Abstract
Objectives
We sought to define the cardiometabolic phenotype associated with rs5068, a genetic variant of the atrial natriuretic peptide (ANP) gene.
Background
ANP and BNP play an important role in cardiorenal homeostasis but also exert metabolic actions.
Methods
We genotyped 1608 randomly selected residents from Olmsted County, Minnesota, USA. Subjects were well characterized.
Results
Genotype frequencies were: AA 89.9%, AG 9.7%, and GG 0.4%; all subsequent analyses were AA vs AG+GG. After adjustment for age and gender the G allele was associated with increased plasma levels of N-terminal-proANP (NT-proANP) (p=0.002). The minor allele was also associated with lower BMI (p=0.006), prevalence of obesity (p=0.002), waist circumference (p=0.021), lower levels of C-reactive protein (p=0.027) and higher values of HDL cholesterol (p=0.019). The AG+GG group had a lower systolic blood pressure (p=0.011) and lower prevalence of myocardial infarction (p=0.042). The minor allele was associated with a lower prevalence of metabolic syndrome (p=0.025). After adjusting for BMI the association between the G allele and HDL cholesterol, C – reactive protein values, myocardial infarction and metabolic syndrome was not significant; the association with systolic blood pressure, BMI, obesity, waist circumference remained significant even after adjusting for NT-proANP.
Conclusions
In a random sample of the general US population the minor allele of rs5068 is associated with a favorable cardiometabolic profile. These findings suggest that rs5068 or genetic loci in linkage disequilibrium may affect susceptibility for cardiometabolic diseases and support the possible protective role of natriuretic peptides by their favorable effects on metabolic function. Replication studies are needed to confirm our findings.
Keywords: atrial natriuretic peptide, metabolic syndrome, lipid metabolism, natriuretic peptides, cardiometabolic disease
INTRODUCTION
Since the discovery of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) over two decades ago, the endocrine role of the heart in the control of salt and water balance and cardiac structure has been clearly established (1-3). Both ANP and BNP are secreted by the heart and are endogenous ligands for the particulate guanylyl cyclase-A receptor, mediating their biological actions via the second messenger 3′,5′ cyclic guanosine monophosphate (cGMP) and consequent activation of cGMP-dependent protein kinase, phosphodiesterases and ion channels. Their actions include vasodilation, natriuresis, suppression of the renin-angiotensin-aldosterone system and inhibition of both cardiomyocyte hypertrophy and cardiac fibroblast activation (4).
The natriuretic peptide precursor A gene (NPPA) lies in tandem with the BNP gene on chromosome 1 and encodes for the prohormone from which ANP and N-terminal-proANP (NT-proANP) are derived in equimolar amounts (5). Most recently, Newton-Cheh and co-workers reported in a seminal study that two common genetic variants in the 3′ untranslated region and 2 kb downstream of NPPA, the single nucleotide polymorphisms (SNPs) rs5068 and rs198358, respectively, are associated with increased circulating levels of NT-proANP, N-terminal-proBNP (NT-proBNP), lower blood pressure (BP), and reduced prevalence of hypertension (6). This recent study underscores the importance of ANP and BNP in BP homeostasis as well as the impact of common genetic variations on cardiovascular function as predicted from the early studies in mouse models in which ANP gene disruption resulted in increased BP (4,7).
Increasing evidence supports the view that both ANP and BNP may also play a fundamental role in metabolic regulation. Indeed, low levels of ANP infusion in healthy volunteers increase free fatty acids mobilization and lipid oxidation (8). In humans with heart failure, ANP infusion results in increased circulating levels of the adipokine adiponectin (9), which is an important regulator of glucose and lipid metabolism and possesses anti-hypertrophic properties in cardiomyocytes (10-12). In mice, activation of the guanylyl cyclase-A receptor has been shown to slow gastric emptying (13). Most recently, Miyashita et al. demonstrated in a mouse model that BNP overexpression prevented the development of obesity in response to a high fat diet (14). Moreover, in humans rs198389, a SNP in the promoter region of the BNP gene which increases circulating BNP, is associated with reduced risk for type 2 diabetes mellitus (15,16).
The impact of rs5068 on the metabolic phenotype in the general population is unknown. In the current study we hypothesized that rs5068, which has previously been associated with higher levels of ANP and BNP and lower BP, is also associated with a protective cardiometabolic phenotype in the general population. To test this hypothesis, we used a subset of a large, well-characterized sample of the general population 45 years and older from Olmsted County, Minnesota both to reconfirm the elegant studies of Newton-Cheh and co-workers on BP and to extend our studies to define the cardiometabolic phenotype of rs5068 in the general community (17).
METHODS
This study was approved by the Mayo Clinic Institutional Review Board.
Study population
We analyzed a subset of clinically well-characterized population-based sample of the general population 45 years or older living in Olmsted County, Minnesota in 1997-2000. This population was first characterized as part of the National Institutes of Health-funded Prevalence of Left Ventricular Dysfunction Study and Cardiac Peptides in Cardiorenal Regulation (RO1 HL55502 and HL36634). The design and selection criteria of the above study as well as the characteristics of the Olmsted County population have been previously described (17,18). This population was characterized clinically, biochemically, and by echocardiography. From the 2027 subjects in this study from whom there were collected DNA samples a total of 1608 subjects were successfully genotyped and were included in this study.
Body mass index (BMI) was measured as kilograms per (meter)2. Obesity was defined as BMI ≥30 kg/m2. Waist circumference was measured in centimeters at the top of the umbilicus. In accordance with the National Cholesterol Education Program Adult Treatment Panel III criteria, metabolic syndrome was defined by the presence of 3 or more of the following criteria: (1) central obesity defined as a waist circumference greater than 102 cm in men and greater than 88 cm in women, (2) triglyceride level higher than 150 mg/dL (to convert to mmol/L, multiply by 0.0113), (3) high-density lipoprotein cholesterol level less than 40 mg/dL (to convert to mmol/L, multiply by 0.0259) in men and less than 50 mg/dL in women, (4) blood pressure of 130/85 mm Hg or higher, and (5) fasting glucose level of 110 mg/dL (to convert to mmol/L, multiply by 0.0555) or higher. Hypertension was diagnosed using Joint National Committee VI criteria (19).
Genotyping
Genotyping of rs5068 was carried out on 1608 subjects using TaqMan (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions, using 10-20ng DNA. Primers and probes were Assay-by Design (Applied Biosystems). Following PCR amplification, end reactions were read on the ABI Prism 7900ht using Sequence Detection Software (Applied Biosystems). The quality value percentage is a quality metric that indicates the reliability of called genotypes generated by the SDS software. The quality value was calculated by using ABI’s proprietary calling algorithm determining how well that sample fits into the cluster. Genotypes less than 95% are located further from their clusters and have a lower reliability. An electronic data file was generated that contains genotypes and the quality value.
Natriuretic peptide assays
Plasma NT-proANP levels were available in a subgroup of 1485 subjects using a radioimmunoassay (Phoenix Pharmaceuticals, Belmont, CA) (20). NT-proBNP values were measured in a subgroup of 1566 subjects using an electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, Indiana) (21).
Doppler echocardiography
All echocardiograms were performed with the same echocardiographic instrument (HP-2500, Palo Alto, California) and were interpreted by a single echocardiologist blinded to clinical data. Two-dimensional and color Doppler imaging were performed to screen for valvular stenosis and regurgitation. In each subject, ejection fraction was measured and diastolic function was classified as mild, moderate, and severe as previously described (17). Left ventricular (LV) dimension and mass and left atrial volume were calculated from M-mode and 2-D measurements, respectively, and were indexed to body surface area (22-24). LV mass was calculated according to the Devereux formula. Presence of LV hypertrophy was defined on the basis of LV mass index greater than 130 g/m2 for men and greater than 100 g/m2 for women (25). Presence of left atrial enlargement was defined as left atrial volume index >33 ml/m2 in men and >30 ml/m2 in females (26).
Statistical Analysis
Data pertaining to patient demographics and clinical characteristics were summarized with descriptive statistics. These included counts and percentages for categorical and ordinal variables, or medians and interquartile ranges for continuous variables. To test for an association with the rs5068 genotype, specifically whether or not a subject had at least one copy of the minor G allele, each clinical factor was modeled as the dependent variable via linear regression (or logistic regression if the factor was binary) with rs5068 genotype as the explanatory variable. All modeling was performed unadjusted and adjusted for potential confounding variables such as age and gender. Further adjustments were done for BMI and NT-proANP. Due to highly skewed distributions of the NT-proANP and NT-proBNP biomarkers, a probit transformation was applied to the ranked values of each, thus producing normal distributional properties. Similarly, other skewed variables including C-reactive protein, serum glucose, insulin and triglycerides levels were transformed to approximate normality using the logarithmic transform. Furthermore, since age and gender are highly correlated with these biomarkers and with several other clinical factors, both were treated as adjusting covariates in the logistic models to control for their potential confounding. Although multiple hypothesis tests were carried out, a nominal 2-sided significance level of 0.05 was used with no formal adjustment for multiple testing. Given that about 30 to 40 variables were evaluated, the expected number of about 1 or 2 nominally significant results by chance alone should be considered in the interpretation of our findings. All analyses were carried out using the SAS statistical software package (Version 8.2, SAS Institute Inc., Cary, NC).
RESULTS
Prevalence of rs5068 and Circulating Natriuretic Peptides
From collected DNA samples on 2027 subjects, a total of 1608 subjects were successfully genotyped and included in this study. Genotype frequencies of rs5068 were AA: 89.9% (n=1445), AG: 9.7% (n=157), and GG: 0.4% (n= 6), corresponding to a minor allele frequency of 5.3%. The distribution was in Hardy-Weinberg equilibrium (p=0.435). Due to the low frequency of homozygotes for the G allele, all analyses were performed assuming a dominant model with AG and GG genotypes combined. The characteristics of the study population are summarized in Table 1. Neither age nor gender were significantly associated with the genotype, though there was a trend toward a higher prevalence of females among those who had at least one minor allele compared to those with none (58% vs 52%, age-adjusted p=0.122). Importantly, the presence of at least one copy of the minor allele was associated with increased plasma levels of NT-proANP (median 2584 vs 2188 pg/mL), both unadjusted (p<0.001), and after adjustment for age, gender and BMI (p=0.006). In contrast, circulating levels of NT-proBNP were not significantly different between genotypes.
Table 1. Characteristics of the Study Population across the rs5068 Genotype.
| Characteristic | AA (n=1445) |
AG + GG (n=163) |
Unadjusted P-value* |
Adjusted P-value † |
Adjusted P-value ‡ |
Adjusted P-value § |
|---|---|---|---|---|---|---|
| Females | 746 (52%) | 95 (58%) | 0.108 | 0.122 | 0.173 | 0.396 |
| Age, yrs | 61.5 (53.2, 70.4) | 62.5 (54.6, 71.6) | 0.182 | 0.208 | 0.300 | 0.998 |
| Age categories | 0.183 | 0.211 | 0.295 | 0.935 | ||
| . 45-54 yrs | 436 (30%) | 41 (25%) | ||||
| . 55-64 yrs | 433 (30%) | 53 (33%) | ||||
| . 65-74 yrs | 367 (25%) | 39 (24%) | ||||
| . 75+ yrs | 209 (14%) | 30 (18%) | ||||
| Systolic blood pressure, mmHg | 132 (116, 147) | 129 (115, 144) | 0.063 | 0.011 | 0.051 | 0.054 |
| Diastolic blood pressure, mmHg | 73 (67, 80) | 72 (66, 77) | 0.063 | 0.132 | 0.247 | 0.262 |
| Creatinine, mg/dL | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.1) | 0.283 | 0.437 | 0.479 | 0.450 |
| NT-proBNP, pg/mL∥ | 67.4 (27.8, 146.9) | 80.2 (37.8, 175.1) | 0.096 | 0.413 | 0.497 | 0.565 |
| NT- proANP, pg/mL ∥ | 2188 (1374, 3238) | 2584 (1693, 3842) | < 0.001 | 0.002 | 0.006 | - |
| Ejection Fraction,% | 65 (60, 68) | 65 (60, 65) | 0.701 | 0.916 | 0.897 | 0.642 |
| Ejection Fraction <40% | 28 (2%) | 1 (1%) | 0.254 | 0.247 | 0.284 | 0.207 |
| Ejection Fraction <50% | 83 (6%) | 6 (4%) | 0.279 | 0.267 | 0.323 | 0.229 |
| Moderate to Severe Diastolic Dysfunction | 106 (7%) | 11 (7%) | 0.627 | 0.458 | 0.432 | 0.173 |
| LV Dimension Index, cm/m2 | 2.6 (2.4, 2.8) | 2.6 (2.4, 2.8) | 0.236 | 0.321 | 0.910 | 0.689 |
| LV mass index, g/m2 | 94 (82, 109) | 93 (82, 107) | 0.297 | 0.315 | 0.565 | 0.337 |
| LA volume index, mL/m2 | 23.3 (19.5, 28.4) | 23.9 (19.2, 27.9) | 0.377 | 0.211 | 0.314 | 0.085 |
Continuous data are summarized with medians and 25th and 75th percentile values, and categorical data with counts and percentages.
N-Terminal proANP, NT-proANP; N-Terminal proBNP, NT-proBNP, left ventricular, LV; left atrial, LA.
P-value obtained from univariate regression model
P-value obtained from regression model adjusting for age and gender
P-value obtained from regression model adjusting for age, gender and BMI
P-value obtained from regression model adjusting for age, gender, BMI and NT-proANP
P-values reflect probit transformation applied to rank-ordered NT-proANP and NT-proBNP values
Cardiovascular Phenotype
Controlling for age and gender, the G allele was significantly associated with lower systolic BP (delta = −4.28 mmHg, p=0.011) but not with diastolic BP (delta = −1.24 mmHg, p=0.132) (Table 1). The effect on SBP remained marginally significant after adjusting for BMI (p=0.051) or further adjusting for NT-proANP (p=0.054). The analysis of left atrial volume, LV structure and function as determined by echocardiography (LV ejection fraction, LV dimensions, LV mass, and LV volume index) did not reveal any significant associations with the rs5068 genotype.
With regard to cardiovascular diseases, in a model adjusted for age and gender the regression analysis showed that fewer minor allele carriers of rs5068 had a history of myocardial infarction (adjusted odd ratio= 0.29, p=0.042) whereas in a model adjusted also for BMI the result was slightly attenuated (odd ratio= 0.32, p=0.061) (Table 2). There was no significant association between genotype and hypertension, coronary artery disease, congestive heart failure, atrial fibrillation, or cerebrovascular accident.
Table 2. Prevalence of Cardiovascular Diseases and Diabetes Mellitus type 2 across the rs5068 Genotype.
| Characteristic | AA (n=1445) |
AG + GG (n=163) |
Odds Ratio (95% CI) [P-value] * |
Odds Ratio (95% CI) [P-value] † |
Odds Ratio (95% CI) [P-value] ‡ |
|---|---|---|---|---|---|
| Verified Hypertension | 425 (29%) | 47 (29%) | 0.97 (0.68, 1.39) [0.879] | 0.91 (0.63, 1.31) [0.600] | 1.01 (0.69, 1.48) [0.950] |
| Coronary artery disease | 185 (13%) | 14 (9%) | 0.64 (0.36, 1.13) [0.124] | 0.57 (0.31, 1.05) [0.071] | 0.61 (0.33, 1.12) [0.109] |
| Myocardial Infarction | 80 (6%) | 3 (2%) | 0.32 (0.10, 1.02) [0.055] | 0.29 (0.09, 0.96) [0.042] | 0.32 (0.10, 1.06) [0.061] |
| Congestive Heart Failure | 31 (2%) | 5 (3%) | 1.44 (0.55, 3.77) [0.453] | 1.40 (0.52, 3.74) [0.504] | 1.57 (0.58, 4.23) [0.373] |
| Atrial Fibrillation | 68 (5%) | 10 (6%) | 1.32 (0.67, 2.63) [0.421] | 1.29 (0.63, 2.64) [0.487] | 1.35 (0.66, 2.77) [0.417] |
| Cerebrovascular Accident | 20 (1%) | 4 (2%) | 1.79 (0.61, 5.31) [0.292] | 1.76 (0.59, 5.26) [0.311] | 1.79 (0.60, 5.37) [0.300] |
| Diabetes Mellitus type 2 | 108 (7%) | 13 (8%) | 1.07 (0.59, 1.95) [0.818] | 1.05 (0.57, 1.93) [0.875] | 1.26 (0.68, 2.35) [0.459] |
Odds Ratio, 95% CI and P-value obtained from logistic regression univariate model
Odds Ratio, 95% CI and P-value obtained from logistic regression model adjusting for age and gender
Odds Ratio, 95% CI and P-value obtained from logistic regression model adjusting for age, gender and BMI
Metabolic Phenotype
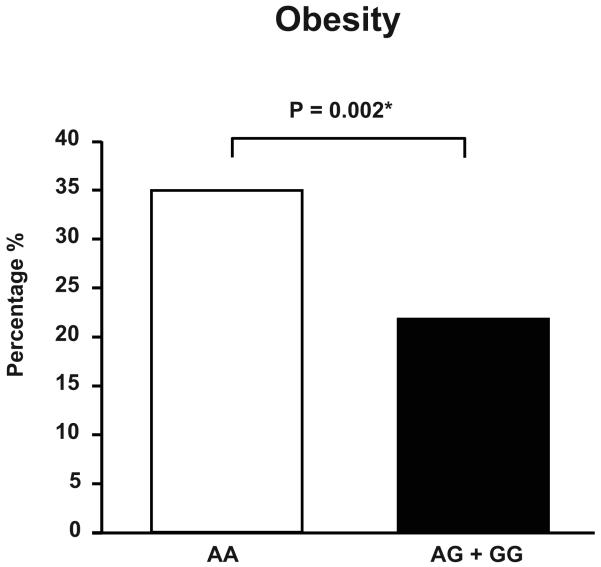
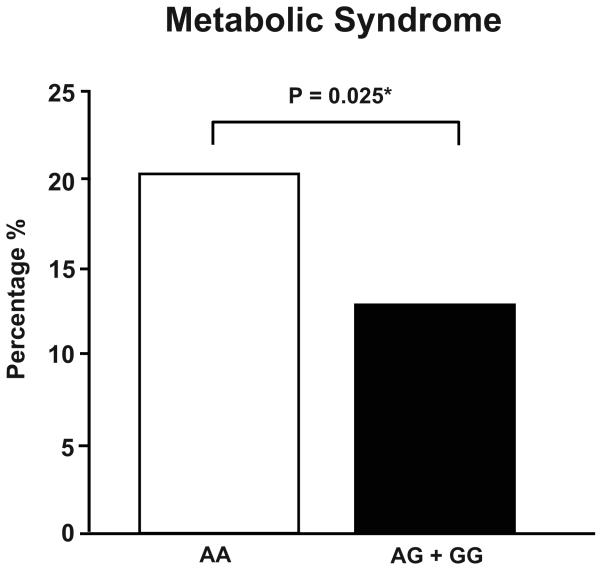
In a regression model adjusted for age and gender the presence of at least one copy of the G allele was associated with lower BMI (delta= −1.22 kg/m2, p=0.006; Table 3) and waist circumference (delta= −2.45 cm, p=0.021), as well as reduced rate of obesity (odds ratio=0.54, p=0.002; Figure 1A). In addition, the minor allele was associated with higher levels of HDL cholesterol (delta= 2.46 mg/dL, p=0.019), lower plasma values of C-reactive protein (0.17 vs 0.20 mg/dL, p=0.027; Table 3) and, although not significant, with lower levels of insulin in subjects free of diabetes mellitus type 1 and 2 (4.7 vs 5.2 μU/mL, p=0.069; Table 3). Moreover, having at least one minor allele was associated with lower prevalence of metabolic syndrome (odds ratio= 0.58, p=0.025; Figure 1B). After including BMI in the regression model the association between the rs5068 minor allele HDL cholesterol, C – reactive protein values and metabolic syndrome failed to remain significant (Table 3). However, the association of the G allele with BMI, obesity and waist circumference remained significant after adjusting for NT-proANP. Genotypes did not differ regarding plasma concentration of total and low-density lipoprotein cholesterol, triglycerides, and fasting glucose (Table 3). Of note, the proportion of subjects with antilipemic treatment was similar between the two genotype groups (17% in both), as was the prevalence of diabetes mellitus type 2 (Table 2).
Table 3. Metabolic Parameters in the Study Population according to the rs5068 Genotype.
| Characteristic | AA (n=1445) |
AG + GG (n=163) |
Unadjusted P-value* |
Adjusted P-value† |
Adjusted P-value ‡ |
Adjusted P-value § |
|---|---|---|---|---|---|---|
| Body Mass Index, kg/m2 | 27.9 (25, 31.6) | 26.7 (24.3, 29.4) | 0.003 | 0.006 | - | 0.006∥ |
| Obesity (BMI ≥ 30 kg/m2) | 503 (35%) | 36 (22%) | 0.001 | 0.002 | - | 0.001∥ |
| Waist Circumference, cm | 93 (82, 101) | 90 (80, 96) | 0.006 | 0.021 | - | 0.025∥ |
| Total Cholestorol, mg/dL | 200 (178, 222) | 207 (183, 231) | 0.190 | 0.274 | 0.301 | 0.336 |
| HDL Cholestorol, mg/dL | 42 (35, 54) | 47 (39, 58) | 0.004 | 0.019 | 0.115 | 0.205 |
| LDL Cholestorol, mg/dL | 125 (105, 146) | 133 (108, 154) | 0.296 | 0.275 | 0.280 | 0.197 |
| Triglycerides, mg/dL¶ | 129 (95, 182) | 123 (88, 169) | 0.102 | 0.096 | 0.295 | 0.234 |
| Serum Glucose, mg/dL¶ | 94 (89, 101) | 93.0 (89, 101) | 0.573 | 0.535 | 0.960 | 0.486 |
| Insulin, μU/mL ¶# | 5.2 (3.6, 7.8) | 4.7 (3.5, 7.2) | 0.059 | 0.069 | 0.446 | 0.469 |
| C-Reactive Protein, mg/dL¶ | 0.20 (0.09, 0.44) | 0.17 (0.07, 0.40) | 0.083 | 0.027 | 0.220 | 0.120 |
| Metabolic Syndrome | 296 (20%) | 21 (13%) | 0.024 | 0.025 | 0.286 | 0.133 |
Continuous data are summarized with medians and 25th and 75th percentile values, and categorical data with counts and percentages.
High density lipoprotein, HDL; low density lipoprotein, LDL.
P-value obtained from univariate regression model
P-value obtained from regression model adjusting for age and gender
P-value obtained from regression model adjusting for age, gender and BMI
P-value obtained from regression model adjusting for age, gender, BMI and NT-proANP
BMI not adjusted for in the model; adjusting factors were age, gender, and NT-proANP only
P-values based on logarithmic transformed variable
Analyzed on subgroup of subjects free of diabetes mellitus type 1 and 2
Figure 1A. Prevalence of Obesity in the Study Population according to Genotype.
Prevalence of obesity in the study population according to genotype.
Obesity was defined as BMI ≥30 kg/m2.
*P value obtained from logistic regression model adjusting for age and gender.
Figure 1B. Prevalence of Metabolic Syndrome in the Study Population according to Genotype.
Prevalence of metabolic syndrome in the study population according to genotype.
*P value obtained from logistic regression model adjusting for age and gender.
DISCUSSION
While ANP and BNP have been known to play a fundamental role in cardiorenal homeostasis, significant metabolic actions of natriuretic peptides have only recently emerged. Here we report for the first time that an ANP genetic variant associated with higher NT-proANP levels is associated with a favorable metabolic profile, primarily via its association with BMI, and that it is also associated with a favorable cardiovascular profile. Specifically, according to a regression analysis adjusted for age and gender the minor allele of the NPPA SNP rs5068 correlates not only with reduced systolic BP and lower prevalence of myocardial infarction but also with lower BMI, prevalence of obesity, waist circumference, higher levels of HDL cholesterol as well as lower values of C-reactive protein. Carriers of the minor allele are also characterized by a lower risk of metabolic syndrome.
In our study of residents in Olmsted County, Minnesota, the genotype frequencies for rs5068 were similar to the recently reported Framingham Heart Study and Malmö Diet and Finrisk97 cohorts (6). Similar to this recent report, we observed that the G allele was associated with increased levels of NT-proANP. This increase in NT-proANP is notable, as Itoh et al reported that it is produced in equimolar amounts with ANP and represent a robust estimator of ANP secretion from the heart (27,28).
While a major stimulus for ANP release is increased atrial stretch, there is no indication that this would explain the higher levels associated with the minor allele in our study as there were no differences in terms of congestive heart failure, atrial fibrillation or left atrial enlargement across genotypes, and moreover, BP levels were lower in the group characterized by the presence of the minor allele. Newton-Cheh et al. speculated that rs5068, which is located in the 3′ untranslated region, could affect transcript stability and result in higher ANP production. These investigators pointed out that this mechanism would not explain the higher BNP levels seen in their study, the levels of which would be expected to decrease in a compensatory manner if the primary mechanism was an increase in ANP production. They propose an alternative explanation that rs5068 resides in a shared enhancer element that coordinately regulates expression of the adjacent NPPA and NPPB genes. However, unlike in the study by Newton-Cheh et al., levels of NT-proBNP were not different between genotypes in our cohort. This discrepancy could be a consequence of the smaller size of the sample analyzed in our study as well as the different assay performed.
Given the known metabolic effects of natriuretic peptides and the increased levels of NT-proANP and NT-proBNP associated with the rs5068 minor allele, it was our main objective to define the metabolic phenotype associated with the rs5068 genotype. Indeed, minor allele carriers not only showed a favorable cardiovascular profile characterized by lower systolic BP and lower prevalence of myocardial infarction, but they also had a favorable metabolic phenotype characterized by lower BMI, waist circumference, and prevalence of obesity. Furthermore, they had higher levels of HDL cholesterol and lower levels of C-reactive protein. The differences in HDL and C-reactive protein cannot be explained by anti-lipemic treatment, as both genotypes were similar in this regard. Consistent with all these findings, the prevalence of the metabolic syndrome in our study population was lower in the minor allele carriers.
These associations support once more the view of a significant, clinically relevant metabolic action of natriuretic peptides more specifically ANP. Interestingly, Miyashita et al. provided recent important findings regarding the critical role of natriuretic peptides and guanylyl cyclase-A receptor stimulation in lipid catabolism and glucose intolerance. Transgenic mice overexpressing BNP were protected against obesity and insulin resistance induced by high fat-diet while transgenic mice overexpressing cGMP-dependent protein kinase revealed a significant reduction in body weight and higher insulin sensitivity compared to wild type mice, even on standard diet. Both models showed augmented muscle mitochondrial biogenesis and fat oxidation (14). Of note, acute infusion of ANP in humans has been reported to promote insulin secretion and inhibit glucagon secretion (29),(30). However, in our study this direct insulin-increasing effect of ANP is likely to be offset by the favorable metabolic status of rs5068 minor allele carriers, who would be expected to have improved insulin sensitivity and thus generally lower insulin levels.
While our study is an association study which cannot establish a causal relationship between increased NT-proANP and cardiovascular-metabolic phenotype, our findings support the emerging metabolic role of guanylyl cyclase-A activation. The capacity of ANP and BNP to promote lipid mobilization and oxidation, slow gastric emptying, decrease blood pressure and body weight, and increase insulin sensitivity provide strong arguments in favor of the natriuretic peptide/guanylyl cyclase-A/cGMP signaling system’s capacity to enhance metabolic function and phenotype (6,8,13,14).
Moreover, our findings support the hypothesis that the presence of at least one copy of the minor allele of rs5068 associated with higher circulating level of NT-proANP may identify individuals with lower risk for cardiovascular and metabolic diseases.
Despite no formal adjustment for multiple comparisons, the consistency of the main significant findings with previous results, as well as the number of significant findings, tend to support the association between the minor allele of rs5068 and a favorable cardiometabolic phenotype. We acknowledge that a potential criticism of the study is that significant associations with multiple comparisons occurred by chance. Based on the null distribution of p-values from the approximately 40 unique associations tested, the expected number of type I errors for this study is 2. That is to say that on average two associations detected as nominally significant at the 0.05 level would have occurred by chance alone. Since there were a total of 6 such associations detected (counting the significant results for BMI, obesity, and waist circumference as only one), more than the expected chance finding, and because several of these associations with the rs5068 genotype reflected a consistent protective effect on cardiometabolic parameters, it is unlikely that chance alone explains the associations. The results may not meet a strict threshold for multiple testing, but given the a priori hypothesis and experimental data, the findings seem plausible.
We also note that after fitting a regression model adjusted for age, gender and BMI, the associations between the G allele and values of HDL cholesterol, C-reactive protein, metabolic syndrome and myocardial infarction were attenuated. These data could reveal how these metabolic associations might be mediated by the primary important correlation between the NPPA SNP and lower BMI. We further performed a regression analysis including NT-proANP in our model. The associations between the minor allele of rs5068 and BMI, prevalence of obesity and values of waist circumference still remained significant, while the associations between genotype and SBP and myocardial infarction remained marginally significant. These findings should be interpreted cautiously. Indeed, our data may indicate that rs5068 or genetic loci in linkage disequilibrium with it may affect susceptibility for cardiometabolic diseases. There is also the possibility that rs5068 alters lifelong levels of ANP, and this effect is not completely accounted for by the measurement of one value of NT-proANP in each individual. Moreover, it is important to remark that a lack of attenuation after assuming a regression model adjusted for NT-proANP does not exclude a possible biological role for ANP in determining the observed favorable metabolic phenotype. With respect to detect a relationship between plasma ANP levels and metabolic phenotype, we were probably limited by the size of our sample, our ability to directly measure ANP due to the short half life of ANP, and instability of the peptide under laboratory conditions. Although we tested for an association, we did not detect any difference between groups with respect to plasma ANP levels.
Our model considered as a confounding variable, not ANP, but NT-proANP, which was significantly higher in the group characterized by the presence of the minor allele. Indeed, NT-proANP is secreted in equimolar amounts with ANP (27). Due to its longer half-life, greater laboratory stability and less variability in plasma concentration, NT-proANP is considered a reliable biomarker to estimate ANP secretion from the heart (27,28) but no evidences are present in literature regarding a biological action of NT-proANP. On the contrary, several findings support the view of ANP as an important metabolic regulator. Infusion of ANP at pharmacological doses into healthy lean men and head down bed rest position, both which increase plasma ANP levels, promote lipid mobilization and utilization (8,31). This lipolytic effect of ANP is independent of insulin or sympathetic nervous system activation and is mediated by a cGMP-dependent pathway that induces the phosphorylation of hormone sensitive lipase and perilipin A via the activation of cGMP-dependent protein kinase I (32,33). Further, in a key Framingham study, NT-proANP and BNP correlated inversely with the metabolic syndrome and its individual components, even after adjustment for BMI (34).
A replication study is clearly needed in order to confirm the validity of our data and the lack of replication in the current study is a limitation. An additional limitation is the low genotyping call rate. In order to support the validity of our findings from a statistical point of view we can assume that the failures occurred in a random fashion unrelated to any phenotype traits. Indeed, the genotype frequencies of rs5068 in our population are similar to the frequencies reported in the HapMap Project regarding a population from UTAH, in the Framingham Heart Study, Malmö Diet and Finrisk cohort.
Our investigation, together with previous experimental data, provide a rationale for the development of ANP or guanylyl cyclase-A agonist/ANP/BNP-like drugs as potential cardiometabolic therapeutics that are able to target the complex metabolic syndrome on several different levels. Indeed, our recent report of the feasibility of orally delivered BNP in an animal model of experimental hypertension supports such a therapeutic direction (35,36). Further studies are required to confirm our findings and to clarify the physiological mechanisms underlying the effects exerted by natriuretic peptides on metabolism.
In summary, our findings in a general community population demonstrate that the minor allele of rs5068 is associated with a favorable cardiometabolic profile characterized by higher levelsof NT-proANP and HDL cholesterol, lower systolic blood pressure, prevalence of myocardial infarction, BMI, waist circumference, C-reactive protein levels and prevalence of metabolic syndrome. Our results, which both confirm previous findings regarding a link to blood pressure and extend the relationship of rs5068 to cardiometabolic homeostasis, suggest that rs5068 or genetic loci in linkage disequilibrium may affect susceptibility for cardiometabolic diseases. Replication studies are needed to confirm our results. These findings support the possible protective role of natriuretic peptides by their favorable effects on metabolic function, including body weight and lipid metabolism with clinical implications in disease prevention and innovative therapeutics.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (RO1 HL55502, RO1 HL36634 and PO1 HL76611).
ABBREVIATIONS
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- cGMP
3′,5′cyclic guanosine monophosphate
- NPPA
natriuretic peptide precursor A gene
- NT-proANP
N-terminal-proANP
- SNP
single nucleotide polymorphism
- NT-proBNP
N-terminal-proBNP
- BP
blood pressure
- BMI
body mass index
- LV
left ventricular
Footnotes
Disclosure: No financial disclosure. No relationship with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Kangawa K, Matsuo H. Purification and complete amino acid sequence of alpha-human atrial natriuretic polypeptide (alpha-hANP) Biochem Biophys Res Commun. 1984;118:131–9. doi: 10.1016/0006-291x(84)91077-5. [DOI] [PubMed] [Google Scholar]
- 3.Holtwick R, Gotthardt M, Skryabin B, et al. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99:7142–7. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbers DL, Chrisman TD, Wiegn P, et al. Membrane guanylyl cyclase receptors: an update. Trends Endocrinol Metab. 2006;17:251–8. doi: 10.1016/j.tem.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemer M, Chamberland M, Sirois D, et al. Gene structure of human cardiac hormone precursor, pronatriodilatin. Nature. 1984;312:654–6. doi: 10.1038/312654a0. [DOI] [PubMed] [Google Scholar]
- 6.Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–53. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smithies O, Kim HS, Takahashi N, Edgell MH. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int. 2000;58:2265–80. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 8.Birkenfeld AL, Boschmann M, Moro C, et al. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J Clin Endocrinol Metab. 2005;90:3622–8. doi: 10.1210/jc.2004-1953. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto O, Fujita M, Kato M, et al. Natriuretic Peptides Enhance the Production of Adiponectin in Human Adipocytes and in Patients with Chronic Heart Failure. J Am Coll Cardiol. 2009;53:2070–7. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 11.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello-Boerrigter LC, Burnett JC., Jr. A new role for the natriuretic peptides: metabolic regulators of the adipocyte. J Am Coll Cardiol. 2009;53:2078–9. doi: 10.1016/j.jacc.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addisu A, Gower WR, Jr., Landon CS, Dietz JR. B-type natriuretic peptide decreases gastric emptying and absorption. Exp Biol Med (Maywood) 2008;233:475–82. doi: 10.3181/0708-RM-216. [DOI] [PubMed] [Google Scholar]
- 14.Miyashita K, Itoh H, Tsujimoto H, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–92. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meirhaeghe A, Sandhu MS, McCarthy MI, et al. Association between the T-381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet. 2007;16:1343–50. doi: 10.1093/hmg/ddm084. Epub 2007 Apr 5. [DOI] [PubMed] [Google Scholar]
- 16.Choquet H, Cavalcanti-Proenca C, Lecoeur C, et al. The T-381C SNP in BNP gene may be modestly associated with type 2 diabetes: an updated meta-analysis in 49 279 subjects. Hum Mol Genet. 2009;18:2495–501. doi: 10.1093/hmg/ddp169. [DOI] [PubMed] [Google Scholar]
- 17.Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 18.McKie PM, Cataliotti A, Lahr BD, et al. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 55:2140–7. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The sixth report of the Joint Natonal Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. published correction appears in Arch Intern Med. 1998;158:573. [DOI] [PubMed] [Google Scholar]
- 20.Burnett JC, Jr., Kao PC, Hu DC, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–7. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 21.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–53. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shub C, Klein AL, Zachariah PK, Bailey KR, Tajik AJ. Determination of left ventricular mass by echocardiography in a normal population: effect of age and sex in addition to body size. Mayo Clin Proc. 1994;69:205–11. doi: 10.1016/s0025-6196(12)61058-1. [DOI] [PubMed] [Google Scholar]
- 23.Murray JA, Kennedy JW, Figley MM. Quantitative angiocardiography. II. The normal left atrial volume in man. Circulation. 1968;37:800–4. doi: 10.1161/01.cir.37.5.800. [DOI] [PubMed] [Google Scholar]
- 24.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–82. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 25.Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;59:956–60. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 26.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 27.Itoh H, Nakao K, Sugawara A, et al. Gamma-atrial natriuretic polypeptide (gamma ANP)-derived peptides in human plasma: cosecretion of N-terminal gamma ANP fragment and alpha ANP. J Clin Endocrinol Metab. 1988;67:429–37. doi: 10.1210/jcem-67-3-429. [DOI] [PubMed] [Google Scholar]
- 28.Thibault G, Murthy KK, Gutkowska J, et al. NH2-terminal fragment of rat pro-atrial natriuretic factor in the circulation: identification, radioimmunoassay and half-life. Peptides. 1988;9:47–53. doi: 10.1016/0196-9781(88)90008-3. [DOI] [PubMed] [Google Scholar]
- 29.Uehlinger DE, Weidmann P, Gnadinger MP, et al. Increase in circulating insulin induced by atrial natriuretic peptide in normal humans. J Cardiovasc Pharmacol. 1986;8:1122–9. doi: 10.1097/00005344-198611000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Verspohl EJ, Bernemann IK. Atrial natriuretic peptide (ANP)-induced inhibition of glucagon secretion: mechanism of action in isolated rat pancreatic islets. Peptides. 1996;17:1023–9. doi: 10.1016/0196-9781(96)00152-0. [DOI] [PubMed] [Google Scholar]
- 31.Moro C, Pillard F, de Glisezinski I, et al. Atrial natriuretic peptide contribution to lipid mobilization and utilization during head-down bed rest in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R612–7. doi: 10.1152/ajpregu.00162.2007. [DOI] [PubMed] [Google Scholar]
- 32.Galitzky J, Sengenes C, Thalamas C, et al. The lipid-mobilizing effect of atrial natriuretic peptide is unrelated to sympathetic nervous system activation or obesity in young men. J Lipid Res. 2001;42:536–44. [PubMed] [Google Scholar]
- 33.Sengenes C, Bouloumie A, Hauner H, et al. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003;278:48617–26. doi: 10.1074/jbc.M303713200. [DOI] [PubMed] [Google Scholar]
- 34.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–53. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 35.Cataliotti A, Chen HH, Schirger JA, et al. Chronic actions of a novel oral B-type natriuretic peptide conjugate in normal dogs and acute actions in angiotensin II-mediated hypertension. Circulation. 2008;118:1729–36. doi: 10.1161/CIRCULATIONAHA.107.759241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cataliotti A, Schirger JA, Martin FL, et al. Oral human brain natriuretic peptide activates cyclic guanosine 3′,5′-monophosphate and decreases mean arterial pressure. Circulation. 2005;112:836–40. doi: 10.1161/CIRCULATIONAHA.105.538520. [DOI] [PubMed] [Google Scholar]