Abstract
Cells can change identity during normal development, in response to tissue damage or defined artificial treatments, or during disease processes such as cancer. Strikingly, not only the reprogramming of tissue cells to an embryonic stem cell-like state, but also the direct conversion from one cell type to another have been described. Direct cell type conversion could represent an alternative strategy for cellular therapies. However, little is known about the actual cellular steps undertaken by a cell as it changes its identity and their possible consequences for the organism. Using an in vivo single-cell system of natural direct reprogramming, in which a C. elegans rectal cell transforms into a motoneuron, we present an in-depth analysis of the cellular transformations involved. We found that the reprogrammed cell transits through intermediate states during direct in vivo reprogramming. We identified and characterised a mutant in the conserved COE transcription factor UNC-3 in which this cellular transformation is blocked. We determined that complete erasure of initial identity first takes place, followed by stepwise, unc-3-dependent, redifferentiation into a motoneuron. Furthermore, unlike in vitro induced reprogramming, reversion to a dedifferentiated identity does not lead to an increase in cellular potential in a natural, in vivo context. Our findings suggest that direct cell type conversion occurs via successive steps, and that dedifferentiation can occur in the absence of cell division. Furthermore, our results suggest that mechanisms are in place in vivo to restrict cell potential during reprogramming, a finding with important implications for regenerative medicine.
Keywords: Cellular reprogramming, Transdifferentiation, Collier/Olf1/Ebf, C. elegans, Rectum, Direct cell type conversion
INTRODUCTION
Over recent decades, the capacity of mature differentiated cells to be reprogrammed and change identity has been exemplified by a number of cases of experimentally induced cellular reprogramming. Nuclear transfer, cell fusion and transfection of a cocktail of transcription factors have each shown that the differentiated identity of a cell can be erased, and even fully reversed to an embryonic stem cell-like state (Gurdon and Melton, 2008; Takahashi and Yamanaka, 2006). Remarkably, not only reversion to an embryonic stem cell-like state (Gurdon and Melton, 2008), but also direct cell type conversion (or transdifferentiation), which involves direct reprogramming of one differentiated cell type into another, have been described (Means et al., 2005; Vierbuchen et al., 2010; Zhou et al., 2008). Direct cell type conversions have been induced in vitro (Gurdon and Melton, 2008; Means et al., 2005; O'Neill et al., 2008; Szabo et al., 2010; Vierbuchen et al., 2010) and in vivo (Del Rio-Tsonis and Tsonis, 2003; Zhou et al., 2008) in many cell lineages. Induction of direct reprogramming, following the forced expression of one or a small number of powerful transcription factors, results in a complete phenotypic switch (e.g. Vierbuchen et al., 2010). Importantly, examples of physiological direct reprogramming have also been described in nature, suggesting that cells have an inherent flexibility. For example, photoreceptor conversion during metamorphosis in the fly (Sprecher and Desplan, 2008), the formation of coronary arteries by developmental reprogramming of venous cells (Red-Horse et al., 2010), the generation of new neurons from astrocytes during adult neurogenesis (Doetsch, 2003), or the switch from a rectal cell to a motoneuron in C. elegans (Jarriault et al., 2008) have all been documented. An understanding of the mechanisms at work in these cell type conversions will thus answer fundamental questions about cell identity and plasticity.
In addition, these studies have opened new avenues for regenerative medicine. One strategy being actively investigated concerns the generation of induced pluripotent stem (iPS) cells through forced reprogramming of patient tissue cells, followed by redifferentiation into the damaged or diseased cells. However, this strategy is associated with some risks for the patient, as iPS cell approaches involve the generation of potentially tumourigenic cells (Miura et al., 2009) and there is an increased probability of accumulating mutations or chromosomal aberrations through the many cell divisions required (Gurdon and Melton, 2008). By contrast, direct cell type conversion may bypass the need for the intermediary cellular stages that could actually pose a health risk. As such, direct cell conversion could hold great promise as an alternative means to generate replacement cells for regenerative medicine.
Nevertheless, the actual cellular mechanisms behind direct cell type conversion are unclear, especially in the context of a physiological setting. Key issues are to determine whether direct conversion involves transition through distinct intermediary identities, if cell division is required, and whether putative intermediate stages are associated with high cellular potency and are thus potentially dangerous for applications in regenerative medicine. It remains a challenge to unambiguously identify single reprogramming events and follow them in vivo at the molecular level in most multicellular organisms. These limitations have hampered the analysis of the cellular transition involved. For example, it has been assumed that the absence of cell division during direct cell type conversion implies that no transition through distinct intermediary steps, such as a reversion to a less differentiated identity, is involved (Zhou et al., 2008).
In C. elegans, we have previously described how it is possible to unambiguously follow and study the cell identity switch of the Y rectal cell into the PDA motoneuron in real time in live animals at the single-cell level (Jarriault et al., 2008). Because single cells can be definitively identified in C. elegans, our system opens a unique window in which to observe and define the cellular transformations that underlie cellular reprogramming. We have identified a mutant background in which the Y-to-PDA transdifferentiation is blocked at an intermediate step, and have used it as a model to elucidate the cellular transformations involved. We have further identified molecular markers for the initial, intermediate and final identities, and have tested their associated plasticity using various cell fate challenge assays. We have found that direct cellular reprogramming in vivo proceeds through an intermediate dedifferentiated stage that is not widely plastic.
MATERIALS AND METHODS
C. elegans strains
The wild-type parent for the strains used in this study is the Caenorhabditis elegans var. Bristol strain N2. The relevant mutations and transgenes used in this study are: unc-3 (e151, fp8, oy85) on LGX, wyIs75 [exp-1p::gfp], bxIs7 [egl-5p::gfp], kuIs36 [egl-26p::gfp], maIs113 [cki-1p::gfp], mcEx177 [che-14::gfp], jcIs1 [ajm-1::gfp], mcIs47 [dlg-1::RFP], gmIs5 [ina-1p::gfp], wdIs4 [unc-4p::gfp], ctIs43 [dbl-1p::gfp], otIs107 [ser-2p::gfp], muIs62 [mig-13p::gfp], ccIs4251 [myo-3p::gfp], arIs99 [dpy-7p::gfp], wIs84 [elt-2p::gfp], krIs6 [unc-47p::DsRed2], kmIs438 [hsp::hlh-1], wIs47 [hsp::end-1], nEx648 [hsp::unc-30], mcIs22 [hs:lin-26], rhIs11 [unc-3::gfp] and fpEx60 [hsp::unc-3].
Isolation and characterisation of the unc-3 mutations
We isolated fp8, which exhibits an Uncoordinated phenotype, in a screen for mutants defective in Y-to-PDA transdifferentiation. The genomic region containing the fp8 mutation was narrowed down, using single nucleotide polymorphism (SNP) mapping (Davis et al., 2005), to a 952.6 kb region on the right arm of chromosome X, between SNPs 17 and 23. This region contains 62 genes, including one, unc-3, that displays an Uncoordinated phenotype similar to fp8. unc-3(e151) mutants did not complement with fp8 animals (not shown). Sequencing of the unc-3 genomic region in fp8 revealed a G-to-A nucleotide substitution in exon 3 that affects a conserved glycine (G) of the DNA-binding domain, changing it into a glutamic acid (E). We also identified the molecular lesions associated with unc-3 (e95 and e121) mutant alleles. Sequencing of the unc-3 genomic region in e95 and e121 revealed a C173F substitution affecting the last cysteine of the zinc-finger (ZnF) coordination motif in the DNA-binding domain, and a G279R substitution in the TIG domain, respectively. unc-3(e95) resulted in a penetrant Y-to-PDA defect (70% ‘no PDA’ defect; n=59), as did other unc-3 alleles affecting the Znf motif: n3413 (Prasad et al., 2008), 66% ‘no PDA’ defect (n=30); n3435 (Prasad et al., 2008), 56.6% ‘no PDA’ defect (n=30). By contrast, unc-3(e121) had no impact on the Y-to-PDA process (0% ‘no PDA’ defect; n=30), similar to the e54 allele (8.5% ‘no PDA’ defect; n=59), which introduces a stop at the end of the first HLH domain helix (Prasad et al., 1998).
Larval staging and scorings
Experiments were conducted at 20°C and nematodes were routinely cultured as described (Brenner, 1974), unless otherwise indicated. Cellular morphology and marker expression were assessed at different time points during the L1 and L2 as well as in the early L3 stages, as follows. Worms were either synchronised by performing 1-hour egg-laying pulses or by hatching an egg preparation on a plate without food. Worms were then stained or scored directly after 0 (newly hatched L1 larvae), 15 or 19 (before Y retraction from rectum), 18 or 23 (1/3rd Y migration), 21 or 27 (2/3rd Y migration) and 24 or 31 (early L3) hours for wild-type (WT) or unc-3 mutant worms (which develop more slowly), respectively. Staging was confirmed by evaluating somatic gonad development. To assess the identity of the Y or PDA cells in WT or unc-3 mutants the following criteria were used: Y identity, characteristic rectal cell morphology by differential interference contrast (DIC) optics, and epithelial and/or Y marker expression as in the early L1 stage; PDA identity, WT final position, PDA marker expression and presence of an axon projecting to the dorsal cord after the rectum, as in WT L3 and older worms. Pairwise one-tailed Student's t-tests were performed to obtain P-values for Fig. 1E and Fig. 4C.
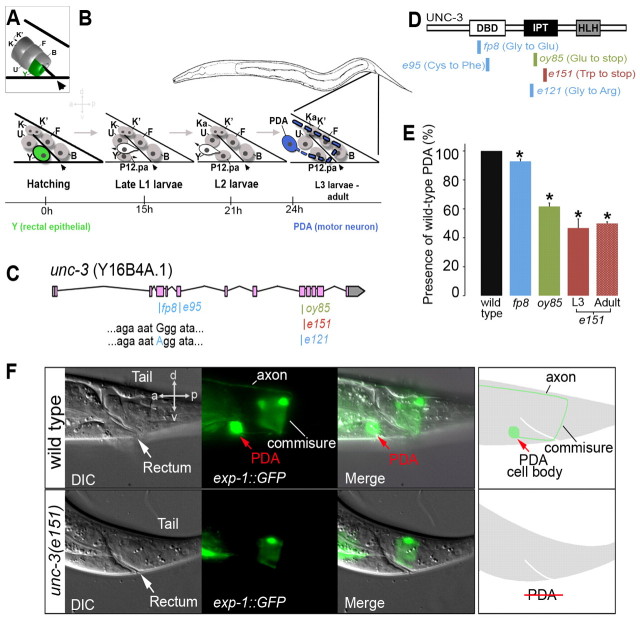
Fig. 1.
The Y-to-PDA cell identity switch is defective in unc-3 mutants. (A) The C. elegans rectal tube is formed by six epithelial cells (Y, B, U, F, K/Ka and K′) that join via apical junctions to form a toroid structure in the hindgut used for defecation. The arrowhead indicates the rectal slit. (B) The Y cell abandons its function during the L2 larval stage, retracts from the rectum, migrates anterodorsally, and changes into the motoneuron PDA. Another cell, P12.pa, replaces the Y cell rectal role and adopts a rectal epithelial identity. Beneath is indicated the corresponding time frame in hours at 20°C. Arrowheads, rectal slit. (C) unc-3 structure, showing allele positions. Molecular lesions identified in this study are in blue. (D) The UNC-3 protein, indicating the changes in the mutant alleles examined. e151 and oy85 bear a stop codon in the IPT domain, e121 is a missense mutation in the IPT domain, fp8 is a missense mutation in the DNA-binding domain and e95 is a missense mutation that affects the zinc-finger coordination motif. DBD, DNA-binding domain; IPT, Immunoglobulin-like fold; HLH, helix-loop-helix. (E) Mutant alleles of unc-3 cause defects in Y-to-PDA transdifferentiation at various penetrance. Note that the same penetrance of defects is seen in unc-3(e151) mutant L3 animals, when the PDA neuron should just have been made, as in adults, which excludes a defect in the maintenance of PDA identity. Error bars indicate s.e.m. (*, P<0.01). (F) DIC, fluorescence and schematics of the wild type (WT) and the unc-3(e151) mutant that lacks PDA (marked with pexp-1::gfp) and its characteristic axon. a, anterior; d, dorsal; p, posterior; v, ventral.
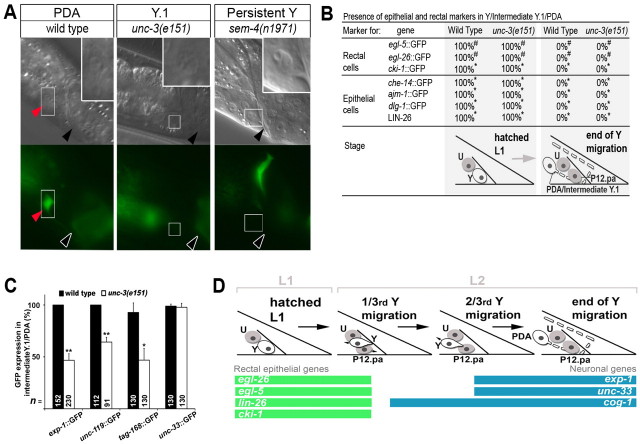
Fig. 4.
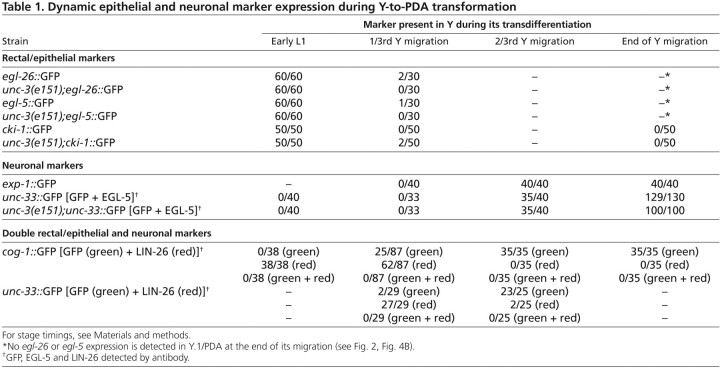
The intermediate cell in Y-to-PDA reprogramming lacks Y and PDA characteristics. (A) Nomarski (top) and fluorescent (bottom) images of the WT PDA, the Y.1 cell in unc-3(e151) mutants, and the persistent Y in sem-4(n1971) mutants. The location of the PDA, Y.1 and persistent Y cells is boxed and enlarged in the DIC panel insets. Black or white arrowheads indicate rectum. Red arrowhead indicates PDA; no PDA is formed in unc-3 and sem-4 mutants. (B) Y has lost expression of rectal and epithelial markers at the L3 larval stage in both WT and e151 animals (#60 or *50 animals were scored). (C) Expression of pan-neuronal markers in intermediate Y.1/WT PDA. An early pan-neural marker, unc-33, is expressed in the intermediate Y.1 cell. Error bars represent s.e.m. (*P<0.01, **P<0.001). The total number of animals scored (n) is indicated for each condition. (D) Transient expression of rectal epithelial and neuronal genes during the course of Y-to-PDA reprogramming in WT (see also Table 1).
Transgenic strains
To obtain fpEx60 [hsp::unc-3] worms, unc-3 cDNA was made from N2 worm RNA with the forward primer 5′-CGGGTACCAGAAAAATGAGTTTGACAGCTCCGCTAAG-3′ and the reverse primer 5′-CGCTATAGTTAAGACAGACGGGACGACGAC-3′. The PCR product was then cloned into the pPD49.78 vector using the incorporated Acc651 and EcoRV restriction sites. Microinjection of unc-3(e151) worms was carried out using 0.1 ng/μl hs::unc-3, 5 ng/μl myo-2::mCherry as a co-injection marker and 100 ng/μl pBluescript as carrier DNA.
Antibody staining
Synchronised WT and unc-3 mutant worms at the relevant stage were stained as previously described (Jarriault et al., 2008) using monoclonal antibodies against GFP (Roche) together with antibodies against LIN-26 (Labouesse et al., 1996) or EGL-5 (Ferreira et al., 1999) and Cy3-conjugated (Jackson ImmunoResearch Laboratories) or Alexa 488-conjugated (Invitrogen) secondary antibodies, together with DAPI. Whereas 13 kb of the egl-5 5′ regulatory region drives expression in the rectal cells but not PDA, EGL-5 protein can be detected in both Y and PDA after antibody staining (Ferreira et al., 1999; Jarriault et al., 2008). We found that the EGL-5 protein could also be detected in the mutant Y cell in the unc-3(e151) mutant, which allowed unambiguous identification of the mutant Y cell following antibody staining. Staining was assessed using spinning disk confocal microscopy (system and/or revolution) and cell morphology was assessed following three-dimensional image rendering.
Cell fate challenge assays
Heat shock pulses, by immersion in a water bath at 33-34°C for 30 minutes (except where indicated otherwise), were administered to WT or unc-3(e151) larvae or embryos carrying each of the specified heat shock-inducible transgenes at the time points indicated in Fig. 5B. These time points correspond to (1) embryonic; (2) early L1; (3) 1 hour prior to Y cell rectal retraction; (4) 1 hour after Y cell rectal retraction; (5) 1/3rd of Y cell migration; and (6) mixed stage embryos subjected to 29°C for 18 hours. In transgenic animals carrying the hs::unc-3 transgene and subjected to heat shock, PDA was present 80.4% (n=82) of the time versus 47.2% (n=127) for non-heat shocked transgenic worms. In all cases, following heat shock animals were incubated at 20°C until they reached adulthood before scoring. Induction of end-1, hlh-1, lin-26 or unc-3 was confirmed after heat shock in animals bearing an hs::end-1, hs::hlh-1, hs::lin-26 or hs::unc-3 transgene as compared with their non-transgenic counterparts by semi-quantitative PCR. act-1 and C30G5.4 were used as control housekeeping genes and the following primers (5′ to 3′) were used: act-1 forward GAGGCCCAATCCAAGAGAGGT and reverse CTTCTCCTTGATGTCACGGACGAT; C30G5.4 forward GGGATGTTATTGCCGTTTTCTCTCAATA and reverse GTGCTTGTTCTCACCATACAAATCA; end-1 forward ATGATTTTGGGCAATACTTTGTT and reverse GCACAACTCGGCTGGTCT; hlh-1 forward CCGCGGAGAACCAAATTGG and reverse TCCCAACGAGGAAGCAATGACC; lin-26 forward TCCATTCGGATTGTAAGCCATATAAGTG and reverse GCTCGAGTTGCTCGGCAA; and unc-3 forward ATCGACACGTCGAATACAAGCA and reverse AACGTCTCATGTCGCGTGGA.
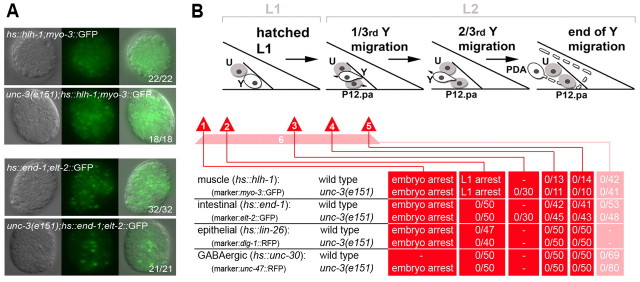
Fig. 5.
Y-to-PDA reprogramming involves successive cellular steps but not a gain of wide cellular potential. (A) Embryonic cell plasticity remains intact in an unc-3(e151) background. Widespread expression of a muscle (top two rows) or an endodermal (bottom two rows) marker is observed after forced expression of hlh-1 and end-1, respectively, at the 4E embryonic stage. Left, DIC image; middle, fluorescent image; right, merge. The number of embryos showing the phenotype over the total number of embryos scored is indicated. (B) Red triangles and the pink bar (labelled 1-6) represent the time points at which heat shock was applied in hs::hlh-1, hs::end-1, hs::lin-26 and hs::unc-30 backgrounds to test Y cell plasticity. Heat shock was administered in: (1) embryos; (2) freshly hatched L1; (3) 1 hour before Y retraction from the rectum; (4) 1 hour after Y retraction; (5) 1/3rd of Y migration; and (6) from embryo to L2 (see Materials and methods). None of these conditions resulted in the adoption by the Y.0 or the Y.1 cell of a different fate (as evidenced by the lack of expression of the GFP cell-type reporters).
Embryonic cell fate challenge assay
Heat shock pulses, by immersion in a water bath at 33-34°C for 30 minutes, were administered to 4E stage embryos (120 minutes after the two-cell stage) of the strains listed in Fig. 5A. Following heat shock, embryos were left overnight at 20°C before scoring for widespread expression of each cell identity marker. Note that both heat shock promoters, hsp16.2 and hsp16.41, were used to drive expression in the Y cell.
RESULTS
The rectum, a vital organ required for defecation, is formed during embryogenesis by six cells in C. elegans. One of these cells, named Y, exhibits an extraordinary behaviour. Until the L2 larval stage, Y is a fully differentiated rectal epithelial cell, which forms with B (another rectal epithelial cell) the posterior ring of the rectal tube (Fig. 1A). However, Y later abandons its function during the L2 larval stage, retracts from the rectum, migrates and changes into the motoneuron PDA (Fig. 1B), a unique behaviour among neighbouring rectal cells and in the development of all 302 other C. elegans neurons. Another cell, P12.pa, replaces the Y cell rectal role (Fig. 1B). Both Y and PDA have distinct identities at the molecular, morphological and functional levels (Jarriault et al., 2008). This direct reprogramming in the absence of cell division uniquely affords the opportunity to use molecular and genetic analyses to probe the mechanisms underlying transdifferentiation.
Y-to-PDA direct reprogramming is blocked in unc-3 mutants
To dissect the mechanisms underlying Y cell conversion, we carried out a mutagenesis screen for mutants unable to successfully reprogram Y into PDA. Our genetic screen provided mutants that fall into different classes based on the morphology and numbers of cells in the rectal area. We focused on one allele, fp8, in which the Y-to-PDA reprogramming may have been initiated, as judged by the number of rectal cells, but lacked a PDA neuron and could thus affect intermediate steps of Y-to-PDA reprogramming. SNP mapping, sequencing and non-complementation between a null allele of the unc-3 gene and our fp8 allele revealed that a conserved residue in the DNA-binding domain of UNC-3 was mutated in fp8, causing the Y-to-PDA reprogramming defect (Fig. 1C,D). UNC-3 is the sole C. elegans member of the conserved COE (Collier/Olf-1/EBF) transcription factor family, which is involved in cell fate determination, including neuronal differentiation (Dubois and Vincent, 2001). Analysis of other unc-3 alleles confirmed that a Y-to-PDA reprogramming defect is observed when the activity of this gene is lowered (Fig. 1E). We found that all unc-3 alleles that resulted in the destruction of the zinc-finger coordination motif of the DNA-binding domain (n3413, n3435 and e95), or in the disruption of the IPT domain (oy85, e151), led to the most penetrant Y-to-PDA defect (Fig. 1E,D; see Materials and methods). By contrast, substitutions (fp8, e121) or late stops (e54) did not appear to strongly alter unc-3 activity during Y-to-PDA transdifferentiation (Fig. 1E; see Materials and methods). We focused on unc-3(e151), the reference allele that has been described as a null allele (Prasad et al., 1998) and which affects Y-to-PDA conversion with high penetrance (Fig. 1D-F).
Y transdifferentiation is initiated normally in unc-3 mutants
How differentiated cells change identity is a fascinating problem in cell biology. There are two potential mechanisms for how this occurs without cell division: (1) concomitant loss and gain of the initial and final cell identities, respectively, with a transient mixed identity stage (Fig. 2A); (2) transition through distinct intermediary cells that lack either of the two identities (Fig. 2A). To determine which mechanism occurs during natural cellular reprogramming in vivo, we examined Y fate in unc-3(e151) animals and sought to determine how the Y-to-PDA process is affected. In unc-3(e151), as in unc-3(fp8) mutants and wild-type (WT) animals, a Y cell is initially born, as assessed by Nomarski optics and expression of an egl-26 transgene (Jarriault et al., 2008) at the L1 stage (Fig. 2B,D). Comparison of the expression of egl-26 and egl-5 transgenes, which are differentially expressed in the rectal cells at different time points of Y-to-PDA reprogramming (Fig. 2D,E), revealed that a Y cell is no longer found in the rectum of unc-3(e151) mutants at later larval stages (L3 and onwards, Fig. 2B-E). These observations suggest that the Y cell develops normally in unc-3(e151) mutants and later moves away from the rectum, as observed when Y conversion is initiated normally in WT animals. However, in unc-3 mutants, this cell never transforms into PDA.
Fig. 2.
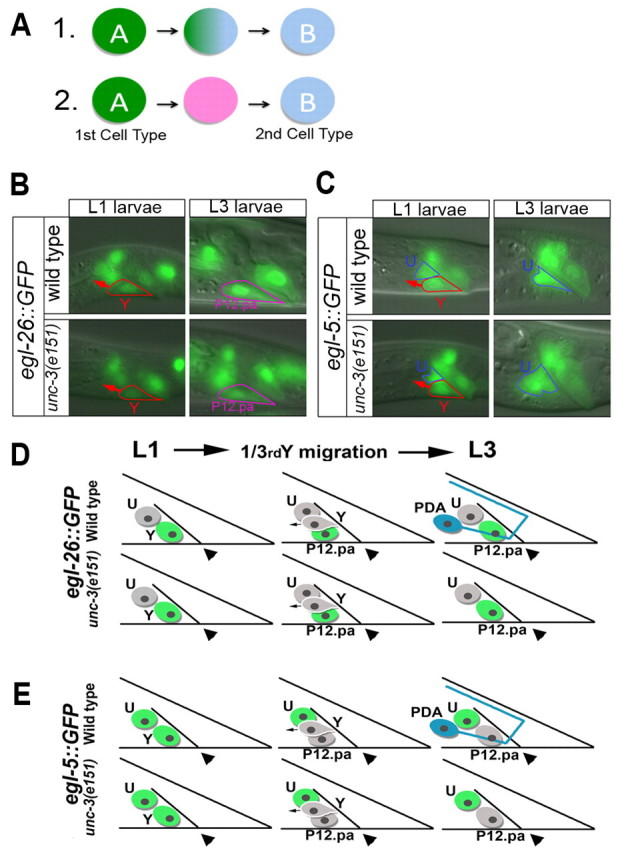
Model for direct reprogramming and initiation of process in unc-3 mutants. (A) Alternative models of cellular reprogramming: (1) the initial (A) and final (B) cell identities overlap during transition; and (2) an intermediate cell state exists that lacks both identities. (B,C) Expression of a Y and P12.pa marker (egl-26 transgene, B) and a Y and U cell marker (egl-5 transgene, C) indicate that a normal Y cell is present in e151 mutants at the L1 larval stage, but is no longer part of the rectum at the L3 larval stage and is replaced by P12.pa. Anterior is to the left and ventral to the bottom. (D,E) Schematics of the egl-5 and egl-26 transgene expression during the L1 to L3 larval stages in WT and unc-3 mutants.
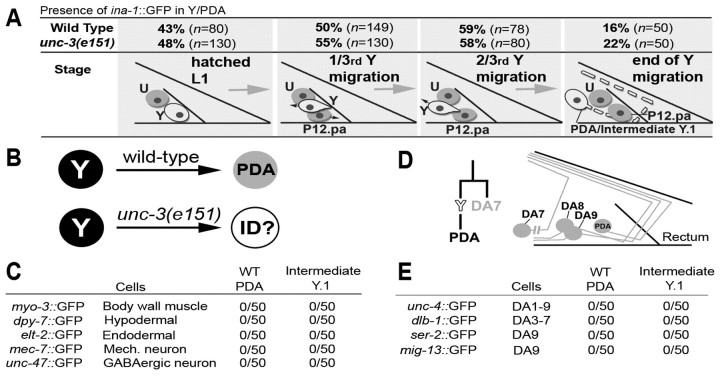
It could be that the Y-to-PDA conversion is blocked mid-process in unc-3 mutants, or that the Y cell migrates to an abnormal position or undergoes cell death. To discount these latter possibilities, we identified a marker [ina-1p::GFP (Baum and Garriga, 1997)] that is expressed in Y and remains expressed during its migration, allowing tracking of the moving cell. Analysis of ina-1p::GFP in WT and unc-3(e151) backgrounds showed that the Y cell correctly migrates to its final position in unc-3 mutants and does not die (Fig. 3A). Nomarski optics also revealed the presence of the abnormal Y cell in unc-3 mutants at its final position (see Fig. 4A). Thus, in unc-3 mutants, the early phase of Y reprogramming (retraction from the rectum and migration) are initiated normally, as in the WT. From now on, we will refer to the cell found at the Y final position in unc-3 mutant L3 larvae as ‘intermediate Y.1’, to distinguish it from the rectal Y found initially in young L1 larvae in both the WT and unc-3 backgrounds.
Fig. 3.
Initiation of Y-to-PDA reprogramming occurs normally in unc-3 animals and does not involve aberrant cellular changes. (A) Presence of ina-1::GFP in Y [expressed as a percentage of total animals scored (n)] as it migrates to its final position. Schematics beneath illustrate Y migration at the different stages examined. (B) Probing of the cellular identity of the intermediate Y.1 cell. (C) Markers of neighbouring cell identities or different neuron subtypes are not expressed in the intermediate Y.1 cell (50 animals scored for each marker). (D) Neuron DA7 is the lineal sister of Y, DA9 is the lineal contralateral homologue of Y, and DA8 is a morphologically related neuron. (E) Intermediate Y.1 did not adopt DA7, DA8 or DA9 identities (50 animals scored for each marker).
Y-to-PDA transdifferentiation is blocked at an intermediate step in unc-3 mutants
As Y appeared to have initiated its transdifferentiation but failed to become a PDA in unc-3 mutants, we next sought to determine whether Y.1 identity represents a transformation into another cell type or a block in the process. We thus asked whether intermediate Y.1 could have adopted an alternative aberrant identity (Fig. 3B), such as that of a neighbouring cell. Analysis of endodermal, hypodermal or muscle markers in the cell suggested that this was not the case (Fig. 3C). Furthermore, intermediate Y.1 did not adopt the identity of a different neuron type such as a mechanosensory or GABAergic neuron (Fig. 3C). Because cells can transform into their sister cell or their contralateral homologues in C. elegans (Horvitz et al., 1983; Shibata et al., 2010; White et al., 1991), we examined whether the intermediate Y.1 cell had adopted the identity of its sister (the DA7 neuron), of its contralateral homologue (DA9) or of a neuron with a similar morphology (DA8) (Fig. 3D). Examination of markers expressed in these three neuronal types showed that this was not the case (Fig. 3E). Thus, in an unc-3(e151) background, the Y-to-PDA process appears to be blocked at an intermediate step of reprogramming without any obvious aberrant cellular changes. Several additional lines of evidence developed below further support this notion that Y-to-PDA reprogramming is blocked mid-way between the initial Y and the final PDA identity in unc-3 mutants. First, not only Y retraction from the rectum and migration but also loss of expression of the rectal egl-26, egl-5, lin-26 and cki-1 transgenes occur with the same timing in WT and unc-3 mutants. Second, a wild-type unc-3 rescue construct can restore Y-to-PDA reprogramming in unc-3 mutants.
Transdifferentiation of Y into PDA is a stepwise process that first involves the erasure of the initial identity
To understand the intermediate stages of direct reprogramming in the absence of cell division or fusion, we further analysed the characteristics of the Y.1 cell in unc-3 mutants. We first examined whether the intermediate Y.1 cell retains any rectal or epithelial characteristics. Morphological analysis using Nomarski optics revealed that it did not exhibit the characteristic ‘fried egg’ epithelial appearance such as is observed in sem-4(n1971) mutants, in which Y-to-PDA transdifferentiation is not initiated and Y remains as a rectal epithelial cell (Jarriault et al., 2008) (Fig. 4A). Furthermore, the intermediate Y.1 cell had lost expression of the egl-26 and egl-5 transgenes (Fig. 2B,C and Fig. 4B) that mark the rectal Y in L1, as well as the expression of a cki-1 transgene, another marker that is expressed in Y but not PDA in the WT (Fig. 4B). Consistent with this, the expression of epithelia-specific markers (che-14, ajm-1, dlg-1 and LIN-26) that are normally expressed in Y was also lost (Fig. 4B). Thus, the intermediate Y.1 cell has lost both the morphological and molecular characteristics of its former rectal epithelial identity.
We next asked whether the intermediate Y.1 cell expresses any neural characteristics. We found three pan-neural markers to be expressed in WT PDA (Fig. 4C, Table 1): unc-119 (Maduro and Pilgrim, 1995), tag-168 (Shioi et al., 2001) and unc-33 (Altun-Gultekin et al., 2001). In unc-3(e151), the penetrance of the ‘No PDA’ phenotype is ~50%. A similar proportion of unc-3(e151) mutants did not express the unc-119 and tag-168 transgenes (Fig. 4C), indicating that the intermediate Y.1 cell does not express these pan-neural markers. The unc-33 transgene, however, the expression of which starts in dividing embryonic neuroblasts (Altun-Gultekin et al., 2001), was expressed in almost all intermediate Y.1 cells in unc-3(e151), suggesting that intermediate Y.1 has begun to acquire early neural properties (Fig. 4C). Accordingly, in rare unc-3(e151) mutants (6%, n=31), the exp-1p::gfp marker could be detected in the Y.1 cell, but no axonal projection from the cell body was observed. This suggests that the intermediate Y.1 cell has not yet adopted neural morphological features. Overall, our evidence indicates that intermediate Y.1 has started to acquire some neural characteristics; however, complete transformation into a neuron is blocked early on.
Table 1.
Dynamic epithelial and neuronal marker expression during Y-to-PDA transformation
The identification of this intermediate cellular stage, which is devoid of rectal characteristics and in which neural characteristics have only just begun to appear, supports a model whereby successive distinct cellular steps are undertaken by a cell that directly reprograms in vivo (Fig. 2A, model 2), even in the absence of cell division. To determine whether this is consistent with the WT scenario, we next examined the transient expression of Y and PDA markers in N2 worms during the cellular transition event. The Y cell rectal epithelial markers LIN-26, egl-26, egl-5 and cki-1 were expressed in Y during the L1 larval stage, but were then permanently switched off as Y began its migration (Table 1, Fig. 4D). The PDA markers unc-33 and exp-1 switched on towards the end of Y migration, whereas cog-1 expression was apparent earlier during migration (Table 1, Fig. 4D). Co-staining of LIN-26 and cog-1::GFP, the earliest PDA marker, as well as LIN-26 and unc-33::GFP, revealed that there was never an overlap in expression between the rectal epithelial marker and the neural markers during Y-to-PDA conversion, similar to the sequence of Y identity changes in unc-3(e151) mutants (Table 1). Thus, just after Y retraction from the rectum, the Y cell appeared to transit through a stage that lacked both initial (Y) and final (PDA) identities, and that preceded the appearance of the Y.1 cell with neural character. The erasure of rectal epithelial characteristics prior to the adoption of neural characteristics in WT animals further supports the model whereby successive distinct cellular steps are undertaken by a cell that directly reprograms in vivo (Fig. 2A, model 2). Furthermore, these results also suggest that Y first undergoes dedifferentiation. This transient dedifferentiated intermediary, which we have termed Y.0, appears to quickly initiate redifferentiation into Y.1 as a migrating early neuronal cell.
Dedifferentiation does not lead to an increase in potency in vivo
Dedifferentiation of tissue cells has been achieved in vitro, yielding cells that are highly plastic (Gurdon and Melton, 2008). We sought to assess the plastic potential of the intermediate Y.1 cell, as well as the transient Y.0 cell, in WT and unc-3 mutants. Multipotent cells in C. elegans, such as early blastomeres, can be forced to adopt alternative fates by the ectopic expression of cell fate determinants (Fukushige and Krause, 2005; Quintin et al., 2001; Zhu et al., 1998). To test whether the dedifferentiated intermediate cell could be forced to differentiate into cell types other than PDA, we took advantage of cell fate determinants necessary for specific neural and non-neural lineages that are able to reprogram early C. elegans blastomeres (Fig. 5A). We delivered pulses of hlh-1 [muscle specification (Fukushige and Krause, 2005)], end-1 [intestinal specification (Zhu et al., 1998)], lin-26 [epithelial specification (Quintin et al., 2001)] or unc-30 [GABAergic neuron specification (Jin et al., 1994)] expression through heat shock at different stages of Y-to-PDA transition in order to induce transgene expression before Y began reprogramming and during the intermediate stages of reprogramming (Fig. 5B; see Materials and methods). We validated that the heat shock promoters used to drive the expression of these fate determinant genes drove the expression of GFP in Y under our heat shock conditions, and that mRNA levels of the cell fate determinants used were indeed increased in the transgenic strains after heat shock (see Materials and methods; data not shown). We also delivered a long-term but lower strength heat shock treatment, which we found sufficient to restore Y-to-PDA transformation in unc-3(e151) mutants carrying a hs:unc-3 rescue construct (see Materials and methods). Furthermore, we confirmed that hlh-1, lin-26 and end-1 were capable of reprogramming embryonic blastomeres in an unc-3(e151) background, as in the WT (Fukushige and Krause, 2005; Zhu et al., 1998) (Fig. 5A). Under these conditions, neither the unc-3 nor WT Y.0 cell, nor the intermediate Y.1, could be forced to change into an alternative identity other than PDA (Fig. 5B). This suggests that in both cases (WT and unc-3), the dedifferentiated cell has limited cellular potential and that the intermediate cellular steps of direct reprogramming do not represent reversion to a more plastic state.
DISCUSSION
To our knowledge, this study represents the first single-cell level analysis of the cellular steps undertaken by a differentiated cell that switches identity in vivo. The tractable nature of this system has allowed us to unambiguously determine the mechanism by which one differentiated postmitotic cell can change into an altogether different cell type. We found that cells undergoing transdifferentiation do not transit through a mixed identity stage. Rather, the converting cell passes through a temporary state that lacks characteristics of both the initial and the final cellular identities. This suggests that a dedifferentiation step precedes redifferentiation into the new cell type, separating the two processes temporally.
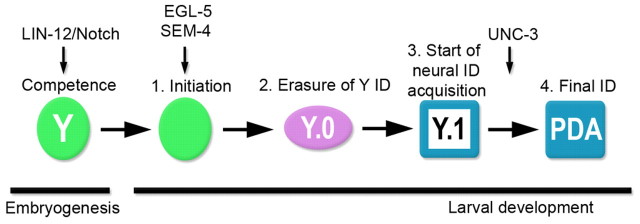
The discrete steps undertaken by Y as it changes into PDA involve: (1) initiation (retraction from the rectum); (2) complete erasure of Y rectal identity; (3) transformation into a cell with neural character; and (4) transformation into a neuron with PDA identity (Fig. 6). We found that UNC-3, the sole C. elegans COE factor, is necessary for Y to transform into PDA. In unc-3(e151) animals, the Y cell is blocked in step 3 of this reprogramming process, supporting the notion that unc-3 activity is needed to complete redifferentiation into a different cell type. UNC-3 has been described as both a transcriptional activator and a repressor (Kim et al., 2005). It is thus revealing that mutations in UNC-3 that destroy the zinc-finger coordination motif in the DNA-binding domain or the integrity of the IPT domain, which is thought to be important for DNA binding, result in the most penetrant Y-to-PDA defects, suggesting that DNA binding is key to UNC-3 activity during Y transdifferentiation. The four mammalian COE factors play a role in various types of tissue differentiation (Dubois and Vincent, 2001; Prasad et al., 2008). In particular, vertebrate COE factors act at different points during neuronal differentiation, from neuronal specification and progenitor migration to neuronal subtype specification (Dubois et al., 1998; Garcia-Dominguez et al., 2003; Jin et al., 2010). Similarly, UNC-3 exerts different activities in a neuron-specific manner. For instance, UNC-3 is necessary to maintain ASI neuron identity and functionality during larval development (Kim et al., 2005), whereas its absence leads to differentiation defects in a subset of ventral cord motoneurons, which then exhibit a mixed identity (Prasad et al., 2008). Both of these roles are consistent with unc-3 being necessary in these neurons to repress the transcription of other neuronal subtype-specific expression programmes (Kim et al., 2005; Prasad et al., 2008). By contrast, we did not detect ectopic expression of other ventral cord neuron markers in the Y.1 cell of unc-3 mutants, suggesting that repression of alternative neural programmes is not the main role of unc-3 during Y-to-PDA conversion. In addition, the PDA defects did not become more severe in later developmental stages in unc-3 mutants, excluding a role for unc-3 in the maintenance of PDA identity (Fig. 1E). Our data point to a role for unc-3 in promoting the terminal differentiation of an early neural cell into a motoneuron, with PDA identity. Such an inductive role is consistent with the ability of unc-3 to transform totipotent germ cells into cholinergic neurons (Tursun et al., 2011). Furthermore, it is conceivable that unc-3 exerts its activity in a cell-autonomous manner, as we could detect unc-3 expression in PDA (J.P.R. and S.J., unpublished).
Fig. 6.
Model for Y-to-PDA transdifferentiation. Previously, we found that a Notch signal is required in the C. elegans embryo to confer Y its competence to change identity, and that EGL-5 and SEM-4 are required to initiate the reprogramming process (Jarriault et al., 2008). Our current results suggest that transdifferentiation occurs through a dedifferentiated intermediate cell (Y.0) that lacks both the initial and final identities. UNC-3 is required to progress with redifferentiation into the PDA motoneuron; unc-3 mutants are stuck in an intermediate cellular stage (Y.1) in which only early neural identity is acquired. Our results support a model of cell type conversion that involves successive distinct cellular steps, rather than the existence of a mixed identity intermediate cell stage.
Our results demonstrate that direct cell type conversion occurs in the absence of cell division as a multistep process. DNA staining and quantification also suggested that this process occurs in the absence of DNA replication (N.F. and S.J., unpublished). How likely is the complete reprogramming of one cellular identity into another to occur in the absence of cell division? This could be a C. elegans-specific feature: indeed, nuclear reprogramming from one cell type-specific gene expression programme to another could be a comparatively simple process in C. elegans because, for example, they lack DNA methylation, which impacts on gene expression (Bird, 2002). However, even though direct evidence is missing, BrdU staining during various cell type conversions in mammals has suggested that they too occur in the absence of cell division (Means et al., 2005; Wu et al., 2010; Zhou et al., 2008), indicating that the complexity of DNA or chromatin modifications is not an obstacle to nuclear reprogramming in the absence of cell division. Consistently, recent findings have shown that elevated cell division does not accelerate iPS derivation by increasing the overall efficiency of the process itself, but rather by increasing the probability of a stochastic reprogramming event (Hanna et al., 2009). Thus, our data and those of others indicate that cell division is not strictly required to erase the initial identity of a cell in vivo. In the context of cellular therapy strategies, this feature could help minimise the risks associated with multiple cell divisions, such as the introduction of chromosomal aberrations.
Our results provide evidence that direct cell type conversion involves a dedifferentiation step, even in the absence of cell division. This is an important insight, as the absence of cell division has led to the assumption that direct cell type conversion does not involve transition through a less differentiated identity (Zhou et al., 2008). Indeed, the prevailing assumption would necessitate a transient intermediate cell that has the features of both cells. This is perhaps a risky state, as some cancer cells have mixed cellular identities (Zhang et al., 2010) that might contribute to their instability. Thus, the first and complete erasure of the initial identity, which is then followed by the acquisition of a new identity, could represent a safer cellular reprogramming mechanism in terms of cell identity stability. In line with this, some evidence indicates that step 2 of our model (Fig. 6), or reversion to a less differentiated intermediary, could be a conserved aspect of direct reprogramming across phyla. In mammals, although the detailed sequence is missing, direct cell type conversion of pancreatic acinar to ductal cells has been suspected to occur through an intermediary cellular stage (Doetsch, 2003; Means et al., 2005; Pastrana et al., 2009; Wu et al., 2010). Similarly, pancreatic cell-to-hepatocyte conversion, the formation of coronary arteries from venous cells and the conversion of astrocytes into neuroblasts during adult neurogenesis are also suspected to occur through an intermediate cell that is less differentiated (Doetsch, 2003; Means et al., 2005; Pastrana et al., 2009; Red-Horse et al., 2010; Wu et al., 2010). Thus, some features of the Y-to-PDA conversion highlighted in this study are most likely relevant to other organisms.
Although our data suggest that natural cellular reprogramming occurs through a dedifferentiated intermediate step, which is also observed when cells are reprogrammed in vitro with iPS cell technology (Takahashi and Yamanaka, 2006), no gain of wide cellular plasticity is observed. The cells generated through the iPS cell reprogramming approach exhibit a wide cellular potency and are potentially tumourigenic (Takahashi and Yamanaka, 2006). Our results suggest that, in a physiological environment, cellular potential is more constrained. This might be especially important since we found that the suspended intermediary cell state encountered in unc-3 mutants was remarkably stable and the cell did not die. Therefore, strategies involving direct cell type conversion for human cellular replacement, which have been suggested recently (Zhou et al., 2008), might represent a safer alternative to iPS cell-based approaches. In addition, recent findings have shown that during regeneration in axolotl in vivo, the proliferative and apparently undifferentiated cells that form a blastema are a heterogeneous population that, rather than being multipotent as initially assumed, seem to retain their lineage-of-origin commitment (Kragl et al., 2009). Thus, the uncoupling of dedifferentiation and multipotency in vivo might represent a common theme during natural direct reprogramming. The control mechanisms in place during Y reprogramming appear to be twofold. We found that reversion back to the initial identity, once the process has been initiated, appears blocked. This argues for an actively maintained shutdown of the initial identity expression programme. In addition, there appears to be tight control of which expression programme can be activated following dedifferentiation. It might be that direct cell type conversion allows the partial maintenance of a mature cell epigenetic make-up, thus restricting cellular potential. Alternatively, the in vivo environment in which the cell being reprogrammed is located might help to restrict cell fate decisions and thus cellular potential. In support to this latter hypothesis, recent studies have suggested that microenvironmental cues can have a key impact on cellular identity, for example by reprogramming thymic stem cells into skin stem cells, or by restricting the potential of adult neural stem cells that are otherwise equivalent in vitro (Bonfanti et al., 2010; Merkle et al., 2007).
A number of salient features of the Y-to-PDA reprogramming are consistent with, and expand upon, our knowledge of cell plasticity events described in vertebrates. The elucidation of the possible intrinsic or extrinsic cues that restrict cellular potential during direct cell type conversion in vivo will be a major challenge for future work. The analysis of defined direct cell conversion events at the single-cell level is a key step towards understanding the detailed cellular mechanisms of cell identity conversion and their impact on cell potential, and for future efforts to employ direct conversion for regenerative purposes.
Acknowledgements
We thank M. de Bono, S. Cameron, S. Emmons, J. McGhee, M. Krause, M. Labouesse, G. Marot, J. Rothman, D. Ron, R. Roy, Y. Jin and the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR), for providing strains and reagents; M. Koch and the IGBMC imaging platform for help with spinning disk imaging; S. DuManoir, O. Hobert, M. Labouesse, O. Pourquié and the members of the S.J. lab for a critical reading of this manuscript. This work was supported by an AFM predoctoral fellowship to J.P.R., a UdS fellowship to S.Z., a FRM fellowship to V.P. and grants from the CNRS, the AFM, the FRM and the ARC to S.J. S.J. is an investigator of the CNRS.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Altun-Gultekin Z., Andachi Y., Tsalik E. L., Pilgrim D., Kohara Y., Hobert O. (2001). A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128,1951-1969 [DOI] [PubMed] [Google Scholar]
- Baum P. D., Garriga G. (1997). Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron 19,51-62 [DOI] [PubMed] [Google Scholar]
- Bird A. (2002). DNA methylation patterns and epigenetic memory. Genes Dev. 16,6-21 [DOI] [PubMed] [Google Scholar]
- Bonfanti P., Claudinot S., Amici A. W., Farley A., Blackburn C. C., Barrandon Y. (2010). Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 466,978-982 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77,71-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. W., Hammarlund M., Harrach T., Hullett P., Olsen S., Jorgensen E. M. (2005). Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6, 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Tsonis K., Tsonis P. A. (2003). Eye regeneration at the molecular age. Dev. Dyn. 226,211-224 [DOI] [PubMed] [Google Scholar]
- Doetsch F. (2003). The glial identity of neural stem cells. Nat. Neurosci. 6,1127-1134 [DOI] [PubMed] [Google Scholar]
- Dubois L., Vincent A. (2001). The COE-Collier/Olf1/EBF-transcription factors: structural conservation and diversity of developmental functions. Mech. Dev. 108,3-12 [DOI] [PubMed] [Google Scholar]
- Dubois L., Bally-Cuif L., Crozatier M., Moreau J., Paquereau L., Vincent A. (1998). XCoe2, a transcription factor of the Col/Olf-1/EBF family involved in the specification of primary neurons in Xenopus. Curr. Biol. 8,199-209 [DOI] [PubMed] [Google Scholar]
- Ferreira H. B., Zhang Y., Zhao C., Emmons S. W. (1999). Patterning of Caenorhabditis elegans posterior structures by the Abdominal-B homolog, egl-5. Dev. Biol. 207,215-228 [DOI] [PubMed] [Google Scholar]
- Fukushige T., Krause M. (2005). The myogenic potency of HLH-1 reveals wide-spread developmental plasticity in early C. elegans embryos. Development 132,1795-1805 [DOI] [PubMed] [Google Scholar]
- Garcia-Dominguez M., Poquet C., Garel S., Charnay P. (2003). Ebf gene function is required for coupling neuronal differentiation and cell cycle exit. Development 130,6013-6025 [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. (2008). Nuclear reprogramming in cells. Science 322,1811-1815 [DOI] [PubMed] [Google Scholar]
- Hanna J., Saha K., Pando B., van Zon J., Lengner C. J., Creyghton M. P., van Oudenaarden A., Jaenisch R. (2009). Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462,595-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R., Sternberg P. W., Greenwald I. S., Fixsen W., Ellis H. M. (1983). Mutations that affect neural cell lineages and cell fates during the development of the nematode Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 48,453-463 [DOI] [PubMed] [Google Scholar]
- Jarriault S., Schwab Y., Greenwald I. (2008). A Caenorhabditis elegans model for epithelial-neuronal transdifferentiation. Proc. Natl. Acad. Sci. USA 105,3790-3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Jiang H., Mo Z., Xiang M. (2010). Early B-cell factors are required for specifying multiple retinal cell types and subtypes from postmitotic precursors. J. Neurosci. 30,11902-11916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Hoskins R., Horvitz H. R. (1994). Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature 372,780-783 [DOI] [PubMed] [Google Scholar]
- Kim K., Colosimo M. E., Yeung H., Sengupta P. (2005). The UNC-3 Olf/EBF protein represses alternate neuronal programs to specify chemosensory neuron identity. Dev. Biol. 286,136-148 [DOI] [PubMed] [Google Scholar]
- Kragl M., Knapp D., Nacu E., Khattak S., Maden M., Epperlein H. H., Tanaka E. M. (2009). Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460,60-65 [DOI] [PubMed] [Google Scholar]
- Labouesse M., Hartwieg E., Horvitz H. R. (1996). The Caenorhabditis elegans LIN-26 protein is required to specify and/or maintain all non-neuronal ectodermal cell fates. Development 122,2579-2588 [DOI] [PubMed] [Google Scholar]
- Maduro M., Pilgrim D. (1995). Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141,977-988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. L., Meszoely I. M., Suzuki K., Miyamoto Y., Rustgi A. K., Coffey R. J., Jr, Wright C. V., Stoffers D. A., Leach S. D. (2005). Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132,3767-3776 [DOI] [PubMed] [Google Scholar]
- Merkle F. T., Mirzadeh Z., Alvarez-Buylla A. (2007). Mosaic organization of neural stem cells in the adult brain. Science 317,381-384 [DOI] [PubMed] [Google Scholar]
- Miura K., Okada Y., Aoi T., Okada A., Takahashi K., Okita K., Nakagawa M., Koyanagi M., Tanabe K., Ohnuki M., et al. (2009). Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 27,743-745 [DOI] [PubMed] [Google Scholar]
- O'Neill K. E., Eberhard D., Tosh D. (2008). Origin of beta-cells in regenerating pancreas. BioEssays 30,617-620 [DOI] [PubMed] [Google Scholar]
- Pastrana E., Cheng L. C., Doetsch F. (2009). Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc. Natl. Acad. Sci. USA 106,6387-6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B., Karakuzu O., Reed R. R., Cameron S. (2008). unc-3-dependent repression of specific motor neuron fates in Caenorhabditis elegans. Dev. Biol. 323,207-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. C., Ye B., Zackhary R., Schrader K., Seydoux G., Reed R. R. (1998). unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O/E family of transcription factors. Development 125,1561-1568 [DOI] [PubMed] [Google Scholar]
- Quintin S., Michaux G., McMahon L., Gansmuller A., Labouesse M. (2001). The Caenorhabditis elegans gene lin-26 can trigger epithelial differentiation without conferring tissue specificity. Dev. Biol. 235,410-421 [DOI] [PubMed] [Google Scholar]
- Red-Horse K., Ueno H., Weissman I. L., Krasnow M. A. (2010). Coronary arteries form by developmental reprogramming of venous cells. Nature 464,549-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y., Takeshita H., Sasakawa N., Sawa H. (2010). Double bromodomain protein BET-1 and MYST HATs establish and maintain stable cell fates in C. elegans. Development 137,1045-1053 [DOI] [PubMed] [Google Scholar]
- Shioi G., Shoji M., Nakamura M., Ishihara T., Katsura I., Fujisawa H., Takagi S. (2001). Mutations affecting nerve attachment of Caenorhabditis elegans. Genetics 157,1611-1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher S. G., Desplan C. (2008). Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature 454,533-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E., Rampalli S., Risueno R. M., Schnerch A., Mitchell R., Fiebig-Comyn A., Levadoux-Martin M., Bhatia M. (2010). Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468,521-526 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126,663-676 [DOI] [PubMed] [Google Scholar]
- Tursun B., Patel T., Kratsios P., Hobert O. (2011). Direct conversion of C. elegans germ cells into specific neuron types. Science 331,304-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T., Ostermeier A., Pang Z. P., Kokubu Y., Sudhof T. C., Wernig M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463,1035-1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N. (1991). On the nature of the undead cells in the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B 331,263-271 [Google Scholar]
- Wu S. Y., Hsieh C. C., Wu R. R., Susanto J., Liu T. T., Shen C. R., Chen Y., Su C. C., Chang F. P., Chang H. M., et al. (2010). Differentiation of pancreatic acinar cells to hepatocytes requires an intermediate cell type. Gastroenterology 138,2519-2530 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Fan H., Shen J., Hoffman R. M., Xing H. R. (2010). Human breast cancer cell lines co-express neuronal, epithelial, and melanocytic differentiation markers in vitro and in vivo. PLoS One 5, e9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D. A. (2008). In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455,627-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. W., Fukushige T., McGhee J. D., Rothman J. H. (1998). Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev. 12,3809-3814 [DOI] [PMC free article] [PubMed] [Google Scholar]