Abstract
No consensus has yet been reached to associate oral bacteria conclusively with the etio-pathogenesis of bisphosphonate-induced osteonecrosis of the jaw (BONJ). Therefore, the present study examined the effects of oral bacteria on the development of BONJ-like lesions in a mouse model. In the pamidronate (Pam)-treated mice, but not control non-drug-treated mice, tooth extraction followed by oral infection with Fusobacterium nucleatum caused BONJ-like lesions and delayed epithelial healing, both of which were completely suppressed by a broad-spectrum antibiotic cocktail. Furthermore, in both in vitro and in vivo experiments, the combination of Pam and Fusobacterium nucleatum caused the death of gingival fibroblasts (GFs) and down-regulated their production of keratinocyte growth factor (KGF), which induces epithelial cell growth and migration. Therefore, in periodontal tissues pre-exposed to bisphosphonate, bacterial infection at tooth extraction sites caused diminished KGF expression in GFs, leading to a delay in the epithelial wound-healing process that was mitigated by antibiotics.
Keywords: bisphosphonate-induced osteonecrosis of the jaw, pamidronate, gingival fibroblast, KGF, wound healing, Fusobacterium nucleatum
Introduction
Bisphosphonate-induced osteonecrosis of the jaw (BONJ) is an emerging oral complication, particularly in patients receiving intravenous aminobisphosphonate (N-BP) therapy for osteoporosis, myeloma, and metastatic tumors in the bone (Coleman, 2008). Recent case-control studies reported the highest risk in the patients receiving intravenous N-BP for more than 2 yrs, accompanied by tooth extraction or local suppuration as an independent risk factor (Barasch et al., 2011), and demonstrated substantial risk-association for oral-bisphosphonate-treated non-cancer patients (Barasch et al., 2011; Fellows et al., 2011). In addition to osteoclast inhibition, retarded epithelial regeneration and diminished vascularity have been implicated as pathogenic features of BONJ (Fournier et al., 2002; Wood et al., 2002; Reid et al., 2007). Bisphosphonate (BP) is also known to cause esophagitis through BP’s toxicity to the epithelial tissues, a phenomenon which has long been known to occur in the digestive tract (Reszka et al., 2001; Moreira et al., 2005; Siris et al., 2009). Therefore, it is theorized that retarded epithelial regeneration may be a pathogenic factor in the occurrence of BONJ, starting with trauma to the alveolus, which results in local release of bound BP into the surrounding epithelium (Reid et al., 2007; Sonis et al., 2009).
Interactions between fibroblasts and keratinocytes are indispensable for epithelial regeneration (Rheinwald and Green, 1975; Smola et al., 1993), because fibroblast-derived soluble growth factors, such as keratinocyte growth factor (KGF), play a pivotal role in the differentiation and growth of keratinocytes (Werner and Smola, 2001). However, few studies thus far have addressed the possible involvement of gingival fibroblasts in the retarded epithelial healing process at the onset of BONJ.
Aseptic or radiation osteonecrosis is known to occur in most bones in the body, yet reported cases of BONJ have been mainly limited to the oral cavity, except for a handful of cases involving other bones (Polizzotto et al., 2006; Longo et al., 2009). As such, the presence of microflora distinct to the oral cavity has been implicated as a factor that may initiate or encourage the progress of BONJ. Biopsies and cultures sampled from BONJ lesions noted the presence of species such as Fusobacterium, Eikenella, Bacillus, Actinomyces, Staphylococcus, and Streptococcus (Marx et al., 2005; Hansen et al., 2006, 2007; Sedghizadeh et al., 2008). Although some of these bacteria were considered biofilm colonizers rather than invasive pathogens, none of the studies has yet elucidated the relevance of oral microflora in the context of BONJ.
Therefore, the aims of the present study were (1) to develop a mouse model of BONJ-like disease induced by a systemic administration with pamidronate (Pam) and (2) to elucidate the possible pathogenic effects mediated by oral bacteria on the onset of BONJ-like lesions.
To induce BONJ-like lesion, we pre-treated the mice with Pam administered subcutaneously or control saline injections for 4 wks, followed by maxillary molar tooth extraction and oral inoculation with or without live Fusobacterium nucleatum (Fn). Furthermore, the wound at the tooth extraction site was analyzed for KGF levels. We further undertook an in vitro experiment to investigate the viability and KGF production of GFs exposed to Fn with or without Pam.
Materials & Methods
Animals
C57BL/6j mice (6- to 8-week-old females; weight ~25 gm) were kept in a conventional room with a 12-hour light-dark cycle at constant temperature. The experimental procedures used were approved by the Forsyth Institutional Animal Care and Use Committee (IACUC).
Reagents
Pamidronate disodium (Pam) was provided by LKT Laboratories (St. Paul, MN, USA). KGF was purchased from R&D Systems (Minneapolis, MN, USA). The dosing level (1 mg/kg) was selected to replicate that used for human oncology dosing.
Bacterial Strain and Culture
Fn (ATCC 10953) was cultured in brain heart infusion medium (Difco Laboratory, Detroit, MI, USA) supplemented with yeast extract (Difco) under anaerobic conditions (5% H2, 10% CO2, and 85% N2).
Induction and Evaluation of BONJ-like Disease in Mice
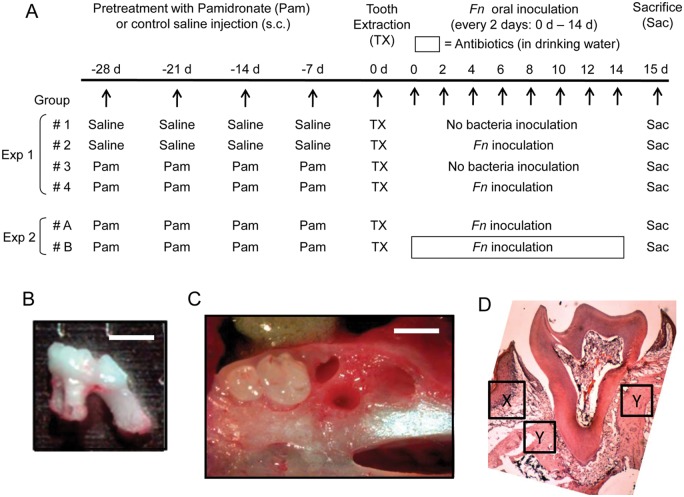
Mice were divided into 4 groups (n = 10/group) as follows: (Group 1) saline (control), (Group 2) Fn, (Group 3) Pam, and (Group 4) Pam+Fn. To Group 3 (Pam) and Group 4 (Pam+Fn), Pam (1 mg/kg/wk in saline) was subcutaneously (s.c.) injected weekly for 4 wks prior to tooth extraction; Group 1 (saline) and Group 2 (Fn) received control saline injection (s.c.) instead of Pam (Fig. 1A, diagram). On Day 0, the first right maxillary molar was extracted under anesthesia (ketamine/xylazine) without causing fracture in alveolar bone or tooth (Figs. 1B, 1C). After tooth extraction, each animal in Group 2 (Fn) or Group 4 (Pam+Fn) was inoculated orally with Fn (109 CFU/50 µL PBS with 5% carboxymethyl cellulose/animal) every other day until termination of the experiment scheduled at Day 15. In some independent experiments (Fig. 1A), Group A (n = 10; Pam+Fn) animals were supplied with control regular drinking water, while Group B (n = 10; Pam+Fn) received drinking water containing an antibiotic cocktail (Rakoff-Nahoum et al., 2004), consisting of ampicillin (1 g/L), metronidazole (1 g/L), vancomycin (0.5 g/L), and neomycin sulfate (1 g/L), after tooth extraction until termination of the animal (Day 0 – Day 15).
Figure 1.
Study design for in vivo experiment to induce BONJ-like lesion. (A) Experiment 1 (Exp 1). To examine the effects of Pam and Fn on the development BONJ-like lesions, we divided the mice into 4 groups (n = 10/group) as described in the “Materials & Methods” section: (Group 1) saline (control), (Group 2) Fn, (Group 3) Pam alone, and (Group 4) Pam+Fn. To Groups 3 and 4, Pam (1 mg/kg/wk in saline) was subcutaneously (s.c.) injected weekly for 4 wks prior to tooth extraction (-28, -21, -14, and -7 days). Group 1 (saline) and Group 2 (Fn) received control saline injection (s.c.) instead of Pam, following the same injection schedule as Pam (-28, -21, -14, and -7 days). On Day 0, the first right maxillary molar was extracted under anesthesia (ketamine/xylazine) without causing fracture in alveolar bone or tooth (B, C). After tooth extraction, each animal in Group 2 (Fn) or Group 4 (Pam+Fn) was inoculated orally with live Fn (109 CFU/50 µL PBS with 5% carboxymethyl cellulose/animal) every other day until termination of the experiment scheduled at Day 15. Experiment 2 (Exp 2). For examination of the effects of antibiotics on the BONJ-like lesions induced by Pam+Fn, both Group A (n = 10; Pam+Fn) and Group B (n = 10; Pam+Fn) received Pam injection (1 mg/kg/wk in saline s.c.) weekly for 4 wks prior to tooth extraction (-28, -21, -14, and -7 days). After tooth extraction, both groups received oral inoculation with live Fn (109 CFU) every other day until termination of the experiment scheduled at Day 15. After tooth extraction until termination (Day 0 to Day 15), Group A animals were supplied with control regular drinking water, while Group B received drinking water containing an antibiotic cocktail, consisting of ampicillin (1 g/L), metronidazole (1 g/L), vancomycin (0.5 g/L), and neomycin sulfate (1 g/L). (B) Image of extracted maxillary first molar is shown. (C) Image of extraction socket is shown. Soft tissue was removed so that structure of alveolar bone can be visualized. Bar = 1 mm. (D) Histological image (H&E staining) of first right maxillary molar of control mice receiving no treatment is shown. The specific location and area where gingival epithelium, connective tissue, and bone were evaluated in Fig. 3 as well as Appendix Fig. 4 are indicated by “X” (gingival epithelium and connective tissue) and “Y” (bone), respectively.
The following post mortem evaluations were undertaken: (A) bone mineral density; (B) clinical features of BONJ at the time of death; (C) histologic evaluation of maxillary tissues at tooth extraction site; (D) measurement of apoptotic cells in periodontal connective tissue, alveolar bone, and gingival epithelium by TUNEL staining; and (E) KGF levels in gingival tissues.
Bone Mineral Density (BMD) Measurements
To confirm the pharmaceutical effects of Pam by BMD, we scanned each animal using a Lunar-PIXImus2 densitometer (GE Healthcare Biosciences, Pittsburgh, PA, USA).
Clinical Observation
The extraction site for each animal was photographed and assessed. The extraction socket overlying epithelium was classified as either completely healed or unhealed.
Histological Evaluation and TUNEL Staining
The maxillae isolated from mice were decalcified in 10% ethylenediaminetetraacetic acid for 2 wks and embedded in OCT compound. Frozen sections (5-µm thickness) cut in a medial-distal direction were analyzed histochemically by Hematoxylin and Eosin (H&E) staining. TUNEL staining was performed by the use of an Apoptag-Plus kit (Millipore, Billerica, MA, USA), and the nuclei were counter-stained with methyl green. TUNEL-positive apoptotic cells were counted in the randomly selected 4 fields (0.01 mm2) in each specimen with an Olympus FSX100 microscope.
KGF ELISA
The concentrations of KGF in gingival tissue homogenates as well as cell culture supernatant were measured with an KGF ELISA kit (R&D).
Cultures of Gingival Fibroblasts and Epithelial Cells
Mouse gingival fibroblasts (GFs) isolated from the gingival explants of C57BL/6j mice were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum and antibiotics (penicillin and streptomycin). Confluent GFs in a 96-well plate were stimulated with or without Fn (106 CFU/mL) and/or Pam (10 nM) for 24 hrs, and the culture supernatant was harvested for ELISA. Mouse gingival epithelial cells (GE-1; RIKEN, Saitama, Japan) were cultured with keratinocyte-SFM (Invitrogen) supplemented with antibiotics. All cell cultures were incubated in a humidified 5% CO2 incubator at 37°C. The following evaluations were undertaken at the end of the in vitro experiments: (A) cell viability of GFs, (B) KGF production from GFs, and (C) wound-healing assay with GE-1.
Cell Viability Assay
After incubation of GFs cultured in 96-well plates with 5 mg/mL of MTT (Promega, Madison, WI, USA) for 3 hrs, the intensity of dissolved blue-formazan released from live cells was measured at a wavelength of 570 nm by means of the Synergy-HT plate reader (BioTek, Winooski, VT, USA).
In vitro Wound-healing Assay
The in vitro epithelial wound-healing assay was described previously (Kajiya et al., 2010). To generate a wound on the gingival epithelial sheet, we scratched the surface of a culture well with confluent GE-1 using a plastic micropipette tip with a small orifice, and then incubated it in keratinocyte-SFM in the presence or absence of KGF (1 ng/mL or 50 ng/mL) for 24 hrs (4 different cultures/group). After the culture image was captured by phase-contrast microscopy, the total number of migrating cells was measured with Image-J software (NIH, Bethesda, MD, USA).
Statistical Analysis
Results were subjected to the Student’s t test or one-way analysis of variance ANOVA, followed by Bonferroni’s post-test. We used Fisher’s exact test to evaluate if there were non-random association between two different groups of mice. Values of p < 0.05 were considered statistically significant.
Results
Bone Mineral Density (BMD) Measurements
At Day 15, the total body BMD of animals that received Pam alone (Group 3) and Pam+Fn (Group 4) significantly increased (53.5 ± 1.4 mg/cm2) and (52.3 ± 2.2 mg/cm2, respectively) compared with saline control (47.8 ± 1.4 mg/cm2) (p < 0.05). In contrast, the BMD of Group 2 (Fn only; 45.0 ± 2.7 mg/cm2) did not demonstrate a statistically significant difference in BMD compared with control Group 1 (p > 0.05). The radiographic images of maxillary jaws isolated from all 4 groups also corresponded to the results of BMD (Appendix, Supplemental Results, section A, and Appendix Fig. 1).
Clinical Evaluation for Presence of BONJ-like Lesions
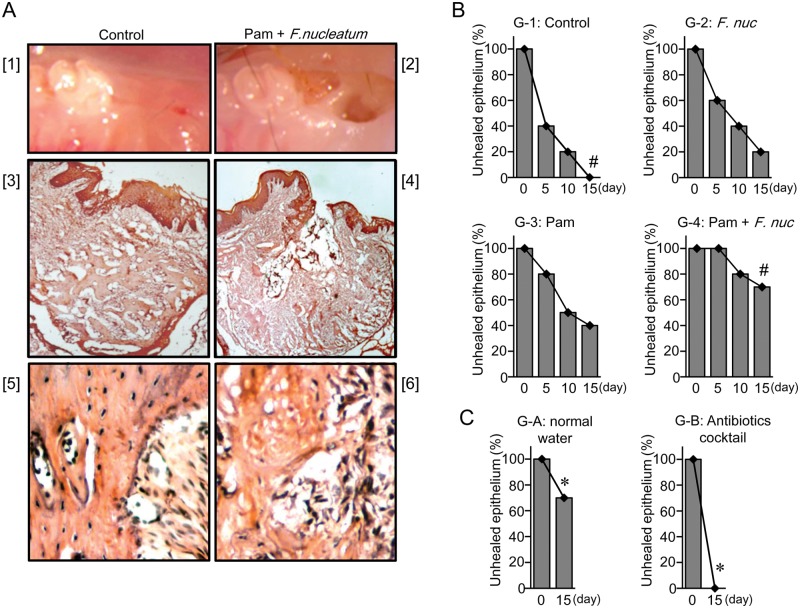
Fifteen days after tooth extraction (Day 15), the control group (Group 1) showed complete healing of the extraction socket (Fig. 2A[1]). However, in Group 4 (Pam+Fn), unhealed epithelial wounds and persistently exposed extraction sockets were observed at Day 15 (Fig. 2A[2]), and such lesions showing unclosed epithelium were found consistently at Day 21 (not shown). Furthermore, Group 1 (control) showed epithelial healing with complete re-epithelialization of the wounds in all animals (Fig. 2B). At Day 15, both Group 2 (Fn) and Group 3 (Pam) showed better healing than Group 4 (70-80% healed; Fig. 2B). More specifically, Group 3 (Pam), but not Group 2, showed a statistically significant difference in unclosed epithelium compared with Group 1 (control) in a larger-scale experiment (Appendix, Supplemental Results, section B, and Appendix Table). Very importantly, unhealed epithelium at extraction sockets caused by the combination of Pam+Fn (Group A) was completely abolished by the administration of a broad-spectrum antibiotics cocktail (Group B; Fig. 2C), suggesting that bacterial infection was, indeed, associated with the development of BONJ-like lesions.
Figure 2.
The combination of oral bacterial infection and Pam treatment caused failure of epithelial healing and BONJ lesions. (A) On Day 15, representative clinical photos ([1] and [2]) and H&E staining pattern (original magnification: [3] and [4], ×50; [5] and [6], ×200) of mice in Group 1 (Control, [1], [3], and [5]), or Group 4 (Pam+Fn, [2], [4], and [6]) are shown. (B) The number of mice with unhealed epithelial tissue at tooth extraction sites was counted every 5 days. The incidence of mice with unhealed epithelium in each group is expressed in percentage (%). These data are representative of 3 independent experiments. #Difference of percentages between two groups (Group 1 and Group 4 at Day 15) is significant (Fisher’s exact test, p < 0.05). (C) The mice (n = 10) treated with the combination of Pam and Fn were supplied with normal water (Group A) or an antibiotics cocktail (Group B) for 15 days after tooth extraction, and the number of mice with unhealed epithelium was counted. The incidence of mice with unhealed epithelium is expressed as a percentage (%). *Difference of percentages between two groups (Group A and Group B at Day-15) is significant (Fisher’s exact test, p < 0.05).
Histological Evaluation of BONJ-like Lesions in Mice
In contrast to the control (Group 1) that exhibited intact epithelium overlying the extraction socket, which was adequately filled with new bone (Fig. 2A[3]), histological evaluation showed Group 4 (Pam+Fn) with unhealed gingival epithelium accompanied by retarded bone regeneration in the extraction socket (Fig. 2A[4]), where sequestra of necrotic bone as well as infiltrations of inflammatory cells (neutrophils and mononuclear cells) and fibroblasts were observed (Fig. 2A[6]). In contrast, Group 2 showed healing comparable with that in Group 1, whereas Group 3 demonstrated less bone fill and epithelialization of the extraction socket than Group 1 (Appendix, Supplemental Results, section C, and Appendix Fig. 2A).
TUNEL-staining Evaluation of BONJ-like Lesions in Mice
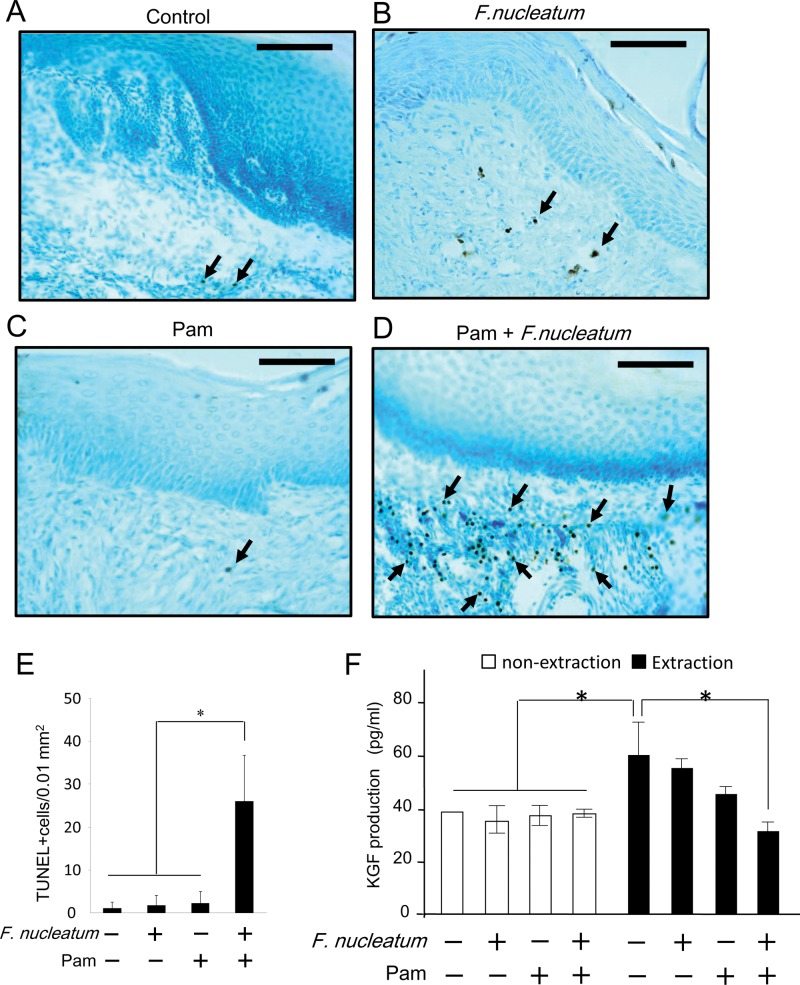
Few TUNEL-positive apoptotic cells were found in the control group (Fig. 3A) or in either the Fn or Pam treatment group alone (Fig. 3C or 3D, respectively). However, the combination of Pam+Fn treatment induced a significantly higher number of TUNEL-positive apoptotic cells in connective tissue (Fig. 3B) compared with control or the Fn or Pam treatment-alone group. Furthermore, there were few TUNEL-positive apoptotic cells in all 4 groups, while a remarkably low number of osteocytes was found in alveolar bone of the Pam+Fn group compared with control or the Fn-alone group (Appendix, Supplemental Results, section D, Appendix Figs. 3 and 4).
Figure 3.
The combination of Fn and Pam induced cell death of GF in vivo and attenuated KGF production from gingival tissue. (A-D) At Day 15 after tooth extraction, the apoptotic cells in gingival tissue isolated from 4 mice in Group 1 (A; control), Group 2 (B; Fn), Group 3 (C; Pam-alone), and Group 4 (D; Pam and Fn) were identified by TUNEL staining. Arrows indicate representative, but not all, TUNEL-positive cells. Original magnification, ×200. Bar = 100 µm. (E) On the gingival tissue samples isolated from all 4 groups at Day 15 after tooth extraction, TUNEL-positive apoptotic cells were counted. Column and bar indicate the mean ± SD of data (TUNEL-positive cells/0.01 mm2 microscopic field) in 3 mice per group. *p < 0.0001. Values differ significantly (ANOVA test). (F) The KGF concentrations in gingival tissue homogenates isolated from the mice killed at Day 15 (n = 6/group) were measured by ELISA. Data indicate the mean ± SD of KGF amounts in gingival tissue (KGF pg/mg gingival tissue). *p < 0.05. Values differed significantly (ANOVA).
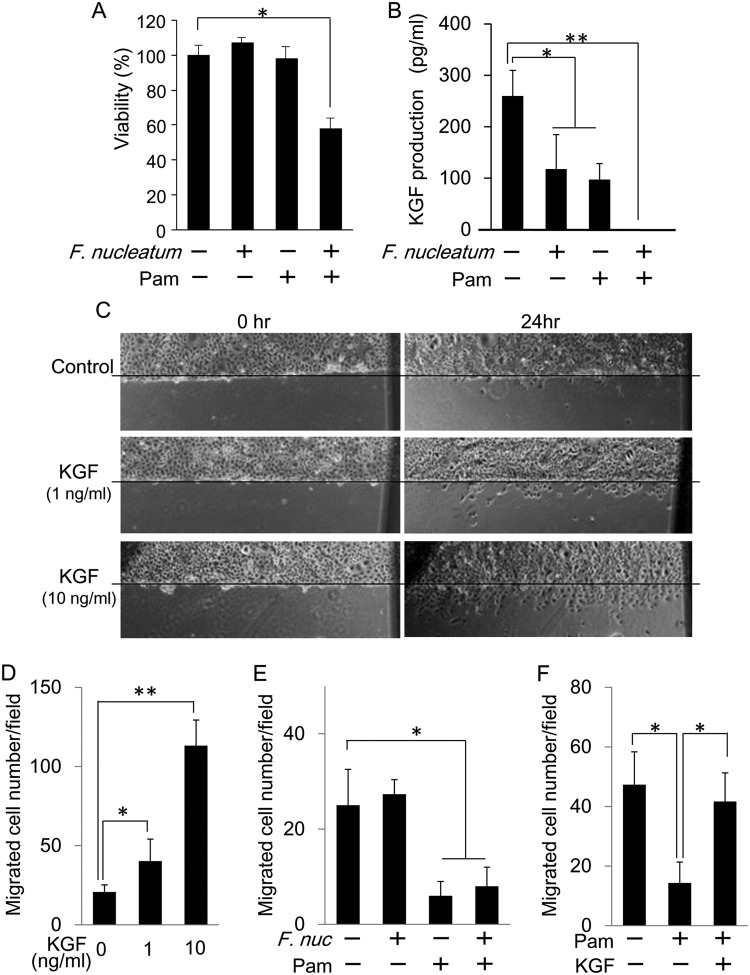
Figure 4.
The combination of Fn and Pam attenuated KGF production, which could otherwise induce epithelial wound-healing. (A) Effects of Fn (106 CFU/mL) and/or Pam (10 nM) on the viability of in vitro mouse GFs were evaluated by MTT assay. Data indicate the mean ± SD of 4 cultures. *p < 0.01. Values differ significantly (t test). (B) Mouse GFs were cultured for 24 hrs with or without Fn (106 CFU/mL) and/or Pam (10 nM) as indicated in the Fig. The KGF concentration in culture supernatant was measured by ELISA. Data indicate the mean ± SD of 4 cultures. *p < 0.05, **p < 0.01. Values differ significantly (t test). (C, D) To generate a wound on an epithelial sheet, we scratched the surface of a culture well with confluent GE-1 using a plastic micropipette tip and treated it with KGF (0, 1, or 10 ng/mL) for 24 hrs. Representative photographs of an epithelial sheet taken after 24-hour incubation are shown (C). The histogram shows the number of epithelial cells migrating out from the edge of the wound line created on the epithelial sheet (D). Values represent means ± SD of 4 cultures. *p < 0.05, **p < 0.01. Values differ significantly (t test). (E, F) After generation of the wound, as noted above, the epithelial sheet of GE-1 cells was incubated with or without Fn (106 CFU/mL), Pam (10 µM), or KGF (10 ng/mL) for 48 hrs. The histogram shows the number of epithelial cells migrating out from the edge of the wound line. Values represent means ± SD of 3 cultures. *p < 0.05, **p < 0.01. Values differ significantly (t test).
Measurement of KGF Production by ELISA
Delayed epithelial regeneration and healing were characteristic of the extraction site in Group 4 (Fig. 2), and this can be related to decreased KGF production. Although tooth extraction by itself significantly increased the levels of KGF in gingiva (Fig. 3D), the combination of Pam+Fn attenuated such gingival KGF production induced by tooth extraction (p < 0.05). However, KGF levels in non-extraction sites of gingiva did not change, regardless of treatments with or without Pam and/or Fn (Fig. 3D).
Combination of Pam+Fn Induced GF Cell Death
It was conceivable that cytotoxicity induced by exposure to Pam+Fn resulted in the diminished KGF production from GFs. Based on the in vitro assay to evaluate the viability of GFs, the combination of Pam+Fn, but not single applications of either Pam or F. nucleatum, significantly reduced the cell viability of GFs (Fig. 4A), indicating that bacterial challenge is required to cause the cell death of GFs in addition to the exposure to Pam.
Combination of Pam+Fn Attenuated Production of KGF from GFs
Similar to the result from in vivo experiments, exposure of cultured GFs to either Pam or Fn alone slightly decreased levels of KGF in GFs, but the combination of Pam+Fn completely suppressed its production (Fig. 4B). To further confirm if KGF can promote epithelial re-growth and migration, we carried out an epithelial wound-healing assay out in vitro using GE-1 cells. As expected, KGF increased the migration of GE-1 cells to the artificial wound region in a dose-dependent manner (Figs. 4C. 4D), suggesting that KGF derived from GFs may play a crucial role in the promotion of epithelial wound-healing.
KGF Can Regain the Pam-mediated Retardation of Epithelial Growth/Migration
To investigate the possible role of KGF in the healing process of Pam-exposed gingival epithelium in the context of BONJ-like lesions, we assessed the impact of Pam and/or Fn on the wound-induced growth/migration of gingival epithelial cells using the in vitro epithelial wound-healing assay. The exposure of GE-1 gingival epithelial cells with Pam significantly suppressed their wound-induced migration (Fig. 4E) regardless of the presence or absence of Fn. Stimulation of GE-1 cells with Fn alone also did not change the wound-induced migration of GE-1 cells. These results suggested that Pam suppressed the wound-induced growth/migration of gingival epithelial cells, and bacteria (Fn) by themselves did not affect such Pam-mediated retardation of epithelial cell growth/migration. Importantly, the addition of KGF to the Pam-exposed GE-1 cells regained the retardation of wound-induced GE-1 cell migration (Fig. 4F), indicating that KGF plays a pivotal role in promoting epithelial cell migration/growth in the context of ONJ-like lesions.
Discussion
A rodent model for BONJ-like disease was recently developed with a combination of dexamethasone with zoledronic acid or zoledronic acid alone (Sonis et al., 2009; Bi et al., 2010; Kobayashi et al., 2010). In this study, we have, for the first time, demonstrated an observation in a mouse model that emulates human BONJ through combining Pamidronate (Pam) with oral trauma (in this case tooth extraction) and bacteria. The combination of oral bacteria and Pam appears to diminish KGF production by promoting death of GFs, which are the major source of KGF. This event, in turn, leads to delayed epithelial healing combined with bone death. The clinical and histological findings in mice demonstrate bony changes that mimic the symptomology of human BONJ, and are unlikely to present dry sockets, the definition of which is alveolar osteitis without bone necrosis or sequestra. Although we did not find necrotic bone exposed outside of epithelium, some sequestra of necrotic bone pieces in the unhealed extraction sockets of Group 4 appeared to face the lumen that is connected to the oral cavity, which emulates the clinical criteria of Stage 0 in human BONJ (Mawardi et al., 2009; Woo et al., 2009). Although we have not determined the direct evidence supporting KGF as solely responsible for epithelial closure at extraction sockets, the diminished level of KGF was strongly associated with unhealed epithelium, and KGF was proven to be a robust factor that can induce not only growth but also migration of gingival epithelial cells. Therefore, this mouse model of BONJ-like lesions is expected to help as a tool for additional studies in the area of BONJ. (More detailed discussions are posted in the Appendix, Supplemental Discussion).
Acknowledgments
We acknowledge the assistance of Hajime Yamazaki in taking x-rays.
Footnotes
This study was supported by NIH grants DE-18499 (TK) and DE-19917 (TK) from the NIDCR.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Barasch A, Cunha-Cruz J, Curro FA, Hujoel P, Sung AH, Vena D, et al. (2011). Risk factors for osteonecrosis of the jaws: a case-control study from the CONDOR dental PBRN. J Dent Res 90:439-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Gao Y, Ehirchiou D, Cao C, Kikuiri T, Le A, et al. (2010). Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol 177:280-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE. (2008). Risks and benefits of bisphosphonates. Br J Cancer 98:1736-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows JL, Rindal DB, Barasch A, Gullion CM, Rush W, Pihlstrom DJ, et al. , DPBRN Collaborative Group (2011). ONJ in two dental practice-based research network regions. J Dent Res 90:433-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, et al. (2002). Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 62:6538-6544 [PubMed] [Google Scholar]
- Hansen T, Kunkel M, Weber A, Kirkpatrick CJ. (2006). Osteonecrosis of the jaws in patients treated with bisphosphonates—histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med 35:155-160 [DOI] [PubMed] [Google Scholar]
- Hansen T, Kunkel M, Springer E, Walter C, Weber A, Siegel E, et al. (2007). Actinomycosis of the jaws—histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Arch 451:1009-1017 [DOI] [PubMed] [Google Scholar]
- Kajiya M, Shiba H, Komatsuzawa H, Ouhara K, Fujita T, Takeda K, et al. (2010). The antimicrobial peptide LL37 induces the migration of human pulp cells: a possible adjunct for regenerative endodontics. J Endod 36:1009-1013 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, et al. (2010). Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab 28:165-175 [DOI] [PubMed] [Google Scholar]
- Longo R, Castellana MA, Gasparini G. (2009). Bisphosphonate-related osteonecrosis of the jaw and left thumb. J Clin Oncol 27:e242-e243 [DOI] [PubMed] [Google Scholar]
- Marx RE, Sawatari Y, Fortin M, Broumand V. (2005). Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63:1567-1575 [DOI] [PubMed] [Google Scholar]
- Mawardi H, Treister N, Richardson P, Anderson K, Munshi N, Faiella RA, et al. (2009). Sinus tracts—an early sign of bisphosphonate-associated osteonecrosis of the jaws? J Oral Maxillofac Surg 67:593-601 [DOI] [PubMed] [Google Scholar]
- Moreira MS, Katayama E, Bombana AC, Marques MM. (2005). Cytotoxicity analysis of alendronate on cultured endothelial cells and subcutaneous tissue. a pilot study. Dent Traumatol 21:329-335 [DOI] [PubMed] [Google Scholar]
- Polizzotto MN, Cousins V, Schwarer AP. (2006). Bisphosphonate-associated osteonecrosis of the auditory canal. Br J Haematol 132:114. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. (2004). Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229-241 [DOI] [PubMed] [Google Scholar]
- Reid IR, Bolland MJ, Grey AB. (2007). Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone 41: 318-320 [DOI] [PubMed] [Google Scholar]
- Reszka AA, Halasy-Nagy J, Rodan GA. (2001). Nitrogen-bisphosphonates block retinoblastoma phosphorylation and cell growth by inhibiting the cholesterol biosynthetic pathway in a keratinocyte model for esophageal irritation. Mol Pharmacol 59:193-202 [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331-343 [DOI] [PubMed] [Google Scholar]
- Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. (2008). Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J Oral Maxillofac Surg 66:767-775 [DOI] [PubMed] [Google Scholar]
- Siris ES, Oster MW, Bilezikian JP. (2009). More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med 360:1791; author reply, 1791-1792 [PubMed] [Google Scholar]
- Smola H, Thiekotter G, Fusenig NE. (1993). Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol 122:417-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC. (2009). Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol 45:164-172 [DOI] [PubMed] [Google Scholar]
- Werner S, Smola H. (2001). Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol 11:143-146 [DOI] [PubMed] [Google Scholar]
- Woo SB, Mawardi H, Treister N. (2009). Comments on “Osteonecrosis of the jaws in intravenous bisphosphonate use: proposal for a modification of the clinical classification”. Oral Oncol 45:740. [DOI] [PubMed] [Google Scholar]
- Wood J, Bonjean K, Ruetz S, Bellahcene A, Devy L, Foidart JM, et al. (2002). Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther 302:1055-1061 [DOI] [PubMed] [Google Scholar]