Abstract
BACKGROUND
Although controversial, evidence suggests statins may reduce the risk of advanced prostate cancer (PC), and recently statin use was associated with prostate-specific antigen (PSA) reductions among men without PC. The authors sought to examine the association between statin use and PSA recurrence after radical prostatectomy (RP).
METHODS
The authors examined 1319 men treated with RP from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Time to PSA recurrence was compared between users and nonusers of statin at surgery using Cox proportional hazards models adjusted for multiple clinical and pathological features.
RESULTS
In total, 236 (18%) men were taking statins at RP. Median follow-up was 24 months for statin users and 38 for nonusers. Statin users were older (P < .001) and underwent RP more recently (P < .001). Statin users were diagnosed at lower clinical stages (P = .009) and with lower PSA levels (P = .04). However, statin users tended to have higher biopsy Gleason scores (P = .002). After adjusting for multiple clinical and pathological factors, statin use was associated with a 30% lower risk of PSA recurrence (hazard ratio “HR”, 0.70; 95% confidence interval “CI”, 0.50–0.97; P = .03), which was dose dependent (relative to no statin use; dose equivalent<simvastatin 20 mg: HR, 1.08; 95% CI, 0.66–1.73; P = .78; dose equivalent = simvastatin 20 mg: HR, 0.57; 95% CI, 0.32–1.00; P = .05; dose equivalent>simvastatin 20 mg: HR, 0.50; 95% CI, 0.27–0.93; P = .03).
CONCLUSIONS
In this cohort of men undergoing RP, statin use was associated with a dose-dependent reduction in the risk of biochemical recurrence. If confirmed in other studies, these findings suggest statins may slow PC progression after RP.
Keywords: prostate neoplasms, radical prostatectomy, hydroxymethylglutaryl-coenzyme A reductase inhibitors, statins
The efficacy of statin medications in improving cholesterol profiles and reducing cardiovascular mortality is well described and backed by high-quality, level I evidence.1 Statins also have excellent safety profiles and are well tolerated,2 and because of this risk-benefit ratio are the most commonly prescribed drug class in the United States.3
Recently, attention has focused on whether statins influence cancer incidence and outcomes. Laboratory studies show statins have antineoplastic properties that are both cholesterol-mediated4,5 and non-cholesterol-mediated.6
Epidemiologic studies examining whether statins lower risk of overall prostate cancer (PC) have been conflicting.1,7–9 However, several large prospective cohort studies found statins may be associated with reduced risk of advanced PC, although the numbers of men with advanced disease are small.10–14 Recently, we found that in men without PC, prostate-specific antigen (PSA) levels declined after starting statins, and this decline was independently proportional to the statin dose and the amount by which cholesterol levels lowered.15 This may represent an objective marker of statins' influence on prostate biology, but the PSA lowering could theoretically complicate cancer diagnosis.
Because of the complexity of these potential PSA changes and the different risks for overall versus advanced PC, it is more feasible to examine the influence of statins in subsets of men, including those already diagnosed and undergoing treatment.
Some studies suggest that statins may reduce biochemical progression in men undergoing external beam radiotherapy,16,17 although not all studies agree.18 One prior study examined statin use among men undergoing radical prostatectomy (RP) and found no change in the risk of biochemical recurrence; however, statin dose, duration of therapy, and other important potential confounding variables were not controlled for.19 Thus, we sought to study the association between statin use and outcomes in men undergoing RP within the Shared Equal Access Regional Cancer Hospital (SEARCH) database.20
MATERIALS AND METHODS
Study Population
After obtaining institutional review board approval from each institution, data from patients undergoing RP between 1988 and 2008 at 5 Veterans Administration (VA) Medical Centers across the country were combined into SEARCH.20 SEARCH does not include patients treated with preoperative androgen deprivation or radiation therapy. Within SEARCH, 2320 patients were available for analysis. However, statin data were unavailable from 1 center and thus, patients from that center were excluded (n = 345). We also excluded 53 (2%) patients in whom PC was diagnosed after transurethral resection of the prostate, 214 (9%) with unknown clinical stage, 39 (2%) with unknown biopsy Gleason scores, 2 (<1%) with unknown race, 46 (2%) with unknown preoperative PSA, and 38 (1%) with missing follow-up data. Finally, we excluded 264 (11%) men treated before 1996, as very few men (6 of 264) before 1996 were on statins, resulting in a population of 1319.
Follow-Up
As this was a retrospective analysis, follow-up protocols were not predetermined and were left to the discretion of the treating physicians at each of the 4 centers. Biochemical recurrence was defined as a single PSA >0.2 ng/mL, 2 concentrations at 0.2 ng/mL, or secondary treatment for detectable postoperative PSA. Men receiving adjuvant therapy ≤6 months after surgery for an undetectable PSA were considered as nonrecurrent at the time of adjuvant therapy, and their follow-up was censored at that point.
Statistical Analysis
Type and dose of statin and duration of use before and after surgery were ascertained by examining the pharmacy database within the VA computerized medical records. Statin use of ≥1 day before surgery was considered sufficient to be classified as a statin user. Doses were translated into dose equivalents (DEs) based on published guidelines, with simvastatin 20 mg being assigned a value of 1.21 Because few patients were on very high (≥4) or very low (<0.5) DEs, DEs were analyzed categorically as <1, 1, and >1. Differences in demographic and clinicopathological factors between statin users and nonusers were examined using t tests and chi-square tests for continuous and categorical variables, respectively. For non-normally distributed continuous variables, rank sum tests were used. Differences in recurrence risk between statin users and nonusers were analyzed using Cox proportional hazards analyses.22 The relationship between statin use and biochemical recurrence was tested to ensure that the assumptions of the proportional hazards model were not violated. With 236 statin users and 1083 nonusers accrued over a 12-year period (1996–2008) with a maximum follow-up of 12 years, using a 2-sided log-rank test of the difference in biochemical recurrence-free survival with a P value of .05, our power is 0.82 to detect a hazard ratio (HR) of 0.70 in favor of statin users.
Analyses predicting recurrence risk were adjusted for either clinical factors (age at surgery, year of surgery, race, body mass index [BMI], biopsy Gleason score, preoperative PSA, center, clinical stage, percentage of biopsy cores containing cancer) or clinical and pathological factors (pathological Gleason score, extracapsular extension, seminal vesicle invasion, positive margins, positive lymph nodes). Center (1–4), BMI (<25, 25.0–29.9, 30–34.9, ≥35 kg/m2), race (white, black, nonwhite-nonblack), clinical stage (T1c, T2/T3), and Gleason score (≤6, 7, ≥8) were analyzed as categorical variables, whereas age, year of surgery, percent of biopsy cores containing cancer, and the natural log of PSA were analyzed as continuous variables.
The association between statin use and time to PSA recurrence adjusted for the multiple clinical and pathological features was illustrated graphically by computing the Kaplan-Meier survival curve23 for the referent group (statin nonusers). For statin users, the curve was estimated by multiplying the survival function of the referent group (statin nonusers) by the HR for statin use obtained from the multivariate Cox model (Multivariate 2). The influence of statin dose on this relationship was modeled by creating a 4-tier categorical variable (no statin use, <1 DE, 1 DE, >1 DE).
As some studies suggest that statins may preferentially affect aggressive prostate cancer,10–14 we determined if the association between statin use and recurrence differed depending on pre- and postoperative risk assessment. We stratified patients first into preoperative risk categories according to D'Amico et al24 (low risk “PSA ≤10, clinical stage T1c/T2a, and Gleason score ≤6”, intermediate risk “PSA >10 but ≤20, clinical stage T2b, or Gleason score 7”, high risk “PSA >20, clinical stage ≤T2c, or Gleason score ≥8”) and postoperatively by whether the disease was organ confined (organ confined “T2, margin negative, lymph node negative” vs non-organ confined “T2 with positive margins, T3a/T3b or lymph node positive”). Similar stratified analyses were done for age (median ≤61 vs >61 years), race (white, black, nonwhite-nonblack), and BMI (<25, 25.0–29.9, 30–34.9, and ≥35 kg/m2).
All statistical analyses were performed using Stata 9.2 (StataCorp, College Station, Tex).
RESULTS
Clinical and Pathological Characteristics
A total of 236 (18%) men were taking statins at the time of surgery. On average, statin users were 2.0 years older (P < .001), yet had significantly lower median PSA levels (P = .04). Statin users had a higher probability of being white (P < .001) and higher BMI (P = .005), presented with lower clinical stage (P = .009), yet higher biopsy Gleason scores (P = .002), and were operated on more recently (P < .001) (Table 1).
Table 1.
Differences in Demographic, Clinical, and Pathological Features Between Statin Users and Nonusers (n = 1319)
| Feature | Statin Use at Surgery | Pa | |
|---|---|---|---|
| No | Yes | ||
| Patients, No. (%) | 1083 (82) | 236 (18) | |
| Mean age at surgery, y ± SD | 60.6 ± 6.6 | 62.6 ± 5.6 | <.001b |
| Type of statin, No. (%) | |||
| Simvastatin | 171 (72) | ||
| Lovastatin | 35 (15) | ||
| Atorvastatin | 12 (5) | ||
| Pravastatin | 4 (2) | ||
| Fluvastatin | 4 (2) | ||
| Rosuvastatin | 1 (<1) | ||
| Unknown | 9 (4) | ||
| Statin dose equivalent, No. (%) | |||
| Less than simvastatin 20 mg | 74 (33) | ||
| Equal to simvastatin 20 mg | 80 (35) | ||
| Greater than simvastatin 20 mg | 73 (32) | ||
| Statin use prior to surgery, median mo (IQR) | 25 (7–49) | ||
| Biopsy-surgery interval, median d (IQR) | 78 (51–120) | 92 (63–129) | <.001c |
| Follow-up, median mo (IQR) | 38 (13–68) | 24 (11–52) | <.001c |
| Race, No. (%) | <.001 | ||
| White | 522 (48) | 125 (53) | |
| Black | 504 (47) | 84 (36) | |
| Nonwhite-nonblack | 57 (5) | 27 (11) | |
| Median year of surgery | 2002 | 2004 | <.001c |
| BMI categories, No. (%) | .005 | ||
| ≤24.9 kg/m2 | 271 (26) | 38 (17) | |
| 25.0–29.9 kg/m2 | 478 (46) | 103 (46) | |
| 30.0–34.9 kg/m2 | 196 (19) | 61 (27) | |
| ≥35.0 kg/m2 | 86 (8) | 22 (10) | |
| Median preoperative PSA (IQR) | 6.9 (4.9–10.5) | 6.2 (4.7–9.1) | .04c |
| Biopsy Gleason score, No. (%) | .001 | ||
| 2–6 | 678 (62) | 118 (50) | |
| 7 | 312 (29) | 88 (37) | |
| 8–10 | 93 (9) | 30 (13) | |
| Clinical stage, No. (%) | .009 | ||
| T1c | 627 (58) | 159 (67) | |
| T2/T3 | 456 (42) | 77 (33) | |
| Median cores positive, % (IQR) | 33 (17–50) | 33 (17–50) | .78c |
| Median prostate weight, g (IQR) | 38 (30–50) | 42 (32–55) | .007c |
| Pathological Gleason score, No. (%) | .003 | ||
| 2–6 | 425 (39) | 66 (28) | |
| 7 | 526 (49) | 141 (60) | |
| 8–10 | 121 (12) | 28 (12) | |
| Positive surgical margins, No. (%) | 475 (45) | 103 (44) | .80 |
| Seminal vesicle invasion, No. (%) | 92 (9) | 22 (9) | .73 |
| Extracapsular extension, No. (%) | 241 (23) | 51 (22) | .74 |
| Positive lymph node metastases, No. (%) | 10 (1) | 4 (2) | .30 |
SD indicates standard deviation; IQR, interquartile range; BMI, body mass index; PSA, prostate-specific antigen.
P value was determined using the chi-square test except when noted.
P value was determined using the Student t test.
P value was determined using the rank sum test.
At surgery, statin users were more likely to have high-grade disease in the pathological specimen (P = .001). However, there were no significant differences in the rates of extracapsular extension, positive margins, seminal vesicle invasion, or lymph node metastases (Table 1). After adjusting for multiple clinical characteristics, the increased risk of high-grade pathological disease was no longer statistically significant (P = .20), and there remained no significant differences in odds of extracapsular extension, positive margins, or seminal vesicle invasion. Overall, 260 (20%) men received radiation therapy after surgery, 158 (12%) received hormonal therapy, and 84 (6%) received both. After stratifying by statin use, there was no difference in the proportion receiving radiation (20% statin users vs 20% nonusers, P = .93) or hormonal therapy (11% statin users vs 12% nonusers; P = .47).
Biochemical Disease Recurrence
Biochemical recurrences occurred among 304 (23%) men: 37 (16%) statin users and 267 (25%) nonusers. Overall, statin use was not associated with biochemical recurrence (HR, 0.90; 95% confidence interval “CI”, 0.68–1.19; log-rank P = .45) (Table 2). However, after adjusting for multiple clinical (HR, 0.70; 95% CI, 0.51–0.98; P = .04) or clinical and pathological features (HR, 0.70; 95% CI, 0.50–0.97; P = .03), statin users had significantly lower biochemical recurrence risk (Fig. 1) (Tables 2 and 3).
Table 2.
Risk of Biochemical Recurrence for Statin Users Compared With Nonusers
| Analysis | HR (95% CI) | P |
|---|---|---|
| Crude | 0.90 (0.45–1.19) | .45 |
| Multivariate 1a | 0.70 (0.51–0.98) | .04 |
| Multivariate 2b | 0.70 (0.50–0.97) | .03 |
HR indicates hazard ratio; CI, confidence interval.
Multivariate 1 was adjusted for clinical characteristics: biopsy Gleason score, preoperative prostate-specific antigen, year of surgery, age at surgery, surgical center, clinical stage, race, body mass index, and percentage of biopsy cores containing cancer.
Multivariate 2 was adjusted for clinical and pathological characteristics: pathological Gleason score, extracapsular extension, seminal vesicle invasion, positive surgical margins, and lymph node metastases.
Figure 1.
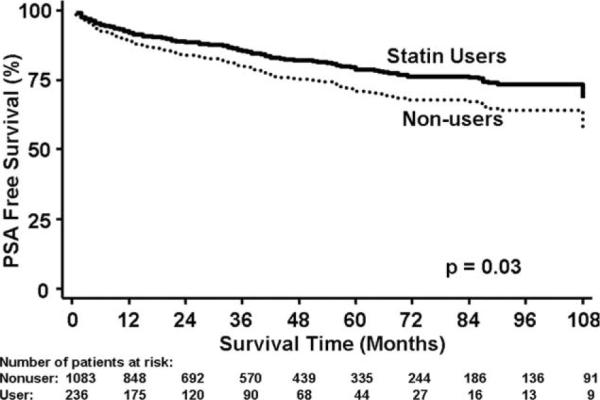
Association between statin medication use and prostate-specific antigen (PSA)-free survival is shown, adjusted for multiple clinical and pathological factors. The curve for the referent group (statin nonusers) represents the Kaplan-Meier survival function. For statin users, the curve is an estimate that was generated by multiplying the survival function of the referent group (statin nonusers) by the hazard ratio obtained from the multivariate Cox model.
Table 3.
Multivariate Model (Multivariate 2) Examining Risk of Biochemical Disease Recurrence for Statin Users Compared With Nonusers Adjusting for Multiple Clinical and Pathological Variables
| Unadjusted HR (95% CI) | Unadjusted P | Adjusted HR (95% CI) | Adjusted P | |
|---|---|---|---|---|
| Statin use | 0.92 (0.67–1.25) | .57 | 0.70 (0.50–0.97) | .03 |
| Age | 1.00 (0.99–1.02) | .58 | 1.01 (0.99–1.03) | .30 |
| Race (Ref = white) | ||||
| Black | 1.26 (1.00–1.58) | .05 | 1.13 (0.89–1.45) | .32 |
| Nonwhite/nonblack | 0.90 (0.56–1.45) | .66 | 1.12 (0.67–0.89) | .67 |
| Center (Ref = Center 1) | ||||
| Center 2 | 0.62 (0.41–0.94) | .02 | 0.57 (0.37–0.89) | .01 |
| Center 3 | 0.96 (0.71–1.30) | .80 | 0.79 (0.56–1.11) | .18 |
| Center 4 | 1.19 (0.92–1.56) | .19 | 0.90 (0.68–1.19) | .45 |
| Biopsy Gleason (Ref: <6) | ||||
| 7 | 2.79 (2.18–3.56) | <.001 | 1.88 (1.43–2.47) | <.001 |
| >7 | 3.89 (2.81–5.30) | <.001 | 2.21 (1.54–3.17) | <.001 |
| Clinical stage | 1.26 (1.00–1.57) | .04 | 1.09 (0.86–1.38) | .46 |
| BMI (Ref: ≤24.9 kg/m2) | ||||
| 25.0–29.9 kg/m2 | 1.34 (0.99–1.81) | .06 | 1.38 (1.01–1.87) | .04 |
| 30.0–34.9 kg/m2 | 1.80 (1.29–2.51) | .001 | 1.91 (1.36–2.69) | <.001 |
| ≥35.0 kg/m2 | 1.79 (1.16–2.77) | .01 | 1.87 (1.19–2.94) | .01 |
| PSAa | 2.06 (1.75–2.41) | <.001 | 1.56 (1.30–1.87) | <.001 |
| % cores positive | 4.26 (2.78–6.53) | <.001 | 1.41 (0.86–2.32) | .17 |
| Year of surgery | 1.01 (0.97–1.05) | .62 | 1.00 (0.96–1.05) | .85 |
| Pathology Gleason (Ref: <6) | ||||
| 7 | 3.55 (2.64–4.79) | <.001 | 1.82 (1.31–2.54) | <.001 |
| >7 | 6.95 (4.85–9.94) | <.001 | 2.76 (1.82–4.18) | <.001 |
| Extracapsular extension | 2.42 (1.93–3.05) | <.001 | 1.23 (0.93–1.62) | .14 |
| Seminal vesicle invasion | 4.16 (3.13–5.53) | <.001 | 2.04 (1.46–2.85) | <.001 |
| Positive margins | 3.06 (2.43–3.86) | <.001 | 2.01 (1.56–2.58) | <.001 |
| Lymph node invasion | 4.21 (1.99–8.92) | <.001 | 0.90 (0.39–2.05) | .80 |
HR indicates hazard ratio; CI, confidence interval; BMI, body mass index; PSA, prostate-specific antigen.
PSA was logarithmically transformed.
To determine which factor had the greatest impact on strengthening the inverse association between statin use and recurrence risk, we added each covariate to the multivariate model (Multivariate 2) 1 at a time. Adding Gleason score (either biopsy or pathological) generated the largest shift in HR for statin use. However, when stratified by pathological Gleason score (≤6, 7, ≥8), statin use on multivariate analysis still trended toward an inverse association with biochemical recurrence across all strata of Gleason scores (data not shown).
A post hoc analysis of the influence of statin dose on biochemical recurrence risk found only doses ≥1 DE were associated with lower risk, whereas a DE <1 was not associated with recurrence (Table 4). Thus, we repeated the full multivariate model categorizing statin use as nonuse/<1 DE versus ≥1 DE. In doing so, ≥1 DE of statin use associated with a 46% reduced risk of biochemical recurrence (HR, 0.54; 95% CI, 0.35–0.83; P = .005). We also found that duration of statin use before surgery was not associated with recurrence (data not shown). As 89% of men had documented statin use at least up to within 3 months of their last PSA follow-up, we could not assess the association between postsurgical statin duration and biochemical recurrence.
Table 4.
Association Between Dose of Statin and Risk of Biochemical Disease Recurrence Adjusted for Multiple Clinical and Pathological Factorsa
| Statin Dose | No. of Patients | HR (95% CI) | Pb |
|---|---|---|---|
| No statin | 1083 | 1.00 | — |
| Statin dose equivalent <1 | 74 | 1.08 (0.66–1.73) | .78 |
| Statin dose equivalent = 1 | 80 | 0.57 (0.32–1.00) | .05 |
| Statin dose equivalent >1 | 73 | 0.50 (0.27–0.93) | .03 |
HR indicates hazard ratio; CI, confidence interval.
As depicted in Table 2.
P values were determined using the Cox proportional hazards model.
We previously found that statin use in men without PC was associated with a 4.1% decline in PSA.15 Thus, for a PSA of 0.2 ng/mL, the cutoff used to define recurrence, a 4.1% decline would translate into an adjusted PSA of 0.192 ng/mL. Given a median PSA doubling time among recurrent patients of 15.1 months within SEARCH,20 this would translate into an approximate 27 day delay in diagnosing recurrence among statin users. After subtracting 27 days from the time to recurrence among all recurrent statin users and repeating the multivariate analysis adjusting for clinical and pathological factors, the results are essentially unchanged (data not shown).
The protective association observed for statin users overall held across strata of D'Amico preoperative risk categories (P-interaction = .47), whether the cancer was organ-confined at surgery (P-interaction = .53), and across race (P-interaction = .50) and age strata (P-interaction = .65). However, statin users in each of the 3 lowest BMI categories (n = 37: <25 kg/m2; n = 101: 25.0–29.9 kg/m2;n = 59: 30–34.9 = kg/m2) had a reduced recurrence risk, whereas statin users in the highest BMI category (n = 22: ≥35.0 kg/m2) had increased recurrence risk. When stratified into <35.0 versus ≥35.0 kg/m2, statin use in the highest BMI category (≥35.0 kg/m2) was associated with increased recurrence risk (HR, 17.3; 95% CI, 3.39–88.1; P = .001) relative to nonusers, whereas statin use among men with BMI <35.0 kg/m2 (HR, 0.60; 95% CI, 0.41–0.86; P = .006) was associated with decreased risk (P-interaction = .001). Similar results were obtained when statin use was categorized as ≥1 DE versus <1 DE/no use. Specifically, statin use ≥1 DE was associated with a 59% reduced risk of biochemical recurrence (HR, 0.41; 95% CI, 0.25–0.67; P < .001) among men with a BMI <35.0 kg/m2, but an increased risk of recurrence among men with a BMI ≥35.0 kg/m2 (HR, 15.7; 95% CI, 3.25–75.4; P = .001) (P-interaction = .001).
Statin users had shorter follow-up. In addition to using time-to-event analyses and controlling for year of surgery in all analyses, we further explored if the protective association observed for statin users was a result of follow-up bias by dividing our patients into groups based up 4-year blocks (1996–1999, 2000–2003, and 2004–2008; Table 5). There were roughly equal numbers of men in each group, and follow-up was similar for statin users and non-users except in the most recent group, wherein statin users had a 5 months shorter median follow-up. When stratified by year of treatment, statin use was associated with lower recurrence risk within each year stratum, although this did not always reach statistical significance because of smaller numbers, and men on higher doses of statins had reduced recurrence risk across all year strata.
Table 5.
Risk of Biochemical Disease Recurrence for Statin Users Stratified by Year of Surgery Adjusted for Clinical and Pathological Factorsa
| Year | No. of Patients | Median Follow-up, mo | Crude HR for Statin Users | Multivariate HR for Statin Users | Multivariate HR for Statin Dose Equivalents ≥20 μg | ||
|---|---|---|---|---|---|---|---|
| Nonuser | User | Nonuser | User | ||||
| 1996–1999 | 348 | 34 | 68 | 69 | 0.85 (0.66) | 0.52 (0.10) | 0.29 (0.06) |
| 2000–2003 | 382 | 77 | 44 | 44 | 0.87 (0.56) | 0.60 (0.06) | 0.47 (0.02) |
| 2004–2008 | 353 | 125 | 24 | 19 | 1.00 (0.97) | 0.73 (0.31) | 0.50 (0.07) |
As depicted in Table 2.
DISCUSSION
Mounting evidence suggests that statins may influence prostate biology, and although controversial, statins may reduce the risk of advanced PC.10–14 However, studying statins as primary prevention for PC poses logistical and financial challenges.25 We sought to examine the influence of statins in men with PC undergoing RP. In our cohort, statin users differed significantly from nonusers at presentation. Statin users had lower PSA and clinical stages, but were older, and had higher BMI and higher biopsy Gleason scores. After adjusting for these and other clinical and pathological variables, statin users were 30% less likely to recur after surgery (P = .03), while men taking ≥1 DE, were 46% less likely to recur (P = .005). If confirmed in other studies, a randomized controlled trial (RCT) of statins among men undergoing prostatectomy may be warranted.
Recent evidence has pointed to statins potentially preventing cancer. Laboratory evidence shows that statins inhibit inflammation,26 angiogenesis,27 cell proliferation,28 migration/adhesion,29 and invasion,30 and promote apoptosis selective for tumor cells.31 Studies with PC cells have also been encouraging, suggesting that statins may inhibit PC growth and development.32 The mechanisms through which statins inhibit cancer processes can be classified into cholesterol-mediated and non-cholesterol-mediated. By altering serum cholesterol levels, statins alter the cholesterol composition of cell membrane domains that are involved in intracellular signaling.5 Some of these signaling pathways have been implicated in PC development and growth.4,33 Statins may influence prostate biology through non-cholesterol-mediated, or direct, pathways too. For example, statins induce apoptosis through inhibition of cell cycle enzymes34 and have antiangiogenic and anti-inflammatory properties that may inhibit PC development and subsequent progression.6
Initial observational studies suggested that statins reduced PC risk.7,35 However, subsequent studies, including meta-analyses of RCTs of statins and cardiovascular outcomes, found no association between statin use and overall PC.1,9,36–44 Recently, 5 large prospective cohort studies confirmed that statins were not associated with overall PC risk, but observed that statins were associated with reduced risk of advanced disease, although the number of cases of advanced cancer were small.10–14 In these studies, the risk of advanced disease declined with longer duration and/or higher dose.
A few studies examined whether statins augment PC therapies. A study of 938 patients undergoing brachytherapy found that statin users (n = 191) had significantly lower PSA levels, a lower percentage of positive biopsy cores, and smaller prostates. Although they tended to have improved PC-specific and overall survival, this was not statistically significant in multivariate analysis.45 A study of 871 men undergoing external beam radiation therapy (EBRT) found improved 10-year PSA-free survival for statin users (76% vs 66%; P=.01), which held after multivariate adjustment (P=.03).16 Another study of 719 men undergoing EBRT found similar benefit for statin users after multivariate adjustment.17 However, a third study of 968 men undergoing EBRT found that statin use, in multivariate analysis, was not significantly predictive of PSA-free survival.18
A recent retrospective cohort study of 1351 men undergoing RP found statin users had lower preoperative PSA levels, tumor volumes, and percentage of prostate involved with cancer.46 Furthermore, statin users had lower rates of adverse pathological features in the RP specimen. However, biochemical recurrence was not evaluated as an outcome in this study.
In our study, despite being older with higher BMI and Gleason scores, 3 adverse risk factors for recurrence, on crude analysis statin users trended toward reduced recurrence risk (HR, 0.90; P=.45). After adjusting for the differing clinical and pathological factors, statin users had a significant 30% lower recurrence risk (P=.03). Of note, another series by Mondul et al also found a generally similar association between statin use and decreased recurrence risk after prostatectomy (unpublished data).
We also observed that statin use at doses <1DE were not associated with recurrence risk, whereas 1 and >1 DE appeared to equally reduce recurrence risk. This apparent dose threshold at simvastatin 20 mg (ie, 1 DE) is identical to the dose relationship we previously observed in our study of statin use and PSA levels.15 Although also a VA-based study, that study cohort was comprised of markedly different men: healthy men without PC. Thus, as we have now seen similar findings across 2 populations, this may reflect a statin dose threshold to influence prostate biology, although more study is needed.
Time to recurrence after surgery correlates with risk of PC progression and death.47 With 35% of men experiencing biochemical recurrence within 10 years after RP, there is a clear potential for statins.48 Given that statins appear to lower PSA values,15 1 potential explanation is statins merely delay the diagnosis of recurrence. However, on average, statin use lowers PSA by only 4%,15 thus in men with PSA values around 0.2 ng/mL, a cutoff for determining biochemical recurrence, it is unlikely that statin use would meaningfully alter observed time to recurrence. Further studies examining the PSA doubling time, time to metastases, or time to death among statin users with recurrent PC are needed.
Although we did not identify any modification of the statin effect across different categories of pre- or postoperative risk, age, or race, we did notice differences across BMI categories. Specifically, for those in the highest BMI category (≥ 35 kg/m2), statins were associated with an increased recurrence risk. This finding may be spurious, as only 22 men in this BMI category used statins. However, a case-control study examining statin use and risk of PC diagnosis found statins protective for lower BMI categories, but for obese men (BMI ≥30 kg/m2) statin use was associated with increased risk (odds ratio, 1.8; 95% CI, 1.1–3.0).49 Another prospective cohort study, however, found no interaction between statin use and BMI in the setting of risk of PC diagnosis.10 Thus, the relationship between statins and PC among obese men warrants further investigation.
Statin users differed significantly from nonusers. Although we controlled for multiple clinical and pathological variables, it is possible that residual confounding could explain the reduced recurrence risk. Specifically, statin users may have had differences in diet, exercise, smoking, and screening behaviors. Unfortunately we were not able to capture these data. Poor diet, lack of exercise, and smoking have all been linked to adverse prostate cancer outcomes. As statin use is indicated for hyperlipidemia and myocardial infarctions, conditions linked with the above lifestyle factors, it is possible that these poor risk lifestyle factors were more prevalent among statin users. Thus, one would expect statin users to be more likely to recur after surgery. Yet we observed the opposite. Pertaining to screening behavior, the prevailing assumption is that men on statins represent a group more likely to seek preventive care and thus more likely to be screened for both hyperlipidemia and PC.10 This theoretically would translate into earlier diagnoses at earlier disease stages. Indeed, statin users were diagnosed at lower clinical stages. However, if we apply the same assumed bias postoperatively, statin users would have greater postoperative surveillance, potentially again biasing against statin users, as the closer patients are followed after surgery, the sooner a recurrence would be detected. Thus, taken together, although we unfortunately cannot capture information on diet, exercise, smoking, and screening behavior, we estimate that if available and adjusted for, these data would only have further strengthened the inverse association between statins and recurrence. In addition, we did not assess if nonusers of statin at surgery subsequently started statins. As such, our control group may be contaminated with statin users, causing a further underestimation of the effect of statins.
Our study did not have sufficient power to examine PC-specific or overall mortality, as too few men met these endpoints. In our cohort, the median follow-up of 3 years was relatively short. However, prior data with 6-year mean follow-up found that nearly 2/3 of recurrences occur within 3 years after surgery, and those recurring after 3 years are less likely to be fatal and thus of unclear clinical significance.47 Thus, we believe we have captured the majority of recurrences. As statin use only became prevalent in the early 2000s, it is expected that nonusers would have longer follow-up and more recurrences overall. However, in addition to using time-to-event analyses and adjusting for year in all of our analyses, when we stratified our results by year of surgery, we observed that the protective association held across all strata of year of surgery.
Conclusions
In this group of men undergoing RP, statin use was significantly associated with a reduced biochemical recurrence risk, which was dose dependent. Our findings require confirmation in other settings and in particular to determine whether statins are associated with a reduction in metastases and/or PC-specific and overall mortality. Further retrospective studies may help elucidate the optimal timing to start a statin, but if the association between statin use and reduced recurrence risk holds regardless of the timing of statin use before surgery, an RCT placing men undergoing RP on statins may be warranted. Given that statins have proven efficacy in preventing cardiovascular mortality, if it is definitively proven that statins reduce recurrence after surgery, then the potential influence statins could have on overall and disease-specific mortality is substantial.
Acknowledgments
CONFLICT OF INTEREST DISCLOSURES Supported by the Department of Defense, Prostate Cancer Research Program (R.J.H., L.L.B., S.J.F.); Department of Veterans Affairs, National Institutes of Health (NIH) grant R01CA100938 (W.J.A.); NIH Specialized Programs of Research Excellence grant P50 CA92131-01A1 (W.J.A.); the Georgia Cancer Coalition (M.K.T.); and the American Urological Association Foundation/Astellas Rising Star in Urology Award (S.J.F.).
REFERENCES
- 1.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–2797. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed April 20, 2010];IMS Top 15 U.S. Pharmaceutical Products by Sales. Available at: http://www.imshealth.com/deployedfiles/imshealth/global/content/staticfile/top_line_data/top%2015%20products% 20by%20u.s.sales.pdf.
- 4.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hager MH, Solomon KR, Freeman MR. The role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab Care. 2006;9:379–385. doi: 10.1097/01.mco.0000232896.66791.62. [DOI] [PubMed] [Google Scholar]
- 6.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 7.Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318–325. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 8.Murtola TJ, Tammela TLJ, Maattanen L, Auvinen A. Statins and prostate cancer among men participating in the Finnish Prostate Cancer Screening Trial. Abstract presented at: American Urological Association Meeting; Anaheim, Calif.. May 19–24, 2007; Abstract 1719. [Google Scholar]
- 9.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 10.Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Bio-markers Prev. 2007;16:2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 12.Flick ED, Habel LA, Chan KA, et al. Statin use and risk of prostate cancer in the California Men's Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2218–2225. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 13.Murtola T, Tammela TLJ, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–2232. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 14.Friedman GD, Flick ED, Udaltsova N, Chan J, Quesen-berry CP, Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100:1511–1518. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 16.Shippy AM, Katz MS, Yamada Y, Feder DJ, Zelefsky MJ. Statin use and clinical outcomes after high dose radiotherapy for prostate cancer “abstract”. Int J Radiat Biol. 2007;69:S113. Abstract 203. [Google Scholar]
- 17.Gutt R, Weichselbaum RR, Liauw SL. Association of statins with biochemical failure after radiotherapy for prostate cancer “abstract”. Int J Radiat Biol. 2008;72(1 suppl):S297. Abstract 2287. [Google Scholar]
- 18.Soto DE, Daignault S, Sandler HM, Ray ME. No effect of statins on biochemical outcomes after radiotherapy for localized prostate cancer. Urology. 2009;73:158–162. doi: 10.1016/j.urology.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 19.Krane LS, Kaul SA, Stricker HJ, Peabody JO, Menon M, Agarwal PK. Men presenting for radical prostatectomy on preoperative statin therapy have reduced serum prostate specific antigen. J Urol. 2010;183:118–124. doi: 10.1016/j.juro.2009.08.151. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton RJ, Aronson WJ, Presti JC, Jr, et al. Race, biochemical disease recurrence, and prostate-specific antigen doubling time after radical prostatectomy: results from the SEARCH database. Cancer. 2007;110:2202–2209. doi: 10.1002/cncr.23012. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed August 1, 2007];Guidelines for the Diagnosis and Management of Dyslipidemia 2003. Available at: http://www.pplusic.com/uploads/media/LIPIDGuide.pdf.
- 22.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–485. [Google Scholar]
- 24.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton RJ, Freedland SJ. Review of recent evidence in support of a role for statins in the prevention of prostate cancer. Curr Opin Urol. 2008;18:333–339. doi: 10.1097/MOU.0b013e3282f9b3cc. [DOI] [PubMed] [Google Scholar]
- 26.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 27.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 28.Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci U S A. 1999;96:7797–7802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nubel T, Dippold W, Kleinert H, Kaina B, Fritz G. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004;18:140–142. doi: 10.1096/fj.03-0261fje. [DOI] [PubMed] [Google Scholar]
- 30.Kusama T, Mukai M, Iwasaki T, et al. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutarylcoenzyme A reductase inhibitors. Cancer Res. 2001;61:4885–4891. [PubMed] [Google Scholar]
- 31.Wong WW, Tan MM, Xia Z, Dimitroulakos J, Minden MD, Penn LZ. Cerivastatin triggers tumor-specific apoptosis with higher efficacy than lovastatin. Clin Cancer Res. 2001;7:2067–2075. [PubMed] [Google Scholar]
- 32.Hoque A, Chen H, Xu XC. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2008;17:88–94. doi: 10.1158/1055-9965.EPI-07-0531. [DOI] [PubMed] [Google Scholar]
- 33.Freeman MR, Cinar B, Lu ML. Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol Metab. 2005;16:273–279. doi: 10.1016/j.tem.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Lee SJ, Ha MJ, Lee J, et al. Inhibition of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase pathway induces p53-independent transcriptional regulation of p21(WAF1/CIP1) in human prostate carcinoma cells. J Biol Chem. 1998;273:10618–10623. doi: 10.1074/jbc.273.17.10618. [DOI] [PubMed] [Google Scholar]
- 35.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 36.Friis S, Poulsen AH, Johnsen SP, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 37.Olsen JH, Johansen C, Sorensen HT, et al. Lipid-lowering medication and risk of cancer. J Clin Epidemiol. 1999;52:167–169. doi: 10.1016/s0895-4356(98)00147-4. [DOI] [PubMed] [Google Scholar]
- 38.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 39.Coogan PF, Rosenberg L, Strom BL. Statin use and the risk of 10 cancers. Epidemiology. 2007;18:213–219. doi: 10.1097/01.ede.0000254694.03027.a1. [DOI] [PubMed] [Google Scholar]
- 40.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635–637. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor ML, Wells BJ, Smolak MJ. Statins and cancer: a meta-analysis of case-control studies. Eur J Cancer Prev. 2008;17:259–268. doi: 10.1097/CEJ.0b013e3282b721fe. [DOI] [PubMed] [Google Scholar]
- 42.Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120:833–843. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 43.Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer. 2008;44:2122–2132. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Boudreau DM, Yu O, Buist DS, Miglioretti DL. Statin use and prostate cancer risk in a large population-based setting. Cancer Causes Control. 2008;19:767–774. doi: 10.1007/s10552-008-9139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moyad MA, Merrick GS, Butler WM, et al. Statins, especially atorvastatin, may improve survival following brachytherapy for clinically localized prostate cancer. Urol Nurs. 2006;26:298–303. [PubMed] [Google Scholar]
- 46.Loeb S, Kan D, Helfand BT, Nadler RB, Catalona WJ. Is statin use associated with prostate cancer aggressiveness? BJU Int. 2010;105:1222–1225. doi: 10.1111/j.1464-410X.2009.09007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 48.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 49.Agalliu I, Salinas CA, Hansten PD, Ostrander EA, Stanford JL. Statin use and risk of prostate cancer: results from a population-based epidemiologic study. Am J Epidemiol. 2008;168:250–260. doi: 10.1093/aje/kwn141. [DOI] [PMC free article] [PubMed] [Google Scholar]


