Abstract
Dickkopf-related protein 1 (DKK1) is a potent inhibitor of Wnt/β-catenin signaling. Dkk1-null mutant embryos display severe defects in head induction. Conversely, targeted expression of Dkk1 in dental epithelial cells leads to the formation of dysfunctional enamel knots and subsequent tooth defects during embryonic development. However, its role in post-natal dentinogenesis is largely unknown. To address this issue, we studied the role of DKK1 in post-natal dentin development using 2.3-kb Col1a1-Dkk1 transgenic mice, with the following key findings: (1) The Dkk1 transgene was highly expressed in pulp and odontoblast cells during post-natal developmental stages; (2) the 1st molar displayed short roots, an enlarged pulp/root canal region, and a decrease in the dentin formation rate; (3) a small malformed second molar and an absent third molar; (4) an increase of immature odontoblasts, few mature odontoblasts, and sharply reduced dentinal tubules; and (5) a dramatic change in Osx and nestin expression. We propose that DKK1 controls post-natal mandibular molar dentin formation either directly or indirectly via the inhibition of Wnt signaling at the following aspects: (i) post-natal dentin formation, (ii) formation and/or maintenance of the dentin tubular system, (iii) mineralization of the dentin, and (iv) regulation of molecules such as Osx and nestin.
Keywords: DKK1, tooth development, odontoblast, mandibular molar, transgenic mice, dentinogenesis
Introduction
Tooth formation begins with a series of reciprocal signaling interactions between the stomodeal ectoderm and the underlying neural-crest-derived ectomesenchyme (Tucker and Sharpe, 2004). The first sign of tooth formation is the thickening of the oral epithelium. This is followed by the invagination of the epithelium into the underlying mesenchyme, which then condenses and forms a tooth bud. In the past two decades, many signaling molecules and transcription factors which are critical for these processes have been identified (Jernvall and Thesleff, 2000; Tucker and Sharpe, 2004; Chai and Maxson, 2006; Denaxa et al., 2009). However, critical factors required for tooth root formation, which occurs mainly post-natally, are largely unknown.
It is known that Wnt/β-catenin signaling in the dental epithelium is critical for dental patterning during multiple stages of early tooth development. The supportive evidence is that constitutive activation of β-catenin in the epithelium causes the formation of large malformed tooth buds and ectopic teeth (Liu et al., 2008). Alternatively, blocking Wnt/β-catenin signaling by the targeted expression of Dkk1 in epithelial and underlying mesenchymal cells led to the formation of blunted molar cusps (i.e., blocking the secondary enamel knots) (Liu et al., 2008). The role of Wnt signaling in the dental mesenchyme during tooth patterning is controversial. Chen et al. showed that the inactivation of β-catenin resulted in tooth arrest at the bud stage in both molars and incisors (Chen et al., 2009). In contrast, a new report showed that the genetic inactivation of β-catenin results in a splitting of the incisal placode. This inactivation forms 2 incisors per incisal placode in the lower jaw in approximately 50% of all mutant embryos (Fujimori et al., 2010).
Recently, Lohi et al. showed that Axin2 lacZ signal, which reflects the canonical Wnt signaling pathway, is expressed in dental pulp and developing odontoblast cells, but not in ameloblast cells post-natally (Lohi et al., 2010), suggesting a potential role for canonical Wnt signaling in post-natal tooth formation.
DKK1 (a secreted protein with 2 cysteine-rich domains, separated by a linker region) is expressed in the tooth and the limb during development (Grotewold et al., 1999; Grotewold and Ruther, 2002; Fjeld et al., 2005; Nie, 2005). Suomalainen and Thesleff showed that Wnt/β-catenin activity and Dkk1 mRNA were detected in incisor mesenchyme (Suomalainen and Thesleff, 2010). DKK1 functions as an antagonist of canonical Wnt signaling through two mechanisms: (1) by binding to the LRP5/6 co-receptor to prevent its interaction with Wnt-Frizzled complexes; and (2) by binding to the cell-surface receptor Kremen1 or Kremen 2 to promote the internalization of LRP5/6 (Williams and Insogna, 2009). Injections of Dkk1 mRNA result in head induction in Xenopus embryos (Glinka et al., 1998), and deletion of Dkk1 leads to embryonic lethality with no anterior head structures in addition to exhibiting limb defects (Mukhopadhyay et al., 2001). Ectopic expression of Dkk1 in K5-expressing epithelium blocks taste papilla development, causing a lack of innervation of the tongue (Liu et al., 2007).
Although Dkk1 mRNA is expressed in the dental papilla, pre-odontoblasts, and odontoblasts (Fjeld et al., 2005), its function in post-natal tooth root formation is largely unknown. In this study, we attempted to address the function of DKK1 in post-natal dentin formation using 2.3-kb Col 1a1-Dkk1 transgenic (Tg) mice (Li et al., 2006) and multiple approaches, including: radiography, µ-CT, histology, TRAP staining, immunohistochemistry, double-fluorochrome labeling for measuring dentin formation rates, and scanning electron microscopy (SEM) for determining dentin properties. Our results demonstrate that overexpression of Dkk1 in pulp and odontoblast cells impaired mandibular molar dentin formation, suggesting that DKK1 plays an active role in post-natal tooth formation either directly or indirectly through the Wnt/β-catenin signaling pathway.
Materials & Methods
Generation of 2.3-kb Col 1a1-Dkk1 Transgenic Mice
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at the institute. The 2.3-kb Col 1a1-Dkk1 transgenic mice were generated as described previously (Li et al., 2006). A C57/B6 strain background was used in this study. The genotypes of the mice were determined by PCR analysis of genomic DNA extracted from tail biopsies. For the Col 1a1-Dkk1 transgene, the forward primer, 5′-CATCCCTGTGACCCCTCC-3′, and the reverse primer, 5′-CTCCAAACCACCCCCCTC-3′, were used to generate a PCR product of 150 bp.
Sample Preparation and Histological Analyses of Dkk1 Expression
Mandibular samples obtained from E16.5, P01, 1-week-, and 1-month-old mice were fixed in 4% paraformaldehyde in 4ºC overnight. These samples were then decalcified with 10% EDTA in a microwave, dehydrated, and embedded in paraffin. They were then sectioned (4-µm thick) and used in immunohistochemistry for DSPP (polyclonal antibody was kindly provided by Dr. Larry Fisher from NIDCR, National Institutes of Health, Bethesda, MD, USA), DKK1 (polyclonal antibody, R&D Systems, Minneapolis, MN, USA), OSX (osterix monoclonal antibody, Abcam, Cambridge, MA, USA), Nestin (mouse monoclonal antibody, Santa Cruz, CA), and TRAP (tartrate-resistant acid phosphatase) staining. Finally, the slides were mounted with permount, and photographed under by light microscopy.
Analysis of Dentin Formation Rate by Double-labeling and Imaging Resin-cast Odontoblast Processes by Scanning Electronic Microscopy (SEM)
To examine the dentin formation rate, we performed double-fluorescence labeling as described previously (Lu et al., 2007). Briefly, a calcein label (5 mg/Kg i.p.; Sigma-Aldrich, St. Louis, MO, USA) was administered to 20-day-old mice, followed by administration of calcein 7 days later. Mice were sacrificed 2 days after the second injection (1 mo old). The non-decalcified mandibles were embedded in resin (methylmethacrylate, MMA), sectioned, and photographed under epifluorescent illumination with a Nikon 800 microscope (Nikon, Melville, NY, USA). Furthermore, the surface of the same blot was polished with different diamond suspensions until smooth and scratch-free before being acid-etched and imaged by SEM as described previously (Feng et al., 2006). The surface was acid-etched with 37% phosphoric acid for 2 to 10 sec, washed twice with water followed by 5% sodium hypochlorite for 5 min, and washed again with water. After being air-dried, the samples were coated with gold and palladium, and examined by FEI/Philips XL30 Field emission environmental SEM.
Radiograph and Micro-CT Imaging of Mandibles from 1-month-old Dkk1 Transgenic Mice
Both the wild-type and Dkk1-Tg mandibles were radiographed with a Faxitron model MX-20 System (Faxitron X-Ray LLC, Lincolnshire, IL, USA), and scanned with a Micro-CT 35 (Scanco Medical AG, Bassersdorf, Switzerland).
Results
The 2.3-kb Col 1a1-Dkk1 Transgene Is Highly Expressed in Odontoblasts and Pulp Cells
To determine whether DKK1 plays a role in odontogenesis, we first compared the DKK1 expression patterns during dentinogenesis in WT and Tg mice starting from E16.5. Analysis of the immunohistochemical data from E16.5 molars showed that DKK1 is largely undetected in both WT and Tg odontoblasts (Fig. 1A). DKK1 was weakly expressed in WT newborn odontoblasts (Fig. 1B, left panel), but had a much higher expression level in newborn Dkk1-Tg pulp cells and odontoblasts within the molars at a low antibody concentration (Fig. 1B, right panel; 1:100 dilution). Higher expression levels were also observed in pulp and odontoblast cells of Dkk1-Tg 1-week mandibular molars (Fig. 1C). A weak DKK1 signal was detected in osteoblast cells, but a much higher level of DKK1 was detected in Tg osteoblasts (Fig. 1D). A clear DKK1 signal was detected in newborn and 1-week WT pulp, odontoblast, and ameloblast cells at a high antibody concentration (Appendix Fig. 1).
Figure 1.
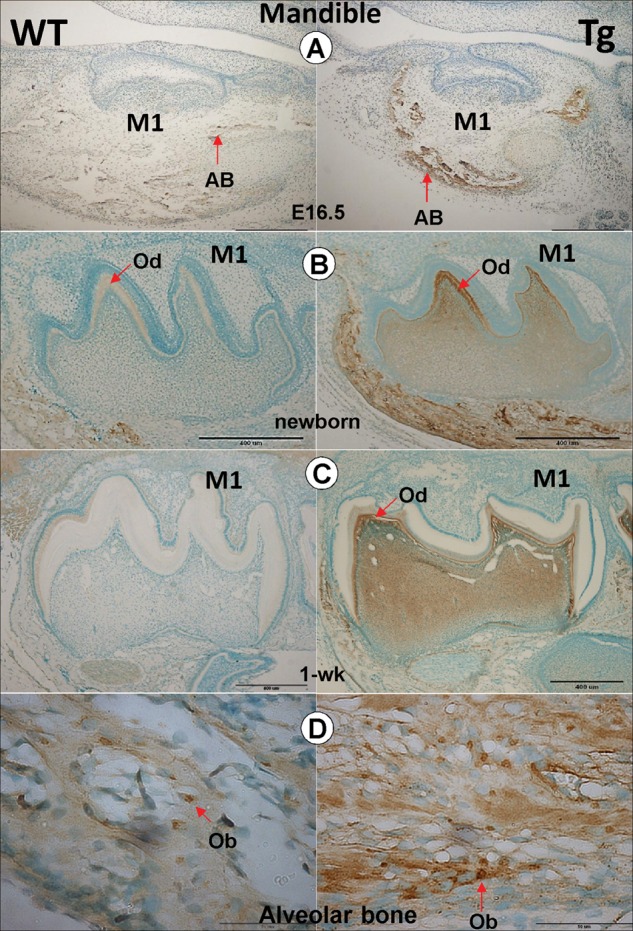
Expression patterns of the 2.3-kb Col1a1-Dkk1 transgene (Tg) during tooth development. Immunohistochemical staining showing DKK1 expression patterns in both WT and Tg mice during different stages of mouse tooth development. (A) At E16.5, DKK1 is highly expressed in the Tg mouse alveolar bone (AB), with no signal in the molars (right panel) compared with the age-matched control (left panel). (B) In the Tg newborn first molars, DKK1 is highly expressed in the pulp and odontoblast cells (right panel), compared with the age-matched control (left panels). (C) In 1-week-old Dkk1-Tg mouse molars, the DKK1 signal was maintained high in both pulp and odontoblast cells. (D) In the newborn alveolar bone, the DKK1 signal was detected in both WT (left panel) and Dkk1 Tg (right panel) osteoblast cells.
The 2.3-kb Col 1a1-Dkk1 Transgenic Mice Display a Striking Molar Phenotype in the Mandible
To address whether overexpression of Dkk1 in mesenchymal cells changes the tooth phenotype, we first screened mandibular samples using x-ray and µCT (Fig. 2A). Unexpectedly, the 3rd molar in all Dkk1 Tg mice was invisible in mice examined at the age of 2 wks (Appendix Fig. 2A and Appendix Table, 6 out of 6) and 4 wks (Appendix Fig. 2B and Appendix Table, 6 out of 6). The radiograph and µ-CT images obtained from the 1st molar also showed a malformed crown which is reduced in size and has short roots and an enlarged pulp/root canal region. The 2nd molar is much smaller than that in the age-matched control (Fig. 2A, right panels). Analysis of quantitative data showed that reduction of the full tooth, the root length, the ratio of tooth root/full tooth length, and dentin thickness in the Dkk1-Tg 1st molar is significant (Fig. 2B).
Figure 2.
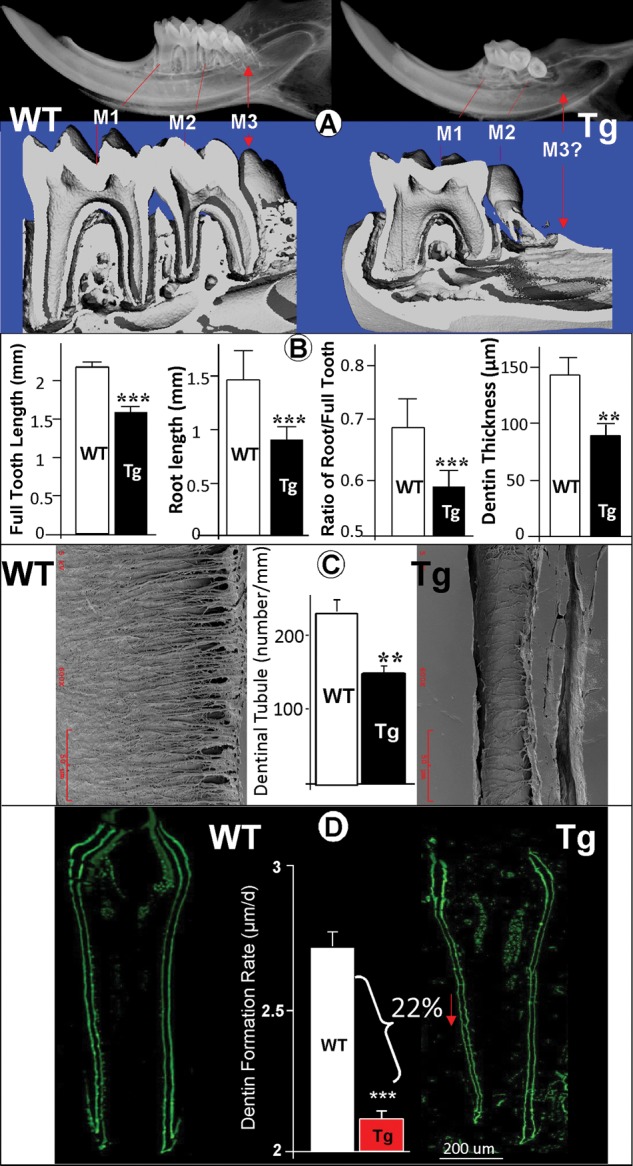
Dkk1-Tg mice display a severe tooth defect in the mandible (1 mo old). (A) Representative radiograph (upper panels) and µCT (lower panels) images reveal a severe molar phenotype in a 1-month-old Dkk1-Tg mandible, including an expanded pulp chamber and root canal region in the 1st molar, a small malformed 2nd molar, and lacking a 3rd molar. (B) Analysis of quantitative data based on radiographs (see Appendix Fig. 2C for the measurement method) showed a significant reduction of dentin root in the Dkk1-Tg 1st molar (data are mean ± SEM from n = 4 samples, **p < 0.01; ***p < 0.001 by Student’s t test). The 1st molar dentin thickness was calculated based on SEM images obtained from 4 WT and 4 Tg 1-month-old samples. (C) Images of an acid-etched resin-embedded 1-month-old 1st molar with a striking difference between the well-organized WT dentin tubules (left panel) and the irregular dentin structure in the Dkk1-Tg mouse (right panel). Analysis of quantitative data showed a significant reduction of dentin tubule numbers in the Dkk1-Tg mouse (middle panel,> data are mean ± SEM from n = 4 samples, **p < 0.01). (D) 1-month-old 1st molar fluorochrome-labeled sections of the lower first molar from WT (left panel) and Dkk1-Tg mice (right panel) unveiled a reduction of the dentin mineralization rate in the Tg mice. Scale bar = 200 µm. Quantitative analysis shows a significant difference in the dentin appositional rate between the WT and Tg first molar (data are mean ± SEM from n = 4 samples, ***p < 0.001 by Student’s t test).
Since the tooth root is embedded in alveolar bone and the Dkk1 transgene is targeted to the osteoblast cells (Fig. 1D), we next asked whether there is an alveolar bone phenotype that could be responsible for the tooth phenotype observed within the Dkk1-Tg mice. There is mild bone loss in the Dkk1-Tg mice, shown by µCT and H&E staining assays (data not shown), although it is very unlikely that bone loss is the major cause of such a severe tooth phenotype.
To determine whether the overexpression of Dkk1 changes the dentin ultrastructure, we next examined the first mandibular molar by SEM using an acid-etched resin-casting technique (Fig. 2C). When comparing the Dkk1-Tg first molars with the age-matched controls, we observed striking differences in the appearance of the dentin tubules, with the tubular processes being sharply reduced and disorganized. Analysis of quantitative data showed a significant reduction of dentinal tubule number in Dkk1-Tg mice (Fig. 2C, middle panel). We also measured the dentin apposition rate using a fluorochrome labeling assay (Miller et al., 1985). The distance between the 2 fluorochrome-labeled lines was used to calculate the dentin formation rate and was shown to be significantly reduced in the Dkk1-Tg mandibular molars (> 20%, Fig. 2D).
The above data indirectly reflect a critical role of Wnt/β-catenin signaling during post-natal molar dentin formation.
The 2.3-kb Col 1a1-Dkk1 Transgenic Mice Exhibit Dentin Erosion and Expanded Periodontal Ligament (PDL)
Because the striking tooth phenotype is displayed in the mandibular molars at 1 mo of age, we further characterized the dentin phenotype using backscattered SEM imaging, which displayed numerous areas of dentin erosion in the Dkk1-Tg mice (Fig. 3A, right panel). To address the cause of dentin erosion, we re-examined DKK1 expression in the first molar. Unexpectedly, DKK1 was detected in the PDL cells and the acellular matrix with the area of dentin erosion which was filled with many cells (Fig. 3B, right panel). Since the osteoclast is the only cell that can resorb the mineralized tissue, we next examined the presence of osteoclast cells using a TRAP staining assay. The results showed that overexpression of DKK1 significantly increased osteoclast numbers (Figs. 3C, 3D, right panels). We also showed that there were few acellular cementoblasts in the Dkk1-Tg mice (Figs. 3B, 3C, right panel), and the µCT image displayed an expanded PDL region (Fig. 3E, right panel). Analysis of these data, taken together, supports the notion that dentin erosion is caused by two factors: an increase in osteoclast number and a defect in acellular cementoblasts within Dkk1-Tg mice.
Figure 3.
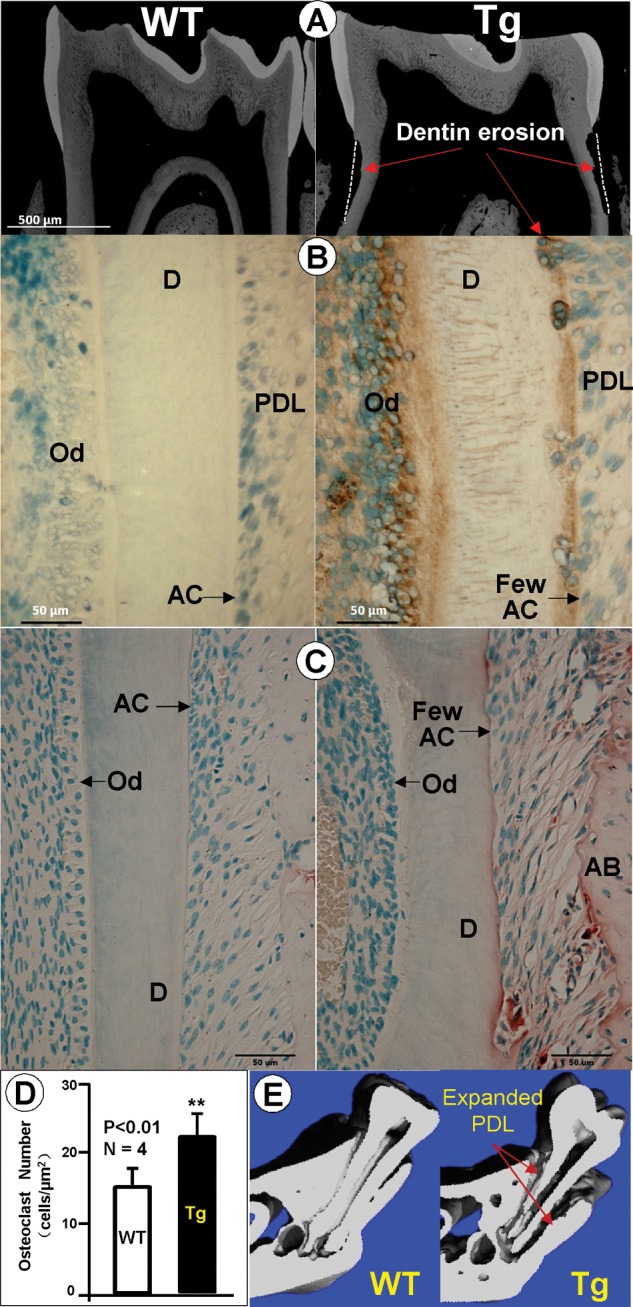
Dentin erosion in the Dkk1-Tg lower first molar. (A) Backscattered SEM images displaying a reduced dentin thickness and areas of dentin erosion in Dkk1-Tg 1-month-old molars (right panel). (B) Immunostained images show a strong DKK1 expression in pulp/odontoblast cells, plus a weak expression of DKK1 in the Dkk1-Tg PDL and acellular cementoblasts in the 1-month-old molars. The number of acellular cementoblasts appears to be reduced (right panel) compared with the age-matched WT control (left panel), exposing the dentin to osteoclast erosion. (C) TRAP (Tartrate-resistant acid phosphatase) -stained images showing more TRAP+ cells in the Dkk1-Tg mouse AB with a few in the eroded dentin and PDL region (right panel). A majority of the PDL region was occupied by fibrous-like cells, with few acellular cementoblast cells in the Dkk1-Tg mouse. Dkk1-Tg mice appear to be lacking a polarized odontoblast layer. (D) Analysis of quantitative TRAP-staining data showed a significant increase in osteoclast numbers in the Dkk1-Tg mouse (data are mean ± SEM from n = 6 replicates, ***p < 0.001 by Student’s t test). (E) The µCT images confirmed the expanded PDL region and eroded root surface in the Dkk1-Tg mouse (right panel, arrows). AC, acellular cementoblasts; D, dentin; Od, odontoblast; PDL, periodontal ligament.
Overexpression of DKK1 Delays the Maturation of Dentinogenesis during Post-natal Development
To study the mechanism by which the targeted overexpression of Dkk1 leads to the above dentin structural changes, we investigated the expression patterns of OSX (osterix), a transcription factor which is critical for tooth formation (Lu et al., 2007), and nestin, a specific marker for odontoblasts reflecting its neural-crest derivation (Terling et al., 1995; Struys et al., 2005). Analysis of immunohistochemical data showed that OSX was mainly expressed in the nucleus of odontoblasts of WT molars (Figs. 4A, 4B, left panels). Conversely, OSX was expressed in a much broader array of cells, including later pulp cells and early odontoblasts, and the Dkk1-Tg molars lacked a defined polarized odontoblast layer (Fig. 3C and Appendix Fig. 4, right panels). In addition, analysis of nestin antibody staining data revealed a sharp reduction of nestin expression levels in Dkk1-Tg odontoblasts (Fig. 4C, right panel). Analysis of these data suggests that DKK1 regulates Osx and nestin in post-natal dentin formation, and that the changes of these molecules could be partly responsible for the immature odontoblast phenotype in the Dkk1 Tg mice.
Figure 4.
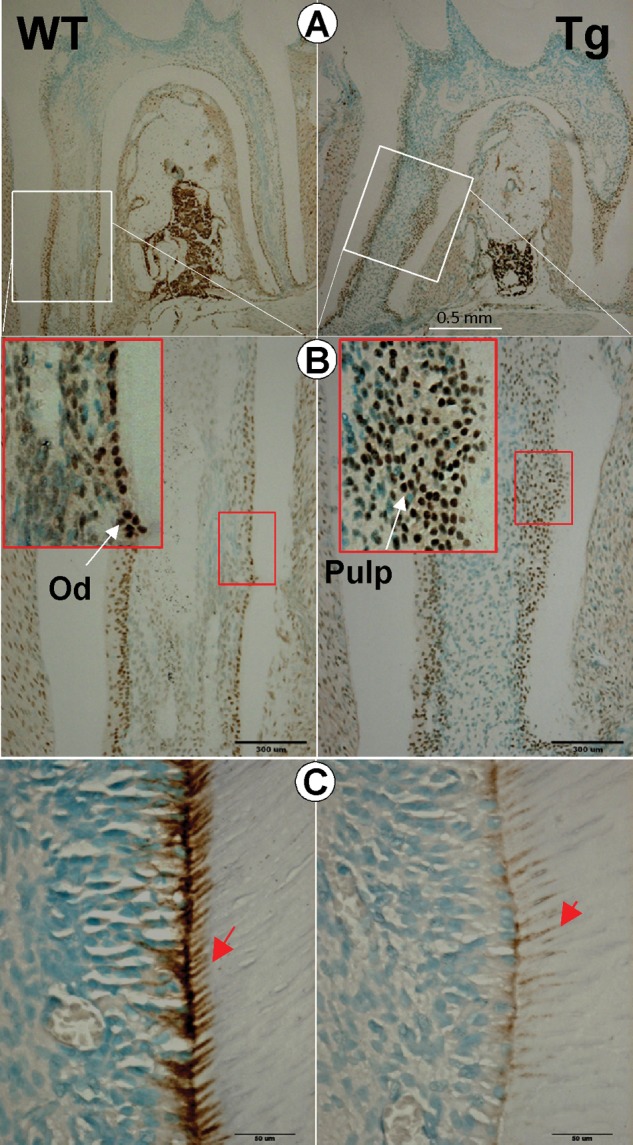
Defects in odontogenesis in the Dkk1-Tg molar and working hypothesis. (A,B) Analysis of immunohistochemical data showed that OSX was mainly expressed in the odontoblast (Od) layer in the wild-type mouse (WT, left panels), whereas OSX was widely expressed in immature odontoblast cells in Dkk1-Tg pulp (right panels). (C) Analysis of immunohistochemical data revealed a marked reduction of nestin in Dkk1-Tg dentin (right panel) compared with the WT (left panel). Pd, predentin.
Discussion
Dkk1, primarily expressed in mesenchymal-derived tissues such as the dental papilla, pre-odontoblasts, and odontoblasts during tooth development, is speculated to be important for dental patterning and crown morphology (Fjeld et al., 2005). In this study, we directly targeted Dkk1 overexpression in pulp odontoblasts to investigate its specific role during post-natal tooth (particularly root) development in vivo. Our main findings (see Appendix Table) are: (1) The Dkk1 transgene, driven by the rat 2.3-kb Col 1a1 promoter, whose activity is observed in both pulp (low level) and odontoblast (high level) cells (Braut et al., 2003) (also see Appendix Fig. 5), is mainly expressed in post-natal pulp and odontoblast cells; (2) Dkk1-Tg mandibular molars display a severe post-natal phenotype, including short roots, an enlarged pulp/root canal region, thin dentin, a considerable reduction in the dentin formation rate, a small malformed second molar, and an absent third mandibular molar; and (3) changes of Osx and nestin expression in odontoblast cells, an increased number of immature odontoblasts, few mature odontoblasts, and a sharply reduced dentinal tubule number. Thus, we propose that DKK1 controls post-natal mandibular molar dentin formation either directly or indirectly via inhibition of Wnt signaling in the following levels: (i) post-natal dentin formation, (ii) formation and/or maintenance of the dentin tubular system, (iii) mineralization of the dentin, and (iv) regulation of molecules such as Osx and nestin.
The actual role of Wnt/β-catenin or DKK1 in post-natal tooth (especially root) formation is not clear, since previous work mainly focused on tooth germ and crown formation during embryonic development. In this work, we directly target DKK1 in pulp and odontoblast cells during the post-natal developmental stage. Analysis of our data clearly showed a striking dentin phenotype (see above), suggesting that Wnt/β-catenin signaling continuously plays a key role during post-natal dentin formation. Interestingly, the 2.3-kb Col 1a1-Dkk1 transgene is active in pulp and odontoblast cells, while there is no sign of the 3rd mandibular molar in all Dkk1-Tg mice examined by radiograph, with 6 samples at the age of 2 wks and 6 samples at the age of 4 wks (Fig. 2 and the Appendix Table; see Appendix). With an H&E staining assay, we noticed an empty cavity within the Dkk1-Tg mandibular alveolar bone where the 3rd molar should be (data not shown), suggesting that the tooth germ was formed but its growth was arrested and then it was subsequently resorbed. Thus, we speculate that the high level of DKK1 released from pulp cells either directly or indirectly (through the inhibition of Wnt/β-catenin signaling) blocks further development of the 3rd molar. At this stage, we do not know whether this inhibitory role is Wnt/β-catenin-dependent or –independent, or both. Future generation of an odontoblast-specific Dkk1 knockout animal model would yield a clearer picture of the roles of DKK1 in post-natal tooth development. In addition, we plan to cross the 2.3-kb Col1a1-Dkk1 Tg mice to the Top-gal mouse line (DasGupta and Fuchs, 1999), to determine whether LacZ expression, which reflects Wnt/β-catenin signaling, is altered in the Dkk1-Tg tooth.
Tooth, unlike bone, is usually not resorbed by osteoclasts, likely because of the presence of anti-resorption factors residing in the PDL region and an intact acellular cementoblast layer for its protection (Andreasen and Andreasen, 1992). Here we showed severe dentin resorption on the Dkk1-Tg root surface (Fig. 3). This defect is most likely caused by an increase in osteoclast numbers and a defect in the acellular cementum. It is known that Wnt signaling up-regulates OPG in the mature osteoblast, which blocks RANKL-induced osteoclastogenesis (Goldring and Goldring, 2007). Overexpression of DKK1 will increase osteoclast numbers via an inhibition of Wnt signaling.
In contrast to the severe tooth phenotype observed in the mandibular molars (Fig. 2), the maxillary molar phenotype is mild (Appendix Fig. 3), suggesting that regulation of tooth patterning is complex. Similar variation in tooth phenotype within other genetic animal models has also been reported. For example, Thomas et al. demonstrated that null mutations of both Dlx-1 and Dlx-2 homeobox genes display no maxillary molars, while the incisors and the mandibular molars develop normally (Thomas et al., 1997). Denaxa et al. showed that a double knockout of homeodomain transcription factors Lhx6 and Lhx7 leads to molar agenesis, with incisors being largely unaffected (Denaxa et al., 2009).
In summary, 2.3-kb Col1a1-Dkk1 transgenic mice display a striking dentin phenotype, which occurs post-natally. We propose that DKK1 in pulp and odontoblast cells directly or indirectly (though the inhibition of Wnt signaling) controls post-natal mandibular molar development at three levels: (i) gene expression (through molecules such as Osx and nestin) and cell maturation; (ii) dentin ultrastructure; and (iii) dentin mineralization.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was partly supported by a US National Institutes of Health grant to J.Q.F. (DE018486), by China State Key Laboratory of Oral Diseases Open Funding to J.Q.F. (SKLODOF 2010-03), and by a National Natural Science Foundation of China grant to X.L.H. (31000419).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Andreasen JO, Andreasen FM. (1992). Root resorption following traumatic dental injuries. Proc Finn Dent Soc 88(Suppl 1):95-114 [PubMed] [Google Scholar]
- Braut A, Kollar EJ, Mina M. (2003). Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a col1a1-2.3-GFP transgene. Int J Dev Biol 47:281-292 [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr (2006). Recent advances in craniofacial morphogenesis. Dev Dyn 235:2353-2375 [DOI] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA, Gao Y, Jiang R. (2009). Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol 334:174-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126:4557-4568 [DOI] [PubMed] [Google Scholar]
- Denaxa M, Sharpe PT, Pachnis V. (2009). The LIM homeodomain transcription factors Lhx6 and Lhx7 are key regulators of mammalian dentition. Dev Biol 333:324-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. (2006). Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeld K, Kettunen P, Furmanek T, Kvinnsland IH, Luukko K. (2005). Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev Dyn 233:161-166 [DOI] [PubMed] [Google Scholar]
- Fujimori S, Novak H, Weissenbock M, Jussila M, Gonçalves A, Zeller R, et al. (2010). Wnt/beta-catenin signaling in the dental mesenchyme regulates incisor development by regulating Bmp4. Dev Biol 348:97-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. (1998). Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391:357-362 [DOI] [PubMed] [Google Scholar]
- Goldring SR, Goldring MB. (2007). Eating bone or adding it: the Wnt pathway decides. Nat Med 13:133-134 [DOI] [PubMed] [Google Scholar]
- Grotewold L, Ruther U. (2002). The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J 21:966-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold L, Theil T, Ruther U. (1999). Expression pattern of Dkk-1 during mouse limb development. Mech Dev 89:151-153 [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. (2000). Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 92:19-29 [DOI] [PubMed] [Google Scholar]
- Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, et al. (2006). Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39:754-766 [DOI] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, et al. (2007). Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet 39:106-112 [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, et al. (2008). Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol 313:210-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi M, Tucker AS, Sharpe PT. (2010). Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn 239:160-167 [DOI] [PubMed] [Google Scholar]
- Lu Y, Ye L, Yu S, Zhang S, Xie Y, McKee MD, et al. (2007). Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol 303:191-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SC, Omura TH, Smith LJ. (1985). Changes in dentin appositional rates during pregnancy and lactation in rats. J Dent Res 64:1062-1064 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, et al. (2001). Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1:423-434 [DOI] [PubMed] [Google Scholar]
- Nie X. (2005). Dkk1, -2, and -3 expression in mouse craniofacial development. J Mol Histol 36:367-372 [DOI] [PubMed] [Google Scholar]
- Struys T, Krage T, Vandenabeele F, Raab WH, Lambrichts I. (2005). Immunohistochemical evidence for proteolipid protein and nestin expression in the late bell stage of developing rodent teeth. Arch Oral Biol 50:171-174 [DOI] [PubMed] [Google Scholar]
- Suomalainen M, Thesleff I. (2010). Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn 239:364-372 [DOI] [PubMed] [Google Scholar]
- Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. (1995). Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol 39:947-956 [PubMed] [Google Scholar]
- Thomas BL, Tucker AS, Qui M, Ferguson CA, Hardcastle Z, Rubenstein JL, et al. (1997). Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development 124:4811-4818 [DOI] [PubMed] [Google Scholar]
- Tucker A, Sharpe P. (2004). The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet 5:499-508 [DOI] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. (2009). Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res 24:171-178 [DOI] [PMC free article] [PubMed] [Google Scholar]


