chitosan. Assessed using porcine buccal mucosa, the best mucoadhesive properties were shown by trimethyl chitosan with high molecular weight and low substitution degree mixed with GP. Such mixture was loaded with benzydamine hydrochloride, an anti-inflammatory drug with antimicrobial properties, and subjected to in vitro drug release and wash away test. The formulation, based on trimethyl chitosan/GP mixture, was able to prolong drug release and to withstand the physiological mechanisms of removal. The antimicrobial properties of both vehicle and formulation were investigated. Also, in the absence of drug, trimethyl chitosan/GP mixture was characterized by antimicrobial properties.
Treatment of hemorrhagic cystitis
Hemorrhagic cystitis is a common problem following cyclophos-phamide (CY) or radiation therapy. Okamura et al. evaluated the safety and efficacy of intravesical chitosan in an animal model of CY cystitis [25]. Hemorrhagic cystitis was induced in female rats by intraperitoneal CY. Sequential examination revealed that chitosan inhibited the occurrence of hemorrhagic cystitis when it was used within 1 h after CY administration. Treatment delayed until after the appearance of the cystitis, especially repeated treatments, appeared to make the CY-induced changes worse.
Table 2 summarizes the animal studies on the antimicrobial effects of chitosan preparations discussed in this section.
Table 2.
Antimicrobial effects of chitosan preparations: a summary of animal studies.
| Chitosan preparations | Models of infection | Animal species | Microorganisms | Major results/conclusions | Ref. |
|---|---|---|---|---|---|
| Chitosan acetate bandage | Open skin wound infection | Mouse | Pseudomonas aeruginosa, Proteus mirabilis and Staphylococcus aureus | Chitosan acetate rapidly kills bacteria in the wounds | [20] |
| Chitosan incorporated with polyphosphate and silver | Open skin wound infection | Mouse | P. aeruginosa and S. aureus | Significantly reduced mortality of mice from 90 to 14.3% | [19] |
| Chitosan acetate bandage | Burn infection | Mouse | P. aeruginosa and P. mirabilis | Significantly reduced mortality of mice | [21] |
| Chitosan gel loaded with triclosan | Surgical infection | Rat | S. aureus | Satisfactory preventive effect against infection presented | [22] |
| Chitosan bar loaded with gentamicin | Osteomyelitis | Rabbit | Not reported | Significant antibacterial activity showed | [23] |
| Trimethyl chitosan/glycerophosphate mixture | Oral mucositis | Porcine | Not reported | Mixture was characterized by antimicrobial properties | [24] |
| Intravesical chitosan | Hemorrhagic cystitis | Rat | Not reported | Used within 1 h of cyclophosphamide administration, chitosan seemed to have the possibility to inhibit the appearance of hemorrhagic cystitis | [25] |
Clinical studies
Akncbay et al. reported the clinical effectiveness of chitosan, both as a carrier in gel form and as an active agent in the treatment of chronic periodontitis (CP) [26]. A total of 15 patients with moderate-to-severe CP were selected for this study. The chitosan gel (1% w/w) incorporated with or without 15% metronidazole was prepared and applied adjunctive to scaling and root planing in comparison to scaling and root planing alone (control group) in CP patients. In all groups, significant improvements were observed in clinical parameters between baseline and week 24. No complications related to the chitosan were observed in patients throughout the study period. The authors suggested that chitosan itself is effective as well as its combination with metronidazole in CP treatment owing to its antimicrobial properties.
In a similar study, Boynuegri et al. evaluated the effects of chitosan on periodontal regeneration. A total of 20 patients with CP were recruited for the study [27]. The chitosan gel (1% w/v) was applied alone or in combination with demineralized bone matrix or collagenous membrane. Radiographic data revealed that, in comparison with the nontreated control group, all treated groups showed statistically significant bone fills when compared with baseline, indicating that chitosan gel alone or its combination with demineralized bone matrix/collagenous membrane is promising for periodontal regeneration.
Wound-healing effects of chitosan preparations
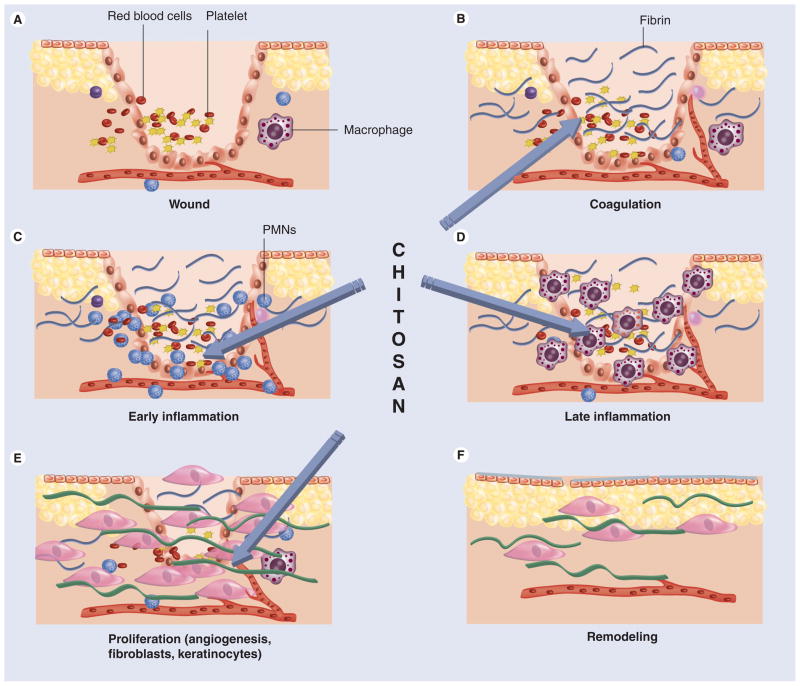
Wound healing is a specific biological process related to the general phenomenon of growth and tissue regeneration. Wound healing progresses through a series of interdependent and overlapping stages in which a variety of cellular and matrix components act together to re-establish the integrity of damaged tissue and replacement of lost tissue [28]. The wound-healing process has been described as comprising five overlapping stages, which involve complex biochemical and cellular processes. These are described as hemostasis, inflammation, migration, proliferation and maturation phases (Figure 4).
Figure 4. The phases of cutaneous wound healing.
(A) Immediately following cutaneous injury, blood elements and amines extravasate from locally damaged blood vessels within the dermis. Vascular permeability is temporarily increased to allow neutrophils (PMNs), platelets and plasma proteins to infiltrate the wound. Vasoconstriction follows, in response to factors released by these cells. (B) Coagulation then occurs as platelets aggregate with fibrin, which is deposited in the wound following its conversion from fibrinogen. (C) Platelets release several factors, including PDGF and TGF-β, which attract PMNs to the wound, signaling the beginning of inflammation. (D) After 48 h, macrophages replace PMNs as the principal inflammatory cell. Together, PMNs and macrophages remove debris from the wound, release growth factors and begin to reorganize the extracellular matrix. (E) The proliferation phase begins at about 72 h as fibroblasts, recruited to the wound by growth factors released by inflammatory cells, begin to synthesize collagen, and angiogenesis and re-epitheliazation occurs. (F) Collagen crosslinking and reorganization occur for months after injury in the remodeling phase of repair. Chitosan has been reported to beneficially influence stages (B–E).
PMN: Polymorphonuclear leukocyte.
Reproduced with permission from [108].
Many studies have been reported on the use of chitosan as a wound-healing accelerator, and in fact there is good evidence that chitosan can beneficially influence every separate stage of wound healing. Chitosan and its derivatives could accelerate wound healing by enhancing the functions of inflammatory cells, such as polymorphonuclear leukocytes (PMN) [4,29–31], macrophages [4,32,33], and fibroblasts [4,34–36] or osteolasts [37]. It has also been reported that chitosan could increase the tensile strength of wounds [38]. The wound-healing effects of chitosan could be affected by the factors of molecular weight [33,39,40], deacetylation degree [35,39,40], as well as the state of chitosan [41].
In vitro studies
Effects on human skin fibroblasts & keratinocytes
In a study presented by Wiegand et al., the cytotoxic effects of two chitosans with a similar DDA but different molecular weight, 120 kDa and 5 kDa, on the human keratinocyte cell line HaCaT were analyzed [34]. The results indicated that chitosans exhibited a molecular-weight-dependent negative effect on HaCaT cell viability and proliferation in vitro. The chitosans tested also stimulated the release of inflammatory cytokines by HaCaT cells depending on incubation time and concentration. Chitosan-120 kDa and chitosan-5 kDa induced apoptotic cell death, which was mediated by activation of the effector caspases 3/7. At least for chitosan-120 kDa, the involvement of both extrinsic and intrinsic signal pathways was shown by activation of caspases 8 and 9.
In another study, Howling et al. examined the effect of chitin and chitosan with different DDA but similar molecular weight on the proliferation of human skin fibroblasts and keratinocytes in vitro [35]. It was reported that chitosans with relatively high DDA (89%) strongly stimulated fibroblast proliferation, while samples with lower DDA showed less activity. The stimulatory effect on fibroblast proliferation required the presence of serum in the culture medium, suggesting that the chitosan may be interacting with growth factors present in the serum and potentiating their effect. In contrast to the stimulatory effects on fibroblasts, chitosans inhibited human keratinocyte mitogenesis. These data demonstrated that high DDA chitosans can modulate human skin cell mitogenesis in vitro.
Chemical and physical modifications of chitosan influence its biocompatibility and biodegradability, but it is unknown as to what degree. Therefore, a study on the determination of the biocompatibility of the chitosan porous skin regenerating templates (PSRTs) using an in vitro toxicology model at the cellular and molecular level on primary normal human epidermal keratinocytes was reported by Lim et al. Chitosan was dissolved in 1% (v/v) acetic acid (PSRT 82 and 108) or 1% (v/v) lactic acid (PSRT 87) to prepare 2% (w/v) chitosan solution [42]. This was followed by an addition of 4 g glycerol as the plasticizer in all PSRTs. All PSRTs were found to be cytocompatible, but only PSRT 108 was capable of stimulating cell proliferation. While all of the PSRTs showed some DNA damage, PSRT 108 showed the least DNA damage, followed by PSRT 87 and 82. PSRT 87 and 82 induced a higher secretion of TNF-α and IL-8 in the keratinocytes cultures than PSRT 108. Based on the experiments, the authors concluded that PSRT 108 is the most biocompatible wound dressing of the three tested.
Effects on osteoblasts
An in vitro study was carried out by Klokkevold et al. to evaluate the effect of chitosan on osteoblast differentiation and bone formation [37]. Mesenchymal stem cells were harvested from fetal Swiss Webster mice calvarias before osteoblast differentiation and calcification. Experimental wells were pretreated with chitosan and were allowed to grow under optimal conditions for 14 days. Histologic cross-sections of representative positively Von Kossa-stained colonies identified osteoblasts and confirmed bone formation. Examination of experimental wells revealed a significantly greater average of colonies per well than the control wells. Computer-assisted image analysis of the average area of bone formed by control colonies was 0.34 ± 0.09 (relative units), while that of experimental colonies was 0.39 ± 0.06 (relative units) per average bone-forming colony. The results of this in vitro experiment suggest that chitosan potentiates the differentiation of osteoprogenitor cells and may facilitate the formation of bone.
Effects on human anterior cruciate ligament cells
Recently, a study was carried out by Shao et al. to evaluate the phenotypic responses of human anterior cruciate ligament (ACL) cells on chitosan and another biodegradable materials, poly(epsilon-caprolactone) (PCL) [43]. It was presented that, compared with PCL, chitosan-stimulated ACL cells to secrete more fibronectin, TGF-β1 and collagen III, but relatively low amounts of fibronectin was adsorbed into the chitosan surface to lead to poor ACL cell adhesion. After coating fibronectin on the surface of chitosan, cell morphology and the mRNA levels of all tested genes had similar levels on PCL- and fibronectin-coated chitosan. Since an ideal scaffold used in ACL tissue engineering is not only for cell attachment but also for extracellular matrix deposition during ligament regeneration, chitosan may be considered as a scaffold for ACL tissue engineering, which can upregulate the expression of specific genes of matrix production and wound healing in human ACL cells to synthesize a greater quantity of fibronectin and TGF-β1 proteins.
Effects on human polymorphonuclear neutrophils
The recruitment and activation of PMNs reflects a primary reaction to foreign bodies. Santos et al. investigated the effect of chitosan-based membranes over the activation of human PMNs [29]. Isolated human PMNs were cultured in the presence of chitosan or chitosan/soy newly developed membranes. The effect of the chitosan on the activation of PMNs was assessed by the quantification of lysozyme and reactive oxygen species (ROS). The results showed that PMNs, in the presence of the chitosan, secrete similar lysozyme amounts, as compared with controls (PMNs without materials), and also showed that the materials do not stimulate the production of ROS. Moreover, PMNs incubated with the chitosan, when stimulated with phorbol 12-myristate 13-acetate (PMA) or formyl-methionyl-leucyl-phenylalanine, showed a lower ROS production to that observed for positive controls (cells without materials and stimulated with PMA), which reflects the maintenance of their stimulation capacity. These data suggest that chitosan-based membranes do not elicit activation of PMNs. These findings reinforce previous statements supporting the suitability of chitosan-based materials for wound-healing applications.
Another study was carried out by Ueno et al. to investigate the production of osteopontin from human PMN treated with chitosan [30]. Osteopontin is a glycosylated phosphoprotein and promotes the attachment or spread of a variety of cell types. In addition, osteopontin may play a role in granulomatous inflammation. The in vitro results showed that PMN stimulated with granulocyte-colony stimulating factor (G-CSF) and chitosan accumulated osteopontin mRNA, and released osteopontin into their culture supernatants. These findings suggest that osteopontin is synthesized by migrating PMN, which plays the novel role of regulating the evolution of wound healing with chitosan treatment at the early phase of healing.
Effects on human macrophages
An investigation presented by Peluso et al. showed that chitosan had an in vitro stimulatory effect on both macrophage nitric oxide (NO) production and chemotaxis [32]. The macrophage NO secretion was attributed to the N-acetylglucosamine unit of the chitosan molecule rather than to the glucosamine residue. Moreover, the immunestimulatory effect of chitosan was very specific, since other glycosaminoglycans, such as N-acetyl-D-mannosamine and N-acetyl-D-galactosamine, had no effects on NO production. In vivo experiments strengthened this hypothesis. Transmission electron microscopy analysis identified the presence of many leukocytes in the specimens after 14-day postimplantation, showing poor healing processes (i.e., fibroblast proliferation and collagen deposition) that characterize the tissue repair at this time in the animal model. Table 3 summarizes the in vitro studies on the wound healing effects of chitosan preparations.
Table 3.
Wound-healing effects of chitosan preparations: a summary of in vitro studies.
| Chitosan preparations | Mammalian cells | Major results/conclusions | Ref. |
|---|---|---|---|
| Chitosan | Keratinocytes | Molecular-weight-dependent induction of apoptotic cell death; release of inflammatory cytokines stimulated | [34] |
| Chitosan | Fibroblasts and keratinocytes | Chitosan-stimulated fibroblast proliferation and inhibited keratinocyte mitogenesis | [35] |
| Chitosan porous skin regeneration templates | Keratinocytes | Cyto-compatible; stimulated cell proliferation; DNA damage induced; secretion of TNF-α and IL-8 observed | [42] |
| Chitosan | Osteoblast | Significantly increased osteoblast differentiation | [37] |
| Chitosan | ACL | Chitosan stimulated ACL cells to secrete more fibronectin, TGF-β1 and collagen III. After coating fibronectin on chitosan surface, ACL cell adhesion was improved | [43] |
| Chitosan-based membranes | PMNs | Chitosan did not elicit activation of PMNs | [29] |
| Chitosan | Polymorphonuclear neutrophils | Chitosan accelerated the production of osteopontin from polymorphonuclear leukocytes | [30] |
| Chitosan | Macrophage | Chitosan had a stimulatory effect on both macrophage NO production and chemotaxis | [32] |
ACL: Anterior cruciate ligament cell; NO: Nitric oxide; PMN: Polymorphonuclear neutrophil.
Animal studies
Effects on healing of open skin wounds
Ueno et al. evaluated the effects of cotton fiber-type chitosan (DDA = 18%) on the acceleration of granulation in experimental open skin wounds on beagles for the early phase of wound healing [31]. It was reported that, on day 3 post-wounding, the chitosan-treated wounds showed histologically severe infiltration of PMN cells and an increase in effusion compared with that in the control. Granulation was more pronounced by the chitosan treatment on day 9 and 15 post-wounding. Immunohistochemical analysis showed an increase of the production of type III collagen in the chitosan group. The appearance of mitotic cells occurred numerously in the control on day 3 post-wounding, and in the chitosan group on post-wounding day 6. These results suggested chitosan can accelerate the infiltration of PMN cells at the early stage of wound healing, as well as the production of collagen by fibroblasts.
A similar study using chitin and chitosan powder on dog wounds was performed by Okamoto et al. [44]. Square, full-thickness wounds of skin (2 × 2 cm2) were created on the both sides of the dorsal midline of each dog and treated every 2 days with chitin powder, chitosan powder or not treated. Macroscopic and histological observations indicated that, at 28 days post-wounding, re-epithelialization tended to be greater in chitin and chitosan groups than in the nontreated control group. The number of inflammatory cells was statistically greater in the control group than in the chitin and chitosan groups. Many rete ridges were observed in the nontreated control group, but very few in the treated groups.
Mi et al. prepared an asymmetric chitosan membrane by immersion-precipitation phase-inversion method and evaluated it as wound covering [45]. The chitosan wound dressing consisted of skin surface on top-layer supported by a macro-porous sponge-like sublayer. The asymmetric chitosan membrane demonstrated controlled evaporative water loss, excellent oxygen permeability and promoted fluid drainage ability, but could inhibit the invasion of exogenous microorganisms owing to the dense skin layer and inherent antimicrobial property of chitosan. Open skin wounds in rats covered with the asymmetric chitosan membrane were hemostatic and healed quickly. Histological examination confirmed that epithelialization rate was increased and the deposition of collagen in the dermis was well organized by covering the wounds with this asymmetric chitosan membrane.
The effect of a chitosan acetate bandage (HemCom bandage) on healing of excisional wounds in mice infected or not infected with S. aureus was investigated by Burkatovskaya et al. [46]. In order to study the conflicting clamping and stimulating effects of chitosan acetate bandage on normal wounds, the bandages were removed from wounds at times after application ranging from 1 h to 9 days. Application for 3 days gave the earliest wound closure, and all application times gave a faster healing slope after removal compared with control wounds. In addition, chitosan acetate bandage reduced the number of inflammatory cells in the wound at days 2 and 4, and had an overall beneficial effect on wound healing, especially during the early period where its antimicrobial effect is most important.
A composite nano-titanium oxide-chitosan with collagen artificial skin (NTCAS) was developed by Peng et al. [47], and its bactericidal efficacy was tested in excisional wound in Sprague-Dawley rats. NTCAS demonstrated uniquely potent bactericidal properties with relatively large values of pseudo first-order kinetic coefficient. In the animal model, NTCAS showed a steady level of TNF-α, while the IL-6 level reached a peak on day 7, although this was significantly lower than in the control and DuoDERM® groups. In addition, NTCAS showed better and faster recovery than the other groups, which can be attributed to the unique bactericidal effect of nanotitanium oxide and the immune-enhancing effect of chitosan.
Effect on healing of incisional skin wounds
Chitosan-alginate polyelectrolyte complex (PEC) membranes, cast from aqueous suspensions of chitosan-alginate coacervates with CaCl2, were developed by Wang et al. and evaluated as potential wound-dressing materials [48]. Compared with conventional gauze dressing, the PEC membranes caused an accelerated healing of incision wounds in a rat model. Histological observations showed mature epidermal architecture with a keratinized surface of normal thickness and a subsided inflammation in the dermis. This was followed by an excellent remodeling phase with organized thicker collagen bundles and mature fibroblasts at 21 days postoperative. Control wounds continued to show signs of an active inflammatory phase under scab on day 21. In addition, methylthiazolyl tertrazolium (MTT) and neutral red (NR) assays suggested that the PEC membranes and their aqueous extracts were nontoxic towards mouse and human fibroblast cells. Cell growth was also not hindered by co-incubation with the membranes.
In another study, Qin et al. studied the effect of application of chitosan green tea polyphenol complex on wound healing in a rat incisional wound model [49]. The results showed that the treatment groups showed significantly enhanced breaking strength in the incision wound compared with control. In addition, the percentage of wound contraction was greater in the treatment group compared with two control group. The complex also demonstrated a gradual increase in the release rate of polyphenols from the initial stage and slow increase at different pH values.
A study using a rat model of incisional skin wound was carried out by Kojima et al. to evaluate the effect of chitin and chitosan on collagen synthesis in wound healing [33]. Collagen synthesis was evaluated by measuring prolyl hydroxylase activity induced within rat granulation tissue. It was found from the study that, compared with chitosan, chitin at the higher concentration (10 mg/ml) induced stable collagen synthesis without scatter in the early wound-healing process. The authors speculated that chitin at higher concentration can stimulate platelets and macrophages, resulting in the release of platelet-derived growth factor and TGF-β.
Effect on healing of surgical wounds
In a combined in-vitro and animal study, the adhesion of laser-activated chitosan films was investigated for the application of suture-less tissue fixation [50]. Flexible and insoluble strips of chitosan films were bonded to sheep intestine using several laser powers at 808-nm wavelength. A natural cross-linker (genipin) was also added to the film and the tissue repair strength compared with the strength of plain films. The adhesive was also bonded in vivo to the sciatic nerve of rats and the thermal damage induced by the laser assessed 4 days postoperatively. The experimental results demonstrated that chitosan adhesives successfully repaired intestine tissue, attaining the maximum repair strength at the laser power of 120 mW.
Effect on healing of burns
In a study using a rat model, Jin et al. compared the effects of chitosan with heparin on early extension of burns [51]. Chitosan powder, heparin powder and the mixture of chitosan and heparin were applied, to the burns created on the backs of rats. Histological examination after 72 h showed that the burn degree of the chitosan-treated group was less severe than that of the control group, and chitosan greatly prevented the extension of burns in early phase. In contrast, heparin had no protective effect on the early extension of burns. Use of chitosan and heparin together attenuated chitosan’s protective effect.
Alsarra investigated the wound-healing efficacy of chitosan with different molecular weight and DDA ranges on rat burns [40]. The highest wound-healing rate was found in the group treated with high-molecular-weight and high-DDA chitosan. Burns treated with high-molecular-weight chitosan had significantly more epithelial tissue, and the best re-epithelialization and fastest wound closure were found with the high-molecular-weight chitosan treatment group. Histological examination and collagenase activity studies revealed advanced granulation tissue formation and epithelialization in wounds treated with high-molecular-weight chitosan.
A study on the evaluation of chitosan gel with 1% silver sulfadiazine for burn wound treatment in rats was undertaken by Nascimento et al. [36]. Chitosan gel was applied to the burns every 48 h. The burns treated with chitosan gel with silver sulfadiazine showed a higher fibroblast production and a better angiogenesis than those treated with chitosan gel without silver sulfadiazine or with 1% silver sulfadiazine cream (which was applied every 24 h). Although no statistical difference was found in the healing time among the groups, the authors suggested that chitosan gels pose advantages over the silver sulfadiazine cream, since the former was applied every 48 h, whereas the latter was applied every 24 h. On the other hand, the presence of silver sulfadiazine in the chitosan gel does not seem to contribute to the epithelialization process.
A chitosan hydrogel was developed by Ribeiro et al. and its applicability as a wound dressing for burn wound in rats was evaluated [52]. The results from the initial in vitro study indicated that chitosan hydrogel was able to promote cell adhesion and proliferation. Cell viability studies showed that the hydrogel and its degradation by-products are noncytotoxic. From macroscopic analysis, the wound beds of the animals treated with chitosan hydrogel were considerably smaller than those of untreated controls. Histological analysis revealed a lack of a reactive or a granulomatous inflammatory reaction in skin lesions with chitosan hydrogel and the absence of pathological abnormalities in the organs obtained by necropsy, which supported the local and systemic histocompatibility of the biomaterial.
In a similar study, bio-inspired bilayered physical hydrogels only constituted of chitosan and water were processed and applied for the treatment of full-thickness burn injuries in dogs [53]. A first layer constituted of a rigid protective gel ensured good mechanical properties and gas exchanges. A second soft and flexible layer allowed the material to follow the geometry of the wound and ensured a good superficial contact. The results showed that chitosan materials were well tolerated and promoted good tissue regeneration. They induced inflammatory cell migration and angiogenic activity favoring a high vascularization of the neotissue. At day 22, type I and IV collagens were synthesized under the granulation tissue and the formation of the dermal–epidermal junction was observed. After 100 days, the new tissue was quite similar to a native skin, especially by its aesthetic aspect and its great flexibility.
Effects on wound healing of subcutaneous wound
A study was carried out by Diegelmann et al. to analyze the effect of chitosan on the wound-healing response to a subcutaneous wound in rats [54]. The polyvinyl alcohol sponge implant model was used as a means to deliver either chitosan or its vehicle to a standardized subcutaneous wound on the backs of rats. It was shown that the presence of chitosan significantly delayed the appearance of macrophages, and also reduced capillary ingrowth, fibroblast infiltration and mature collagen fiber deposition. In addition, there were significantly reduced amounts of collagen deposited as determined by hydroxy-L-proline content in the chitosan-treated wounds.
Okamoto et al. investigated the effects of chitosan on experimental abscesses with S. aureus in dogs [55]. The abscesses were treated with suspensions of finely granulated chitosan with varying doses (0.01, 0.1 or 1.0 mg). It was found that, in comparison with the ampicillin-treated and nontreated control group, the group treated with chitosan at doses of 0.1 and 1.0 mg had a faster wound-healing rate. Histologically, the granulation tissue formed had abundant vascularization in the 0.1 and 1.0 mg chitosan groups on day 8.
Effects on mucosal wound healing
Using a sheep model of chronic rhinosinusitis, Athanasiadis et al. compared the effects of a chitosan-dextran derivative gel (CD) on adhesion formation and wound healing with those of two commercial topical agents: recombinant tissue factor (rTF) and PEG [56]. CD significantly decreased lateral nasal wall and ethmoidal adhesions compared with rTF. There was a noticeable trend toward decreased adhesions on the lateral nasal wall and ethmoids in the PEG group and the CD group compared with controls. The CD group had a significantly greater percentage of re-epithelialization at day 28 and day 84 compared with the rTF group. At day 28, the CD group was significantly more ciliated than control and rTF. This difference between CD and rTF reciliazation remained significant at day 56. In addition, the mean cilial grade for CD at day 112 was significantly better than control. The authors concluded that in the sheep model of chronic sinusitis, CD significantly improves microscopic wound healing and reduces adhesion formation after endoscopic sinus surgery.
Effects on wound healing in urogenital tissue
A preliminary study using a dog model was reported by Bartone et al. [57]. Wounds were made in the kidney, ureter and penile foreskin. Chitosan caused no adverse effects on urogenital wound healing. A decrease in fibrosis was seen in the wounds treated with chitosan in all tissues studied. The authors suggested that the morbidity of urogenital surgery may be decreased by treating the wounds with chitosan.
Effects on corneal wound healing
In a study using a rabbit model, Sall et al. applied chitosan topically to the eyes of rabbits after they had sustained central corneal wounds [58]. Group rabbits were treated for 7, 14, 17 and 21 days. For all time sets, there were no apparent statistical differences in corneal wound tensile strength between the chitosan-treated eyes and control eyes.
Effects on liver tissue
Wang et al. compared the biocompatibility and biodegradation of gelatin (a denatured, biodegradable protein obtained by acid and alkaline processing of collagen) and gelatin/chitosan gels following implantation in rat livers for periods of up to 16 weeks [59]. It was found that the gelatin/chitosan gel was more efficient in inducing fibrin formation and vascularization at the implant–host interface. The degrees of inflammatory reaction for the gelatin/chitosan gel were significantly stronger than the gelatin gel. Advanced biodegradation of the gelatin gels was observed.
Effects on healing of spine cord injury
A study was conducted by Wang et al. on the effects of bone marrow mesenchymal stem cells (MBSCs) seeded in chitosan-alginate scaffolds for repairing spinal cord injury in rats [60]. At 2, 4, and 6 weeks after operation, the functional recovery score of the hind limbs was higher in the group implanted with tissue-engineered spine cord than in the control groups. After 6 weeks of operation, wheat germ agglutinin-horseradish peroxidase retrograde tracing indicated that there was no regenerated nerve fiber through both stumps of spinal cord injury in each group. Histological analysis revealed that host spinal cord and tissue-engineering spinal cord linked very compactly, no scar tissue grew and a large number of neurofilament 200-positive fibers and neuron-specific enolase positive cells were detected in the wounded area in the group implanted with tissue-engineered spine cord. In the group implanted with chitosan-alginate scaffold alone, a small quantity of scar tissue intruded into nondegradative chitosan-alginate scaffold at the lesion area edge, and a small degree of neuron-specific enolase fluorescence or NF-200 fluorescence was observed at the junctional zone. The group implanted with MBSCs alone and the nontreated control group were filled with a large number of scar tissue, and neuron-specific enolase-positive cells or NF-200-positive cells were not detected. In the rats of the nontreated control group, porosis was observed at the spinal cord injury.
Similar animal studies on the wound-healing effects of chi-tosan preparations were also carried out by Lu et al. [61], Hirose et al. [62], Kim et al. [63], Kiyozumi et al. [64], Kweon et al. [65], Degim et al. [38], Sung et al. [66], Kang et al. [67], Hong et al. [68] and Khan et al. [69]. Table 4 shows the summary of animal studies investigating the wound-healing effects of chitosan preparations.
Table 4.
Wound-healing effects of chitosan preparations: a summary of animal studies.
| Chitosan preparations | Models of wound | Animal species | Major results/conclusions | Ref. |
|---|---|---|---|---|
| Cotton-fiber chitosan | Open skin wound | Dog | Chitosan accelerated the infiltration of PMN cells and the production of collagen by fibroblasts | [31] |
| Chitosan power | Open skin wound | Dog | Re-epithelialization tended to be greater; fewer number of inflammatory cells; fewer rete ridges were observed | [44] |
| Asymmetric chitosan membrane | Open skin wound | Rat | Wounds were hemostatic and healed quickly. Histological examination confirmed that the epithelialization rate was increased and the deposition of collagen in the dermis was well organized | [45] |
| Chitosan acetate bandage | Open skin wound | Mouse | Chitosan acetate bandage reduced the number of inflammatory cells in the wound, and had an overall beneficial effect on wound healing, especially during the early period | [46] |
| NTCAS | Open skin wound | Rat | NTCAS showed a steady level of TNF-α, while the IL-6 level reached a peak on day 7. In addition, NTCAS showed better and faster recovery than the other groups | [47] |
| Chitosan-alginate polyelectrolyte complex | Incisional skin wound | Rat | Accelerated healing of incision wounds; an excellent remodeling phase with organized thicker collagen bundles and mature fibroblasts; chitosan-alginate PEC membranes were nontoxic towards fibroblast cells | [48] |
| Chitosan green tea polyphenol complex | Incisional skin wound | Rat | Chitosan complex significantly enhanced the breaking strength in the wound | [49] |
| Laser-activated chitosan adhesive | Surgical wound | Rat | Chitosan adhesives successfully repaired intestine tissue, attaining the maximum repair strength at the laser power of 120 mW | [50] |
| Chitosan powder | Burn | Rat | The burn degree of the chitosan-treated group was less severe than the control group, and chitosan greatly prevented the extension of burns in early phase | [51] |
| Chitosan | Burn | Rat | Burns treated with high-molecular-weight chitosan had significantly more epithelial tissue, the best re-epithelialization and the fastest wound closure; advanced granulation tissue formation and epithelialization in wounds | [40] |
| Chitosan gel with 1% silver sulfadiazine | Burn | Rat | Higher fibroblast production and a better angiogenesis; the presence of silver sulfadiazine in the chitosan gel does not seem to contribute to the epithelialization process | [36] |
| Chitosan hydrogel | Burn | Rat | Promotes cell adhesion and proliferation; noncytotoxic; smaller wound bed; lack of a reactive or a granulomatous inflammatory reaction in skin lesions; and the absence of pathological abnormalities in the organs obtained by necropsy | [52] |
| Bilayered chitosan hydrogel | Burn | Dog | Well tolerated and promoted a good tissue regeneration; induced inflammatory cell migration and angiogenic activity | [53] |
| Chitosan | Subcutaneous wound | Rat | Significantly delayed the appearance of macrophages; reduced capillary ingrowth, fibroblast infiltration and mature collagen fiber deposition; significantly reduced amounts of collagen deposited | [54] |
| Granulated chitosan suspension | Abscess | Dog | Faster wound healing; abundant vascularization | [55] |
| Chitosan-dextran derivative gel | Mucosal wound | Sheep | Significantly decreased lateral nasal wall and ethmoidal adhesions; greater percentage of re-epithelialization | [56] |
| Chitosan | Urogenital Wound | Dog | No adverse effect on urogenital wound healing; a decrease in fibrosis was seen | [57] |
| Chitosan | Central corneal wound | Rabbit | No apparent effect on wound healing | [58] |
| Gelatin-chitosan gel | Wound in liver tissue | Rat | More efficient in inducing fibrin formation and vascularization | [59] |
| Chitosan-alginate scaffolds | Spine cord wound | Rat | Higher functional recovery score of hind limbs | [60] |
NTCAS: Nano-titanium oxide-chitosan; PEC: Polyelectrolyte complex; PMN: Polymorphonuclear neutrophil.
Clinical studies
In patients undergoing plastic surgery, Biagini et al. treated the donor sites with soft pads of freeze-dried N-carboxybutyl chitosan to promote ordered tissue regeneration [70]. It was found that, compared with control donor sites, better histoarchitectural order, better vascularization and the absence of inflammatory cells were observed at the dermal level, whilst fewer aspects of proliferation of the Malpighian layer were reported at the epidermal level. Accordingly, it was suggested that N-carboxybutyl chitosan leads to formation of regularly organized cutaneous tissue and reduces anomalous healing.
Stone et al. evaluated the healing at skin graft donor sites dressed with chitosan [71]. A total of 20 patients requiring a split-skin graft during the 7-month period were entered into the study. The skin graft donor sites were dressed half with chitosan and half with a conventional dressing. Chitosan proved to be an easy dressing material to apply and maintain and was painless to remove. Histologically, skin occluded by the chitosan dressing showed marked differences to skin occluded by the conventional dressing at the newly-healed time point. Chitosan biopsies showed a looser connective tissue stroma in the papillary dermis, which was richer in both glycosaminoglycan matrix and capillaries than control biopsies. Small dermal nerve fibers were also more numerous in chitosan biopsies and showed marked differences to skin occluded by the conventional dressing at the newly healed time point. In addition, digital color separation analysis of donor site scars demonstrated an earlier return to normal skin color at chitosan-treated areas.
Another similar clinical study was conducted by Azad et al. to investigate a design for chitosan membrane as a wound-healing dressing [41]. Membranes were prepared from shrimp chitosan (DDA = 75%) with less than 1% protein and mineral content and a molecular weight of 1.5 × 106 ± 0.1 Dalton. The chito-san membranes, in mesh or non-mesh form, were applied to dress the fresh wound that resulted at a skin graft donor site after removal of the skin layer of 0.010–0.015 inches. Half of the wound was dressed with chitosan membrane and the other half with the control Bactigras®, a chlorhexidine acetate impregnated tulle gras. The clinical data indicated that the mesh chitosan membrane promotes efficient adherence, hemostasis, healing and re-epithelialization of the wound. Itching and pain sensitivity were less. The histopathology preparations showed that under the chitosan membrane, cells stimulate repair of the skin cell layers and help to re-establish tissue architecture. The application of non-mesh membrane was not advised. The use of non-mesh chitosan membranes resulted in accumulation of blood under the membrane.
A randomized controlled study aimed to determine the efficacy of a chitosan/dextran gel on hemostasis and wound healing after endoscopic sinus surgery was carried out by Valentine et al. [72]. A total of 40 patients undergoing endoscopic sinus surgery for chronic rhinosinusitis were involved in the study. Chitosan/dextran gel achieved rapid hemostasis with the mean time to hemostasis at 2 min compared with 10 min for the control. There were significantly less adhesions at all time points with chitosan/dextran gel versus control. However, no significant difference was found between chitosan/dextran gel and control with respect to crusting, mucosal edema, infection, or granulation tissue formation. Table 5 summarizes the clinical studies on the wound healing effects of chitosan preparations.
Table 5.
Wound-healing effects of chitosan preparations: a summary of clinical studies.
| Chitosan preparations | No. of patients | Wound types | Major results/conclusions | Ref. |
|---|---|---|---|---|
| N-carboxybutyl chitosan | Not reported | Skin graft donor sites | Better histoarchitectural order, better vascularization and the absence of inflammatory cells were observed at the dermal level, whilst fewer aspects of proliferation of the Malpighian layer at the epidermal level | [70] |
| Chitosan | 20 | Skin graft donor sites | Looser connective tissue stroma in the papillary dermis; more numerous nerve fibers; earlier return to normal skin color | [71] |
| Mesh chitosan membrane | Not reported | Skin graft donor sites | Promoted efficient adherence, hemostasis, healing and re-epithelialization of the wound. Itching and pain sensitivity were less | [41] |
| Chitosan/dextran gel | 40 | Surgical wound | Less adhesion; no significant improvement in granulation tissue formation | [72] |
Chitosan dressing used as a drug-delivery device for enhanced antimicrobial or wound-healing effects
Chitosan and its derivatives have been a subject of interest for application to wounds and burns not only because of their intrinsic antimicrobial and wound-healing effects, but also owing to their properties as versatile drug delivery vehicles that can enhance antimicrobial and wound-healing effects. Studies that have been carried out include the use of chitosan and its derivatives for the delivery of antimicrobials [18,73–81], growth factors [82–85] and other drugs [86,87].
Chitosan for antimicrobial drug delivery
There have been many studies of the ability of chitosan to act as a delivery vehicle for antimicrobial drugs, particularly in view of the fact that compromised wound sites contain avascular zones that can prevent the delivery of systemic antibiotics to the infected tissue. While whole-body dosing can also lead to systemic toxicity, local drug-delivery systems can achieve high local concentrations of drug while lowering the overall serum concentrations.
Noel et al. evaluated chitosan film as a potential localized drug delivery carrier, which does not require later removal (possible by additional surgery) owing to the biodegradability of chitosan [73]. The data from the elution study suggested effective release of amikacin and daptomycin. The activity studies indicated the eluants inhibited the growth of S. aureus. As a result, the authors suggested that incorporating antibiotic in chitosan could provide alternative methods of treating musculoskeletal infections.
In another study performed by the same group, the authors investigated if an adaptable, porous chitosan matrix could absorb and elute antibiotics for potential use as an adjunctive therapy to debridement and lavage; and if the sponges could elute levels of antibiotic that would inhibit growth of S. aureus and P. aeruginosa [74]. The results showed that amikacin concentration was 881.5 μg/ml after 1 h with a gradual decline to 13.9 μg/ml after 72 h. Vancomycin concentration was 1007.4 μg/ml after 1 h with a decrease to 48.1 μg/ml after 72 h. A turbidity assay testing the activity of released amikacin and vancomycin indicated inhibitory levels of elution from the chitosan sponge.
Wound dressings based on chitosan hydrochloride, 5-methylpyrrolidinone chitosan and their mixtures with an anionic polymer, hyaluronic acid, were prepared by Rossi et al. for the release of chlorhexidine diacetate in skin ulcer therapy [18]. While all wound dressings were characterized for drug-release properties, the addition of hyaluronic acid to chitosans leads to a modulation of drug release.
A preliminary study to evaluate the ability of chitosan film loaded with daptomycin and vancomycin to lessen or prevent infections in bone fractures was executed by Smith et al. [75]. The film was designed to be applied to musculoskeletal fixation devices or implant surfaces. Films with 61, 71 and 80% DDA made using lactic or acetic acid solvents were analyzed for various properties including their antibiotic uptake, elution, adhesive strength and degradation. Chitosan films after 1 min of rehydration were able in a simulated, clinical setting to maintain mechanical integrity and adhesive strength to be applied to bone fracture fixation devices or implant surfaces. The film percent degradation increased with DDA increasing from 61 to 80%, but film degradation rate decreased in the presence of antibiotics. 80% DDA chitosan films were optimal for absorbing and eluting antibiotics. Antibiotics eluted by the films were active against S. aureus.
A porous chitosan-silver nanocomposite for increased areas of application in wound dressing and antibacterial application was developed by Vimala et al. [76]. The entire process of development consists of three steps including silver ion-PEG matrix preparation, addition of chitosan matrix, and removal of PEG from the film matrix. Both PEG and chitosan played vital roles in the reduction of metal ions into nanoparticles, and also provided good stability to the formed nanoparticles. The embedded nanoparticles (AgNPs) were clearly observed throughout the film in scanning electron microscopy, and the extracted AgNPs from the porous chitosan-silver nanocomposite showed an average size of approximately 12 nm in transmission electron microscopy. Improved mechanical properties were observed for porous chitosan-silver nanocomposite than for chitosan blend and chitosan-silver nano-composite films. The examined antibacterial activity results of these films revealed that porous chitosan-silver nanocomposite films exhibited superior inhibition.
A similar synthesis approach was presented by Thomas et al. [77]. In their study, chitosan/silver nanoparticle films were synthesized by a simple photochemical method of reduction of silver ions in an acidic solution of AgNO3 and chitosan. The presence of silver nanoparticles was confirmed from the transmission electron microscopy, x-ray diffraction and thermogravimetric analysis of the film. The surface plasmon resonance obtained at 400 nm also confirmed the presence of nanosilver in the chitosan film. The developed chitosan-nanosilver films demonstrated excellent antibacterial action against E. coli and Bacillus.
In a preliminary study, Greene et al. investigated if a chitosan coating either unloaded or loaded with an antibiotic, gentamicin, could lessen or prevent stainless steel screws (for fracture fixation) from becoming an initial nidus for infection [78]. It was demonstrated that the gentamicin eluted from the coating at a detectable level during 72–96 h. The coating was retained at the 90% level in simulated bone screw fixation and the unloaded and loaded chitosan coatings had encouraging in vitro biocompatibility with fibroblasts and stem cells and were bacteriostatic against at least one strain of S. aureus. The authors finally suggested that the use of an antibiotic-loaded chitosan coating on stainless steel bone screws and internal fixation devices in contaminated bone fracture fixation may be considered.
Tunney et al. investigated whether the addition of chitosan to gentamicin-loaded Palacos R bone cement increased antibiotic release and prevented bacterial adherence and biofilm formation by Staphylococcus spp. clinical isolates [79]. It was found that the addition of chitosan to gentamicin-loaded Palacos R bone cement significantly decreased gentamicin release and did not increase the efficacy of the bone cement at preventing bacterial colonization and biofilm formation. Therefore, incorporating chitosan into Palacos R + G bone cement for use in revision surgery has no clinical antimicrobial benefit.
Mi et al. developed a bilayer chitosan membrane, consisting of a dense upper layer (skin layer) and a sponge-like lower layer, and used it as a topical delivery of silver sulfadiazine for the control of wound infections [80]. Physical characterization of the bilayer wound dressing demonstrated that it has excellent oxygen permeability, that it controls the water vapor transmission rate and that it promotes water uptake capability. The release of sulfadiazine from the bilayer chitosan dressing displayed a burst release on the first day and then tapered off to a much slower release. However, the release of silver from the bilayer chitosan dressing displayed a slow release profile with a sustained increase of silver concentration. In vitro studies using the cultures of P. aeruginosa and S. aureus showed effective antimicrobial activity for 1 week. In vivo antibacterial tests using male Wistar rats confirmed that this wound dressing was effective for long-term inhibition of the growth of P. aeruginosa and S. aureus at an infected excisional wound.
A chitosan-polyurethane film dressing loaded with minocycline was also developed by Aoyagi et al. for the treatment of severe burns [81]. In the in vitro studies, sustained release of minocycline was observed from chitosans with DDAs of 67 and 83%. In the in vivo studies, various formulations were applied to severe burn wounds in rats in the early stage and chitosan films with DDA of 83% loaded with minocycline showed an excellent effect in accelerating wound healing.
Chitosan for growth factor delivery
Moreover, chitosan (and its preparations) has also been widely used as a vehicle to deliver growth factors into wounds to encourage healing. Growth factors have long been perceived to have a problem with sustained bioavailability in wounds, and many types of slow-release formulations have been developed with the idea of prolonging the period of activity.
Whilst antibacterial agents prevent or treat infections and can aid in wound healing, they do not necessarily take an active physiological part in the wound-healing process. Growth factors are involved with cell division, migration, differentiation, protein expression and enzyme production. Growth factors affect the inflammatory, proliferation and migratory phases of wound healing. A variety of growth factors have been reported which participate in the process of wound healing including: EGF, PDGF, FGF, TGF-β1, IGF-1, human growth hormone and granulocyte–macrophage colony-stimulating factor.
Mizuno et al. studied the stability of basic fibroblast growth factor (bFGF) incorporated into a chitosan film as a delivery vehicle for providing sustained release of bFGF [82]. The therapeutic effect of this system on wound healing in genetically diabetic mice was determined as a model for treating clinically impaired wound healing. Growth factor was incorporated into chitosan films before drying by mixing bFGF solution with the hydroxypropyl-chitosan solution. Chitosan film or bFGF-chitosan film was applied to full-thickness wounds created on the backs of diabetic mice. Results showed that the wounds were smaller in day 20 in the bFGF-chitosan group than in chitosan alone group. Proliferation of fibroblasts and an increase in the number of capillaries were observed in both groups, but granulation tissue was more abundant in the bFGF-chitosan group. The investigators suggested that chitosan itself facilitates wound repair and bFGF incorporated into chitosan film is a stable delivery vehicle for accelerating wound healing.
In a similar study, chitosan scaffolds loaded with bFGF contained in gelatin microparticles were developed and tested for treating pressure ulcers in an aged mouse model, mimicking the situations in an elderly population [83]. It was demonstrated that both chitosan and chitosan-bFGF scaffolds significantly accelerated wound closure compared with gauze control. By day 10, all wounds achieved similar closure. Delivery and angiogenic function of bFGF was verified through ELISA and histology. Elevated neutrophil levels were observed in chitosan and chitosan-bFGF groups. Since neutrophil elastase contributes to the proteolytic environments of pressure ulcers, the effect of chitosan on elastase was assessed. In vitro, chitosan inhibited elastase activity. In vivo, elastase protein levels in wounds were reduced with chitosan-bFGF scaffolds by day 10. These results suggest that chitosan is an effective material for growth factor delivery and can help to heal chronic ulcers.
In another study, Alemdaroglu et al. aimed to develop an effective chitosan gel formulation containing EGF, and to determine the effect on healing of second-degree burn wounds in rats [84]. In the in vitro study to investigate release of EGF from the formulations, the release rate was 97.3% after 24 h. In the in vivo studies, the EGF formulations were repeatedly applied on the burned areas for 14 days (one application per day). When the results were evaluated immunohistochemically, there were significant increases in cell proliferation observed in the group who had EGF-containing gel applied. The histochemical results showed that the epithelialization rate in the group who had gel containing EGF applied was the highest compared with the group who had non-EGF-containing gel applied. The histological results indicated and supported these findings. The authors concluded that EGF-containing gel could result in a better and faster epithelialization compared with the other control groups.
Obara et al. evaluated the accelerating effect on wound healing of a photocrosslinkable chitosan hydrogel containing FGF-2 [85]. Full-thickness skin incisions were made on the backs of healing-impaired diabetic (db/db) mice and their normal (db/+) lit-termates. Histological analysis indicated that application of the chitosan hydrogel significantly advanced the rate of contraction on days 0 to 2 in db/db and db/+ mice. Although the addition of FGF-2 into the chitosan hydrogel in db/+ mice had little effect, application of the chitosan hydrogel-containing FGF-2 further accelerated the adjusted tissue filling rate (days 2 to 4 and days 4 to 8) in db/db mice. Furthermore, the chitosan hydrogel-containing FGF-2 markedly increased the number of CD-34-positive vessels in the wound areas of db/db mice on day 4. Thus, the application of chitosan hydrogel-containing FGF-2 onto a healing-impaired wound induces significant wound contraction and accelerates wound closure and healing.
Chitosan for delivery of other drugs
A biodegradable sponge, composed of chitosan and sodium alginate, was developed by Dai et al. for curcumin delivery in order to enhance the wound-healing effects [86]. The release of curcumin from the sponges could be controlled by the crosslink-ing degree. Curcumin could be released from the sponges in an extended period for up to 20 days. An in vivo animal test using Sprague-Dawley rats showed that sponge had a better effect than cotton gauze, and adding curcumin into the sponge enhanced the therapeutic healing effect.
Jayakumar et al. developed chitosan-based materials in drug-delivery systems possessing covalent attachment of thiol moieties [87]. Thiol-containing chitosan (TCS), found to be soluble in water, was synthesized by the graft copolymerization technique. The TCS beads were prepared by using tripolyphoshate, at pH 4.0. The release of indomethicin from TCS beads was higher at increasing pHs in the dissolution medium. The release rate of indomethicin at pH 7.4 was higher than the release rate at pH 1.4 owing to ionization of thiol groups and high solubility of indomethicin in an alkaline medium. These results indicated that the TCS beads may become a delivery system for the controlled release of different drugs wherever pH-sensitive mechanics might be useful. This is especially applicable in cases when it is important to minimize drug release in acidic sites, such as in the stomach.
Table 6 summarizes the studies on using chitosan dressing for drug delivery in the management of wound.
Table 6.
Chitosan dressing used for drug delivery: a summary.
| Chitosan preparations | Study type | Antimicrobials/growth factor/drug | Major results/conclusions | Ref. |
|---|---|---|---|---|
| Delivery of antimicrobials | ||||
| Chitosan hydrochloride, 5-methyl-pyrrolidinone chitosan | In vitro | Chlorhexidine diacetate | The wound dressings were also characterized for drug- release properties | [18] |
| Chitosan bar loaded with gentamicin | In vitro and animal (rabbit tibia) | Gentamicin | The gentamicin released from the bar showed significant antibacterial activity. The bar implanted in the proximal portion of the rabbit tibia produced a much higher concentration in local bone and in the hematoma | [23] |
| Minocycline-loaded chitosan hydrogel | In vitro and animal (open wounds in rats) | Minocycline | Incorporation of minocycline did not affect the gel properties, and chitosan hardly affected drug release and protein adsorption. Faster healing of the wound was observed | [66] |
| Chitosan film | In vitro | Amikacin, daptomycin | Effective release of antibiotics which inhibited Staphylococcus aureus | [73] |
| Porous chitosan matrix | In vitro | Amikacin, vancomycin | Effective release of antibiotics which inhibited S. aureus and Pseudomonas aeruginosa | [74] |
| Chitosan film | In vitro | Daptomycin, vancomycin | Antibiotics eluted from the films were active against S. aureus | [75] |
| Porous chitosan-silver nanocomposite | In vitro | Silver | Superior inhibition of bacteria was revealed | [76] |
| Chitosan/silver nanoparticle films | In vitro | Silver nanoparticle | Excellent antibacterial action against Escherichia coli and Bacillus spp. | [77] |
| Chitosan loaded with gentamicin | In vitro | Gentamicin | Bacteriostatic against S. aureus. | [78] |
| Addition of chitosan to gentamicin-coated bone cement | In vitro | Gentamicin | Efficacy at preventing bacterial colonization and biofilm not increased | [79] |
| Bilayer chitosan membrane | In vitro and animal (open wounds in rats) | Silver sulfadiazine | Effective antimicrobial activity in vitro for 1 week; effective for long-term inhibition of S. aureus and P. aeruginosa in open-skin wounds in rats | [80] |
| Chitosan film | In vitro and animal (burns in rats) | Minocycline | Sustained release of minocycline in vitro; excellent effect in accelerating wound healing | [81] |
| Delivery of growth factors | ||||
| Chitosan film | Animal (open wounds in mice) | bFGF | bFGF incorporated into chitosan film is a stabile delivery vehicle for accelerating wound healing | [82] |
| Chitosan scaffolds | In vitro and animal (pressure ulcer in aged mice) | bFGF | In vitro, chitosan inhibited elastase activity; in vivo, elastase protein levels in wounds were reduced; chitosan is an effective material for growth factor delivery | [83] |
| Chitosan gel | In vitro and animal (burns in rats) | Epidermal growth factor | In vitro, the release rate was 97.3% after 24 h. In vivo, significant increase in cell proliferation; better and faster epithelialization rate | [84] |
| Photo-cross linkable chitosan hydrogel | Animal (incisional wounds in mice) | Fibroblast growth factor-2 | Chitosan hydrogel significantly advanced the rate of wound contraction. Increased numbers of CD34-positive vessels were observed in healing-impaired mice | [85] |
| Delivery of other drugs | ||||
| Chitosan-sodium alginate sponge. | In vitro and animal (open skin wounds in rats) | Curcumin | Curcumin could be released from the sponges in an extended period for up to 20 days | [86] |
| TCS beads | In vitro | Indomethicin | The release amounts of indomethicin from TCS beads were higher with increasing pHs in the dissolution medium | [87] |
bFGF: Basic fibroblast growth factor; TCS: Thiol-containing chitosan.
Physical & biological characterizations of chitosan preparations
Many formulations and derivatives of chitosan have been investigated, and some of them are illustrated in Box 1. The physical and biological properties of wound dressings influence their ultimate performance and contribute to satisfying the desirable properties of dressings. The specific property to be characterized will depend on both the type of wound dressing, the nature of the surface to which the dressing will be applied and any secondary dressings that may be involved. Chitosan film, as a wound dressing, should be durable, stress resistant, flexible, pliable and elastic. It should be easy to apply and remove without incurring any trauma during dressing changes. Chitosan films should possess reasonable tensile properties that could bear the stresses exerted by different parts of the body having varying contours. Moreover, the dressing must be rapidly and uniformly adherent and conform to wound bed topography and contour to prevent air or fluid pocket formation. Furthermore, the dressing is preferably permeable to water vapor to the extent that a moist exudate under the dressing is maintained without pooling, but excess fluid absorption and evaporation leading to desiccation of the wound bed is prevented.
Box 1. Classification of chitosan derivatives and formulations.
Chitosan derivatives
Chitosan formulations
Peh et al. compared the mechanical, bioadhesive strength, and biological properties of two chitosan films prepared using different solvents, acetic acid (Chitosan-AA) and lactic acid (Chitosan-LA), with a commercial polyurethane dressing, Omiderm™ [88]. The three preparations differed significantly in the mechanical and bioadhesive strength properties. Chitosan-LA exhibited a lower tensile strength, but was more flexible and bioadhesive than Chitosan-AA. Chitosan films were found to be permeable to water vapor. Chitosan-LA and Omiderm were nonirritant and did not cause any skin allergic reaction in rabbits. In contrast, Chitosan-AA films inflicted adverse skin reactions. Nevertheless, no gross sign of toxicity was encountered from the systemic injection of the extracts of the three preparations.
Rossi et al. tested the mechanical properties of wound dressings, based on chitosan hydrochloride, 5-methyl-pyrrolidinone chitosan, and their mixtures with an anionic polymer, hyaluronic acid [18]. The dressings were prepared by freeze-drying. It was shown that all the wound dressings were characterized by mechanical resistance suitable for skin application. The addition of hyaluronic acid to chitosans led to a reduction in wound dressing hydration properties. The wound dressing based on MPC was characterized by the highest elastic properties and by the best scavenger activity. Antimicrobial activity against bacteria and C. albicans was also shown by the chitosan dressing in the absence of chlorhexidine.
A hydrogel cross-linked hyaluronan with glycol chitosan was developed by Wang in aqueous solution using water-soluble car-bodiimide at nearly neutral pH and room temperature [89]. The hydrogel could be easily formulated into injectable gels, films, membranes and sponges for soft-tissue augmentation, visco-supplementation, drug delivery, preventing adhesion after the application of chitosan, wound dressing and tissue-engineering scaffolds. In addition, the hydrogel had high water adsorption properties and biostability. Rheololgical results of the gel showed a soft and viscoelastic structure. Fourier transform infrared spectra further confirmed the formation of amide bonds between carboxyl groups of hyaluronan and amine groups of glycol chitosan, and no N-acylurea and other derivatives were identified.
Wittaya-areekul et al. developed chitosan films and their blends with cornstarch and dextran to improve the films’ physical strength [90]. Polypropylene glycol at various concentrations was added to improve the films’ flexibility. Properties of wound dressing, such as liquid adsorption, vapor and oxygen penetration, bioadhesiveness and film elasticity, were examined. Chitosan films demonstrated the highest liquid adsorption and the adsorption tended to decrease with the addition of cornstarch and dextran. Moisture vapor and oxygen were found to be able to penetrate through all film formulations, and those films with cornstarch and dextran demonstrated increased penetration rates through the films. The bioadhesiveness test using a pig gut model did not show significantly different bioadhesive properties with the addition of cornstarch and dextran. The film elasticity of the formulation containing only chitosan exhibited the lowest elongation of the film at a force of 2N, but increased with the addition of cornstarch and dextran, respectively.
In a review, Jayakumar et al. discussed the chemical modification of chitin and chitosan with sulfate to generate new bifunctional materials [91]. As the modification would not change the fundamental skeleton of chitin and chitosan, it would keep the original physicochemical and biochemical properties, and finally would bring new or improved properties. The sulfated chitin and chitosan have a variety of applications, such as adsorbing metal ions, in drug-delivery systems, blood compatibility and in the antibacterial field.
Similar studies on the characterization of physical and biological properties of chitosan preparations were also reported by Altiok et al. [17], Kim et al. [63], Sung et al. [66], Keong et al. [92], Lu et al. [93] and Meng et al. [94].
Newest developments concerning chitosan
In recent years, new forms of chemically modified chitosan have been developed in order to improve the properties of chitosan for various biological activities, and these substances have gained increasing attention. Representative members of these novel polymers include ammonium chitosans, carboxymethyl chitosan and derivatives.
Ammonium chitosan
One factor that limits the application of native chitosan is its non-solubility in neutral and alkaline aqueous solutions. As a result, chitosan derivatives containing quaternary ammonium salts, such as N,N,N-trimethyl chitosan, N-propyl-N,N-dimethyl chitosan and N-furfuryl-N,N-dimethyl chitosan have been investigated for improved solubility in water and subsequently improved biological activities. Studies have shown that all quaternary ammonium chitosan derivatives were highly water-soluble at acidic, basic and neutral pH [95–100]. Compared with native chitosan, ammonium chitosan demonstrated enhanced antimicrobial properties [95,98,99] and drug-delivery abilities [96].
Carboxymethyl chitosan
Carboxymethyl chitosan (CMC) is another modification of chitosan formed by attaching carboxymethyl groups to the chitosan backbone. Depending on the location of the carboxymethyl group attachment, CMC can be referred to as ‘N’ when the carboxymehthyl group attaches to the amine, ‘O’ when it attaches to the primary hydroxyl group or N,O,-carboxymethyl chitosan when attached to both [101]. CMC has the advantage of a greater solubility range than native chitosan. CMC has now been extensively studied for its activities for drug delivery [102,103], hemostasis [104], antimicrobial action [105–107] and the stimulation of wound healing [41].
Expert commentary
The main goals of wound care and management are prevention of infection, maintenance of a moist environment, protection of the wound and achievement of rapid and complete healing with the minimum scar formation. Chitosan, as a cationic natural polymer, has been widely used as a topical dressing in wound management owing to its hemostatic, stimulation of healing, antimicrobial, nontoxic, biocompatible and biodegradable properties. In this review, we covered the antimicrobial and wound-healing effects of chitosan preparations for wounds and burns.
With respect to the antimicrobial effects, in-vitro studies have shown that chitosan as well as its derivatives and complexes are active against fungal species such as Candida spp., Gram-positive and Gram-negative bacteria [10–13,76–79]. Generally, chitosan shows stronger antimicrobial effects against Candida spp. and Gram-positive bacteria than it does against Gram-negative bacteria [13]. The proposed antimicrobial mechanisms in Gram-positives include the binding of chitosan to teichoic acids, coupled with a potential extraction of membrane lipids [11], while in Gram-negatives the cationic structure can displace divalent cations resulting in disruption of lipopolysaccharide binding and permeabilization of the outer membrane [13]. Both these sequences of events (e.g., cell wall disruption) ultimately lead to microbial cell death.
Various animal studies on using chitosan to treat or prevent different types of wound infections have been carried out. The data showed that chitosan rapidly killed the microbial cells in wounds and reduced the mortality of the animals in case of fatal infections. Clinical studies on using chitosan for treating chronic periodontitis reported that chitosan significantly improved the clinical parameters.
With respect to wound-healing effects, it has been indicated from in vitro studies that chitosan enhances the functions of PMN, macrophages and fibroblasts. As a result, chitosan promotes granulation and organization. Most of the animal and clinical studies reported that chitosan preparations accelerate the wound healing. The infiltration of PMN cells and production of fibroblasts are promoted. The number of inflammatory cells in the wound is reduced. In addition, chitosans are nontoxic to normal cells. However, side effects of some chitosan preparations were also reported [19,34], and chitosan was also found to be ineffective in corneal wound healing [58].
With respect to the physical and biological properties, it was concluded from the studies in this review that chitosan, as a wound dressing, must be rapidly and uniformly adherent and conform to wound bed topography and contours to prevent air or fluid pocket formation. The dressing is preferably permeable to water vapor so that a moist exudate under the dressing is maintained without pooling.
The large number of publications in this area suggests that chitosan will continue to be an important agent in the management of wounds and burns.
Five-year view
The relentless growth and increasing geographical expansion of antibiotic resistance amongst numerous species of pathogenic bacteria is causing international concern. Coupled with the lack of discovery of new classes of antibiotics, fears are growing that serious wounds and burns may again become life-threatening, as they were in the days before antibiotics were discovered. These concerns have driven a major research effort in both academic laboratories and life science companies to develop alternative antimicrobial strategies and products to which it is hypothesized bacteria will be unable to develop resistance. Topical antimicrobials are a large part of this effort and antimicrobial dressings for wounds and burns that can be used both prophylactically and therapeutically are especially valuable. Since this is precisely the area where the particular qualities of chitosan discussed in the present review are most effective, we see the future potential of chitosan to prevent and treat wound and burn infections as strong. The large and increasing number of publications in this area suggests that chitosan will continue to be an important agent in the management of wounds and burns.
Key issues.
Chitosan and its derivatives and complexes are active against fungi, Gram-positive and Gram-negative bacteria.
Chitosan demonstrates efficacy in treating or preventing different types of wound infections.
Chitosan enhances the functions of polymorphonuclear neutrophils, macrophages and fibroblasts. As a result, chitosan promotes granulation and organization.
Chitosan, as a wound dressing, must be rapidly and uniformly adherent and conform to wound bed topography and contours to prevent air or fluid pocket formation. The dressing is preferably permeable to water vapor so that a moist exudate under the dressing is maintained without pooling.
Chitosan and its derivatives are promising materials for controlled drug delivery.
Footnotes
Financial & competing interests disclosure
Research in the Hamblin laboratory is supported by NIH grant R01AI050875, Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), CDMRP Program in TBI (W81XWH-09-1-0514) and Air Force Office of Scientific Research (FA9950-04-1-0079). Tianhong Dai was partly supported by a Bullock Wellman Fellowship and an Airlift Research Foundation Extremity Trauma Research Grant (grant 109421). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Labrude P, Becq C. Pharmacist and chemist Henri Braconnot. Rev Hist Pharm (Paris) 2003;51(337):61–78. [PubMed] [Google Scholar]
- 2.Kozen BG, Kircher SJ, Henao J, et al. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15(1):74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 3•.Millner RW, Lockhart AS, Bird H, et al. A new hemostatic agent: initial life-saving experience with Celox (chitosan) in cardiothoracic surgery. Ann Thorac Surg. 2009;87(2):e13–e14. doi: 10.1016/j.athoracsur.2008.09.046. Demonstrates the mechanism of antimicrobial effects of chitosan. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52(2):105–115. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 5•.Rabea EI, Badawy ME, Stevens CV, et al. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457–1465. doi: 10.1021/bm034130m. Demonstrates the mechanism of antimicrobial effects of chitosan. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Poon YF, Li W, et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat Mater. 2011;10(2):149–156. doi: 10.1038/nmat2915. [DOI] [PubMed] [Google Scholar]
- 7.Tang H, Zhang P, Kieft TL, et al. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 2010;6(7):2562–2571. doi: 10.1016/j.actbio.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77(3):637–650. doi: 10.1016/s0039-6109(05)70572-7. [DOI] [PubMed] [Google Scholar]
- 9••.Kong M, Chen XG, Xing K, et al. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. Latest and comprehensive review on the antimicrobial properties of chitosan and mode of action. [DOI] [PubMed] [Google Scholar]
- 10.Andres Y, Giraud L, Gerente C, et al. Antibacterial effects of chitosan powder: mechanisms of action. Environ Technol. 2007;28(12):1357–1363. doi: 10.1080/09593332808618893. [DOI] [PubMed] [Google Scholar]
- 11.Raafat D, von Bargen K, Haas A, et al. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol. 2008;74(12):3764–3773. doi: 10.1128/AEM.00453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.No HK, Park NY, Lee SH, et al. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74(1–2):65–72. doi: 10.1016/s0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- 13••.Muzzarelli R, Tarsi R, Filippini O, et al. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob Agents Chemother. 1990;34(10):2019–2023. doi: 10.1128/aac.34.10.2019. Comprehensive study for evaluating the efficacy of N-carboxybutyl chitosan against 298 strains of Gram-positive bacteria, Gram-negative bacteria and Candida spp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seyfarth F, Schliemann S, Elsner P, et al. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int J Pharm. 2008;353(1–2):139–148. doi: 10.1016/j.ijpharm.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni AR, Kulkarni VH, Keshavayya J, et al. Antimicrobial activity and film characterization of thiazolidinone derivatives of chitosan. Macromol Biosci. 2005;5(6):490–493. doi: 10.1002/mabi.200400207. [DOI] [PubMed] [Google Scholar]
- 16.Tsai GJ, Zhang SL, Shieh PL. Antimicrobial activity of a low-molecular-weight chitosan obtained from cellulase digestion of chitosan. J Food Prot. 2004;67(2):396–398. doi: 10.4315/0362-028x-67.2.396. [DOI] [PubMed] [Google Scholar]
- 17.Altiok D, Altiok E, Tihminlioglu F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J Mater Sci Mater Med. 2010;21(7):2227–2236. doi: 10.1007/s10856-010-4065-x. [DOI] [PubMed] [Google Scholar]
- 18.Rossi S, Marciello M, Sandri G, et al. Wound dressings based on chitosans and hyaluronic acid for the release of chlorhexidine diacetate in skin ulcer therapy. Pharm Dev Technol. 2007;12(4):415–422. doi: 10.1080/10837450701366903. [DOI] [PubMed] [Google Scholar]
- 19.Ong SY, Wu J, Moochhala SM, et al. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29(32):4323–4332. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 20••.Burkatovskaya M, Tegos GP, Swietlik E, et al. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27(22):4157–4164. doi: 10.1016/j.biomaterials.2006.03.028. First study to evaluate the efficacy of acetate bandage on bacterial wound infections by using bioluminescent pathogenic bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai T, Tegos GP, Burkatovskaya M, et al. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother. 2009;53(2):393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cakmak A, Cirpanli Y, Bilensoy E, et al. Antibacterial activity of triclosan chitosan coated graft on hernia graft infection model. Int J Pharm. 2009;381(2):214–219. doi: 10.1016/j.ijpharm.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 23.Aimin C, Chunlin H, Juliang B, et al. Antibiotic loaded chitosan bar. An in vitro, in vivo study of a possible treatment for osteomyelitis. Clin Orthop Relat Res. 1999;(366):239–247. [PubMed] [Google Scholar]
- 24.Rossi S, Marciello M, Bonferoni MC, et al. Thermally sensitive gels based on chitosan derivatives for the treatment of oral mucositis. Eur J Pharm Biopharm. 2010;74(2):248–254. doi: 10.1016/j.ejpb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Okamura T, Masui T, St John MK, et al. Evaluation of effects of chitosan in preventing hemorrhagic cystitis in rats induced by cyclophosphamide. Hinyokika Kiyo. 1995;41(4):289–296. [PubMed] [Google Scholar]
- 26.Akncbay H, Senel S, Ay ZY. Application of chitosan gel in the treatment of chronic periodontitis. J Biomed Mater Res B Appl Biomater. 2007;80(2):290–296. doi: 10.1002/jbm.b.30596. [DOI] [PubMed] [Google Scholar]
- 27.Boynuegri D, Ozcan G, Senel S, et al. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: a pilot study. J Biomed Mater Res B Appl Biomater. 2009;90(1):461–466. doi: 10.1002/jbm.b.31307. [DOI] [PubMed] [Google Scholar]
- 28.Boateng JS, Matthews KH, Stevens HN, et al. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97(8):2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 29.Santos TC, Marques AP, Silva SS, et al. In vitro evaluation of the behaviour of human polymorphonuclear neutrophils in direct contact with chitosan-based membranes. J Biotechnol. 2007;132(2):218–226. doi: 10.1016/j.jbiotec.2007.07.497. [DOI] [PubMed] [Google Scholar]
- 30.Ueno H, Murakami M, Okumura M, et al. Chitosan accelerates the production of osteopontin from polymorphonuclear leukocytes. Biomaterials. 2001;22(12):1667–1673. doi: 10.1016/s0142-9612(00)00328-8. [DOI] [PubMed] [Google Scholar]
- 31.Ueno H, Yamada H, Tanaka I, et al. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999;20(15):1407–1414. doi: 10.1016/s0142-9612(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 32.Peluso G, Petillo O, Ranieri M, et al. Chitosan-mediated stimulation of macrophage function. Biomaterials. 1994;15(15):1215–1220. doi: 10.1016/0142-9612(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 33.Kojima K, Okamoto Y, Kojima K, et al. Effects of chitin and chitosan on collagen synthesis in wound healing. J Vet Med Sci. 2004;66(12):1595–1598. doi: 10.1292/jvms.66.1595. [DOI] [PubMed] [Google Scholar]
- 34.Wiegand C, Winter D, Hipler UC. Molecular-weight-dependent toxic effects of chitosans on the human keratinocyte cell line HaCaT. Skin Pharmacol Physiol. 2010;23(3):164–170. doi: 10.1159/000276996. [DOI] [PubMed] [Google Scholar]
- 35.Howling GI, Dettmar PW, Goddard PA, et al. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials. 2001;22(22):2959–2966. doi: 10.1016/s0142-9612(01)00042-4. [DOI] [PubMed] [Google Scholar]
- 36.Nascimento EG, Sampaio TB, Medeiros AC, et al. Evaluation of chitosan gel with 1% silver sulfadiazine as an alternative for burn wound treatment in rats. Acta Cir Bras. 2009;24(6):460–465. doi: 10.1590/s0102-86502009000600007. [DOI] [PubMed] [Google Scholar]
- 37.Klokkevold PR, Vandemark L, Kenney EB, et al. Osteogenesis enhanced by chitosan (poly-N-acetyl glucosaminoglycan) in vitro. J Periodontol. 1996;67(11):1170–1175. doi: 10.1902/jop.1996.67.11.1170. [DOI] [PubMed] [Google Scholar]
- 38.Degim Z, Celebi N, Sayan H, et al. An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids. 2002;22(2):187–198. doi: 10.1007/s007260200007. [DOI] [PubMed] [Google Scholar]
- 39.Minagawa T, Okamura Y, Shigemasa Y, et al. Effects of molecular weight and deacetylation degree of chitin/chitosan on wound healing. Carbohydrate Polymers. 2007;67(4):640–644. [Google Scholar]
- 40.Alsarra IA. Chitosan topical gel formulation in the management of burn wounds. Int J Biol Macromol. 2009;45(1):16–21. doi: 10.1016/j.ijbiomac.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Azad AK, Sermsintham N, Chandrkrachang S, et al. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater. 2004;69(2):216–222. doi: 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- 42.Lim CK, Yaacob NS, Ismail Z, et al. In vitro biocompatibility of chitosan porous skin regenerating templates (PSRTs) using primary human skin keratinocytes. Toxicol In Vitro. 2010;24(3):721–727. doi: 10.1016/j.tiv.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Shao HJ, Chen CS, Lee YT, et al. The phenotypic responses of human anterior cruciate ligament cells cultured on poly(epsilon-caprolactone) and chitosan. J Biomed Mater Res A. 2010;93(4):1297–1305. doi: 10.1002/jbm.a.32629. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto Y, Shibazaki K, Minami S, et al. Evaluation of chitin and chitosan on open would healing in dogs. J Vet Med Sci. 1995;57(5):851–854. doi: 10.1292/jvms.57.851. [DOI] [PubMed] [Google Scholar]
- 45.Mi FL, Shyu SS, Wu YB, et al. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials. 2001;22(2):165–173. doi: 10.1016/s0142-9612(00)00167-8. [DOI] [PubMed] [Google Scholar]
- 46.Burkatovskaya M, Castano AP, Demidova-Rice TN, et al. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16(3):425–431. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng CC, Yang MH, Chiu WT, et al. Composite nano-titanium oxide-chitosan artificial skin exhibits strong wound-healing effect-an approach with anti-inflammatory and bactericidal kinetics. Macromol Biosci. 2008;8(4):316–327. doi: 10.1002/mabi.200700188. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Khor E, Wee A, et al. Chitosan-alginate PEC membrane as a wound dressing: assessment of incisional wound healing. J Biomed Mater Res. 2002;63(5):610–618. doi: 10.1002/jbm.10382. [DOI] [PubMed] [Google Scholar]
- 49.Qin Y, Wang HW, Karuppanapandian T, et al. Chitosan green tea polyphenol complex as a released control compound for wound healing. Chin J Traumatol. 2010;13(2):91–95. [PubMed] [Google Scholar]
- 50.Lauto A, Stoodley M, Marcel H, et al. In vitro and in vivo tissue repair with laser-activated chitosan adhesive. Lasers Surg Med. 2007;39(1):19–27. doi: 10.1002/lsm.20418. [DOI] [PubMed] [Google Scholar]
- 51.Jin Y, Ling PX, He YL, et al. Effects of chitosan and heparin on early extension of burns. Burns. 2007;33(8):1027–1031. doi: 10.1016/j.burns.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Ribeiro MP, Espiga A, Silva D, et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009;17(6):817–824. doi: 10.1111/j.1524-475X.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- 53.Boucard N, Viton C, Agay D, et al. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials. 2007;28(24):3478–3488. doi: 10.1016/j.biomaterials.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 54.Diegelmann RF, Dunn JD, Lindblad WJ, et al. Analysis of the effects of chitosan on inflammation, angiogenesis, fibroplasia, and collagen deposition in polyvinyl alcohol sponge implants in rat wounds. Wound Repair Regen. 1996;4(1):48–52. doi: 10.1046/j.1524-475X.1996.40109.x. [DOI] [PubMed] [Google Scholar]
- 55.Okamoto Y, Tomita T, Minami S, et al. Effects of chitosan on experimental abscess with Staphylococcus aureus in dogs. J Vet Med Sci. 1995;57(4):765–767. doi: 10.1292/jvms.57.765. [DOI] [PubMed] [Google Scholar]
- 56•.Athanasiadis T, Beule AG, Robinson BH, et al. Effects of a novel chitosan gel on mucosal wound healing following endoscopic sinus surgery in a sheep model of chronic rhinosinusitis. Laryngoscope. 2008;118(6):1088–1094. doi: 10.1097/MLG.0b013e31816ba576. In vivo study using a large animal model (sheep) [DOI] [PubMed] [Google Scholar]
- 57.Bartone FF, Adickes ED. Chitosan: effects on wound healing in urogenital tissue: preliminary report. J Urol. 1988;140(5 Pt 2):1134–1137. doi: 10.1016/s0022-5347(17)41980-x. [DOI] [PubMed] [Google Scholar]
- 58.Sall KN, Kreter JK, Keates RH. The effect of chitosan on corneal wound healing. Ann Ophthalmol. 1987;19(1):31–33. [PubMed] [Google Scholar]
- 59.Wang X, Yu X, Yan Y, et al. Liver tissue responses to gelatin and gelatin/chitosan gels. J Biomed Mater Res A. 2008;87(1):62–68. doi: 10.1002/jbm.a.31712. [DOI] [PubMed] [Google Scholar]
- 60.Wang D, Wen Y, Lan X, et al. Experimental study on bone marrow mesenchymal stem cells seeded in chitosan-alginate scaffolds for repairing spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24(2):190–196. [PubMed] [Google Scholar]
- 61.Lu S, Gao W, Gu HY. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns. 2008;34(5):623–628. doi: 10.1016/j.burns.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Hirose K, Onishi H, Sasatsu M, et al. In vivo evaluation of Kumazasa extract and chitosan films containing the extract against deep skin ulcer model in rats. Biol Pharm Bull. 2007;30(12):2406–2411. doi: 10.1248/bpb.30.2406. [DOI] [PubMed] [Google Scholar]
- 63.Kim IY, Yoo MK, Seo JH, et al. Evaluation of semi-interpenetrating polymer networks composed of chitosan and poloxamer for wound dressing application. Int J Pharm. 2007;341(1–2):35–43. doi: 10.1016/j.ijpharm.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 64.Kiyozumi T, Kanatani Y, Ishihara M, et al. The effect of chitosan hydrogel containing DMEM/F12 medium on full-thickness skin defects after deep dermal burn. Burns. 2007;33(5):642–648. doi: 10.1016/j.burns.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Kweon DK, Song SB, Park YY. Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomaterials. 2003;24(9):1595–1601. doi: 10.1016/s0142-9612(02)00566-5. [DOI] [PubMed] [Google Scholar]
- 66.Sung JH, Hwang MR, Kim JO, et al. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm. 2010;392(1–2):232–240. doi: 10.1016/j.ijpharm.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 67.Kang YO, Yoon IS, Lee SY, et al. Chitosan-coated poly(vinyl alcohol) nanofibers for wound dressings. J Biomed Mater Res B Appl Biomater. 2010;92(2):568–576. doi: 10.1002/jbm.b.31554. [DOI] [PubMed] [Google Scholar]
- 68.Hong HJ, Jin SE, Park JS, et al. Accelerated wound healing by smad3 antisense oligonucleotides-impregnated chitosan/alginate polyelectrolyte complex. Biomaterials. 2008;29(36):4831–4837. doi: 10.1016/j.biomaterials.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 69.Khan TA, Peh KK. A preliminary investigation of chitosan film as dressing for punch biopsy wounds in rats. J Pharm Pharm Sci. 2003;6(1):20–26. [PubMed] [Google Scholar]
- 70.Biagini G, Bertani A, Muzzarelli R, et al. Wound management with N-carboxybutyl chitosan. Biomaterials. 1991;12(3):281–286. doi: 10.1016/0142-9612(91)90035-9. [DOI] [PubMed] [Google Scholar]
- 71.Stone CA, Wright H, Clarke T, et al. Healing at skin graft donor sites dressed with chitosan. Br J Plast Surg. 2000;53(7):601–606. doi: 10.1054/bjps.2000.3412. [DOI] [PubMed] [Google Scholar]
- 72•.Valentine R, Athanasiadis T, Moratti S, et al. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am J Rhinol Allergy. 2010;24(1):70–75. doi: 10.2500/ajra.2010.24.3422. Clinical study of 40 patients aimed to determine the efficacy of a chitosan/dextran gel on the homeostasis and wound healing after endoscopic surgery. This is the largest published clinical study on the wound-healing effect of chitosan. [DOI] [PubMed] [Google Scholar]
- 73.Noel SP, Courtney H, Bumgardner JD, et al. Chitosan films: a potential local drug delivery system for antibiotics. Clin Orthop Relat Res. 2008;466(6):1377–1382. doi: 10.1007/s11999-008-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noel SP, Courtney HS, Bumgardner JD, et al. Chitosan sponges to locally deliver amikacin and vancomycin: a pilot in vitro evaluation. Clin Orthop Relat Res. 2010;468(8):2074–2080. doi: 10.1007/s11999-010-1324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith JK, Bumgardner JD, Courtney HS, et al. Antibiotic-loaded chitosan film for infection prevention: a preliminary in vitro characterization. J Biomed Mater Res B Appl Biomater. 2010;94(1):203–211. doi: 10.1002/jbm.b.31642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Vimala K, Mohan YM, Sivudu KS, et al. Fabrication of porous chitosan films impregnated with silver nanoparticles: a facile approach for superior antibacterial application. Colloids Surf B Biointerfaces. 2010;76(1):248–258. doi: 10.1016/j.colsurfb.2009.10.044. Recent study on the combination of chitosan and silver nanoparticles for antimicrobial applications. [DOI] [PubMed] [Google Scholar]
- 77.Thomas V, Yallapu MM, Sreedhar B, et al. Fabrication, characterization of chitosan/nanosilver film and its potential antibacterial application. J Biomater Sci Polym Ed. 2009;20(14):2129–2144. doi: 10.1163/156856209X410102. [DOI] [PubMed] [Google Scholar]
- 78.Greene AH, Bumgardner JD, Yang Y, et al. Chitosan-coated stainless steel screws for fixation in contaminated fractures. Clin Orthop Relat Res. 2008;466(7):1699–1704. doi: 10.1007/s11999-008-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tunney MM, Brady AJ, Buchanan F, et al. Incorporation of chitosan in acrylic bone cement: effect on antibiotic release, bacterial biofilm formation and mechanical properties. J Mater Sci Mater Med. 2008;19(4):1609–1615. doi: 10.1007/s10856-008-3394-5. [DOI] [PubMed] [Google Scholar]
- 80•.Mi FL, Wu YB, Shyu SS, et al. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J Biomed Mater Res. 2002;59(3):438–449. doi: 10.1002/jbm.1260. Study on the development of a bilayer chitosan membrane, consisting of a dense upper layer (skin layer) and a sponge-like lower layer, as a topical delivery of silver sulfadiazine for the control of wound infections. [DOI] [PubMed] [Google Scholar]
- 81.Aoyagi S, Onishi H, Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm. 2007;330(1–2):138–145. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 82.Mizuno K, Yamamura K, Yano K, et al. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res A. 2003;64(1):177–181. doi: 10.1002/jbm.a.10396. [DOI] [PubMed] [Google Scholar]
- 83•.Park CJ, Clark SG, Lichtensteiger CA, et al. Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater. 2009;5(6):1926–1936. doi: 10.1016/j.actbio.2009.03.002. Recent study on the use of chitosan scaffolds to deliver basic fibroblast growth factor to wounds in mice. [DOI] [PubMed] [Google Scholar]
- 84.Alemdaroglu C, Degim Z, Celebi N, et al. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32(3):319–327. doi: 10.1016/j.burns.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 85.Obara K, Ishihara M, Fujita M, et al. Acceleration of wound healing in healing-impaired db/db mice with a photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2. Wound Repair Regen. 2005;13(4):390–397. doi: 10.1111/j.1067-1927.2005.130406.x. [DOI] [PubMed] [Google Scholar]
- 86.Dai M, Zheng X, Xu X, et al. Chitosan-alginate sponge: preparation and application in curcumin delivery for dermal wound healing in rat. J Biomed Biotechnol. 2009;2009:595126. doi: 10.1155/2009/595126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jayakumar R, Reis RL, Mano JF. Synthesis and characterization of pH-sensitive thiol-containing chitosan beads for controlled drug delivery applications. Drug Deliv. 2007;14(1):9–17. doi: 10.1080/10717540600739872. [DOI] [PubMed] [Google Scholar]
- 88.Peh K, Khan T, Ch’ng H. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J Pharm Pharm Sci. 2000;3(3):303–311. [PubMed] [Google Scholar]
- 89.Wang W. A novel hydrogel crosslinked hyaluronan with glycol chitosan. J Mater Sci Mater Med. 2006;17(12):1259–1265. doi: 10.1007/s10856-006-0600-1. [DOI] [PubMed] [Google Scholar]
- 90.Wittaya-areekul S, Prahsarn C. Development and in vitro evaluation of chitosan-polysaccharides composite wound dressings. Int J Pharm. 2006;313(1–2):123–128. doi: 10.1016/j.ijpharm.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 91.Jayakumar R, Nwe N, Tokura S, et al. Sulfated chitin and chitosan as novel. Biomaterials Int J Biol Macromol. 2007;40(3):175–181. doi: 10.1016/j.ijbiomac.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 92.Keong LC, Halim AS. In vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management. Int J Mol Sci. 2009;10(3):1300–1313. doi: 10.3390/ijms10031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu G, Ling K, Zhao P, et al. A novel in situ-formed hydrogel wound dressing by the photocross-linking of a chitosan derivative. Wound Repair Regen. 2010;18(1):70–79. doi: 10.1111/j.1524-475X.2009.00557.x. [DOI] [PubMed] [Google Scholar]
- 94.Meng X, Tian F, Yang J, et al. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J Mater Sci Mater Med. 2010;21(5):1751–1759. doi: 10.1007/s10856-010-3996-6. [DOI] [PubMed] [Google Scholar]
- 95.Sajomsang W, Tantayanon S, Tangpasuthadol V, et al. Quaternization of N-aryl chitosan derivatives: synthesis, characterization, and antibacterial activity. Carbohydr Res. 2009;344(18):2502–2511. doi: 10.1016/j.carres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Zambito Y, Di Colo G. Thiolated quaternary ammonium–chitosan conjugates for enhanced precorneal retention, transcorneal permeation and intraocular absorption of dexamethasone. Eur J Pharm Biopharm. 2010;75(2):194–199. doi: 10.1016/j.ejpb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 97.Belalia R, Grelier S, Benaissa M, et al. New bioactive biomaterials based on quaternized chitosan. J Agric Food Chem. 2008;56(5):1582–1588. doi: 10.1021/jf071717+. [DOI] [PubMed] [Google Scholar]
- 98.Sajomsang W, Gonil P, Tantayanon S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: preparation and characterization. Int J Biol Macromol. 2009;44(5):419–427. doi: 10.1016/j.ijbiomac.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Jia Z, Shen D, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res. 2001;333(1):1–6. doi: 10.1016/s0008-6215(01)00112-4. [DOI] [PubMed] [Google Scholar]
- 100.Rassu G, Gavini E, Jonassen H, et al. New chitosan derivatives for the preparation of rokitamycin loaded microspheres designed for ocular or nasal administration. J Pharm Sci. 2009;98(12):4852–4865. doi: 10.1002/jps.21751. [DOI] [PubMed] [Google Scholar]
- 101.Song Q, Zhang Z, Gao J, et al. Synthesis and property studies of N-carboxymethyl chitosan. J Appl Polym Sci. 2011;119(6):3282–3285. [Google Scholar]
- 102.Di Colo G, Zambito Y, Zaino C. Polymeric enhancers of mucosal epithelia permeability: synthesis, transepithelial penetration-enhancing properties, mechanism of action, safety issues. J Pharm Sci. 2008;97(5):1652–1680. doi: 10.1002/jps.21043. [DOI] [PubMed] [Google Scholar]
- 103.Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 2001;52(2):117–126. doi: 10.1016/s0169-409x(01)00231-9. [DOI] [PubMed] [Google Scholar]
- 104.Pusateri AE, McCarthy SJ, Gregory KW, et al. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma. 2003;54(1):177–182. doi: 10.1097/00005373-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 105.Zhong Z, Li P, Xing R, et al. Antimicrobial activity of hydroxylbenzenesulfonailides derivatives of chitosan, chitosan sulfates and carboxymethyl chitosan. Int J Biol Macromol. 2009;45(2):163–168. doi: 10.1016/j.ijbiomac.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 106.Zhong Z, Chen R, Xing R, et al. Synthesis and antifungal properties of sulfanilamide derivatives of chitosan. Carbohydr Res. 2007;342(16):2390–2395. doi: 10.1016/j.carres.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 107.Muzzarelli RAA, Muzzarelli C, Tarsi R, et al. Fungistatic activity of modified chitosans against Saprolegnia parasitica. Biomacromolecules. 2000;2(1):165–169. doi: 10.1021/bm000091s. [DOI] [PubMed] [Google Scholar]
- 108.Beanes SR, Dang C, Soo C, Ting K. Skin repair and scar formation: the central role of TGF-β. Expert Rev Mol Med. 2003;5(8):1–22. doi: 10.1017/S1462399403005817. [DOI] [PubMed] [Google Scholar]