Abstract
In vertebrates, canonical Wnt signaling controls posterior neural cell lineage specification. Although Wnt signaling to the neural plate is sufficient for posterior identity, the source and timing of this activity remain uncertain. Furthermore, crucial molecular targets of this activity have not been defined. Here, we identify the endogenous Wnt activity and its role in controlling an essential downstream transcription factor, Meis3. Wnt3a is expressed in a specialized mesodermal domain, the paraxial dorsolateral mesoderm, which signals to overlying neuroectoderm. Loss of zygotic Wnt3a in this region does not alter mesoderm cell fates, but blocks Meis3 expression in the neuroectoderm, triggering the loss of posterior neural fates. Ectopic Meis3 protein expression is sufficient to rescue this phenotype. Moreover, Wnt3a induction of the posterior nervous system requires functional Meis3 in the neural plate. Using ChIP and promoter analysis, we show that Meis3 is a direct target of Wnt/β-catenin signaling. This suggests a new model for neural anteroposterior patterning, in which Wnt3a from the paraxial mesoderm induces posterior cell fates via direct activation of a crucial transcription factor in the overlying neural plate.
Keywords: Meis3, Wnt3a, Neural patterning, Dorsal-lateral marginal zone (DLMZ), Paraxial mesoderm, Xenopus
INTRODUCTION
Secreted signals act via transcription factors to regulate the anteroposterior (AP) pattern in vertebrate nervous system development. Yet, little is known about how these signaling factors integrate in time and space to specify different cell types. In Xenopus, BMP antagonists secreted from the Spemann organizer activate transcription factors to trigger anterior neural induction. A subsequent caudalizing step re-specifies anterior cells to posterior fates of midbrain, hindbrain and spinal cord, which also includes primary neurons and neural crest. Three signaling pathways caudalize neuroectoderm in embryos or explants: retinoic acid (RA), fibroblast growth factors (FGFs) and Wnts (Durston et al., 1989; Sive et al., 1990; Sharpe, 1991; Cox and Hemmati-Brivanlou, 1995; Lamb and Harland, 1995; McGrew et al., 1995; Papalopulu and Kintner, 1996; Holowacz and Sokol, 1999; Fletcher et al., 2006). Integration of these pathways is complicated; different ligands of the same family could act in varying regional and temporal zones to induce cell fates. How these different pathways act in concert to activate transcription factors or subsequent signaling events is still an enigma.
In Xenopus, the canonical Wnt signaling pathway is a key regulator of the embryonic neural AP pattern. Activating Wnt/β-catenin signaling in neural tissue induces posterior cell fates (McGrew et al., 1995; Kiecker and Niehrs, 2001), while repressing anterior fates (Glinka et al., 1998). An endogenous inhibitor of this pathway, Dkk1 is expressed in anterior neural regions, and its activity is required to maintain this Wnt-free zone (Glinka et al., 1998; Kazanskaya et al., 2000). Ectopic Dkk1 expression blocks posterior cell fate formation, further supporting a role for Wnt activity in the posterior neural plate (Kazanskaya et al., 2000; Kiecker and Niehrs, 2001). Wnt3a is a good candidate for this caudalizing activity; when ectopically expressed, it induces posterior tissue in vivo and in neuralized explants (McGrew et al., 1995; Kiecker and Niehrs, 2001). Initially expressed in dorsal mesodermal regions of gastrulae, Wnt3a mRNA becomes localized to the neural plate at early neurula stages (McGrew et al., 1997; Faas and Isaacs, 2009). Accordingly, an endogenous AP gradient of nuclear localized β-catenin was observed in the Xenopus neural plate; in Xenopus and chick explants, exogenous Wnt3a induced posterior-neural-marker expression in a gradient-like manner (Kiecker and Niehrs, 2001; Nordstrom et al., 2002). However, the exact role and timing of endogenous Wnt3a activity in neural caudalization has not been rigorously examined.
The temporal and regional locale of the endogenous neural caudalizing signal is still a mystery. Although Wnt3a is expressed in the posterior neural plate, onset of expression in neuroectoderm occurs relatively late (McGrew et al., 1997; Faas and Isaacs, 2009). To address this, we hypothesized that the early mesodermal Wnt3a expression domain might significantly contribute to patterning overlying neural tissue. In chick and zebrafish embryos, paraxial mesoderm explants were shown to have neural caudalizing capacity (Muhr et al., 1997; Woo and Fraser, 1997), but neither FGF nor RA can substitute for this activity (Nordstrom et al., 2002). The comparable tissue in Xenopus is a specialized mesodermal region, the paraxial-fated dorsolateral marginal zone (DLMZ). This region is required to induce neural crest in Xenopus (Bonstein et al., 1998). Wnt3a is indeed expressed in the DLMZ region of gastrula Xenopus and zebrafish embryos (McGrew et al., 1997; Shimizu et al., 2005; Faas and Isaacs, 2009). Thus, this secreted factor may be well positioned to direct transcription factor targets in the overlying neural plate.
Meis3, a TALE-family homeobox protein, is expressed in the posterior neuroectoderm in mid-late gastrula stages; by neurula stages, expression is fixed in the hindbrain and the anterior spinal cord (Salzberg et al., 1999). We showed that Meis3 is required for Xenopus posterior neural cell fate specification. In the absence of Meis3 activity, embryos lose numerous posterior neural cell fates, such as of the hindbrain, primary neuron and neural crest, but not the spinal cord; the forebrain is posteriorly expanded; pan-neural marker expression is normal (Dibner et al., 2001; Gutkovich et al., 2010). We also showed that Meis3 induces posterior neural cell fates non-cell autonomously, perhaps by way of other secreted factors (Aamar and Frank, 2004). In zebrafish embryos, Meis3 protein was also required for hindbrain formation (Vlachakis et al., 2001; Waskiewicz et al., 2001). Thus, Meis3 plays a crucial role in the developing posterior neural plate.
Dkk1 gain-of-function and Meis3-morphant phenotypes are very similar (Kazanskaya et al., 2000; Dibner et al., 2001), suggesting an interaction between canonical Wnt signaling and Meis3 protein. In this study, we show that canonical Wnt signaling is essential for activating Meis3 gene expression in the neural plate. We show that the Meis3 gene is a direct target of the canonical Wnt signaling pathway. As determined by chromatin immunoprecipitation (ChIP) analysis, β-catenin protein is bound to the Meis3 promoter region at the appropriate time. In transgenic frogs, these β-catenin–TCF binding sites are required to drive reporter gene expression in vivo. In addition, Meis3 is sufficient to direct posterior neural fates in the absence of Wnt signaling. Either Dkk1 protein overexpression or Wnt3a knockdown leads to a loss of posterior neural fates, including of the hindbrain, neural crest and primary neurons; this phenotype is rescued by ectopic Meis3 expression. Explant and ablation experiments show that the source of the caudalizing signal is localized to the DLMZ region and not to axial mesoderm. Furthermore, we show that Wnt3a is required in the DLMZ to activate Meis3 expression in adjacent neuroectoderm cells. Taken together, our data suggest a new model for neural pattern formation, in which the earliest expression of Wnt3a protein in paraxial mesoderm directly activates Meis3 in the overlying neuroectoderm to induce posterior fates.
MATERIALS AND METHODS
Xenopus embryos
Ovulation, in vitro fertilization, embryo culture, dissections and explant culture were as described (Re'em-Kalma et al., 1995; Bonstein et al., 1998).
RNA, DNA and morpholino oligonucleotide (MO) injections
Capped sense in vitro transcribed full-length mRNA, BMPR1A dominant-negative receptor (DNR), Meis3 (Salzberg et al., 1999), Dkk1 (Glinka et al., 1998) and the glucocorticoid-inducible Tcf3-VP13 (THVGR) (Wu et al., 2005) were injected into embryos at one- or two-cell stages. Mouse Wnt3 (pCS105) and Xenopus Wnt3a (pCS107) plasmids were also injected in zygotic expression assays. MOs used for Wnt3a (Gene Tools) were:
translational blocking, GAGCAAATATCCAAAGCAGCCCATC;
splice blocking, intron-exon, TCTAAGATCGACTGGAAACAAAATG; and
exon-intron, AGAAAAGTAACTTACTGTTCTGCCT.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was carried out with digoxigenin/flourescein-labeled probes (Harland, 1991): XAG1, Otx2, Krox20, HoxB3, Meis3 (Dibner et al., 2001), HoxD1 (Dibner et al., 2004), N-Tubulin (N-Tub), Slug, FoxD3 (Gutkovich et al., 2010), muscle actin (mAct) (Keren et al., 2005), Gbx2 (Kiecker and Niehrs, 2001). β-Galactosidase (β-Gal) was used as a lineage tracer (Dibner et al., 2004), using light blue (X-Gal) or bright pink (Rose D-X-Gal) colored substrates.
Semi-quantitative (sq) RT-PCR analysis
sqRT-PCR was performed (Snir et al., 2006). Primers: XHis4, Xbra, MyoD, Krox20, HoxB9, Otx2, XAG1 (Snir et al., 2006), EF1a, Meis3, HoxD1, FoxD3, HoxB3, FGF3, FGF8 (Gutkovich et al., 2010), XANF1, Xnr3, Slug, N-Tub, mAct, NeuroD (Harland Laboratory database). In all sqRT-PCR experiments performed, multiple numbers of independent experiment repeats were typically performed, as mentioned in the figure legends (n=X). In all experiments, each sample was routinely assayed a minimum of two times for each marker.
CHX explant assay
Embryos at the one-cell stage were injected with THVGR mRNA. Animal cap (AC) explants were removed at blastula stages for culture. At mid-late blastula stages, cyclohexamide (CHX, 5 μM) was added. After 1.5 hours, dexamethasone (DEX, 1 μM) was added at gastrula stages 10-10.25. Explants were cultured until mid-late gastrula and total RNA was isolated for sqRT-PCR analysis.
ChIP and frog transgenesis
For each immunoprecipitation (IP), 50 late-gastrula stage embryos were fixed and processed according to the ChIP protocol (Blythe et al., 2009) using anti-N-terminal Xenopus β-catenin antiserum or normal rabbit serum as a negative control. Samples were analyzed by SYBR-Green QPCR with the Meis3 (–1813, see Fig. S3 in the supplementary material) and XMLC2 (–118) primers (Blythe et al., 2009). For cloning Meis3 genomic sequences, a X. laevis BAC library was screened with a DNA probe overlapping the Meis3 mRNA sequence: 6.5 kb of the BAC was sequenced, which included 3.0 kb upstream to the start of transcription, the 5′UTR, the first exon, and part of the first intron. A 2.7 kb genomic Meis3 region was amplified using Pfu DNA polymerase and sub-cloned into a pGL-3 basic (promoter-less) luciferase (luc) reporter vector (Promega). Mutagenesis of the two β-catenin/TCF sites was performed by the Dpn method (Stratagene), ACAAAG was converted to ACGCGT. Transgenesis was performed (Rogers et al., 2008); luc gene expression was detected by in situ hybridization.
RESULTS
Wnt activity induces Meis3 expression in vivo and in explants
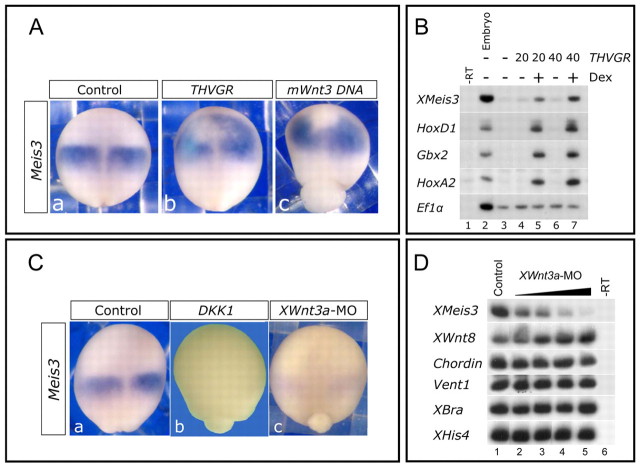
In Xenopus embryos, canonical Wnt signaling and Meis3 protein activities share similar overlapping features as neural caudalizers. We wanted to determine the connection between canonical Wnt signaling and Meis3 expression. Two molecular tools were used to determine if zygotic Wnt activity induces Meis3 expression in embryos or AC explants. We injected RNA encoding a constitutively activated, dexamethasone-inducible TCF protein (THVGR) or a CMV-promoter plasmid containing a Wnt3 (mWnt3) cDNA that is expressed only at the onset of zygotic transcription at mid-blastula. Both tools circumvent the early maternal-Wnt gain-of-function phenotype of axis duplication, and consistently activate high expression levels of posterior neural homeobox genes, such as HoxD1, HoxA2 and Gbx2, in late gastrula-early neurula embryos and ectodermal AC explants (Fig. 1B). Accordingly, high ectopic levels of Meis3 mRNA were also induced (Fig. 1A,B). In mid-late neurula stages, using either approach, there was a sharp decrease in anterior marker expression with a concomitant expansion of posterior neural markers of the hindbrain, primary neuron and neural crest (Fig. 3A, not shown).
Fig. 1.
The canonical Wnt pathway is necessary and sufficient for activating Meis3 gene expression. (A) In situ hybridization of late gastrula/early neurula embryos injected at the one-cell stage with either THVGR mRNA (10-20 pg, b) or mWnt3 DNA (100 pg, c). THVGR was activated by 1 μM DEX at the onset of gastrulation. Meis3 expression is anteriorly expanded in embryos injected with THVGR (113/121 embryos) or with mWnt3 (40/56 embryos). (B) sqRT-PCR of pools of 18 mid-gastrula stage AC explants dissected from embryos injected with THVGR mRNA (20, 40 pg) at the one-cell stage. THVGR was activated by 1 μM DEX at the onset of gastrulation. Ef1α was used as a loading control. No reverse transcriptase (–RT) control PCR was performed on RNA isolated from control embryos in all shown experiments. Posterior markers were not induced in THVGR DEX-untreated ACs (lanes 4, 6; n=5 experiments). (C) In situ hybridization of late gastrula/early neurula embryos injected at the one-cell stage with either Dkk1 mRNA (25 pg, b) or Wnt3a-MO (30 ng, c). Meis3 expression is eliminated in Dkk1 (98/98 embryos) and Wnt3a-morphant (37/37 embryos) embryos. (D) sqRT-PCR of pools of ten late-gastrula embryos injected with Wnt3a-MO either marginally or animally (10, 20, 40, 80 ng) at the one-cell stage. XHis4 was used as a loading control. Mesoderm marker expression is unaltered in Wnt3a-morphant embryos (n=5 experiments).
Fig. 3.
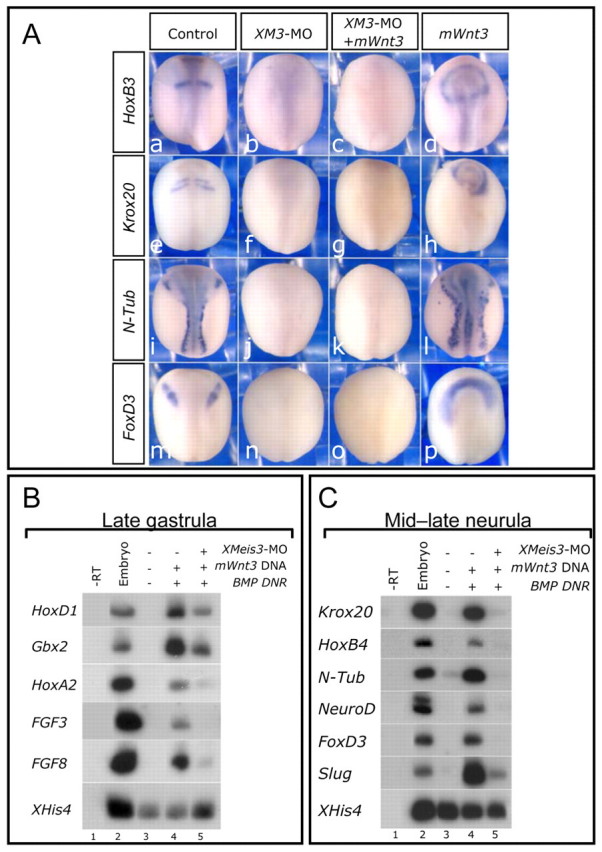
Wnt signaling cannot caudalize in the absence of Meis3 protein activity. (A) In situ hybridization of mid-late neurula embryos injected separately at the one-cell stage with either Meis3 MO (20 ng; b,f,j,n), or mWnt3 DNA (70 pg; d,h,l,p), or both (c,g,k,o). Posterior neural marker expression of HoxB3, Krox20, N-Tub and FoxD3 is highly inhibited in Meis3 morphants (b, 25/25 embryos; f, 40/40 embryos; j, 36/36 embryos; n, 16/16 embryos). mWnt3 co-expression did not rescue the expression of these markers (c, 40/41 embryos; g, 71/75 embryos; k, 63/63 embryos; o, 29/29 embryos). Ectopic mWnt3a levels alone expand posterior marker expression (d, 20/20 embryos; h, 48/49 embryos; l, 33/36 embryos; p, 18/18 embryos). (B) sqRT-PCR of pools of 18 mid-late gastrula AC explants dissected from embryos injected at the one-cell stage with mWnt3 DNA (200 pg) and BMP DNR mRNA (100 pg), and/or separately with Meis3 MO (33 ng) (n=5 experiments). (C) sqRT-PCR of pools of 18 mid-late neurula AC explants from siblings of embryos in B (n=5 experiments).
Zygotic Wnt activity is required for Meis3 expression in late-gastrula/early-neurula stage embryos
To determine whether Wnt was required for Meis3 expression, we depleted canonical Wnt signaling by either ectopic Dkk1 protein expression or Wnt3a morpholino oligonucleotide (MO) injection. To validate Wnt3a MO specificity, both translation- and splice-blocking MOs were used (see Fig. S1 in the supplementary material). These two MOs synergized when injected at low sub-phenotypic concentrations to induce a characteristic anteriorized phenotype, with neural fold inhibition (see Fig. S1F-G in the supplementary material). In addition, rescue experiments were performed in which the morphant phenotype was rescued by co-expression of a mouse Wnt3 RNA/DNA instead of a Xenopus Wnt3a RNA/DNA (see Fig. S1C-E in the supplementary material). Ectopic Dkk1 protein expression or Wnt3a-MO injection severely inhibited the initial activation of Meis3 expression in these embryos (Fig. 1C). Wnt3a-MO inhibition of Meis3 expression was dose dependent (Fig. 1D). By contrast, the Wnt3a-MO did not alter the expression of a wide panel of mesoderm marker genes, such as the pan-mesodermally expressed XBra, the dorsally expressed chordin and goosecoid (not shown), and the ventrolaterally expressed Wnt8 and vent1 genes (Fig. 1D). Consistent with loss of Meis3 expression, Wnt loss of function prevented the expression of a number of early expressed homeobox genes (HoxD1, Gbx2 and HoxA2) that are crucial for determining posterior neural cell fates (Fig. 2C; data not shown). Anterior Otx2 marker expression was also expanded in Dkk1-injected embryos (Fig. 2C). When sibling embryos were cultured to later stages, a typical canonical Wnt loss-of-function phenotype was observed, in which hindbrain, primary neuron and neural crest cell fates were lost with either of the Wnt-inhibitory treatments (Fig. 2A,B). In these same embryos, expression of anterior markers such as XAG1 or XANF1 was expanded (Fig. 2A,B). Interestingly, in the spinal cord, HoxB9 expression levels are typically normal in both Dkk1-injected and Meis3-morphant embryos (Dibner et al., 2001), with only occasional minor perturbations of AP expression levels (not shown). The striking similarity of the Wnt and Meis3 knockdown phenotypes, and the requirement of the Wnts for Meis3 gene expression, strongly suggested that the Meis3 protein could be a downstream mediator of the Wnt pathway in specifying posterior neural cell fates of the hindbrain, neural crest and primary neuron lineages, but not the spinal cord.
Fig. 2.
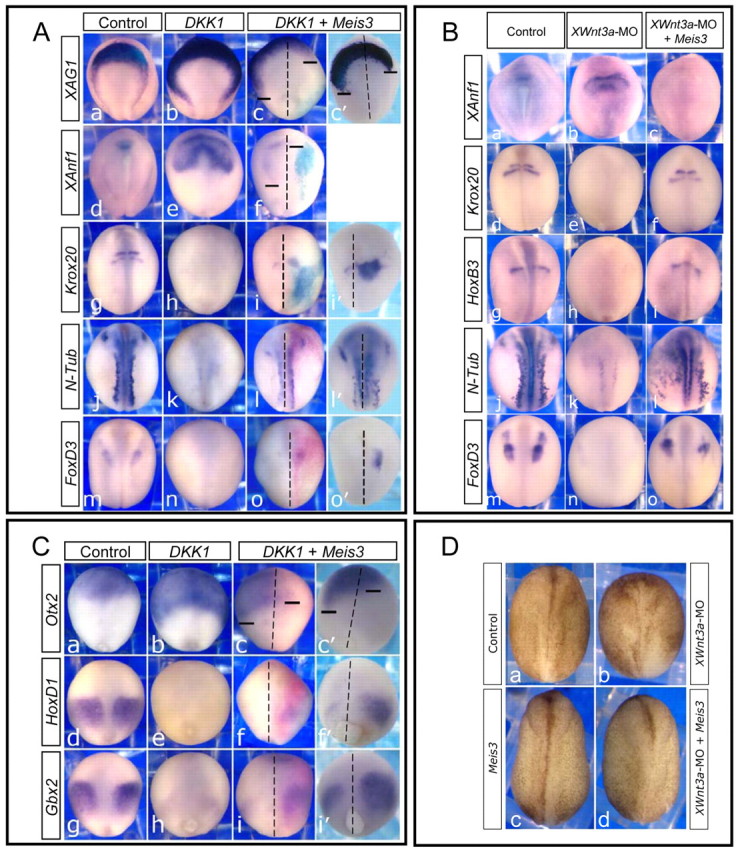
Meis3 restores posterior neural cell fates in the absence of canonical Wnt signaling. (A) In situ hybridization of mid-late neurula embryos injected at the one-cell stage with Dkk1 mRNA (35 pg; b,e,h,k,n) or, additionally, with Meis3 (0.25 ng) and β-gal (25 pg) mRNAs into one blastomere at the two-cell stage (c,f,i,l,o). All embryos are viewed dorsally, and oriented with anterior at the top, posterior at the bottom. The Meis3-injected side is on the right, as traced by the light blue/bright pink X-Gal staining. Dashed lines indicate the dorsal midline; bars indicate the posterior limits of gene expression on either side of the embryo. c′, i′, l′ and o′, show more clearly the signal in embryos treated as in c, i, l and o, respectively, but with no β-gal. XAG1 and XAnf1 expression undergoes robust posterior expansion in Dkk1 embryos (b, 113/115 embryos; e, 30/30 embryos), which is inhibited by Meis3 co-expression (c,c′, 73/81 embryos; f, 32/39 embryos). Posterior neural marker expression, Krox20, N-Tub and FoxD3, is inhibited in Dkk1 embryos (h, 78/80 embryos; k, 40/41 embryos; n, 51/52 embryos), but is strongly rescued by Meis3 co-expression (i,i′, 90/106 embryos; l,l′, 43/62 embryos; o,o′, 36/63 embryos). (B) In situ hybridization of mid-late neurula embryos injected at the one-cell stage with either Wnt3a-MO (50-60 ng; b,e,h,k,n), or, separately, with both Wnt3a-MO (50-60 ng) and Meis3 mRNA (0.5 ng; c,f,i,l,o). XAnf1 expression is posteriorly expanded in Wnt3a-morphants (b, 31/31 embryos), and suppressed by Meis3 co-expression (c, 55/55 embryos). Expression of the posterior neural markers Krox20, HoxB3, N-Tub and FoxD3 is inhibited in Wnt3a-morphants (e, 31/33 embryos; h, 31/31 embryos; k, 51/52 embryos; n, 50/51 embryos), but rescued by Meis3 co-expression (f, 29/33 embryos; i, 10/29 embryos; l, 22/39 embryos; o, 11/38 embryos). (C) In situ hybridization of late gastrula-early neurula siblings of embryos in A. All embryos are viewed dorsally, and oriented with anterior at the top, posterior at the bottom. The Meis3 injected side is on the right (bright pink X-Gal staining). Dashed lines and bars are as in A. Panels c′, f′, i′, embryos treated as in c, f, i, respectively, but with no β-gal. Otx2 expression undergoes robust posterior expansion in Dkk1 embryos (b, 49/51 embryos), which is inhibited by Meis3 co-expression (c,c′, 48/64 embryos). Expression of the posterior neural markers HoxD1 and Gbx2 is inhibited in Dkk1 embryos (e, 53/53 embryos; h, 51/53 embryos), but is strongly rescued by Meis3 co-expression (f,f′, 58/72 embryos; i,i′, 41/64 embryos). (D) Morphology of mid-late neurula embryos injected at the one-cell stage with either Wnt3a-MO (b, 70 ng), or Meis3 mRNA (c, 0.7 ng), or both (d). Neural folding and convergence extension is inhibited in Wnt3a morphants (b, 60/64 embryos); Meis3 co-expression rescued this phenotype (d, 48/49 embryos).
Meis3 can restore posterior neural fates in the absence of Wnt
To determine Meis3-Wnt epistasis, Meis3 protein was ectopically co-expressed in embryos injected with either Dkk1 mRNA or the Wnt3a-MO. In these Wnt loss-of-function phenotypes, ectopic Meis3 protein expression robustly rescued posterior cell fates (Fig. 2A,B). In the Dkk1 rescue experiments (Fig. 2A), Dkk1 mRNA was injected at the one-cell stage followed by Meis3 mRNA injection into one blastomere at the two-cell stage. Meis3 was co-injected with β-galactosidase (β-gal) mRNA as a lineage tracer (light blue/bright pink stain, depending on the substrate used). Over 94% of the embryos were β-gal positive on the injected side and only these embryos were scored. In these co-injected embryos, expression of hindbrain [Krox20, HoxB3 (not shown)], primary neuron (N-Tub) and neural crest [FoxD3, Slug (not shown)] markers was rescued (Fig. 2A). In Dkk1 rescued embryos, the somewhat disorganized regional Krox20 expression in the hindbrain was the typical phenotype (Fig. 2A, parts i,i′). In some cases, gene expression was rescued on both sides of the embryo (N-Tub), despite injecting Meis3 RNA into only one blastomere at the two-cell stage (Fig. 2A, parts l,l′). Lineage tracing studies suggested that cells are not migrating across the mid-line (Fig. 2A,C). Thus, this observation is consistent with previously demonstrated Meis3 non-cell autonomous activity that induces primary neuron fate (Aamar and Frank, 2004). However, for most genes, expression rescue was restricted to the Meis3-injected side.
The Wnt3a-MO is a more specific tool than the Dkk1 protein, because it exclusively inhibits Wnt3a protein translation. The Wnt3a-MO efficiently inhibits posterior neural marker expression (Fig. 2B). In one-cell-stage embryos injected separately with the Wnt3a-MO and Meis3 RNA, there was a significant rescue of Krox20, HoxB3, N-Tub and FoxD3 expression (Fig. 2B).
In late-gastrula stages, reducing Meis3 protein expression levels by either Wnt loss of function, or the Meis3-MO also strongly inhibited expression of early homeobox genes such as HoxD1, Gbx2 and HoxA2 (Fig. 2C; see also Fig. S2 in the supplementary material). At these early stages, HoxD1 and Gbx2 expression are robustly rescued by Meis3 (Fig. 2C); early Otx2 expression is expanded by Dkk1 and strongly inhibited by Meis3 (Fig. 2C).
In Dkk1-injected neurula stage embryos, the anterior markers XAG1, XANF1 and Otx2 were typically expanded (Fig. 2A, parts b,e; data not shown); ectopic Meis3 expression antagonized their expression. Note the striking posterior expansion of XANF1 and XAG-1 in Dkk1-injected embryos, and the strong inhibition by Meis3 co-expression (Fig. 2A, parts c,c′,f; compare the bars on either side). When using cement gland size as an index of AP levels in the embryo, cement glands were typically 50-60% larger in Dkk1-expressing embryos versus controls; in embryos injected solely with Meis3, cement glands were 30% smaller versus controls, yet in Meis3/Dkk1 co-injected embryos, cement glands were less than 5% larger than controls (not shown). Similar results were seen in embryos injected with the Wnt3a-MO (Fig. 2B). The XANF1 marker is expanded in Wnt3a-morphant embryos and Meis3 co-expression completely inhibits its expression (Fig. 2B, compare part b with c).
Another typical phenotype associated with a loss of posterior neural cell fates is the inhibition of neural plate folding and convergence extension (CE). Similar to Meis3 morphants (Gutkovich et al., 2010), in Wnt3a morphants, neural folding and CE is perturbed (Fig. 2D, compare part b with part a). Exogenous Meis3 significantly rescues this phenotype: embryos undergo normal neural folding and CE (Fig. 2D, compare part b with part d).
To confirm that Meis3 is downstream of Wnt3a, we expressed Wnt3a in the Meis3-morphant background (Dibner et al., 2001). Ectopic Wnt3a expression never rescued the Meis3-morphant phenotype (Fig. 3A). In gastrula and neurula stage AC explants, co-expression of the BMP dominant-negative receptor protein and the Wnt3 plasmid induces optimal Meis3 expression levels (not shown), as well as a wide array of posterior neural markers (Fig. 3B,C). These explants likely mimic the in vivo embryonic state, in which both neuralizing and caudalizing pathways act in concert to specify cell fates along the AP axis. The early activation of posterior specifying Hox and FGF gene expression is blocked in these Meis3-morphant explants (Fig. 3B), as is the subsequent high activation of Krox20, HoxB4, N-Tub, NeuroD, FoxD3 and slug expression (Fig. 3C). In complementary experiments, identical results were observed when Meis3-morphant AC explants were alternatively neuralized/caudalized by noggin/Wnt3a mRNA co-injection (not shown). Thus, in sensitized explants, as in embryos, the activation of posterior neural markers by canonical Wnt signaling requires Meis3 protein.
These results show that Meis3 is a downstream mediator of Wnt caudalizing activity in Xenopus embryos. Wnt3a protein appears to be the crucial Wnt ligand, as in its absence, Meis3 expression is highly inhibited and posterior neural cell fates are compromised. However, in the whole embryo, it is difficult to pinpoint the exact regional and temporal function of the Wnt3a protein in neural caudalization.
The dorsolateral paraxial mesoderm is crucial for Meis3 expression and subsequent posterior specification
Wnt3a is only detected in the neural plate at early neurula stages, but posterior neural plate induction occurs at earlier mid-gastrula stages, when Meis3 expression in the presumptive neural plate precedes detectable Wnt3a expression in this region (Y.M.E. and D.F., unpublished). However, prior to expression in the neural plate, Wnt3a mRNA is detected in the dorsolateral paraxial mesoderm regions of gastrula stage Xenopus and zebrafish embryos (McGrew et al., 1997; Shimizu et al., 2005; Faas and Isaacs, 2009); this Wnt3a expression phase precedes Meis3 expression. Our previous studies showed that gastrula-stage DLMZ regions are required for neural crest induction, but not for general neural induction in embryos; by contrast, embryos ablated for the dorsal marginal zone (DMZ) induce neural crest normally (Bonstein et al., 1998). Gastrula-stage DLMZ explants also efficiently induce neural crest in juxtaposed AC explants (Bonstein et al., 1998). Because of its location adjacent to the neurectoderm, the DLMZ could also be a more generic inducer of posterior neural cell fates. In this case, a non-neural source of Wnt3a protein expressed in the DLMZ region could be secreted to overlying neural ectoderm, where it would caudalize the tissue by inducing Meis3 expression.
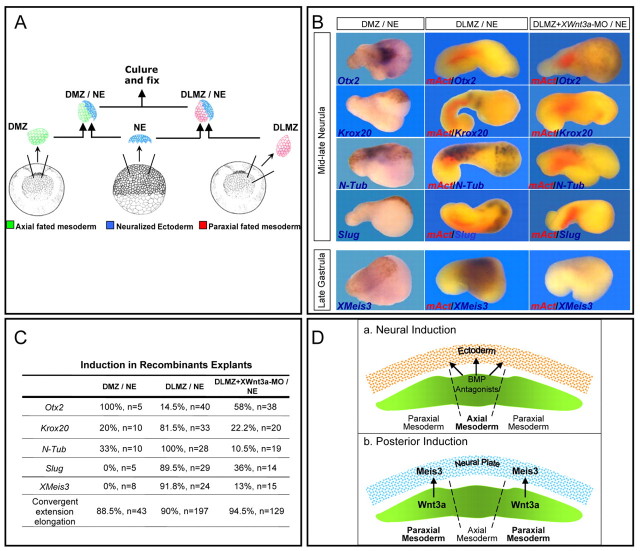
To address this possibility, we cultured embryos to early gastrula stages and dissected out both DLMZs; in a parallel control group, the single DMZ was removed (Fig. 4A). Embryos were cultured to late gastrula or mid-late neurula stages for analysis. DLMZ ablation but not DMZ ablation led to a loss of posterior fates, including hindbrain (Krox20, HoxB3) and primary neurons (N-Tub), as well as neural crest (slug; Fig. 4B). Meis3 expression was also highly reduced in DLMZ-ablated embryos (Fig. 4C). As a control for dissection accuracy, mAct expression was compared in DLMZ- and DMZ-ablated embryos (Fig. 4B). To determine whether Meis3 protein suffices for neural caudalization in this assay, embryos destined for DLMZ ablation were injected with ectopic Meis3 mRNA at the one-cell stage (Fig. 4B). In Meis3-injected/DLMZ-ablated embryos, there was a striking recovery of all of the lost posterior neural cell fate markers (Fig. 4B); this was independent of mesoderm, as mAct expression was not rescued in these embryos (Fig. 4B). Furthermore, ectopic Wnt3a DNA expression rescued Meis3 expression in these DLMZ-depleted embryos (Fig. 4C); again this was independent of paraxial mesoderm formation, as MyoD expression was not rescued in these embryos (Fig. 4C). These results show that the DLMZ is required in the embryo to induce generic posterior neural cell fates, and that Wnt3a or Meis3 can replace the DLMZ in this process. In embryos lacking DMZs, posterior neural markers are expressed at fairly normal levels (Fig. 4B). Supporting the observations in DLMZ-depleted embryos, the induction of posterior neural markers in DMZ-depleted embryos is a canonical Wnt-dependent process, as ectopic Dkk1 levels eliminated their expression (Fig. 4B).
Fig. 4.
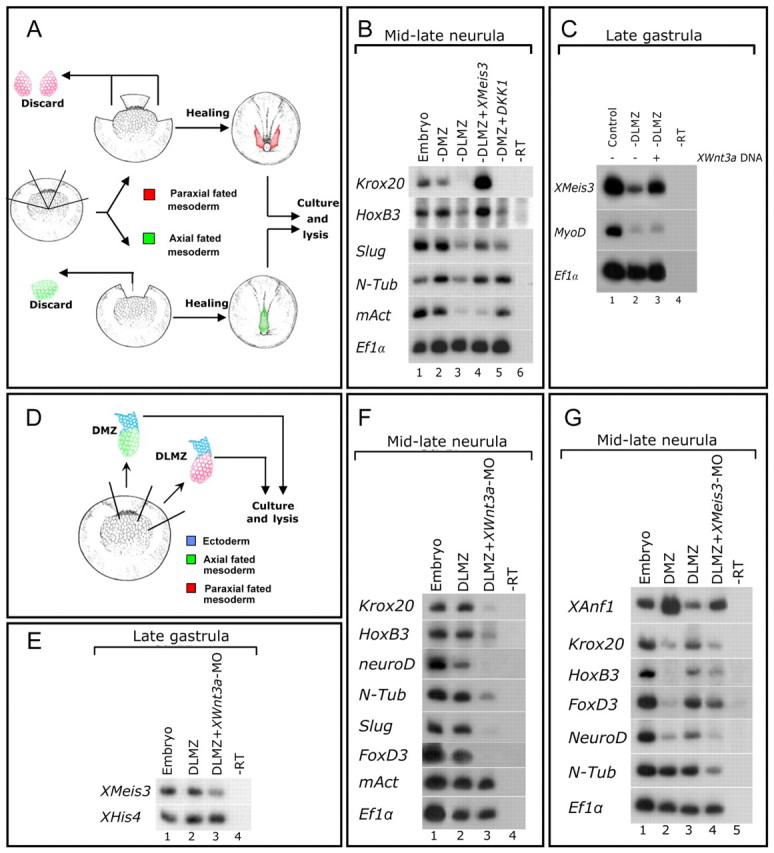
Paraxial-fated DLMZ mesoderm caudalizes neuroectoderm by inducing Meis3 expression in a Wnt3a-dependant manner. (A) The DLMZ ablation assay. In early-gastrula stage 10.25 embryos, either the two DLMZ regions (red) or the one DMZ region (green) of approximately 60° radius were removed. Embryos were cultured to gastrula and neurula stages. (B) sqRT-PCR of pools of nine mid-late neurula DLMZ- or DMZ-ablated embryos, either uninjected, or injected at the one-cell stage with either Meis3 (0.75 ng) or Dkk1 (50 pg) mRNAs. mAct expression controls for accurate ablation of DLMZ regions (n=5 experiments). (C) sqRT-PCR of pools of nine late-gastrula, DLMZ-ablated embryos, either uninjected, or injected at the one-cell stage with mWnt3 DNA (50 pg). MyoD expression controls for accurate ablation of DLMZ regions and the lack of mesoderm induction by mWnt3 (n=4 experiments). (D) The “dirty” DLMZ explant assay. In gastrula stage 10.25 embryos, DLMZ (red) and DMZ (green) regions of approximately 60° radius juxtaposed to adjacent ectoderm (blue) were removed. Explants were cultured to gastrula and neurula stages. (E) sqRT-PCR of pools of nine mid-late gastrula “dirty” DLMZ explants dissected from embryos either uninjected, or marginally injected into two blastomeres at the two-cell stage with Wnt3a-MO (20 ng per blastomere) (n=4 experiments). (F) sqRT-PCR of pools of nine mid-late neurula “dirty” DLMZ explants from siblings of embryos shown in E. mAct expression controls for the lack of mesoderm perturbation by the Wnt3a-MO (n=5 experiments). (G) sqRT-PCR of pools of 12-18 mid-late neurula (stage 17) “dirty” DMZ and DLMZ explants dissected from embryos either uninjected, or injected at the one-cell stage with Meis3-MO (20 ng; n=4 experiments).
Wnt3a expression in the dorsolateral mesoderm induces posterior-neural cell fates via Meis3
Because the DLMZ is crucial for Meis3 expression and neural caudalization, we examined its posterior-neural induction potential. Early-gastrula stage DLMZ explants were removed, in which a flap of juxtaposed cells from the ectodermal region remained attached to the mesoderm. This is an analogous DLMZ version of a Keller DMZ explant, allowing us to examine tissue interactions between the mesoderm and ectoderm ex vivo (Fig. 4D). We call these “dirty” DLMZ explants, because they deliberately contain this residual ectodermal tissue, as opposed to the standard pristine DLMZs, which are predominantly mesodermal in origin. Meis3 expression was induced (Fig. 4E) and posterior neural marker expression was robust in “dirty” DLMZ versus “dirty” DMZ explants (Fig. 4F-G). DMZ explants did not express high levels of Meis3 mRNA (not shown). In Wnt3a-morphant “dirty” DLMZ explants, posterior neural marker expression was inhibited (Fig. 4F), including early Meis3 expression (Fig. 4E). In Meis3-morphant “dirty” DLMZ explants, there was a strong inhibition of all of the posterior neural markers (including early HoxD1 expression; data not shown), with a subsequent increase in the anterior markers Otx2 (not shown) and XANF1 (Fig. 4G), suggesting that the loss of Meis3 expression in the explant prevents its caudalization and drives it to a more anterior neural fate. Mesoderm formation as assayed by mAct expression was normal in the Meis3- (data not shown) and Wnt3a-morphant explants (Fig. 4F).
To fully pinpoint the site of embryonic Wnt3a activity, recombinant explant assays were performed in which DLMZ explants were recombined with AC explants neuralized (NE) using a BMP dominant-negative receptor (Fig. 5A). To lineage trace the cell sources in the explants, two parallel techniques were used. In some assays, we recombined pigmented mesodermal explants with albino NEs, using the albinism as a lineage trace for the NE tissue (Fig. 5B, DMZ/NE explant, far left panel). In other cases, we performed the same experiment on bleached pigmented explants; however, in these experiments, double in situ hybridization was performed for m-Act (red) and the neural marker (purple) of choice in order to distinguish between the mesodermal and neural portions of the explant (Fig. 5B). In mid-late neurula-stage recombinant explants (Fig. 5B, center explant panels), the DLMZ induced the expression of hindbrain (Krox20), primary neuron (N-Tub) and neural crest (slug) markers. Expression of the anterior marker Otx2 (expressed in the NE) was repressed by the DLMZ compared with the DMZ (Fig. 5B, compare left and center explant panels). In late-gastrula stage sibling explants, Meis3 expression in the NE was strongly induced by the DLMZ (Fig. 5B, center explant panel). Typically, neural gene expression patterns in the recombinant explants strikingly recapitulate their spatial patterns in the embryo, a broad band for Meis3, thin stripes for Krox20, punctate dots/stripes for N-Tub and lateral bands for slug (Fig. 5B, compare explant expression patterns in the center panel to embryos in Fig. 2). Meis3 and later posterior markers were not induced in the DMZ recombinant explants (Fig. 5B, compare left and center panels). To confirm that Wnt3a plays a key signaling role in the mesoderm, we recombined the Wnt3a-MO-injected DLMZs with NEs. In this case, the DLMZ failed to induce either early Meis3 or later posterior neural marker expression in the NE portion of the recombinant explant (Fig. 5B, compare right and center explant panels); the anterior Otx2 marker expression was not repressed. The statistics describing recombinant explant results are summarized in Fig. 5C. The injected Wnt3a-MO concentrations did not perturb mesoderm formation in the embryo or in explants, as determined by XBra (Fig. 1D) and mAct gene expression (Fig. 4F, Fig. 5B). These results demonstrate that disruption of Wnt3a protein expression in late-gastrula stage dorsolateral mesoderm prevents Meis3 expression in the early neural plate, thus triggering a loss of posterior cell fates in the developing nervous system.
Fig. 5.
Wnt3a expression in paraxial-fated mesoderm is required to induce Meis3 expression and posterior-neural cell fates. (A) The recombinant DLMZ/neuroectoderm explant assay. In gastrula stage 10.25 embryos, DLMZ (red) and DMZ (green) regions of approximately 60° radius are juxtaposed to AC ectoderm (NE, blue) removed from blastula stage 8-9 embryos injected with 100 pg BMP DNR mRNA. Recombinant explants are cultured to gastrula and neurula stages. (B) Double in situ hybridization of bleached DLMZ/NE explants recombined from uninjected or Wnt3a-MO injected (20 ng) DLMZs. High levels of Krox20, N-Tub, Slug and Meis3 are induced in DLMZ/NE recombinants, with repression of Otx2 expression (purple, center) and typical mAct expression (red). In Wnt3a-morphant DLMZ/NE recombinants, Otx2 expression is recovered and Krox20, N-Tub, Slug and Meis3 expression is not detected (purple, right), whereas mAct expression is normal (red). DMZ/NE explants were recombined from pigmented DMZ and albino NE (left). Otx2 is highly expressed in DMZ/NE recombinants; posterior markers are not detected (purple, left). (C) Summary of the results in B. All scored DLMZ/NE recombinant explants express mAct normally. (D) Model: Wnt3a from the paraxial-fated DLMZ region induces Meis3 expression and posterior cell fates in the neural plate. (a) Neural induction by axial mesoderm. (b) Posterior neural induction by paraxial mesoderm.
Meis3 is a direct target of β-catenin
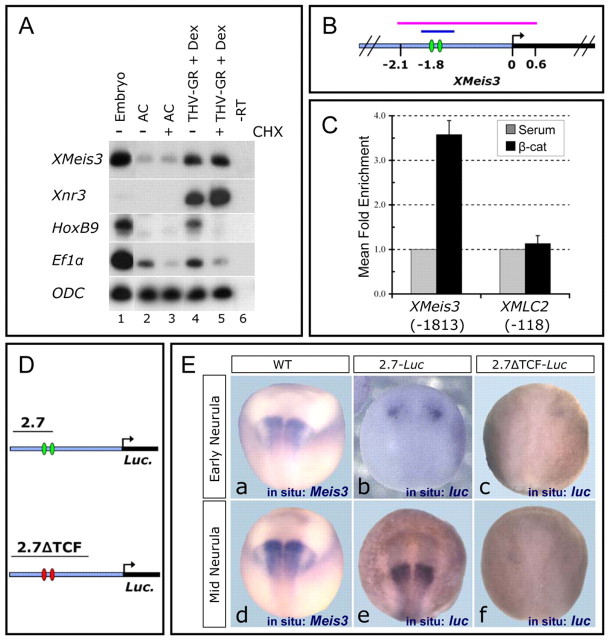
Our data raised the possibility that Meis3 might be a direct early target of Wnt signaling. To address this, we examined Wnt-dependent induction of Meis3 transcription in the absence of protein synthesis. In AC explants expressing inducible TCF (THVGR), treatment with cycloheximide (CHX) prior to dexamethasone (DEX) induction still resulted in robust Meis3 expression (Fig. 6A), as with the positive control, Xnr3, a known direct target of Wnt. As a negative control, CHX-treatment inhibited THVGR induction of HoxB9 expression (Fig. 6A).
Fig. 6.
Meis3 is a direct target of β-catenin. (A) CHX AC assay (Materials and methods). sqRT-PCR of pools of 18 mid-late gastrula ACs were dissected from embryos injected with 20 pg THVGR mRNA at the one-cell stage. ACs were treated with CHX, DEX or CHX+DEX. Ef1α expression serves as a positive control for CHX activity and ODC as a loading control (n=2 experiments). (B) The XMeis3 genomic locus. Green ovals indicate β-catenin/TCF-binding sites, the blue line indicates the ChIP enriched fragment detected in C; the pink line indicates the 2.7 kb used for the constructs in D and E. (C) ChIP for β-catenin in late-gastrula embryos. A region 1.8 kb upstream of the Meis3 transcriptional start site is specifically enriched. Data are plotted as mean fold enrichment by β-catenin IP (black bars) over control serum IP (gray bars) for Meis3 (–1813) or a negative control locus XMLC2 (–118). Error bars show s.e.m. (n=3). (D) Reporter constructs used in E. The 2.7-luc construct contains wild-type β-catenin/TCF-binding sites (green ovals), whereas these are mutated in the 2.7ΔTCF-luc construct (red ovals). (E) Meis3 promoter activity is dependent on two β-catenin/TCF-binding sites. In situ hybridization of early- and mid-neurula 2.7-luc (b,e) and 2.7ΔTCF-luc (c, f) transgenic embryos, and of wild-type embryos (a,d).
To further investigate this regulation, we examined the Meis3 gene promoter. We cloned a 6.5 kb Meis3 gene fragment. This region contains a putative TATA-less promoter initiator-INR sequence (see Fig. S3 in the supplementary material), as mapped by the 5′ RACE procedure (data not shown). Two putative β-catenin/TCF-binding sites were found in close proximity, 1.8 kb upstream of the start of Meis3 gene transcription (Fig. 6B; see also Fig. S3 in the supplementary material). To confirm the β-catenin interaction, we performed ChIP analysis; this region was strongly bound by β-catenin protein in chromatin isolated from late-gastrula stage embryos (Fig. 6C), when Meis3 expression is maximally activated in the embryo. Two similarly located putative β-catenin/TCF-binding sites were also detected in the X. tropicalis Meis3 locus, in a region conserved between X. laevis and X. tropicalis (data not shown).
To complement the ChIP analysis, we generated transgenic frogs using a Meis3 genomic fragment of 2.7 kb driving luciferase (luc) expression (see Materials and methods). This construct (2.7-luc) contains 2.1 kb of sequence upstream to the start of transcription and 0.6 kb of transcribed UTR sequence (Fig. 6B,D; see also Fig. S3 in the supplementary material). In early- to mid-neurula stage embryos, luc expression driven by the 2.7-luc construct strongly resembled the endogenous Meis3 hindbrain expression domain (Fig. 6E; early-neurula: panel b, 22/98 embryos, compare with panel a; mid-neurula: panel e, 11/48 embryos, compare with panel d). To determine whether these two β-catenin/TCF sites bound in ChIP are required for Meis3 promoter activity, we examined luc expression driven by the 2.7ΔTCF-luc construct, in which both of these sites are mutated (Fig. 6D; see also Fig. S3 in the supplementary material). luc expression driven by the 2.7ΔTCF construct was barely detected in early- to mid-neurula stage embryos (Fig. 6E; early neurula: panel c, 3/155 embryos; mid-neurula: panel f, 3/46 embryos). Double mutation of both TCF sites was necessary to fully inhibit transgene expression; mutation of either TCF site did not significantly alter luc expression (not shown). Low luc expression in the mutant construct does not appear to result from poor transgenesis. First, the constructs are nearly identical, varying by only four nucleotide point mutations in each TCF-binding site. In any given experiment, transgenics with 2.7-luc or 2.7ΔTCF-luc were made in the same fertilization batches, under the same experimental conditions. In addition, all transgenics expressed low background levels of luc in the epidermal ectoderm at the same frequency, regardless of whether it was the 2.7-luc or 2.7ΔTCF-luc construct (data not shown). These results show that these β-catenin/TCF sites are obligatory for Meis3 promoter activity.
DISCUSSION
Many studies have shown a wide range of roles for neural caudalizing molecules such as FGF3/FGF8, RA and Wnt3a in inducing spinal cord, hindbrain, neural crest and primary neuron cell fates. Scant data is available connecting downstream transcription factors to these pathways. Many of these signaling pathway components are expressed simultaneously in multiple temporal and regional zones, with apparent overlap in their abilities to induce different cell fates. A major challenge has been to decipher which regionally distinct signaling ligands activate specific transcription factors that act downstream to specify embryonic cell fates. In this study, we have taken advantage of the Xenopus embryo to make these direct connections. We show that signals regulating posterior neural fates emanate from a specialized mesodermal region, the DLMZ (see model in Fig. 5D). Careful examination of previous studies in Xenopus and our own work show that Wnt3a is indeed expressed in paraxial-fated DLMZ (McGrew et al., 1997). This specialized region is required for posterior neural fate induction. We have then shown that mesodermal Wnt3a directly induces expression of the transcription factor Meis3 in the overlying neural plate. Meis3 is necessary and sufficient for neural caudalization.
Using tissue specific ablations, tissue recombination and explant assays, we have clarified the requirements of mesodermal Wnt signaling for patterning the neural plate. In chick and zebrafish embryos, paraxial-fated mesoderm explants caudalize the neural plate, transforming forebrain to more posterior cell types (Muhr et al., 1997; Woo and Fraser, 1997). This endogenous chick caudalizing ligand was not identified, but neither FGF nor RA activity could replace paraxial tissue in caudalizing assays (Muhr et al., 1997; Muhr et al., 1999). In chick (like Xenopus), the addition of ectopic Wnt protein caudalized forebrain explants. Conversely, when soluble Wnt-blocking Frz8 receptor protein was added to caudal neural explants, posterior cell fates were lost (Nordstrom et al., 2002). However, these experiments did not distinguish between mesodermal and neural sources of the functional Wnt signal.
Studies in zebrafish morphants and mutants suggest that Wnt8 and Wnt3a might be functionally redundant; mesodermal sources of either molecule could act as a neural caudalizer. However, these embryos also suffered severe mesoderm perturbations (Erter et al., 2001; Lekven et al., 2001), so it is difficult to conclude whether neural patterning defects are specific, or the indirect result of losing paraxial mesoderm fates. In zebrafish, the Wnt8-MO consistently gave a stronger mesoderm perturbation phenotype than did the Wnt3a-MO, and co-injection of both gave a more severe additive phenotype, which included neural mis-patterning (Shimizu et al., 2005); thus the potential for two overlapping but non-identical roles for Wnt8 and Wnt3a in mesodermal and/or neural patterning cannot be ruled out.
In Xenopus and zebrafish, the disruption of Wnt8 activity perturbs mesoderm pattern: dorsal mesoderm is expanded and more ventrolateral regions, including the paraxial-fated mesoderm, are reduced (Christian and Moon, 1993; Hoppler et al., 1996; Hoppler and Moon, 1998; Lekven et al., 2001; Ramel and Lekven, 2004; Ramel et al., 2005). These observations are not seen for Wnt3a in this study. In Wnt3a-morphant embryos, the organizer is not expanded, ventrolateral markers are expressed normally and paraxial mesoderm forms muscle (Fig. 1D, Fig. 4F, Fig. 5B). Moreover, we show that the Wnt3a-MO blocks the induction of posterior neural cell fates without altering either Wnt8 expression (Fig. 1D) or activity (see Fig. S1C in the supplementary material). The timing of expression in both Xenopus and zebrafish show that Wnt8 is detected before Wnt3a (Christian et al., 1991; Smith and Harland, 1991; Krauss et al., 1992; Kelly et al., 1995; McGrew et al., 1997; Shimizu et al., 2005), which suggests that Wnt8 patterns the mesoderm and that Wnt3a could act downstream to supplement this process by patterning neuroectoderm. We cannot completely rule out a role for Wnt3a in mesoderm patterning. Yet under our experimental conditions, we show that Wnt3a knockdown does not alter mesoderm, but severely perturbs posterior neural cell fates, showing that Wnt3a is the crucial DLMZ ligand required for neural patterning.
We have identified a required direct target of Wnt signaling in the neurectoderm. Previous studies had suggested Wnt signaling acts as a caudalizing morphogen to activate homeobox gene expression in the Xenopus hindbrain (Kiecker and Niehrs, 2001). Furthermore, in chick explants, paraxial mesoderm induced homeobox gene expression in anterior neural ectoderm (Itasaki et al., 1996; Grapin-Botton et al., 1997). Our data demonstrate that Wnt3a directly controls Meis3 expression; in turn, Meis3 protein regulates proper homeobox gene expression levels, thus mediating Wnt morphogenetic activity to induce posterior cell fates. Meis3 protein could optimally activate early homeobox gene expression by simultaneously cooperating with other signaling pathways. For example, HoxD1 is a Meis3 direct target gene that is also a direct target of RA (Dibner et al., 2004) and canonical Wnt signaling (Y.M.E. and D.F., unpublished) pathways. In Xenopus, Gbx2 homeobox protein is required for neural crest induction; Gbx2 is also a direct target of canonical Wnt signaling (Li et al., 2009). No doubt, there is much cross-talk between Meis3 protein and the various caudalizing pathways, as well as with other early transcription factors. These interactions likely ensure the optimal temporal and spatial pattern of early homeobox gene expression in the developing neural plate.
It has recently been proposed that a highly conserved Wnt-Cdx-Hox gene hierarchy acts to pattern the most posterior/tail regions in developing bilaterian embryos (Martin and Kimelman, 2009). Possibly, the activation of more anteriorly expressed Hox genes is dependent on a similar Wnt-Meis-Hox hierarchy. Interestingly, cdx and Meis3 loss-of-function embryos share mirror image-like phenotypes (Isaacs et al., 1998; Dibner et al., 2004; Skromne et al., 2007; Faas and Isaacs, 2009). Cdx protein deficiency causes spinal cord defects (Isaacs et al., 1998; Skromne et al., 2007; Faas and Isaacs, 2009), whereas Meis3 knockdown causes severe hindbrain perturbations, without significantly disrupting spinal cord formation (Dibner et al., 2004).
Is canonical Wnt activation of Meis gene expression a conserved phenomenon? In C. elegans, the PSA-3/Meis protein is required for daughter cell fates after asymmetric cell division, and it is a direct target of the Wnt pathway (Arata et al., 2006). POP-1/TCF protein binds the psa-3/Meis promoter and its binding site is required for expression. In Drosophila, the wingless (Wg/Wnt) protein activates expression of the sole Meis protein homolog, the homothorax (hth) gene, which is required for wing hinge development (Casares and Mann, 2000). In both beetles and spiders, hth expression seems to be regulated by Wg during appendage development (Prpic et al., 2003). Thus, in diverged invertebrate systems, Wnt signaling controls Meis/hth family gene expression. This study shows the first evidence for Wnt regulation of Meis gene expression in vertebrates.
The canonical Wnt, FGF and RA signaling pathways are required for posterior CNS formation in Xenopus (Blumberg et al., 1997; Franco et al., 1999; Hardcastle et al., 2000; Villanueva et al., 2002; Shiotsugu et al., 2004; Monsoro-Burq et al., 2005; Wu et al., 2005). In a unifying model, we suggest that Wnt3a signaling from the DLMZ triggers Meis3 expression in the overlying neural plate (Fig. 5D). Meis3 protein directly activates FGF3 and FGF8 gene expression (Aamar and Frank, 2004; Gutkovich et al., 2010), and Meis3 protein cannot induce posterior cell fates in the absence of downstream FGF signaling (Ribisi et al., 2000; Aamar and Frank, 2004). Supporting these data, in caudalized Xenopus explants, FGF acts downstream of canonical Wnt signaling (Domingos et al., 2001). We show that FGF3 and FGF8 expression in Wnt-caudalized explants is dependent on Meis3 protein activity (Fig. 3B). RA signaling also interacts with Meis3 protein to optimize Hox gene expression in the early neural plate to fine-tune the hindbrain pattern (Dibner et al., 2004). Thus, in a regulatory network controlling posterior neural cell fates, Meis3 acts downstream to canonical Wnt, upstream to FGF, and in concert with RA signaling to activate gene expression.
What is the organizer's role in neural patterning? Clearly the DMZ is indispensable for neural induction, which is a prerequisite for neural patterning. In explants, BMP antagonism does not induce Meis3 expression but, in combination with Wnt signaling, BMP antagonism optimally induces Meis3 expression. BMP antagonism induces competent neuroectoderm that is responsive to caudalizing signals from the DLMZ. Optimal Meis3 expression in the embryo depends on Zic1 protein activity (Gutkovich et al., 2010), and Zic gene family expression requires BMP antagonism. While the organizer does not caudalize neuroectoderm, it provides the competence for the Wnt pathway to activate Meis3 gene transcription (Fig. 5D).
There are still many open questions. In the presumptive hindbrain region of gastrula embryos, Meis3-expressing cells act as a hindbrain-inducing center by inducing posterior neural cell fates non-cell autonomously (Aamar and Frank, 2004). At gastrula stages, when this center is active, the target cells are clustered in close proximity. How does Meis3 induce different cell fates in such proximal, but distinct, embryonic regions? How are the different posterior neural cell types, such as hindbrain, primary neuron and neural crest specified to such exact regions by similar signaling pathways and transcription factors during overlapping time windows? Our future challenge is to determine how Meis3 protein acts with these signaling pathways and other transcription factors to generate multiple nervous system cell fates.
Supplementary Material
Acknowledgements
D.F. was supported by grants from the Israel Science Foundation (197/05, 658/09).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.044750/-/DC1
References
- Aamar E., Frank D. (2004). Xenopus Meis3 protein forms a hindbrain-inducing center by activating FGF/MAP kinase and PCP pathways. Development 131, 153-163 [DOI] [PubMed] [Google Scholar]
- Arata Y., Kouike H., Zhang Y., Herman M. A., Okano H., Sawa H. (2006). Wnt signaling and a Hox protein cooperatively regulate psa-3/Meis to determine daughter cell fate after asymmetric cell division in C. elegans. Dev. Cell 11, 105-115 [DOI] [PubMed] [Google Scholar]
- Blumberg B., Bolado J., Jr, Moreno T. A., Kintner C., Evans R. M., Papalopulu N. (1997). An essential role for retinoid signaling in anteroposterior neural patterning. Development 124, 373-379 [DOI] [PubMed] [Google Scholar]
- Blythe S. A., Reid C. D., Kessler D. S., Klein P. S. (2009). Chromatin immunoprecipitation in early Xenopus laevis embryos. Dev. Dyn. 238, 1422-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonstein L., Elias S., Frank D. (1998). Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Dev. Biol. 193, 156-168 [DOI] [PubMed] [Google Scholar]
- Casares F., Mann R. S. (2000). A dual role for homothorax in inhibiting wing blade development and specifying proximal wing identities in Drosophila. Development 127, 1499-1508 [DOI] [PubMed] [Google Scholar]
- Christian J. L., Moon R. T. (1993). Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 7, 13-28 [DOI] [PubMed] [Google Scholar]
- Christian J. L., McMahon J. A., McMahon A. P., Moon R. T. (1991). Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 111, 1045-1055 [DOI] [PubMed] [Google Scholar]
- Cox W. G., Hemmati-Brivanlou A. (1995). Caudalization of neural fate by tissue recombination and bFGF. Development 121, 4349-4358 [DOI] [PubMed] [Google Scholar]
- Dibner C., Elias S., Frank D. (2001). XMeis3 protein activity is required for proper hindbrain patterning in Xenopus laevis embryos. Development 128, 3415-3426 [DOI] [PubMed] [Google Scholar]
- Dibner C., Elias S., Ofir R., Souopgui J., Kolm P. J., Sive H., Pieler T., Frank D. (2004). The Meis3 protein and retinoid signaling interact to pattern the Xenopus hindbrain. Dev. Biol. 271, 75-86 [DOI] [PubMed] [Google Scholar]
- Domingos P. M., Itasaki N., Jones C. M., Mercurio S., Sargent M. G., Smith J. C., Krumlauf R. (2001). The Wnt/beta-catenin pathway posteriorizes neural tissue in Xenopus by an indirect mechanism requiring FGF signalling. Dev. Biol. 239, 148-160 [DOI] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. (1989). Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature 340, 140-144 [DOI] [PubMed] [Google Scholar]
- Erter C. E., Wilm T. P., Basler N., Wright C. V., Solnica-Krezel L. (2001). Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development 128, 3571-3583 [DOI] [PubMed] [Google Scholar]
- Faas L., Isaacs H. V. (2009). Overlapping functions of Cdx1, Cdx2, and Cdx4 in the development of the amphibian Xenopus tropicalis. Dev. Dyn. 238, 835-852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R. B., Baker J. C., Harland R. M. (2006). FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development 133, 1703-1714 [DOI] [PubMed] [Google Scholar]
- Franco P. G., Paganelli A. R., Lopez S. L., Carrasco A. E. (1999). Functional association of retinoic acid and hedgehog signaling in Xenopus primary neurogenesis. Development 126, 4257-4265 [DOI] [PubMed] [Google Scholar]
- Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. (1998). Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357-362 [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A., Bonnin M. A., Le Douarin N. M. (1997). Hox gene induction in the neural tube depends on three parameters: competence, signal supply and paralogue group. Development 124, 849-859 [DOI] [PubMed] [Google Scholar]
- Gutkovich Y. E., Ofir R., Elkouby Y. M., Dibner C., Gefen A., Elias S., Frank D. (2010). Xenopus Meis3 protein lies at a nexus downstream to Zic1 and Pax3 proteins, regulating multiple cell-fates during early nervous system development. Dev. Biol. 338, 50-62 [DOI] [PubMed] [Google Scholar]
- Hardcastle Z., Chalmers A. D., Papalopulu N. (2000). FGF-8 stimulates neuronal differentiation through FGFR-4a and interferes with mesoderm induction in Xenopus embryos. Curr. Biol. 10, 1511-1514 [DOI] [PubMed] [Google Scholar]
- Harland R. M. (1991). In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 36, 685-695 [DOI] [PubMed] [Google Scholar]
- Holowacz T., Sokol S. (1999). FGF is required for posterior neural patterning but not for neural induction. Dev. Biol. 205, 296-308 [DOI] [PubMed] [Google Scholar]
- Hoppler S., Moon R. T. (1998). BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech. Dev. 71, 119-129 [DOI] [PubMed] [Google Scholar]
- Hoppler S., Brown J. D., Moon R. T. (1996). Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 10, 2805-2817 [DOI] [PubMed] [Google Scholar]
- Isaacs H. V., Pownall M. E., Slack J. M. (1998). Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3. EMBO J. 17, 3413-3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N., Sharpe J., Morrison A., Krumlauf R. (1996). Reprogramming Hox expression in the vertebrate hindbrain: influence of paraxial mesoderm and rhombomere transposition. Neuron 16, 487-500 [DOI] [PubMed] [Google Scholar]
- Kazanskaya O., Glinka A., Niehrs C. (2000). The role of Xenopus dickkopf1 in prechordal plate specification and neural patterning. Development 127, 4981-4992 [DOI] [PubMed] [Google Scholar]
- Kelly G. M., Greenstein P., Erezyilmaz D. F., Moon R. T. (1995). Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development 121, 1787-1799 [DOI] [PubMed] [Google Scholar]
- Keren A., Bengal E., Frank D. (2005). p38 MAP kinase regulates the expression of XMyf5 and affects distinct myogenic programs during Xenopus development. Dev. Biol. 288, 73-86 [DOI] [PubMed] [Google Scholar]
- Kiecker C., Niehrs C. (2001). A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189-4201 [DOI] [PubMed] [Google Scholar]
- Krauss S., Korzh V., Fjose A., Johansen T. (1992). Expression of four zebrafish wnt-related genes during embryogenesis. Development 116, 249-259 [DOI] [PubMed] [Google Scholar]
- Lamb T. M., Harland R. M. (1995). Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development 121, 3627-3636 [DOI] [PubMed] [Google Scholar]
- Lekven A. C., Thorpe C. J., Waxman J. S., Moon R. T. (2001). Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 1, 103-114 [DOI] [PubMed] [Google Scholar]
- Li B., Kuriyama S., Moreno M., Mayor R. (2009). The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development 136, 3267-3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L., Kimelman D. (2009). Wnt signaling and the evolution of embryonic posterior development. Curr. Biol. 19, R215-R219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew L. L., Lai C. J., Moon R. T. (1995). Specification of the anteroposterior neural axis through synergistic interaction of the Wnt signaling cascade with noggin and follistatin. Dev. Biol. 172, 337-342 [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Hoppler S., Moon R. T. (1997). Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech. Dev. 69, 105-114 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A. H., Wang E., Harland R. (2005). Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell 8, 167-178 [DOI] [PubMed] [Google Scholar]
- Muhr J., Jessell T. M., Edlund T. (1997). Assignment of early caudal identity to neural plate cells by a signal from caudal paraxial mesoderm. Neuron 19, 487-502 [DOI] [PubMed] [Google Scholar]
- Muhr J., Graziano E., Wilson S., Jessell T. M., Edlund T. (1999). Convergent inductive signals specify midbrain, hindbrain, and spinal cord identity in gastrula stage chick embryos. Neuron 23, 689-702 [DOI] [PubMed] [Google Scholar]
- Nordstrom U., Jessell T. M., Edlund T. (2002). Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 5, 525-532 [DOI] [PubMed] [Google Scholar]
- Papalopulu N., Kintner C. (1996). A posteriorising factor, retinoic acid, reveals that anteroposterior patterning controls the timing of neuronal differentiation in Xenopus neuroectoderm. Development 122, 3409-3418 [DOI] [PubMed] [Google Scholar]
- Prpic N. M., Janssen R., Wigand B., Klingler M., Damen W. G. (2003). Gene expression in spider appendages reveals reversal of exd/hth spatial specificity, altered leg gap gene dynamics, and suggests divergent distal morphogen signaling. Dev. Biol. 264, 119-140 [DOI] [PubMed] [Google Scholar]
- Ramel M. C., Lekven A. C. (2004). Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development 131, 3991-4000 [DOI] [PubMed] [Google Scholar]
- Ramel M. C., Buckles G. R., Baker K. D., Lekven A. C. (2005). WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation. Dev. Biol. 287, 237-248 [DOI] [PubMed] [Google Scholar]
- Re'em-Kalma Y., Lamb T., Frank D. (1995). Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development. Proc. Natl. Acad. Sci. USA 92, 12141-12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribisi S., Jr, Mariani F. V., Aamar E., Lamb T. M., Frank D., Harland R. M. (2000). Ras-mediated FGF signaling is required for the formation of posterior but not anterior neural tissue in Xenopus laevis. Dev. Biol. 227, 183-196 [DOI] [PubMed] [Google Scholar]
- Rogers C. D., Archer T. C., Cunningham D. D., Grammer T. C., Casey E. M. (2008). Sox3 expression is maintained by FGF signaling and restricted to the neural plate by Vent proteins in the Xenopus embryo. Dev. Biol. 313, 307-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg A., Elias S., Nachaliel N., Bonstein L., Henig C., Frank D. (1999). A Meis family protein caudalizes neural cell fates in Xenopus. Mech. Dev. 80, 3-13 [DOI] [PubMed] [Google Scholar]
- Sharpe C. R. (1991). Retinoic acid can mimic endogenous signals involved in transformation of the Xenopus nervous system. Neuron 7, 239-247 [DOI] [PubMed] [Google Scholar]
- Shimizu T., Bae Y. K., Muraoka O., Hibi M. (2005). Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev. Biol. 279, 125-141 [DOI] [PubMed] [Google Scholar]
- Shiotsugu J., Katsuyama Y., Arima K., Baxter A., Koide T., Song J., Chandraratna R. A., Blumberg B. (2004). Multiple points of interaction between retinoic acid and FGF signaling during embryonic axis formation. Development 131, 2653-2667 [DOI] [PubMed] [Google Scholar]
- Sive H. L., Draper B. W., Harland R. M., Weintraub H. (1990). Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 4, 932-942 [DOI] [PubMed] [Google Scholar]
- Skromne I., Thorsen D., Hale M., Prince V. E., Ho R. K. (2007). Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord. Development 134, 2147-2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. (1991). Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67, 753-765 [DOI] [PubMed] [Google Scholar]
- Snir M., Ofir R., Elias S., Frank D. (2006). Xenopus laevis POU91 protein, an Oct3/4 homologue, regulates competence transitions from mesoderm to neural cell fates. EMBO J. 25, 3664-3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva S., Glavic A., Ruiz P., Mayor R. (2002). Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 241, 289-301 [DOI] [PubMed] [Google Scholar]
- Vlachakis N., Choe S. K., Sagerstrom C. G. (2001). Meis3 synergizes with Pbx4 and Hoxb1b in promoting hindbrain fates in the zebrafish. Development 128, 1299-1312 [DOI] [PubMed] [Google Scholar]
- Waskiewicz A. J., Rikhof H. A., Hernandez R. E., Moens C. B. (2001). Zebrafish Meis functions to stabilize Pbx proteins and regulate hindbrain patterning. Development 128, 4139-4151 [DOI] [PubMed] [Google Scholar]
- Woo K., Fraser S. E. (1997). Specification of the zebrafish nervous system by nonaxial signals. Science 277, 254-257 [DOI] [PubMed] [Google Scholar]
- Wu J., Yang J., Klein P. S. (2005). Neural crest induction by the canonical Wnt pathway can be dissociated from anterior-posterior neural patterning in Xenopus. Dev. Biol. 279, 220-232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.