Abstract
UNC-51 is a serine/threonine protein kinase conserved from yeast to humans. The yeast homolog Atg1 regulates autophagy (catabolic membrane trafficking) required for surviving starvation. In C. elegans, UNC-51 regulates the axon guidance of many neurons by a different mechanism than it and its homologs use for autophagy. UNC-51 regulates the subcellular localization (trafficking) of UNC-5, a receptor for the axon guidance molecule UNC-6/Netrin; however, the molecular details of the role for UNC-51 are largely unknown. Here, we report that UNC-51 physically interacts with LET-92, the catalytic subunit of serine/threonine protein phosphatase 2A (PP2A-C), which plays important roles in many cellular functions. A low allelic dose of LET-92 partially suppressed axon guidance defects of weak, but not severe, unc-51 mutants, and a low allelic dose of PP2A regulatory subunits A (PAA-1/PP2A-A) and B (SUR-6/PP2A-B) partially enhanced the weak unc-51 mutants. We also found that LET-92 can work cell-non-autonomously on axon guidance in neurons, and that LET-92 colocalized with UNC-51 in neurons. In addition, PP2A dephosphorylated phosphoproteins that had been phosphorylated by UNC-51. These results suggest that, by forming a complex, PP2A cooperates with UNC-51 to regulate axon guidance by regulating phosphorylation. This is the first report of a serine/threonine protein phosphatase functioning in axon guidance in vivo.
Keywords: C. elegans, Axon guidance, PP2A, Serine/threonine protein kinase, Serine/threonine protein phosphatase
INTRODUCTION
In the development of the nervous system, neurons extend their axons to precise targets. In this process, axon guidance molecules, expressed on cell membranes or in the extracellular milieu, provide positional information (Tessier-Lavigne and Goodman, 1996; Yu and Bargmann, 2001; Dickson, 2002; Chilton, 2006; Killeen and Sybingco, 2008). To do this, the axon guidance molecules bind to receptors on the growth cone, a specialized structure at the growing axonal tip, and induce cytoskeletal changes in the growth cone.
A conserved axon guidance molecule, UNC-6/Netrin, is required for dorsoventral axon guidance in C. elegans (Hedgecock et al., 1990; Ishii et al., 1992; McIntire et al., 1992; Hao et al., 2001) and is expressed by ventral cells (Wadsworth et al., 1996; Asakura et al., 2007). Two C. elegans UNC-6 receptors are UNC-5 and UNC-40/DCC, which belong to the immunoglobulin superfamily. Each has a single transmembrane domain (Leung-Hagesteijn et al., 1992; Chan et al., 1996), and both are required for ventral UNC-6 to repulse axons that are fated to extend dorsally (Wadsworth, 2002). Ventrally extending axons, however, are attracted to UNC-6 and require only the UNC-40 receptor for this response. The dorsoventral guidance of C. elegans axons is also regulated by a conserved axon guidance molecule, SLT-1/Slit (Hao et al., 2001). SLT-1 is expressed by dorsal muscles and some ventrally extending axons are repelled by it. Two of the C. elegans SLT-1 receptors are SAX-3/Robo and EVA-1. Each has a single transmembrane domain (Zallen et al., 1998; Fujisawa et al., 2007), and SAX-3 belongs to the immunoglobulin superfamily. EVA-1 has two lectin-like galactose binding domains in its ectodomain. UNC-6 and SLT-1 act partially redundantly in ventrally directed axon guidance (Hao et al., 2001; Fujisawa et al., 2007).
UNC-51 and UNC-14 are essential for the axon guidance of many neurons in C. elegans (Hedgecock et al., 1985; Desai et al., 1988; McIntire et al., 1992; Mörck et al., 2003; Lai and Garriga, 2004; Siddiqui and Culotti, 2007). UNC-51 is a conserved serine/threonine protein kinase that is homologous to yeast Atg1 and human ULK (Ogura et al., 1994; Matsuura et al., 1997; Straub et al., 1997; Yan et al., 1998). All three homologs are required for autophagy, that is, the catabolic vesicle trafficking that is required to survive starvation (Matsuura et al., 1997; Straub et al., 1997; Meléndez et al., 2003; Hara et al., 2008). The function of these UNC-51 homologs in axon guidance is also conserved from C. elegans to mammals (Ogura et al., 1994; Tomoda et al., 1999; Tomoda et al., 2004; Zhou et al., 2007; Ahantarig et al., 2008; Toda et al., 2008). Because, in C. elegans, knocking-down of other autophagy genes does not cause axon guidance defects, the axon-guidance function of UNC-51 is probably effected through a different mechanism than its autophagy function (Ogura and Goshima, 2006). The binding partner of UNC-51, UNC-14, is a novel protein that contains a RUN domain (Ogura et al., 1997). Although the function of the RUN domain in UNC-14 is not known, RUN domains are predicted to play important roles in Rap and Rab family GTPase signaling pathways in vesicle trafficking (Callebaut et al., 2001). UNC-51 and UNC-14 together regulate the subcellular localization (trafficking) of the UNC-6 receptor UNC-5 (Ogura and Goshima, 2006). However, the molecular functions of UNC-51 and UNC-14 are largely unknown.
Serine/threonine protein phosphatase 2A (PP2A) is highly conserved from yeast to mammals (Millward et al., 1999; Lechward et al., 2001; Sontag, 2001; Janssens et al., 2008). A 36 kD catalytic subunit (PP2A-C) and a 65 kD regulatory A subunit (PP2A-A) comprise the core structure of PP2A, to which one of a variety of regulatory B subunits can bind, to confer distinct properties on the heterotrimeric holoenzyme. Extensive analysis using PP2A mutants or PP2A inhibitors in cultured cells has revealed the involvement of PP2A in many cellular functions, including cell division, development and apoptosis, and in pathological conditions, such as cancer. For example, in C. elegans, PP2A is involved in vulval differentiation (Kao et al., 2004) and mitotic spindle assembly (Schlaitz et al., 2007). However, the early lethality of the PP2A mutation makes it difficult to analyze the in vivo function of PP2A in neural cells.
Here, we report that LET-92, the catalytic subunit of the C. elegans protein phosphatase 2A (PP2A-C), physically interacts with UNC-51 and that the genes encoding the catalytic and regulatory subunits of PP2A interact genetically with unc-51 to influence axon guidance phenotypes. We also found that LET-92 can work cell-non-autonomously on axon guidance in neurons and colocalized with UNC-51 in neurons. In addition, PP2A dephosphorylated phosphoproteins that had been phosphorylated by UNC-51. These results suggest that PP2A functions in cooperation with UNC-51 to regulate axon guidance by regulating phosphorylation. This is the first report of a serine/threonine protein phosphatase having an in vivo function in axon guidance.
MATERIALS AND METHODS
Worms
Bristol strain N2 was used as the standard wild-type strain. The worms were handled as described by Brenner (Brenner, 1974). The analyzed strains were made by the crossing or transformation of the original strains shown as follows: I, unc-14(e57), sur-6(sv30), zdIs5(mec-4::gfp), hT2; II; III, paa-1(tm655), hT2; IV, let-92(s504), let-92(s677), unc-22(s7), nT1; V, unc-51(e369), unc-51(ks38::Tc1), nT1; X, unc-6(ev400), slt-1(eh15), oxIs12(unc-47::gfp), lin-15(n765ts), sax-3(ky123).
Two-hybrid screening
The C. elegans two-hybrid cDNA library was kindly provided by Robert Barstead (Oklahoma Medical Research Foundation, OK, USA). AH109 (TaKaRa, 630444) was used as the host strain. pGBK-T7 (TaKaRa, 630443) was used to drive the expression of the UNC-51 (276-856) and full-length UNC-14 baits. Library screening was performed as described by the manufacturer (TaKaRa, 630303). let-92 cDNAs were isolated in both screenings.
Isolation of a deletion mutant
The paa-1(tm655) mutant was isolated as described by Gengyo-Ando et al. (Gengyo-Ando et al., 2000). tm655 lacked 1428 base pairs (bp) that included 70.7% of the coding region of the paa-1 gene (http://www.wormbase.org/db/gene/gene?name=WBGene00003901;class=Gene), resulting in a putative null allele.
Genetic analysis
The DD and VD neurons were labeled with oxIs12 (unc-47::gfp) (McIntire et al., 1997). The AVM neuron was labeled with by zdIs5 (mec-4::gfp) (Clark and Chiu, 2003). Each animal was mounted on a 5% agarose pad in M9 buffer containing 5% sodium azide and observed using a fluorescence microscope (Axioplan 2, Carl Zeiss). The number of DD and VD axons that reached more than 80% of the way to the dorsal nerve cord in L4 larvae or young adults was counted. Because we enumerated the DD and VD axons exhibiting defective pathfinding, we used the Bonferroni correction for the statistical analysis. For AVM axon guidance, we used a binary, YES/NO, evaluation mode in which an axon was counted as having a defect if it did not reach the ventral nerve cord, or if the axon extended anteriorly or posteriorly before reaching the ventral nerve cord. Therefore, we used a chi-square test for the statistical analysis of these results.
Imaging
A confocal microscope (LSM510, Carl Zeiss) was used to obtain images.
GST pull-down analysis
Glutathione Sepharose 4B beads (GE Healthcare, 17-0756-01), extracts of E. coli that expressed GST::LET-92 (Ogura et al., 2003), and reticulocytes (Promega, L1170) that expressed each of the MYC-tagged proteins were mixed in cold buffer [25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM DTT, 1 mM MgCl2, 0.2% NP-40]. For the expression of MYC-tagged proteins in reticulocytes, we used a pGBK-T7 vector (TaKaRa, 630443). EST clones yk668c8 (unc-51), yk459e7 (unc-14) and yk678c6 (unc-115) (Lundquist et al., 1998) were used for the constructs. The beads were washed several times with the same cold buffer. Each sample was fractionated by 8% SDS-PAGE and analyzed by western blotting using ECL Advance (GE Healthcare, RPN2135). We used an anti-MYC antibody (Santa Cruz, sc-789) for the primary antibody and an HRP-conjugated anti-rabbit IgG (GE Healthcare, NA 934) for the secondary antibody.
Molecular analysis
A 4.3-kb HindIII fragment (bp 20862-25420 of the cosmid F38H4) containing only F38H4.9(let-92) was sub-cloned into pBluescript SK+ (Stratagene), resulting in a let-92 rescue clone (pL92H3).
We used KOD-Plus (Toyobo, KOD-201) for our PCR experiments. A 2014 bp let-92 promoter region was PCR-amplified from a cosmid clone F38H4. The DNA fragment was inserted into pPD95.77, resulting in a let-92p::GFP construct (pgEL92P). A 3070 bp paa-1 promoter region was PCR-amplified from a cosmid clone F48E8. The DNA fragment was inserted into a Venus (Nagai et al., 2002) expression vector p77-CV, resulting in a paa-1p::Venus construct (ppa1P-CV). A 4123 bp sur-6 promoter region was PCR-amplified from N2 genomic DNA. The DNA fragment was inserted into p77-CV, resulting in a sur-6p::Venus construct (psu6P-CV).
A let-92 open reading frame (ORF) was amplified from pPBP345, which was previously isolated in our two-hybrid screening as a POS-1 bait (Ogura et al., 2003). The let-92 ORF was inserted into the unc-25 promoter (Jin et al., 1999)::Venus expression vector pu25P-NV, resulting in an unc-25p::Venus::let-92 construct (pu25PV-L92). The unc-51 ORF was amplified from yk668c8 and was inserted into an unc-25 promoter::mCherry (Shaner et al., 2004; McNally et al., 2006) expression vector pu25P-CmCH, resulting in an unc-25p::unc-51::mCherry construct (pu25P-u51mCH). An src-2 myristoylation signal ORF corresponding to MGSCIGK (Adler et al., 2005) was inserted into pu25P-CmCH, resulting in an unc-25p::src-2 myristoylation signal::mCherry construct (pu25P-mymCH).
PCR-amplified tissue-specific promoters [a mec-7 promoter (Hamelin et.al., 1992), an H20 promoter (Shioi et al., 2001), a myo-3 promoter (Okkema et al., 1993), an unc-4 promoter (Miller and Niemeyer, 1995), a ceh-12 promoter (Von Stetina et al., 2007) and an inx-18 promoter (Bülow et al., 2004)] were inserted into C. elegans expression vector p77-T2. The let-92 ORF was inserted into the tissue-specific expression vectors, resulting in mec-7p::let-92(pm7P-L92), H20p::let-92(pH20P-L92), myo-3p::let-92(pmy3P-L92), unc-4p::let-92(pu4P-L92), ceh-12p::let-92 (pce12P-L92) and inx-18p::let-92 (pix18P-L92) constructs.
Transformation of C. elegans
Transformation was performed as described by Mello et al. (Mello et al., 1991). For the let-92 rescue experiments, pRF4, a rol-6 marker (Kramer et al., 1990) was used; 10 ng/μl of the let-92 rescue clone (pL92H3) and 90 ng/μl of pRF4 were injected into the adult [unc-22(s7) let-92(s504)/nT1] gonad. For the expression of let-92p::GFP(pgEL92P), paa-1p::Venus(ppa1P-CV) or sur-6p::Venus(psu6P-CV), MT8189 [lin15(n765ts) X] was used as the transformation strain, and pJM23 was used as the transformation marker (Huang et al., 1994). Each sample of 10 ng/μl DNA and 90 ng/μl pJM23 was injected into the adult gonad.
For other transformation analyses, myo-2p::mRFP (pmy2P-mR) (Campbell et al., 2002) was used as the marker (10 ng/μl). pBluescript SK+ was used to equalize the amount of DNA in the transformations. For experiments to evaluate cell autonomy, constructs [pBluescript SK+ (80 ng/μl), pmy2P-mR (10 ng/μl) and pL92H3 (10 ng/μl)], [pBluescript SK+ (70 ng/μl), pmy2P-mR (10 ng/μl), pm7p-dr2 (mec-7p::dsRed2, 5 ng/μl) and pm7P-L92 (10 ng/μl)], [pBluescript SK+ (60 ng/μl), pmy2P-mR (10 ng/μl), pu4P-L92 (10 ng/μl), pce12P-L92 (10 ng/μl) and pix18P-L92 (10 ng/μl)], [pBluescript SK+ (80 ng/μl), pmy2P-mR (10 ng/μl) and pmy3P-L92 (10 ng/μl)] or [pBluescript SK+ (80 ng/μl), pmy2P-mR (10 ng/μl) and pH20P-L92 (10 ng/μl)] were injected into the adult [unc-22(s7) let-92(s504)/nT1; unc-51(ks38::Tc1): zdIs5] gonad. For the colocalization analysis, the constructs [pBluescript SK+ (70 ng/μl), pmy2P-mR (10 ng/μl), pu25PV-L92 (10 ng/μl) and pu25P-u51mCH (10 ng/μl)] were injected into the adult N2 gonad. To detect Venus::LET-92 in VD growth cones, the constructs [pBluescript SK+ (30 ng/μl), pmy2P-mR (10 ng/μl), pu25P-mymCH (5 ng/μl) and pu25PV-L92 (50 ng/μl)] were injected into the adult N2 gonad.
Biochemical analyses
CMVp::unc-51::FLAG, CMVp::unc-51 (ΔAIKAI, a kinase dead form)::FLAG and CMVp::unc-14::HA constructs were kindly provided by Gian Garriga (Lai and Garriga, 2004). pEGFP-N3 (Clontech, 632313) was used for GFP expression. CMVp::HA::let-92 and CMVp::vab-8::HA constructs were made by inserting an HA::let-92 ORF or a vab-8::HA ORF into a mammalian expression vector pcDNA3.1/myc-His C (Invitrogen, V800-20). The vab-8 ORF was amplified by RT-PCR. HEK293T cells were transfected by using the FuGENE 6 Transfection Reagent (Roche, 1 814 443). Cells were harvested in cold buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% Triton X-100, protease inhibitor (complete, Roche, 1 836 153)]. For the VAB-8 analysis, we added phosphatase inhibitor (PhosSTOP, Roche, 4 906 845). The samples were used directly for western blotting or immunoprecipitation (IP). For IP, an anti-FLAG M2 antibody (Sigma, F3165) or an anti-HA antibody (F7, Santa Cruz, sc-7392) was used. Protein G Sepharose (GE Healthcare, 17-0618-01) was used for capturing the protein-antibody complex. For detection, the primary antibody was the anti-FLAG M2 antibody, an anti-HA antibody (3F10, Roche, 1 867 423) or an anti-GFP antibody (Santa Cruz, sc-8334). An HRP-conjugated anti-mouse IgG (GE Healthcare, NA931), anti-rat IgG (GE Healthcare, NA935) or anti-rabbit IgG (GE Healthcare, NA934) was used as the secondary antibody.
λ-PPase (NEB, P0753S) was used for the non-specific dephosphorylation control. PP2A-AC (Millipore, 14-111), which contains the catalytic and the regulatory A subunits of human PP2A, was used for the PP2A dephosphorylation assay. IP protein samples were incubated at 30°C for 30 minutes with λ-PPase (400 units) in reaction buffer [50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 0.1 mM EGTA, 2 mM DTT, 0.01% Brij 35, 2 mM MnCl2], PP2A (0.02 units) in reaction buffer [20 mM HEPES (pH 7.0), 1 mM DTT, 1 mM MnCl2, 100 μg/ml BSA] (Ito et al., 2000) or in the PP2A reaction buffer only.
RESULTS
The catalytic subunit of protein phosphatase 2A (PP2A-C) interacts physically with UNC-51 and UNC-14
To analyze the function of UNC-51 and UNC-14 of C. elegans, we screened for their interacting proteins using the yeast two-hybrid system. From the screenings, we identified the F38H4.9 cDNA, which encodes the catalytic subunit of protein phosphatase 2A (PP2A-C; Fig. 1A). We confirmed that F38H4.9 interacted directly with both UNC-51 and UNC-14 in a GST pull-down assay (Fig. 1B).
Fig. 1.
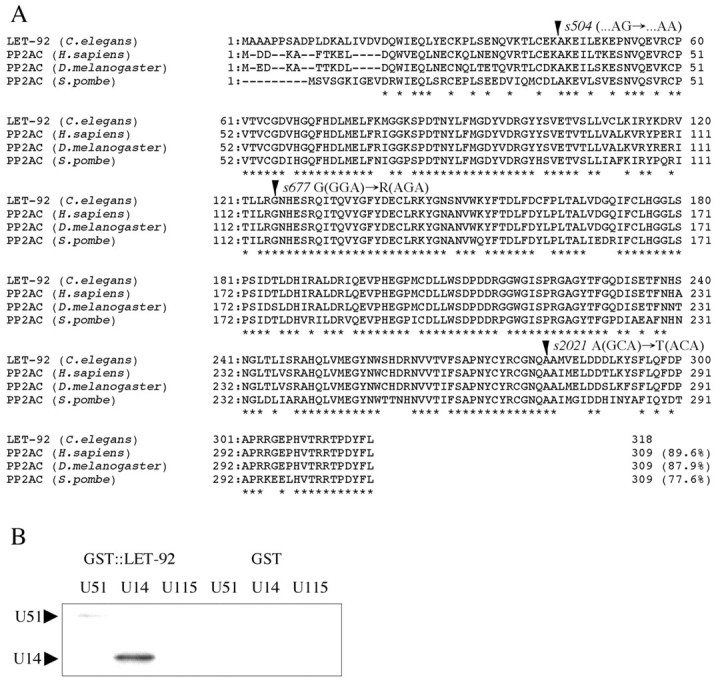
Homology analysis of LET-92/PP2A-C. (A) The putative amino acid sequence of LET-92 in C. elegans is shown aligned with the PP2A-C of human (PP2AC-beta isoform) (Khew-Goodall et al., 1991), D. melanogaster (Orgad et al., 1990) and S. pombe (Ppa1) (Kinoshita et al., 1990). Asterisks indicate conserved amino acids. Amino acids that are altered by the let-92 mutations are indicated by arrowheads. The number of amino acid residues and overall identity between LET-92 and the other PP2A-Cs are shown at the ends of each amino acid sequence. The cDNA sequence of let-92 has been deposited in the DNA Data Bank of Japan (DDBJ) under accession number AB108533. (B) LET-92 interacted directly with both UNC-51 and UNC-14 in a GST pull-down assay. Abbreviations: U51, MYC::UNC-51; U14, MYC::UNC-14; U115, MYC::UNC-115. Arrowheads indicate the physically interacting proteins.
F38H4.9 was previously identified as a POS-1-interacting protein (PIP) (Ogura et al., 2003). We found that the coding sequence for F38H4.9 corresponded to let-92 (Rogalski and Baillie, 1985) for the following reasons: (1) F38H4.9 mapped physically close to let-92, (2) a 4.3 kb HindIII fragment (bp 20862-25420 of the cosmid F38H4) containing only F38H4.9 rescued the larval lethal phenotype of let-92, and (3) three alleles of let-92 had mutations in the F38H4.9 region (Fig. 1A). Two of these alleles, s677 (125 G to R) and s2021 (282 A to T), had single mis-sense mutations at highly conserved amino acids. The third, s504, had a mutation at the splice acceptor site of the first intron. We found that let-92 was expressed in almost all cells, including many neurons, at all stages (Fig. 2A).
Fig. 2.
Expression patterns of GFP or Venus from the reporter constructs. (A) let-92 promoter::GFP. (B) paa-1 promoter::Venus. (C) sur-6 promoter::Venus. These fusion genes were ubiquitously expressed. GFP from let-92 promoter::GFP was not expressed in the germ cells (black region), which is a typical result for transgenic gene expression in C. elegans. Anterior is to the right. Dorsal is to the top. Scale bars: 100 μm.
A low allelic dose of LET-92 suppresses the DD and VD axon guidance defects of unc-51 mutants
To analyze the functional relationship among UNC-51, UNC-14 and LET-92, we examined their genetic interactions in dorsally directed DD and VD axon guidance (Fig. 3A), as unc-51 and unc-14 mutants show axon-guidance defects in these neurons (McIntire et al., 1992; Ogura and Goshima, 2006). All of the alleles (s504, s677 and s2021) of let-92 are completely lethal in early larvae (Rogalski and Baillie, 1985). We found that the let-92(s504) homozygous mutants had minor DD and VD axon guidance defects (data not shown); however, the dead let-92 worms showed the ‘clear’ phenotype, which was probably caused by abnormal osmotic regulation. Therefore, we could not determine whether the axon guidance defects were primary defects or if they were secondary to the larval lethality. In addition, although let-92(s504) homozygotes die as early larvae, let-92(RNAi) resulted in early embryonic lethality (data not shown), indicating that the maternal product functions during embryogenesis. As DD neurons are born in the embryo (Sulston et al., 1983), their axon guidance could be affected by the maternal product. To avoid these issues, we examined the effect of a low allelic dose of the let-92 gene. Heterozygotic let-92 mutants were healthy and active, appeared identical to wild-type worms and had no axon guidance defects (Fig. 4). The low allelic dose of LET-92 produced in let-92(s504/+) and let-92(s677/+) heterozygotes partially suppressed the axon guidance defects of a weak unc-51 allele, unc-51(ks38::Tc1; Fig. 3C; Fig. 4). However, let-92(s504/+) did not suppress the defects of the severe unc-51(e369) allele (Fig. 4). These results suggest that LET-92 negatively regulates the functions of UNC-51 in dorsally directed DD and VD axon guidance, and that some level of UNC-51 function is required for the LET-92 function. We also analyzed the effect of low allelic dose let-92 on animals carrying the null allele unc-14(e57) (Ogura et al., 1997). However, we did not detect a genetic interaction (Fig. 4). Some level of UNC-14 function also may be required for the LET-92 function.
Fig. 3.
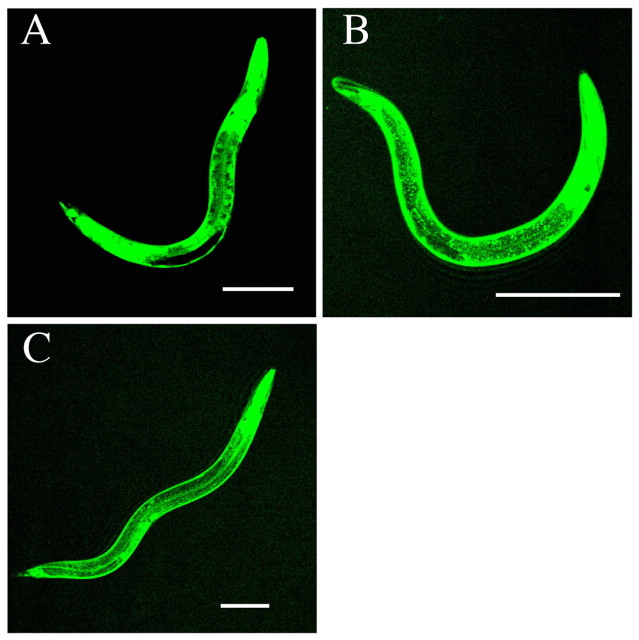
let-92(s504/+) partially suppresses DD and VD axon guidance defects in a weak unc-51 mutant. (A) Wild-type animal. (B) unc-51(ks38::Tc1); unc-22(s7)/nT1. (C) unc-51(ks38::Tc1); unc-22(s7) let-92(s504)/nT1. All animals shown are L4 larvae. DD and VD neurons were labeled with oxIs12 (unc-47::gfp) (McIntire et al., 1997). The white triangles indicate axons that reached the dorsal nerve cord. In the wild-type worm shown (A), 16 axons reach the dorsal nerve cord. As the balancer nT1 includes qIs51, which expresses GFP in the pharynx, we could not analyze one axon that passed around the pharynx, as indicated by the yellow triangle. We therefore excluded this axon from all of our analyses. In the unc-51(ks38::Tc1); unc-22(s7)/nT1 worm shown (B), 11 axons reached the dorsal nerve cord. In the unc-51(ks38::Tc1); unc-22(s7) let-92(s504)/nT1 worm shown (C), 15 axons reached the dorsal nerve cord. Anterior is to the right. Dorsal is to the top. Scale bar: 100 μm.
Fig. 4.
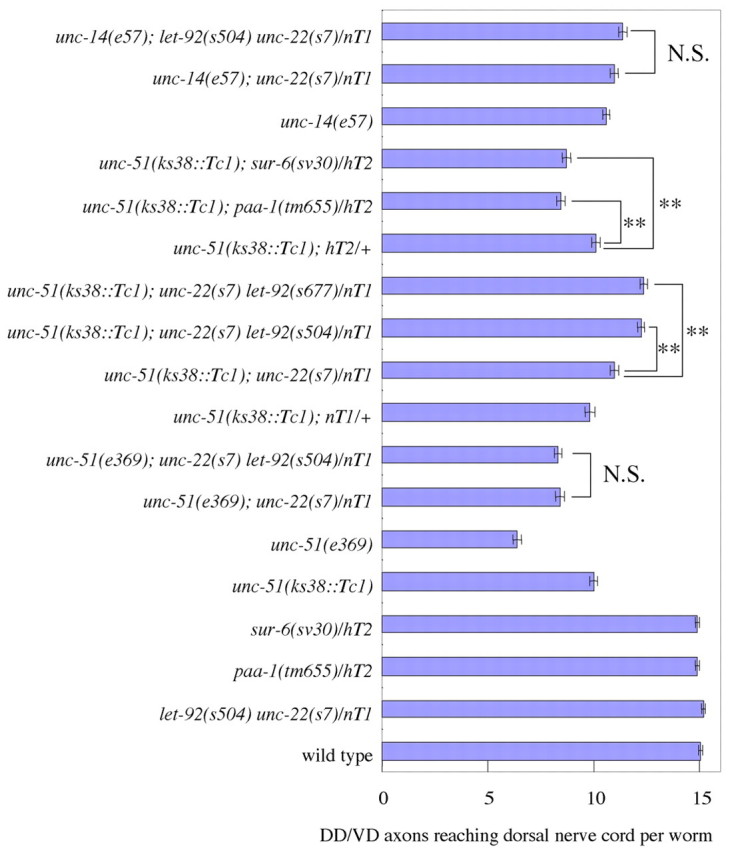
Statistical analyses of the genetic interactions among unc-51, unc-14, let-92, paa-1 and sur-6 in DD and VD axon guidance. The number of axons that reached the dorsal nerve cord in animals of the indicated phenotypes is shown. Eighty worms were counted for each analysis. nT1 and hT2 were balancer chromosomes used for maintaining the heterozygotes. unc-22(s7) was used as a marker for let-92(s504) and let-92(s677) inclusion. The low allelic dose of let-92 suppressed the axon pathfinding defects of the weak unc-51 mutants, but the low allelic dose of paa-1 or sur-6 enhanced them. Error bars show the standard error. **, P<0.01, Bonferroni correction. N.S., not significant.
In the course of the study, we noticed that heterozygotic mutants for unc-22 [unc-22(s7/+)], which encodes muscle protein Twichin (Waterston et al., 1980; Benian et al., 1989), also suppressed the DD and VD axon guidance defects in unc-51(ks38::Tc1) and unc-51(e369) (P<0.01, Bonferroni correction). Homozygotic unc-22(s7/s7) suppressed the axon guidance defects of unc-51(ks38::Tc1) more strongly (data not shown). unc-22 mutants have defects in muscle organization (Waterston et al., 1980; Benian et al., 1989); therefore, we think that muscle disorganization suppresses the DD and VD axon guidance defects of the unc-51 mutants by a novel mechanism. We will report the details of these findings in a future paper.
Low allelic dose of PP2A regulatory subunits (PAA-1/PP2A-A and SUR-6/PP2A-B) enhances the unc-51 defects in DD and VD axon guidance
The heterotrimeric PP2A holoenzyme contains one catalytic (PP2A-C) and two regulatory (PP2A-A and PP2A-B) subunits (Millward et al., 1999; Lechward et al., 2001; Sontag, 2001; Janssens et al., 2008). In the C. elegans genome, a single gene encodes the PP2A catalytic subunit (let-92/PP2A-C) and several encode the PP2A regulatory subunits, which include one PP2A regulatory subunit A gene (paa-1/PP2A-A) and one PP2A regulatory subunit B gene (sur-6/PP2A-B). To analyze the effect of these genes on UNC-51 function, we analyzed the genetic interactions among paa-1, sur-6 and unc-51.
No mutants of the paa-1 gene were available; therefore, we made a deletion-null allele, paa-1(tm655), which caused complete early larval lethality, just as in homozygous let-92 mutations. Like the dead let-92 worms, the dead paa-1(tm655) larvae showed the ‘clear’ phenotype and paa-1(tm655)(RNAi) caused early embryonic death (data not shown). Therefore, we examined the effect of a low allelic dose of the paa-1 gene. The paa-1(tm655/+) heterozygotes were healthy and active, appeared identical to wild-type worms and had no axon guidance defects (Fig. 4). The only reported null allele of the sur-6 gene, sur-6(sv30) (Kao et al., 2004), shows maternal effect lethality. That is, sur-6(sv30) homozygotes of sur-6(sv30/+) heterozygotic mothers grow to adulthood. However, the homozygote eggs never hatch, showing that the maternal product functions during embryogenesis. As with let-92, because the DD neurons are born during embryogenesis (Sulston et al., 1983), DD axon guidance could be affected by the maternal product. To avoid this issue, we used the same strategy of examining animals expressing a low allelic dose of the sur-6 gene. The sur-6(sv30/+) heterozygotes were identical to wild-type animals and had no axon guidance defects (Fig. 4).
We found that the low allelic dose of paa-1 and sur-6 partially enhanced the axon guidance defects of DD and VD motor neurons in the unc-51(ks38::Tc1) mutants (Fig. 4), suggesting that these regulatory subunits positively regulate UNC-51. PAA-1 and SUR-6 probably inhibit LET-92 activity, indirectly causing the upregulation of UNC-51 activity in the DD and VD axons. Both paa-1 and sur-6 were expressed almost ubiquitously, including in many neurons at all stages, similar to the expression of let-92 (Fig. 2B,C).
A low allelic dose of LET-92 partially suppresses the AVM guidance defect of the unc-51 mutant
UNC-51 and UNC-14 are required for the axon guidance of many neurons (Hedgecock et al., 1985; Desai et al., 1988; McIntire et al., 1992; Mörck et al., 2003; Lai and Garriga, 2004; Siddiqui and Culotti, 2007). Furthermore, UNC-51, UNC-14 and LET-92 are expressed in many neurons (Fig. 2A) (Ogura et al., 1994; Ogura et al., 1997). Therefore, we hypothesized that PP2A might regulate UNC-51 and UNC-14 in other neurons in addition to the dorsally directed DD and VD neurons. We next looked for genetic interactions among let-92, unc-51 and unc-14 by assessing the pathfinding phenotype of the AVM axon. The AVM is a mechanosensory neuron that resides in the lateral region of C. elegans (White et al., 1986). The axon of the AVM neuron first extends ventrally and then, after reaching the ventral nerve cord, extends anteriorly (Fig. 5A).
Fig. 5.
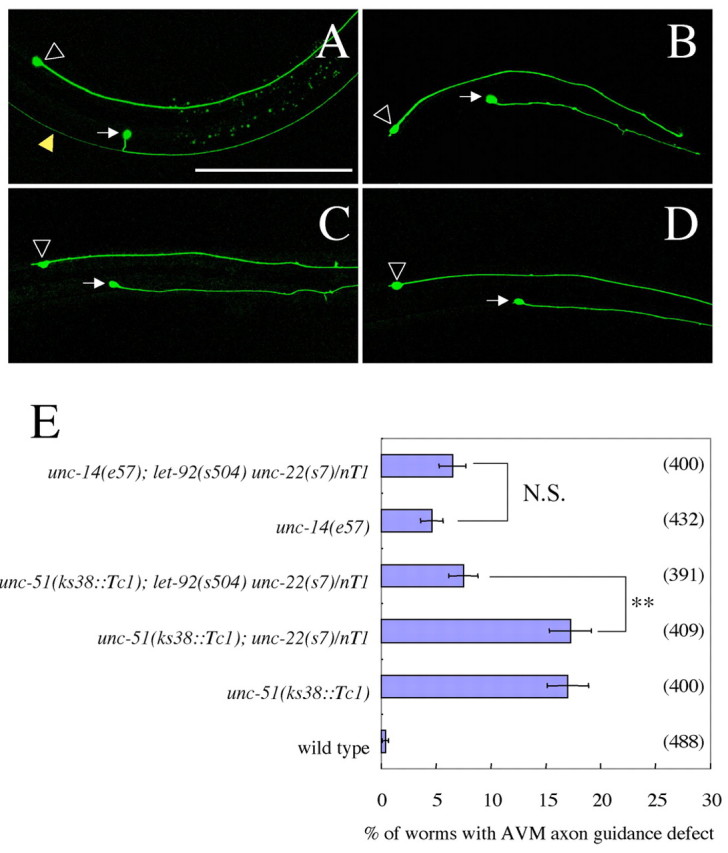
let-92(s504/+) partially suppresses AVM axon guidance defects of a weak unc-51 mutant. (A) Wild-type worm. (B) unc-51(ks38::Tc1) worm. (C) unc-14(e57) worm. (D) slt-1(eh15) worm. The AVM neuron was labeled with zdIs5 (mec-4::GFP) (Clark and Chiu, 2003). White arrows indicate AVM cell bodies. Open arrowheads indicate ALM R cell bodies. Yellow arrowhead in A indicates a PVM axon. (A-D) unc-51(ks38::Tc1) and unc-14(e57) mutants show defects in pathfinding by ventrally directed AVM axons. The phenotype was very similar to that of slt-1(eh15). Anterior is to the right. Dorsal is to the top. Scale bar: 100 μm. (E) Statistical analysis of the genetic interactions among unc-51, unc-14 and let-92 in AVM axon guidance, showing the percentage of animals with aberrant AVM axons. Error bars show the standard error. Parentheses show the number of worms counted. nT1 is a balancer chromosome used for maintaining the heterozygotes. unc-22(s7) was used as a marker for let-92(s504) inclusion. **, P<0.01, chi-square test. N.S., not significant.
We first found that unc-51 and unc-14 mutants had defects in the ventral guidance of the AVM axon (Fig. 5B,C). As we expected, let-92(s504/+) heterozygotes partially suppressed the AVM axon guidance defect in the unc-51(ks38::Tc1) mutant (Fig. 5E). However, let-92(s504/+) did not affect the AVM axon guidance defect in the unc-14(e57)-null mutant (Fig. 5E). Therefore, some UNC-14 function may be required for LET-92 to act in axon guidance.
Mutations in both unc-51 and unc-14 enhance the unc-6 mutant phenotype, but suppress the slt-1 mutant phenotype, in AVM axon guidance
The molecules involved in the ventral guidance of the AVM axon in C. elegans have been extensively studied (Walthall and Chalfie, 1988; Hao et al., 2001; Yu et al., 2002; Gitai et al., 2003; Chang et al., 2004; Chang et al., 2006; Quinn et al., 2006; Fujisawa et al., 2007; Quinn et al., 2008). Ventrally expressed UNC-6 attracts the AVM axon ventrally, and the dorsally expressed SLT-1 repels it. These pathways are partially redundant; approximately 50% of the animals carrying the null mutations unc-6(ev400) or slt-1(eh15) showed abnormal AVM axon pathfinding (Fig. 5D; Fig. 6) and nearly 100% of unc-6(ev400); slt-1(eh15) double mutants showed such defects (Fig. 6).
Fig. 6.
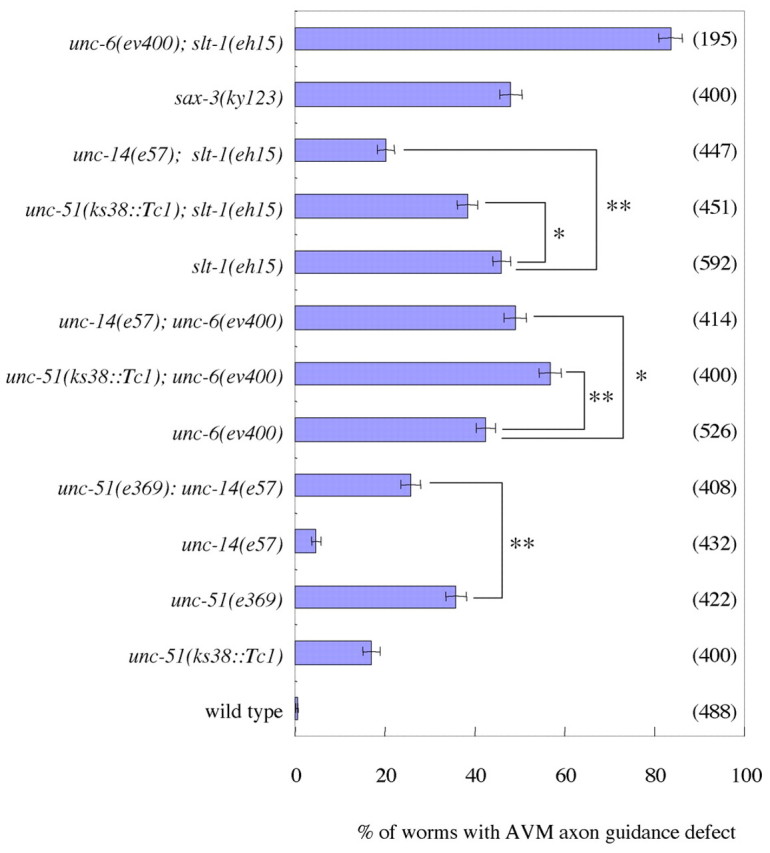
Statistical analysis of the genetic interactions among unc-51, unc-14, unc-6 and slt-1 in AVM axon guidance. The percentage of animals exhibiting defects in AVM axon guidance is shown. The AVM neuron was labeled with zdIs5 (mec-4::GFP) (Clark and Chiu, 2003). Parentheses show the number of worms counted. unc-51(ks38::Tc1) and unc-14(e57) enhanced the defects of unc-6(ev400) and suppressed the defects of slt-1(eh15). unc-14(e57) suppressed the defects of unc-51(e369). Error bars show the standard error. *, P<0.05, **, P<0.01, chi-square test.
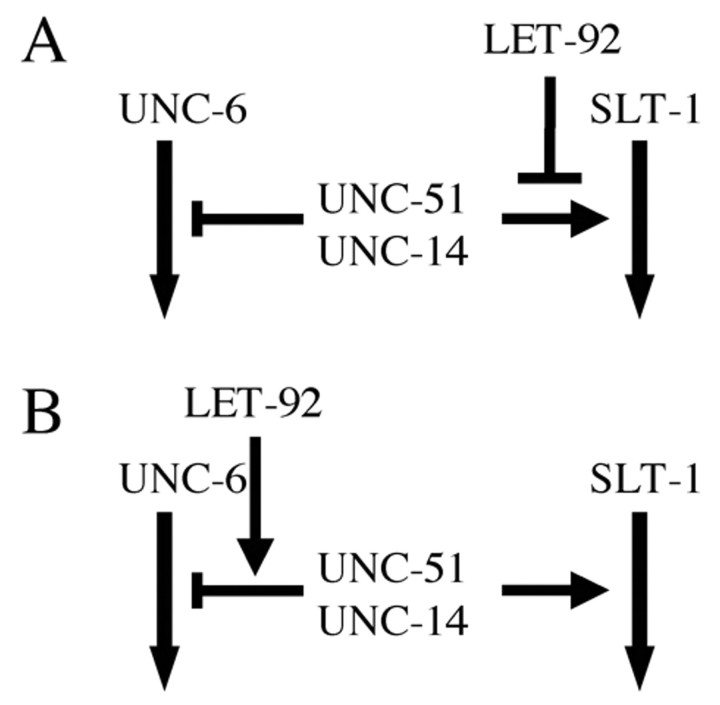
To determine whether LET-92, UNC-51 and UNC-14 function in the UNC-6 or SLT-1 pathways, we examined the genetic interactions among unc-51, unc-14, unc-6 and slt-1. We found that both the unc-51(ks38::Tc1) and unc-14(e57) mutations partially enhanced the AVM guidance defect of the unc-6(ev400) mutant (Fig. 6), indicating that both UNC-51 and UNC-14 positively regulate the SLT-1 pathway. Conversely, both the unc-51(ks38::Tc1) and unc-14(e57) mutations partially suppressed the guidance defect of the slt-1(eh15) mutant (Fig. 6), indicating that both UNC-51 and UNC-14 negatively regulate the UNC-6 pathway. These results indicate that the AVM axon guidance phenotypes observed in the unc-51(ks38::Tc1) and unc-14(e57) mutants result from the additive effects of inhibiting the SLT-1 pathway and enhancing the UNC-6 pathway, and that the abnormal AVM guidance phenotypes in the unc-51 and unc-14 mutants (Fig. 5B,C) result from defects in the SLT-1 pathway. As let-92 partially suppressed the phenotypes of unc-51(ks38::Tc1), LET-92 may negatively regulate the function of UNC-51 in the SLT-1 pathway (Fig. 7A) or positively regulate its function in the UNC-6 pathway (Fig. 7B).
Fig. 7.
Models of UNC-51, UNC-14 and LET-92 contributions to the UNC-6 or SLT-1 pathways in AVM axon guidance. UNC-51 and UNC-14 inhibited the UNC-6 pathway and activated the SLT-1 pathway. (A) LET-92 negatively regulates the UNC-51 function in the SLT-1 pathway. (B) LET-92 positively regulates the UNC-51 function in the UNC-6 pathway.
We also found that the unc-14(e57) mutation partially suppressed the AVM guidance phenotype caused by unc-51(e369), a severe mutant allele of unc-51 (Fig. 6), suggesting that UNC-14 can negatively regulate the UNC-6 pathway without UNC-51.
LET-92 can function cell-non-autonomously in AVM axon guidance
As UNC-51 functions cell-autonomously in neuronal axon outgrowth (Lai and Garriga, 2004), we next examined the cell autonomy of let-92 effects on AVM axon guidance. We found that the expression of let-92 in the AVM neurons did not rescue the phenotype (Fig. 8), suggesting that LET-92 can function cell-non-autonomously in the axon guidance. Recently, we found that unc-51 regulated localization of UNC-6 in ventral neurons (VA, VB and AVG) (T. Asakura, unpublished data). Therefore, we examined the let-92 cell autonomy in the ventral neurons. We found that expression of let-92 in the ventral neurons (VA, VB and AVG) did not rescue the phenotype (Fig. 8). We examined the cell autonomy in body wall muscles in which SLT-1 and UNC-6 are expressed (Hao et al., 2001; Asakura et al., 2007). We found that the expression of let-92 in the body wall muscles did not rescue the phenotype (Fig. 8). Finally, we found that pan-neuronal expression of let-92 rescued the phenotype (Fig. 8). These results suggest that unc-51 and let-92 can cell-non-autonomously work in some neurons in the AVM axon guidance.
Fig. 8.
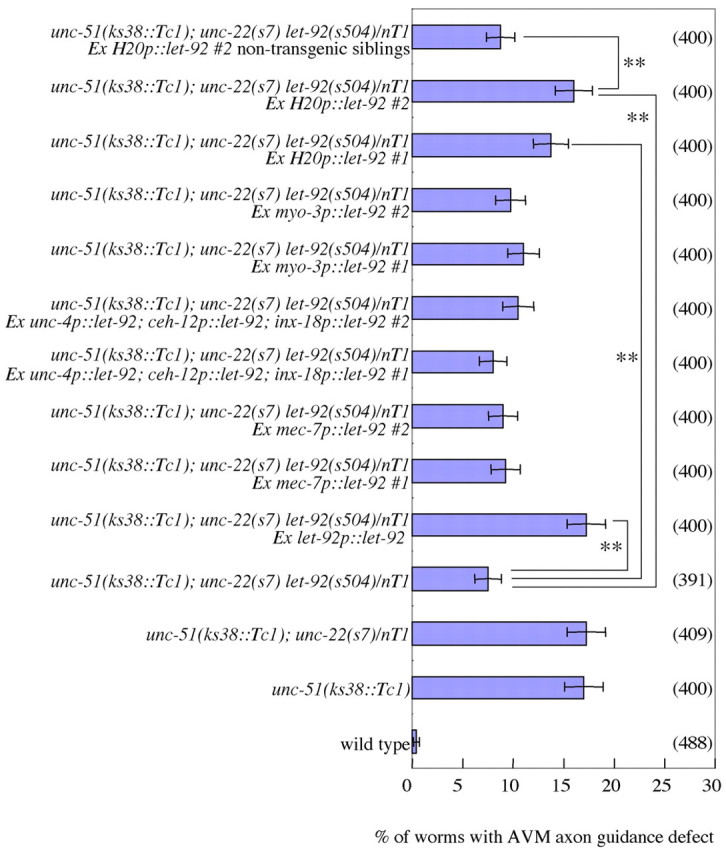
let-92 can act cell-non-autonomously in AVM axon guidance. The percentage of animals exhibiting defects in AVM axon guidance is shown. The AVM neuron was labeled with zdIs5 (mec-4::GFP) (Clark and Chiu, 2003). Parentheses show the number of worms counted. ‘Ex’ represents an extra-chromosomal array that includes a transgenic gene. #1 and #2 represent different lines. let-92p::let-92 represents a let-92 genomic gene. The mec-7 promoter drives in-touch receptor neurons including AVM. Near the AVM neuron, unc-6 is expressed in ventral neurons VA, VB and AVG and in body wall muscles. The unc-4 promoter drives in VA and DA neurons. The ceh-12 promoter drives in the VB neuron. The inx-18 promoter drives in the AVG neuron. Therefore, a mixture of unc-4, ceh-12 and inx-18 promoter constructs drives in the ventral neurons in which unc-6 is expressed. The myo-3 promoter drives in body wall muscles. The H20 promoter drives in pan-neurons. The let-92 genomic and H20 promoter::let-92 fusion genes rescued the phenotype suppressed by let-92(s504/+); however, others did not. Non-transgenic siblings of Ex H20p::let-92 were not rescued. Error bars show the standard error. **, P<0.01, chi-square test.
PP2A dephosphorylates phosphoproteins phosphorylated by UNC-51
How do UNC-51 and LET-92 cooperate to regulate the axon guidance in neurons? We tested two hypotheses. First, as UNC-51 is a serine/threonine kinase and LET-92 is a serine/threonine protein phosphatase, we hypothesized that LET-92 dephosphorylates phosphoproteins that are phosphorylated by UNC-51 and that the balance in phosphorylation created by these enzymes is important for axon guidance. Second, we hypothesized that the activity of LET-92 is regulated by phosphorylation by UNC-51. Given that UNC-51 phosphorylates UNC-14, VAB-8 and UNC-51 itself in cultured cells and in vitro (Lai and Garriga, 2004), we first examined whether PP2A could dephosphorylate the phosphoproteins that were phosphorylated by UNC-51 in vitro. We found that the phosphoproteins UNC-14, VAB-8 and UNC-51, which were phosphorylated by UNC-51, were dephosphorylated by human PP2A-AC, which included the catalytic C and regulatory A subunits (Fig. 9A-D), suggesting that LET-92 regulates their activity by its dephosphorylation activity. By contrast, we did not detect phosphorylation of LET-92 or GFP by UNC-51 in vitro (Fig. 9E,F), suggesting that UNC-51 does not regulate LET-92 activity, and that UNC-51 does not randomly phosphorylate proteins. These results supported the hypothesis that LET-92 dephosphorylates phosphoproteins that are phosphorylated by UNC-51, and that the phosphorylation balance generated by these enzymes may be important for axon guidance.
Fig. 9.
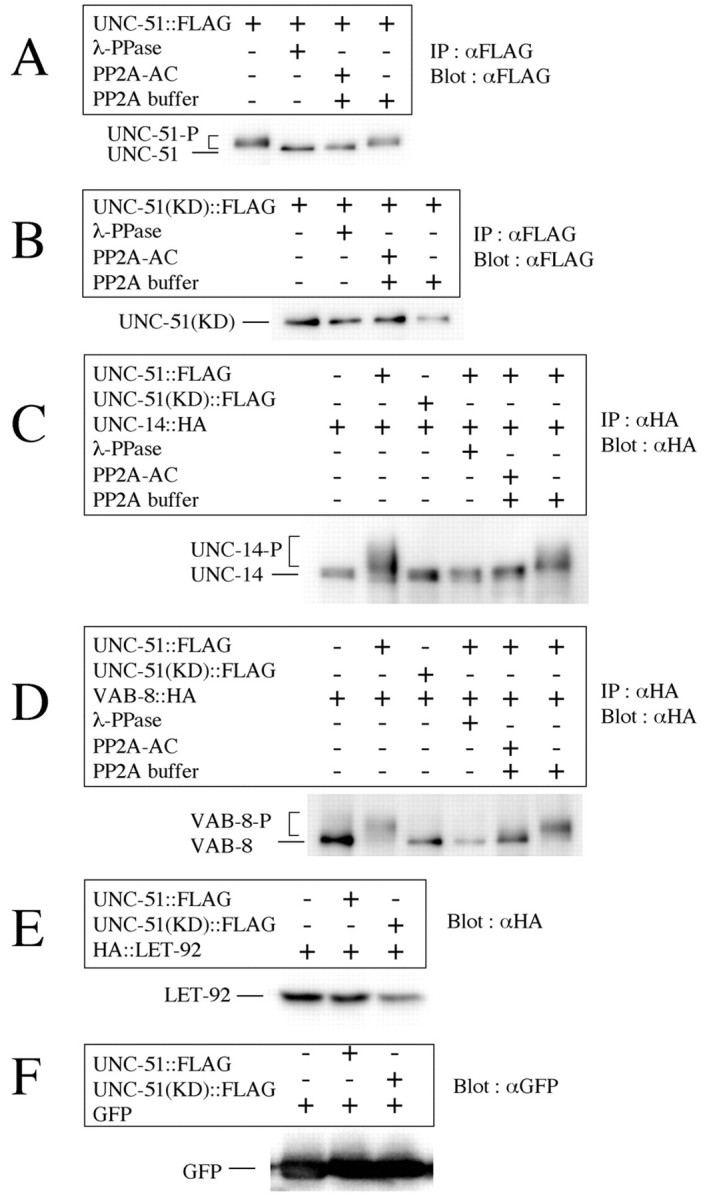
PP2A dephosphorylates phosphoproteins that have been phosphorylated by UNC-51. Proteins were expressed in HEK293T cells. λ-PPase was used as the non-specific dephosphorylation control. PP2A-AC contains catalytic and regulatory A subunits of human PP2A. (A) PP2A-AC dephosphorylated auto-phosphorylated UNC-51. λ-PPase shifted the UNC-51-P band lower, indicating that the higher bands (labeled UNC-51-P) represented phosphorylated UNC-51. PP2A-AC also shifted the UNC-51-P band lower, indicating that PP2A-AC also dephosphorylated phosphorylated UNC-51. The band shift was not observed in PP2A buffer only, indicating that the immunoprecipitation (IP) product did not contain this phosphatase activity. (B) A kinase-dead form of UNC-51, UNC-51(KD) was not phosphorylated in HEK293T cells, confirming that the native UNC-51-P shown in A was autophosphorylated. (C) PP2A-AC dephosphorylated phospho-UNC-14 that was phosphorylated by UNC-51. UNC-51 shifted the UNC-14 band up and λ-PPase shifted it down, indicating that the slower bands (UNC-14-P) represented phospho-UNC-14 phosphorylated by UNC-51 (Lai and Garriga, 2004). PP2A-AC also down-shifted the UNC-14-P, indicating that PP2A-AC also dephosphorylated the UNC-14-P that was phosphorylated by UNC-51. No band shift was observed in PP2A buffer only. (D) As with UNC-14, PP2A-AC dephosphorylated phospho-VAB-8 that was phosphorylated by UNC-51. (E) LET-92 was not phosphorylated by UNC-51 as assayed. However, we cannot rule out the possibility that UNC-51 phosphorylates LET-92, which is then immediately dephosphorylated by the auto-phosphatase activity of LET-92. (F) GFP was not phosphorylated by UNC-51, indicating that UNC-51 did not phosphorylate proteins indiscriminately.
LET-92 colocalizes with UNC-51 in the DD and VD neurons
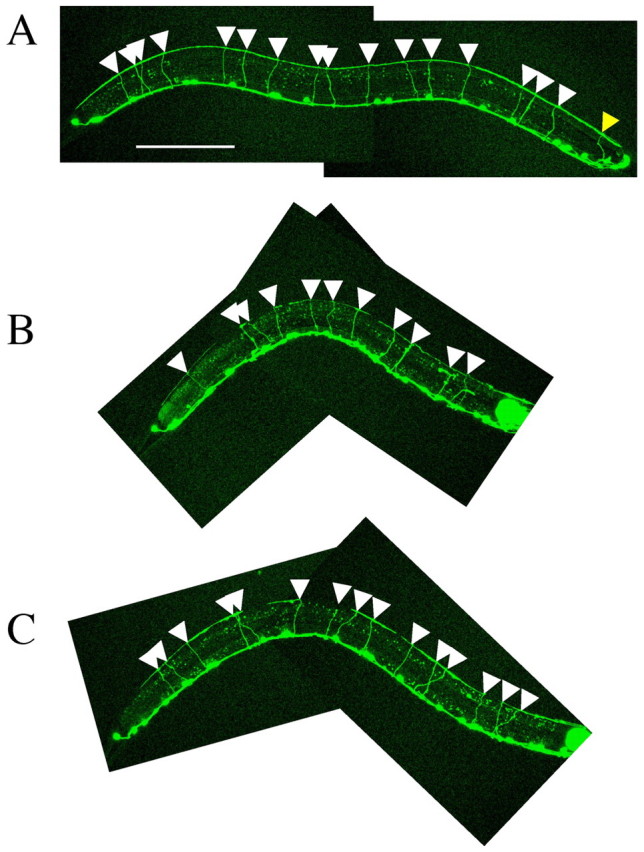
To examine their subcellular localizations, we expressed Venus::LET-92 and UNC-51::mCherry in DD and VD neurons. We found that Venus::LET-92 and UNC-51::mCherry were largely colocalized in the cell bodies and axons in the DD and VD neurons (Fig. 10A-C). Their punctate appearance in the axons may reflect their localization to small transport vesicles. In addition, we found that Venus::LET-92 localized to the margins of the VD growth cones (Fig. 10D-F), where receptors of axon guidance molecules should be located. As UNC-51 regulates the localization of UNC-5, a receptor of UNC-6 (Ogura and Goshima, 2006), LET-92 may cooperate with UNC-51 to regulate the localization of such receptors at the margins of the VD growth cones.
Fig. 10.
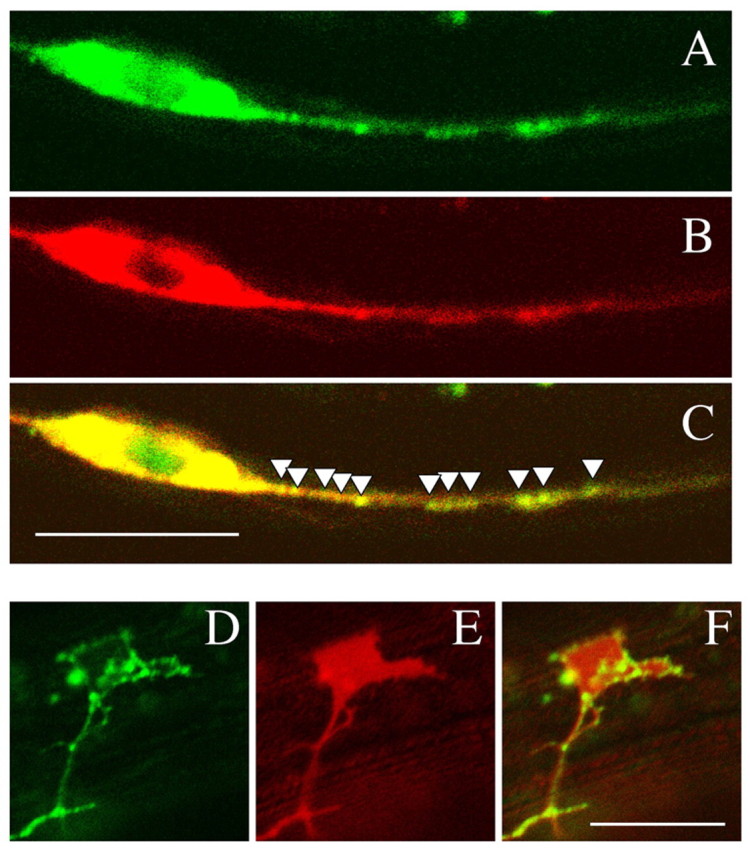
Localization of LET-92. (A-C) Colocalization of Venus::LET-92 and UNC-51::mCherry in DD and VD neurons. (A) Venus::LET-92. (B) UNC-51::mCherry. (C) Merged image of A and B. The white triangles indicate molecule colocalization in the axon. Venus::LET-92 was strongly colocalized with UNC-51::mCherry in the DD and VD neurons. (D-F) Localization of Venus::LET-92 in the VD growth cone. (D) Venus::LET-92. (E) SRC-2 myristoylation signal::mCherry. (F) Merged image of D and E. Venus::LET-92 was expressed along the margins of the VD growth cone. Anterior is to the right. Dorsal is to the top. Scale bars: 10 μm.
DISCUSSION
PP2A regulates the function of UNC-51, probably by forming a complex with it
Protein kinases and phosphatases play important roles in signal transduction pathways (Hunter, 2000). UNC-51 is a serine/threonine protein kinase and its kinase activity is essential for axon guidance (Ogura et al., 1994). PP2A is a serine/threonine protein phosphatase (Millward et al., 1999; Lechward et al., 2001; Sontag, 2001; Janssens et al., 2008). Thus, UNC-51 and PP2A have antagonistic enzymatic activities. We found that UNC-51 physically and genetically interacted with PP2A, and that PP2A dephosphorylated phosphoproteins that were phosphorylated by UNC-51, including UNC-51. In addition, we found that LET-92 colocalized with UNC-51 in axons, and that LET-92 could act in neurons.
PP2A interacts with several serine/threonine protein kinases and regulates their kinase activity by dephosphorylating them (Millward et al., 1999). For example, PP2A forms a stable complex with Ca2+-calmodulin-dependent protein kinase IV (CaMKIV) and negatively regulates its activity by dephosphorylation (Westphal et al., 1998). In this case, PP2A elicits a rapid downregulation of CaMKIV after its activation. Thus, the physical interaction between these proteins probably speeds up the enzymatic reaction of PP2A. By contrast, PP2A also forms a stable complex with the serine/threonine protein kinase Raf-1 and positively regulates its activity by dephosphorylation (Abraham et al., 2000). In this case, PP2A removes an inhibitory phosphate from Ser 259 of Raf-1. Thus, PP2A can positively regulate kinase activity as well. Therefore, the simplest hypothesis for the UNC-51 and LET-92 interaction is that the kinase activity of UNC-51 is regulated by the dephosphorylation activity of LET-92 in a complex that includes these two proteins (Fig. 11A). Similar to the case of CaMKIV, rapid dephosphorylation by PP2A may be important for downregulating UNC-51 function. As UNC-51 has autophosphorylation activity, a kinase that phosphorylates UNC-51 may be UNC-51 itself.
Fig. 11.
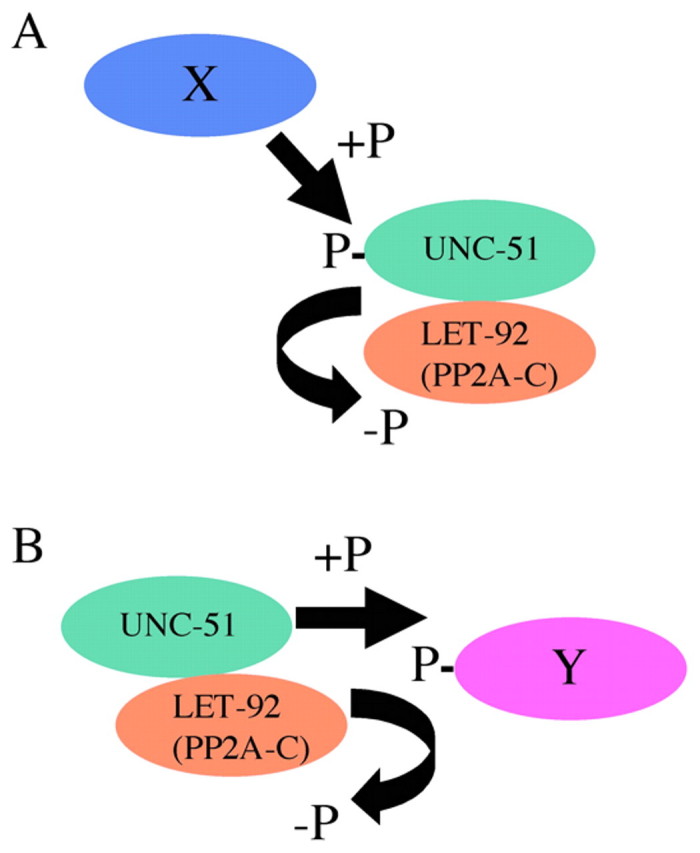
Models for the regulation of UNC-51 function by LET-92. (A) The activity of UNC-51 may be regulated by phosphorylation via the dephosphorylation activity of LET-92 in the complex. X is an unknown kinase that phosphorylates UNC-51. (B) The balance between the kinase activity of UNC-51 and the phosphatase activity of LET-92 in the complex may be important for the function of an unknown substrate (Y) required for axon guidance.
Alternatively, by forming a complex, UNC-51 and LET-92 may rapidly regulate the phosphorylation level (and thus the activity) of an unidentified in vivo substrate required for axon guidance (Fig. 11B). UNC-14 and VAB-8 (a kinesin-like molecule) are strong candidates for such a substrate, as UNC-14 and VAB-8 physically interact with UNC-51 and are phosphorylated by UNC-51 in vitro (Ogura et al., 1997; Lai and Garriga, 2004). In addition, we showed that mammalian PP2A-AC dephosphorylated phospho-UNC-14 and phospho-VAB-8 that had been phosphorylated by UNC-51. Although we did not detect a genetic interaction between unc-14 and let-92 in our study, we cannot rule out this possibility, as we used the null unc-14(e57) allele in the analysis. Thus, some level of UNC-14 functioning may be required for LET-92 to function in axon guidance. As there is no hypomorphic unc-14 mutant that affects axon guidance (Ogura et al., 1997), we cannot examine this possibility at present.
We showed that a low allelic dose of the PP2A regulatory subunits (PAA-1 and SUR-6) enhanced the unc-51 defects in DD and VD axon guidance, suggesting that PAA-1 and SUR-6 positively regulate the function of UNC-51 in DD and VD axon guidance. PP2A-A acts as a scaffolding protein, forming a core dimer with PP2A-C (Millward et al., 1999; Lechward et al., 2001; Sontag, 2001; Janssens et al., 2008). By binding to PP2A-A, PP2A-B is thought to regulate the activity or localization of the PP2A-AC core dimer. In DD and VD axon guidance, SUR-6 may inhibit the activity of the PP2A-AC core dimer or regulate its localization. PAA-1 may be required for SUR-6 function. Therefore, we think that SUR-6 and PAA-1 indirectly promote UNC-51 activity by negatively regulating LET-92.
Possible functions of PP2A and UNC-51 in DD and VD axon guidance
UNC-5 is a receptor for the axon guidance molecule UNC-6 (Leung-Hagesteijn et al., 1992) and is required for dorsally directed axon guidance in C. elegans; for example, that of the DD and VD axons (Hedgecock et al., 1990; McIntire et al., 1992). In this case, UNC-6 is expressed on ventral cells, where it acts as a repellent, and UNC-5 is expressed on the axons, where it mediates the transduction of positional information. Recently, we reported that UNC-51 and UNC-14 regulate the subcellular localization of UNC-5, probably by regulating its trafficking (Ogura and Goshima, 2006). We proposed that the subcellular localization of UNC-5 is correlated with its functional activity.
Our genetic results showed that, in DD and VD axon guidance, LET-92 negatively regulated the function of UNC-51 and that its regulatory subunits, PAA-1 and SUR-6, positively regulated UNC-51. As PP2A dephosphorylated the auto-phosphorylated UNC-51, a simple and attractive hypothesis is that PP2A regulates the effect of UNC-51 on the subcellular localization of UNC-5 by dephosphorylating UNC-51. In D. melanogaster, UNC-51 binds and phosphorylates UNC-76, which is a kinesin heavy chain adaptor protein necessary for kinesin-1-mediated axonal transport, to control the motor-cargo assembly for synaptic vesicle transport (Toda et al., 2008). The phosphorylation of UNC-76 by UNC-51 is important for the binding of UNC-76 to Synaptotagmin-1, a synaptic vesicle protein. In C. elegans, the binding partner of UNC-51, UNC-14, acts as an adaptor protein for kinesin-1-dependent synaptic vesicle transport (Sakamoto et al., 2005). As PP2A dephosphorylated phospho-UNC-14 that was phosphorylated by UNC-51, UNC-51 and PP2A may regulate UNC14 in its role as an adaptor protein for the unidentified motor protein that transports UNC-5. As Venus::LET-92 localized at the margins of the VD growth cones, LET-92 may cooperate with UNC-51 to regulate the UNC-5 localization at the margins of the VD growth cones.
Possible functions of PP2A and UNC-51 in AVM axon guidance
Ventrally expressed UNC-6 attracts the AVM axon ventrally, and the dorsally expressed SLT-1 repels it (Hao et al., 2001). The UNC-6 and SLT-1 pathways have partially redundant functions in AVM axon guidance. In the UNC-6 pathway, an UNC-6 receptor, UNC-40, receives the UNC-6 information, which is transduced via CED-10/Rac, UNC-115/abLIM) and UNC-34/Enabled (Gitai et al., 2003). CED-10 acts upstream of UNC-115, and UNC-34 functions in another pathway. The UNC-40–UNC-34 pathway is inhibited by CLR-1, a receptor protein phosphatase (RPTP) (Chang et al., 2004). MIG-10/lamellipodin and AGE-1/PI3K probably contribute to the neuronally asymmetric axon formation through UNC-40 and CED-10 (Chang et al., 2006; Quinn et al., 2006; Quinn et al., 2008).
In the SLT-1 pathway, SLT-1 binds to the co-receptors SAX-3 and EVA-1 (Zallen et al., 1998; Fujisawa et al., 2007). The information is then partly transmitted by UNC-34 (Yu et al., 2002). Conversely, RPM-1 and CLEC-38 negatively regulate the expression levels of the receptors SAX-3 and UNC-40, respectively (Li et al., 2008; Kulkarni et al., 2008).
Our genetic results suggest that UNC-51 and UNC-14 negatively regulate the UNC-6 pathway, but positively regulate the SLT-1 pathway. Similar to DD and VD axon guidance, in which UNC-51 and UNC-14 regulate the localization of UNC-5 (Ogura and Goshima, 2006), UNC-51 and UNC-14 may regulate the localization (or trafficking) of molecules important for the signal transduction pathways of UNC-6 and SLT-1. Recently, we found that UNC-51 and UNC-14 regulated localization (secretion) of UNC-6 in ventral neurons (T. Asakura, unpublished data), suggesting that UNC-51 and UNC-14 can cell-non-autonomously act on the AVM axon guidance. Our results also suggest that UNC-51 with LET-92 can cell-non-autonomously act on the AVM axon guidance in some neurons that are different from the UNC-6 secretory cells. UNC-51 and UNC-14 probably participate in multiple functions on the AVM axon guidance.
Our genetic results suggest that, in AVM axon guidance, UNC-14 can negatively regulate the UNC-6 pathway without UNC-51. This is different from the DD and VD neurons, in which UNC-14 cannot work without UNC-51 (Ogura and Goshima, 2006). Although UNC-51 and UNC-14 physically interact and regulate the axon guidance of many neurons in C. elegans, their molecular mechanisms for axon guidance may differ from neuron to neuron.
Because the low allelic dose of LET-92 partially suppressed the abnormal pathfinding of the AVM axon in the weak unc-51 mutant, we propose two possible functions for LET-92 in AVM axon guidance: (1) LET-92 negatively regulates the UNC-51 function for the SLT-1 pathway (Fig. 7A), and (2) LET-92 positively regulates the UNC-51 function for the UNC-6 pathway (Fig. 7B). These two models are not necessarily mutually exclusive. That is, LET-92 may participate in both pathways. We found that let-92 cell-non-autonomously worked in some neurons in the AVM axon guidance. In the neurons, unc-51 and let-92 may regulate secretion of an unknown factor that regulates activity of UNC-6, SLT-1 or their receptors. As we only analyzed the effects of the low allelic dose of LET-92, we cannot exclude cell-autonomous function of LET-92 in axon guidance.
Future work
In this paper, we report that PP2A cooperates with UNC-51 to regulate axon guidance in C. elegans. However, some of the molecular details of these axon guidance mechanisms are still unclear. Among the remaining questions are, how do PP2A and UNC-51 regulate axon guidance in DD and VD neurons? Does PP2A participate in localization of UNC-5, which is regulated by UNC-51? How do PP2A, UNC-51 and UNC-14 regulate UNC-6 and SLT-1 signaling in the AVM neuron? Our future research will seek the answers to these questions. Finally, analysis of the mammalian homologs of UNC-51 and PP2A will reveal whether PP2A has evolutionarily conserved roles in axon guidance.
Acknowledgements
We thank Robert Barstead for the two-hybrid cDNA library, Gian Garriga for the plasmid clones used in the in vitro experiments, Takeshi Ishihara for the Venus vector and the H20 promoter clone, Roger Y. Tsien for the mRFP clone, Jon Audhya for the mCherry clone, Andrew Fire for the C. elegans expression vectors, Meera Sundaram for sur-6(sv30), Erik M. Jorgensen for oxIs12, Taro Asakura for the helpful discussion and members of the Goshima laboratory for suggestions and helpful discussion. Some nematode strains used in this study were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. This work was supported by CREST (Core Research for Evolutional Science and Technology) of JST (Japan Science and Technology Agency), Grants-in-Aid for Scientific Research in a Priority Area from the Ministry of Education, Science, Sports and Culture to K.O. and Y.G.; D.L.B. holds a Canada Research Chair and is supported by research grants from NSERC and CIHR.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Abraham D., Podar K., Pacher M., Kubicek M., Welzel N., Hemmings B. A., Dilworth S. M., Mischak H., Kolch W., Baccarini M. (2000). Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275, 22300-22304 [DOI] [PubMed] [Google Scholar]
- Adler C. E., Fetter R. D., Bargmann C. I. (2006). UNC-6/Netrin includes neuronal asymmetry and defines the site of axon formation. Nat. Neurosci. 9, 511-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahantarig A., Chadwell L. V., Terrazas I. B., Garcia C. T., Nazarian J. J., Lee H. K., Lundell M. J., Cassill J. A. (2008). Molecular characterization of Pegarn: a Drosophila homolog of UNC-51 kinase. Mol. Biol. Rep. 36, 1311-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T., Ogura K., Goshima Y. (2007). UNC-6 expression by the vulval precursor cells of Caenorhabditis elegans is required for the complex axon guidance of the HSN neurons. Dev. Biol. 304, 800-810 [DOI] [PubMed] [Google Scholar]
- Benian G. M., Kiff J. E., Neckelmann N., Moerman D. G., Waterston R. H. (1989). Sequence of an unusually large protein implicated in regulation of myosin activity in C. elegans. Nature 342, 45-50 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülow H. E., Boulin T., Hobert O. (2004). Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron 42, 367-374 [DOI] [PubMed] [Google Scholar]
- Callebaut I., de Gunzburg J., Goud B., Mornon J. P. (2001). RUN domains: a new family of domains involved in Ras-like GTPase signaling. Trends Biochem. Sci. 26, 79-83 [DOI] [PubMed] [Google Scholar]
- Campbell R. E., Tour O., Palmer A. E., Steinbach P. A., Baird G. S., Zacharias D. A., Tsien R. Y. (2002). A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877-7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. S., Zheng H., Su M. W., Wilk R., Killeen M. T., Hedgecock E. M., Culotti J. G. (1996). UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87, 187-195 [DOI] [PubMed] [Google Scholar]
- Chang C., Yu T. W., Bargmann C. I., Tessier-Lavigne M. (2004). Inhibition of netrin-mediated axon attraction by a receptor protein tyrosine phosphatase. Science 305, 103-106 [DOI] [PubMed] [Google Scholar]
- Chang C., Adler C. E., Krause M., Clark S. G., Gertler F. B., Tessier-Lavigne M., Bargmann C. I. (2006). MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr. Biol. 16, 854-862 [DOI] [PubMed] [Google Scholar]
- Chilton J. K. (2006). Molecular mechanisms of axon guidance. Dev. Biol. 292, 13-24 [DOI] [PubMed] [Google Scholar]
- Clark S. G., Chiu C. (2003). C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development 130, 3781-3794 [DOI] [PubMed] [Google Scholar]
- Desai C., Garriga G., McIntire S. L., Horvitz H. R. (1988). A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature 336, 638-646 [DOI] [PubMed] [Google Scholar]
- Dickson B. J. (2002). Molecular mechanisms of axon guidance. Science 298, 1959-1964 [DOI] [PubMed] [Google Scholar]
- Fujisawa K., Wrana J. L., Culotti J. G. (2007). The slit receptor EVA-1 coactivates a SAX-3/Robo mediated guidance signal in C. elegans. Science 317, 1934-1938 [DOI] [PubMed] [Google Scholar]
- Gengyo-Ando K., Mitani S. (2000). Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 269, 64-69 [DOI] [PubMed] [Google Scholar]
- Gitai Z., Yu T. W., Lundquist E. A., Tessier-Lavigne M., Bargmann C. I. (2003). The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron 37, 53-65 [DOI] [PubMed] [Google Scholar]
- Hamelin M., Scott I. M., Way J. C., Culotti J. G. (1992). The mec-7 beta-tubulin gene of Caenorhabditis elegans is expressed primarily in the touch receptor neurons. EMBO J. 11, 2885-2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J. C., Yu T. W., Fujisawa K., Culotti J. G., Gengyo-Ando K., Mitani S., Moulder G., Barstead R., Tessier-Lavigne M., Bargmann C. I. (2001). C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron 32, 25-38 [DOI] [PubMed] [Google Scholar]
- Hara T., Takamura A., Kishi C., Iemura S., Natsume T., Guan J. L., Mizushima N. (2008). FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J. Cell Biol. 181, 497-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Thomson J. N., Perkins L. A. (1985). Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111, 158-170 [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H. (1990). The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4, 61-85 [DOI] [PubMed] [Google Scholar]
- Huang L. S., Tzou P., Sternberg P. W. (1994). The lin-15 locus encodes two negative regulators of Caenorhabditis elegans vulval development. Mol. Biol. Cell 5, 395-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. (2000). Signaling-2000 and beyond. Cell 100, 113-127 [DOI] [PubMed] [Google Scholar]
- Ishii N., Wadsworth W. G., Stern B. D., Culotti J. G., Hedgecock E. M. (1992). UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9, 873-881 [DOI] [PubMed] [Google Scholar]
- Ito A., Kataoka T. R., Watanabe M., Nishiyama K., Mazaki Y., Sabe H., Kitamura Y., Nojima H. (2000). A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J. 19, 562-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V., Longin S., Goris J. (2008). PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem. Sci. 33, 113-121 [DOI] [PubMed] [Google Scholar]
- Jin Y., Jorgensen E., Hartwieg E., Horvitz H. R. (1999). The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19, 539-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao G., Tuck S., Baillie D., Sundaram M. V. (2004). C. elegans SUR-6/PR55 cooperates with LET-92/protein phosphatase 2A and promotes Raf activity independently of inhibitory Akt phosphorylation sites. Development 131, 755-765 [DOI] [PubMed] [Google Scholar]
- Khew-Goodall Y., Mayer R. E., Maurer F., Stone S. R., Hemmings B. A. (1991). Structure and transcriptional regulation of protein phosphatase 2A catalytic subunit genes. Biochemistry 30, 89-97 [DOI] [PubMed] [Google Scholar]
- Killeen M. T., Sybingco S. S. (2008). Netrin, Slit and Wnt receptors allow axons to choose the axis of migration. Dev. Biol. 323, 143-151 [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. (1990). Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell 63, 405-415 [DOI] [PubMed] [Google Scholar]
- Kramer J. M., French R. P., Park E. C., Johnson J. J. (1990). The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol. Cell. Biol. 10, 2081-2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni G., Li H., Wadsworth W. G. (2008). CLEC-38, a transmembrane protein with C-type lectin-like domains, negatively regulates UNC-40-mediated axon outgrowth and promotes presynaptic development in Caenorhabditis elegans. J. Neurosci. 28, 4541-4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T., Garriga G. (2004). The conserved kinase UNC-51 acts with VAB-8 and UNC-14 to regulate axon outgrowth in C. elegans. Development 131, 5991-6000 [DOI] [PubMed] [Google Scholar]
- Lechward K., Awotunde O. S., Swiatek W., Muszynska G. (2001). Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim. Pol. 48, 921-933 [PubMed] [Google Scholar]
- Leung-Hagesteijn C., Spence A. M., Stern B. D., Zhou Y., Su M. W., Hedgecock E. M., Culotti J. G. (1992). UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell 71, 289-299 [DOI] [PubMed] [Google Scholar]
- Li H., Kulkarni G., Wadsworth W. G. (2008). RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity. J. Neurosci. 28, 3595-3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist E. A., Herman R. K., Shaw J. E., Bargmann C. I. (1998). UNC-115, a conserved protein with predicted LIM and actin-binding domains, mediates axon guidance in C. elegans. Neuron 21, 385-392 [DOI] [PubMed] [Google Scholar]
- Matsuura A., Tsukada M., Wada Y., Ohsumi Y. (1997). Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192, 245-250 [DOI] [PubMed] [Google Scholar]
- McIntire S. L., Garriga G., White J., Jacobson D., Horvitz H. R. (1992). Genes necessary for directed axonal elongation or fasciculation in C. elegans. Neuron 8, 307-322 [DOI] [PubMed] [Google Scholar]
- McIntire S. L., Reimer R. J., Schuske K., Edwards R. H., Jorgensen E. M. (1997). Identification and characterization of the vesicular GABA transporter. Nature 389, 870-876 [DOI] [PubMed] [Google Scholar]
- McNally K., Audhya A., Oegema K., McNally F. J. (2006). Katanin controls mitotic and meiotic spindle length. J. Cell Biol. 175, 881-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez A., Tallóczy Z., Seaman M., Eskelinen E. L., Hall D. H., Levine B. (2003). Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301, 1387-1391 [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. (1991). Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959-3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., 3rd, Niemeyer C. J. (1995). Expression of the unc-4 homeoprotein in Caenorhabditis elegans motor neurons specifies presynaptic input. Development 121, 2877-2886 [DOI] [PubMed] [Google Scholar]
- Millward T. A., Zolnierowicz S., Hemmings B. A. (1999). Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24, 186-191 [DOI] [PubMed] [Google Scholar]
- Mörck C., Axäng C., Pilon M. (2003). A genetic analysis of axon guidance in the C. elegans pharynx. Dev. Biol. 260, 158-175 [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. (2002). A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological application. Nat. Biotechnol. 20, 87-90 [DOI] [PubMed] [Google Scholar]
- Ogura K., Goshima Y. (2006). The autophagy-related kinase UNC-51 and its binding partner UNC-14 regulate the subcellular localization of the Netrin receptor UNC-5 in Caenorhabditis elegans. Development 133, 3441-3450 [DOI] [PubMed] [Google Scholar]
- Ogura K., Wicky C., Magnenat L., Tobler H., Mori I., Muller F., Ohshima Y. (1994). Caenorhabditis elegans unc-51 gene required for axonal elongation encodes a novel serine/threonine kinase. Genes Dev. 8, 2389-2400 [DOI] [PubMed] [Google Scholar]
- Ogura K., Shirakawa M., Barnes T. M, Hekimi S., Ohshima Y. (1997). The UNC-14 protein required for axonal elongation and guidance in Caenorhabditis elegans interacts with the serine/threonine kinase UNC-51. Genes Dev. 11, 1801-1811 [DOI] [PubMed] [Google Scholar]
- Ogura K., Kishimoto N., Mitani S., Gengyo-Ando K., Kohara Y. (2003). Translational control of maternal glp-1 mRNA by POS-1 and its interacting protein SPN-4 in Caenorhabditis elegans. Development 130, 2495-2503 [DOI] [PubMed] [Google Scholar]
- Okkema P. G., Harrison S. W., Plunger V., Aryana A., Fire A. (1993). Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135, 385-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgad S., Brewis N. D., Alphey L., Axton J. M., Dudai Y., Cohen P. T. (1990). The structure of protein phosphatase 2A is as highly conserved as that of protein phosphatase 1. FEBS Lett. 275, 44-48 [DOI] [PubMed] [Google Scholar]
- Quinn C. C., Pfeil D. S., Chen E., Stovall E. L., Harden M. V., Gavin M. K., Forrester W. C., Ryder E. F., Soto M. C., Wadsworth W. G. (2006). UNC-6/netrin and SLT-1/slit guidance cues orient axon outgrowth mediated by MIG-10/RIAM/lamellipodin. Curr. Biol. 16, 845-853 [DOI] [PubMed] [Google Scholar]
- Quinn C. C., Pfeil D. S., Wadsworth W. G. (2008). CED-10/Rac1 mediates axon guidance by regulating the asymmetric distribution of MIG-10/lamellipodin. Curr. Biol. 18, 808-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski T. M., Baillie D. L. (1985). Genetic organization of the unc-22 IV gene and the adjacent region in Caenorhabditis elegans. Mol. Gen. Genet. 201, 409-414 [DOI] [PubMed] [Google Scholar]
- Sakamoto R., Byrd D. T., Brown H. M., Hisamoto N., Matsumoto K., Jin Y. (2005). The Caenorhabditis elegans UNC-14 RUN domain protein binds to the kinesin-1 and UNC-16 complex and regulates synaptic vesicle localization. Mol. Biol. Cell 16, 483-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaitz A. L., Srayko M., Dammermann A., Quintin S., Wielsch N., MacLeod I., de Robillard Q., Zinke A., Yates J. R., 3rd, Müller-Reichert T., et al. (2007). The C. elegans RSA complex localizes protein phosphatase 2A to centrosomes and regulates mitotic spindle assembly. Cell 128, 115-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567-1572 [DOI] [PubMed] [Google Scholar]
- Shioi G., Shoji M., Nakamura M., Ishihara T., Katsura I., Fujisawa H., Takagi S. (2001). Mutations affecting nerve attachment of Caenorhabditis elegans. Genetics 157, 1611-1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S. S., Culotti J. G. (2007). Examination of neurons in wild type and mutants of Caenorhabditis elegans using antibodies to horseradish peroxidase. J. Neurogenet. 21, 271-289 [DOI] [PubMed] [Google Scholar]
- Sontag E. (2001). Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell. Signal. 13, 7-16 [DOI] [PubMed] [Google Scholar]
- Straub M., Bredschneider M., Thumm M. (1997). AUT3, a serine/threonine kinase gene, is essential for autophagocytosis in Saccharomyces cerevisiae. J. Bacteriol. 179, 3875-3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M., Goodman C. S. (1996). The molecular biology of axon guidance. Science 274, 1123-1133 [DOI] [PubMed] [Google Scholar]
- Toda H., Mochizuki H., Flores R., 3rd, Josowitz R., Krasieva T. B., Lamorte V. J., Suzuki E., Gindhart J. G., Furukubo-Tokunaga K., Tomoda T. (2008). UNC-51/ATG1 kinase regulates axonal transport by mediating motor-cargo assembly. Genes Dev. 22, 3292-3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda T., Bhatt R. S., Kuroyanagi H., Shirasawa T., Hatten M. E. (1999). A mouse serine/threonine kinase homologous to C. elegans UNC51 functions in parallel fiber formation of cerebellar granule neurons. Neuron 24, 833-846 [DOI] [PubMed] [Google Scholar]
- Tomoda T., Kim J. H., Zhan C., Hatten M. E. (2004). Role of Unc51.1 and its binding partners in CNS axon outgrowth. Genes Dev. 18, 541-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina S. E., Fox R. M., Watkins K. L., Starich T. A., Shaw J. E., Miller D. M., 3rd (2007). UNC-4 represses CEH-12/HB9 to specify synaptic inputs to VA motor neurons in C. elegans. Genes Dev. 21, 332-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth W. G. (2002). Moving around in a worm: netrin UNC-6 and circumferential axon guidance in C. elegans. Trends Neurosci. 25, 423-429 [DOI] [PubMed] [Google Scholar]
- Wadsworth W. G., Bhatt H., Hedgecock E. M. (1996). Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16, 35-46 [DOI] [PubMed] [Google Scholar]
- Walthall W. W., Chalfie M. (1988). Cell-cell interactions in the guidance of late-developing neurons in Caenorhabditis elegans. Science 239, 643-645 [DOI] [PubMed] [Google Scholar]
- Waterston R. H., Thomson J. N., Brenner S. (1980). Mutants with altered muscle structure of Caenorhabditis elegans. Dev. Biol. 77, 271-302 [DOI] [PubMed] [Google Scholar]
- Westphal R. S., Anderson K. A., Means A. R., Wadzinski B. E. (1998). A signaling complex of Ca2+-calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science 280, 1258-1261 [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S. (1986). The structure of the nervous system of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1-340 [DOI] [PubMed] [Google Scholar]
- Yan J., Kuroyanagi H., Kuroiwa A., Matsuda Y., Tokumitsu H., Tomoda T., Shirasawa T., Muramatsu M. (1998). Identification of mouse ULK1, a novel protein kinase structurally related to C. elegans UNC-51. Biochem. Biophys. Res. Commun. 246, 222-227 [DOI] [PubMed] [Google Scholar]
- Yu T. W., Bargmann C. I. (2001). Dynamic regulation of axon guidance. Nat. Neurosci. 4, 1169-1176 [DOI] [PubMed] [Google Scholar]
- Yu T. W., Hao J. C., Lim W., Tessier-Lavigne M., Bargmann C. I. (2002). Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat. Neurosci. 5, 1147-1154 [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Yi B. A., Bargmann C. I. (1998). The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell 92, 217-227 [DOI] [PubMed] [Google Scholar]
- Zhou X., Babu J. R., da Silva S., Shu Q., Graef I. A., Oliver T., Tomoda T., Tani T., Wooten M. W., Wang F. (2007). Unc-51-like kinase 1/2-mediated endocytic processes regulate filopodia extension and branching of sensory axons. Proc. Natl. Acad. Sci. USA 104, 5842-5847 [DOI] [PMC free article] [PubMed] [Google Scholar]