Abstract
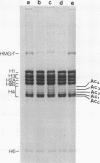
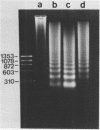
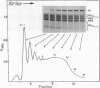
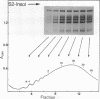
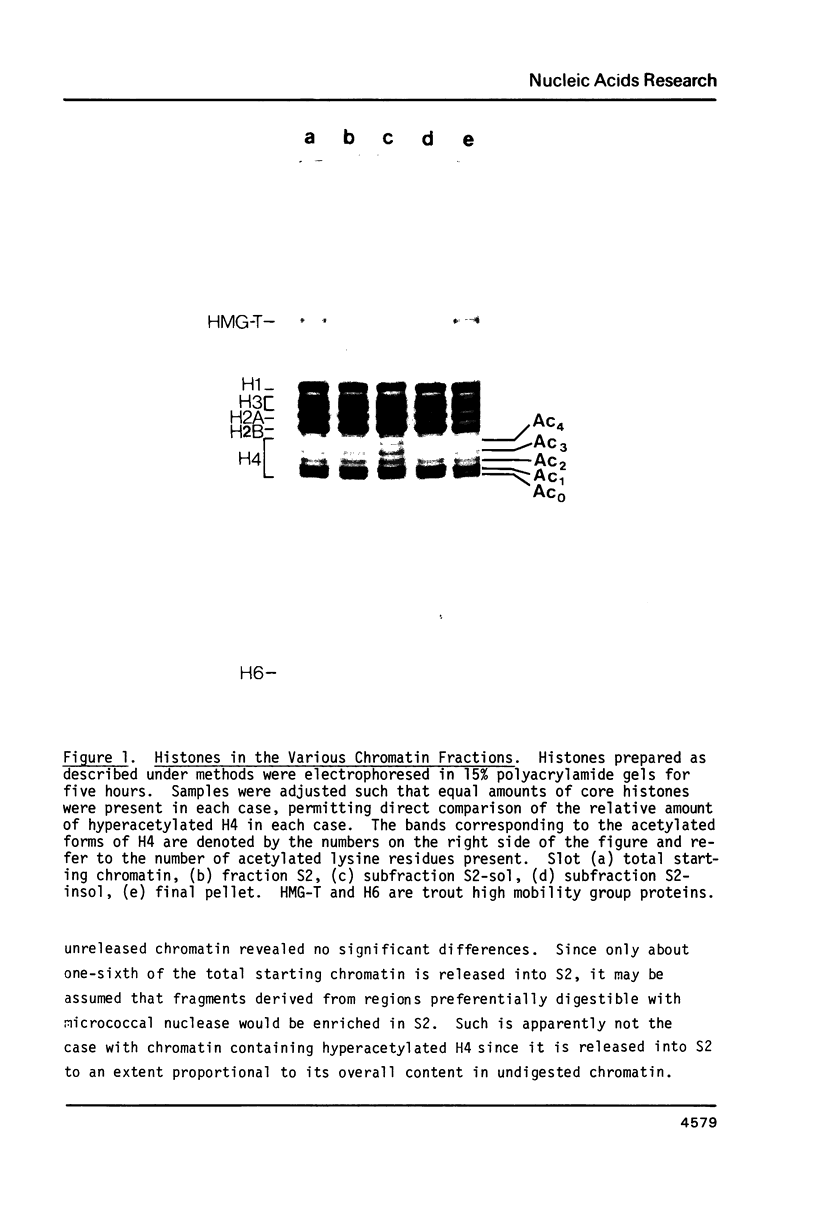
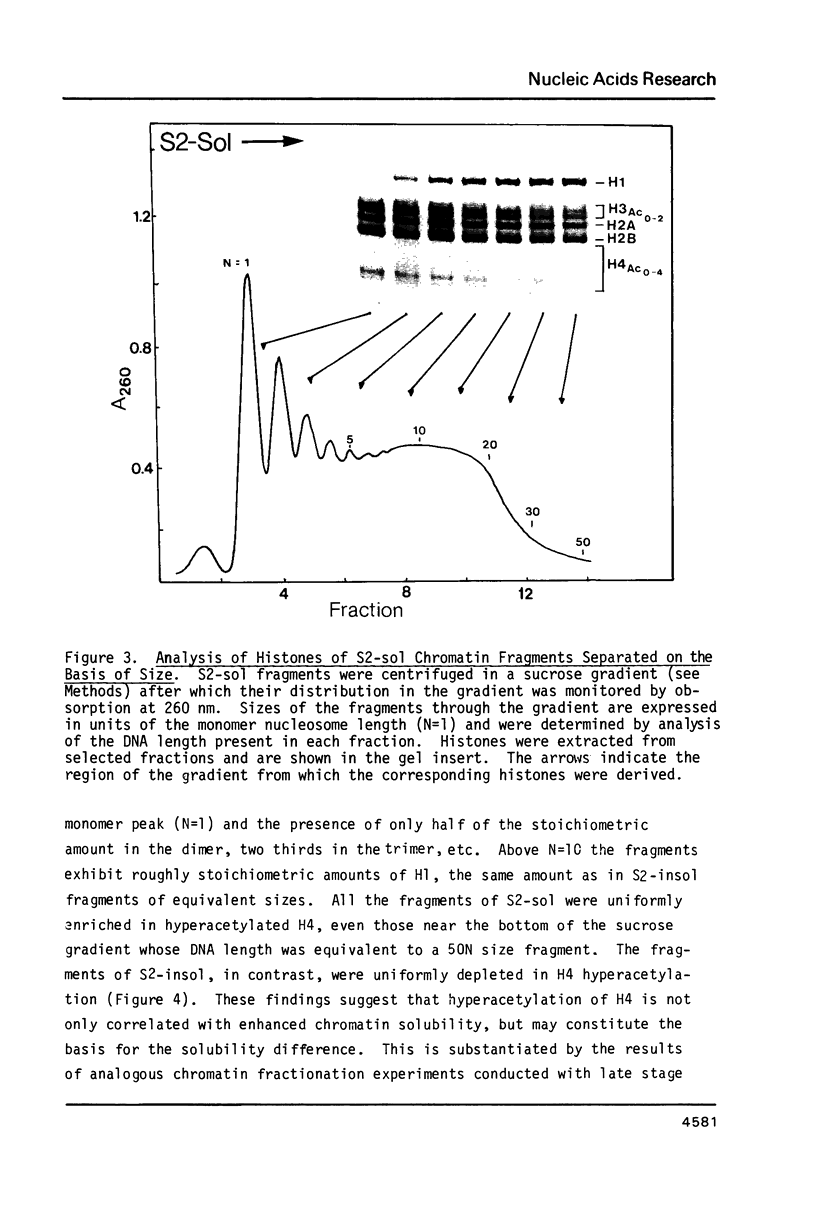
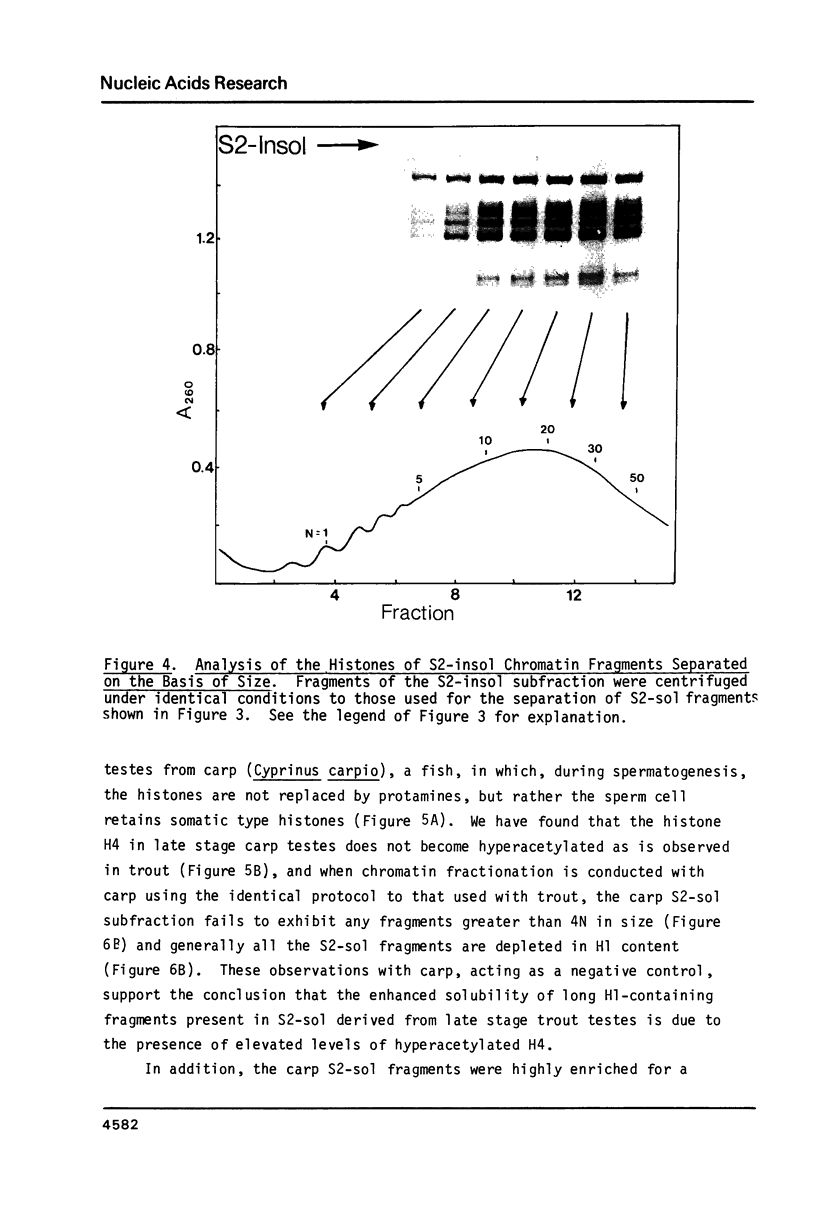
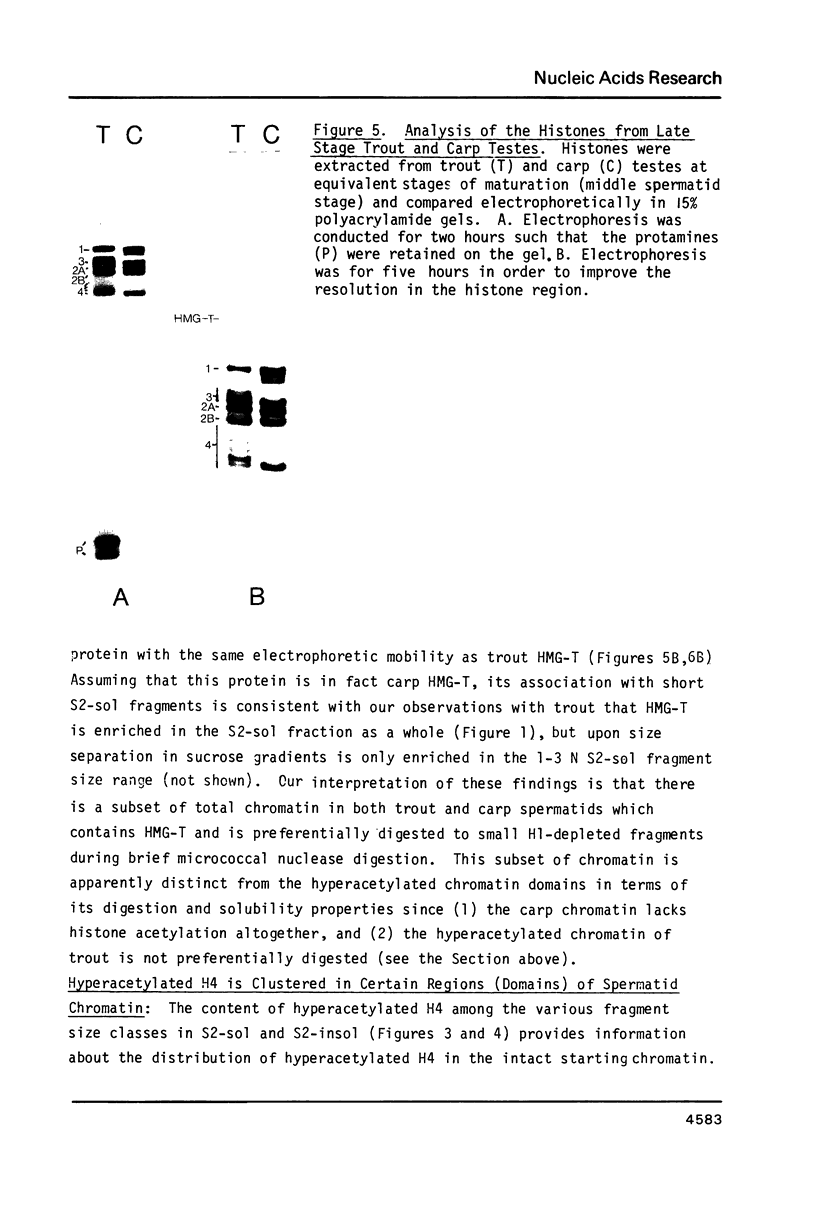
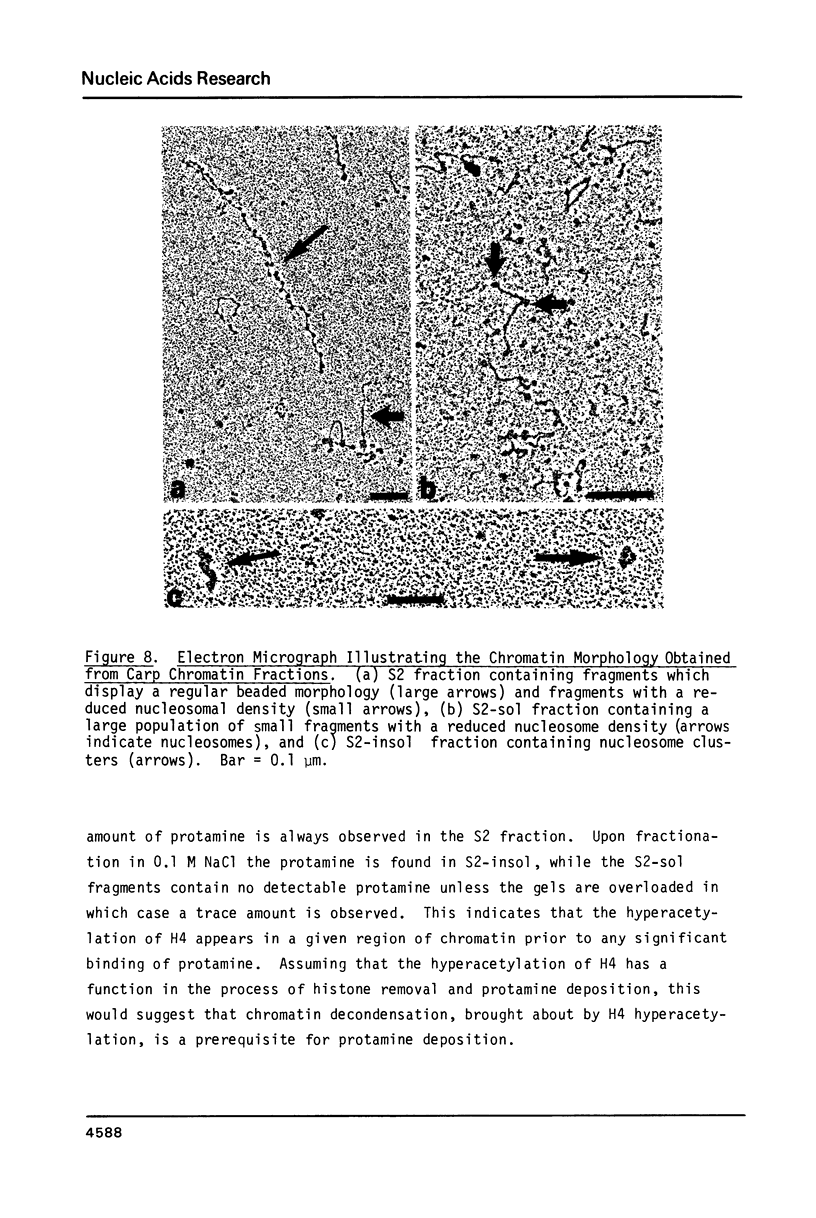
During the final stages of spermatogenesis in rainbow trout a dramatic increase in the level of histone H4 hyperacetylation is observed which is closely correlated with the replacement of histones by protamines. In order to understand further how H4 hyperacetylation might assist in protamine replacement of the histones, we have investigated the effect of H4 hyperacetylation on chromatin structure in trout testes actively undergoing the replacement process. Long chromatin fragments enriched in hyperacetylated H4 have been isolated and characterized. Evidence is presented that hyperacetylated H4 is clustered in certain regions (domains) of late stage testis chromatin and within these domains the chromatin exhibits an altered, highly relaxed structure which is believed to be the result of the extensive hyperacetylation. These domains, which are nearly devoid of protamine, are postulated to represent an initial structural transition which is necessary for the proper histone removal and protamine replacement process to take place.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annunziato A. T., Seale R. L. Histone deacetylation is required for the maturation of newly replicated chromatin. J Biol Chem. 1983 Oct 25;258(20):12675–12684. [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Bode J., Gómez-Lira M. M., Schröter H. Nucleosomal particles open as the histone core becomes hyperacetylated. Eur J Biochem. 1983 Feb 15;130(3):437–445. doi: 10.1111/j.1432-1033.1983.tb07170.x. [DOI] [PubMed] [Google Scholar]
- Bode J., Henco K., Wingender E. Modulation of the nucleosome structure by histone acetylation. Eur J Biochem. 1980 Sep;110(1):143–152. doi: 10.1111/j.1432-1033.1980.tb04849.x. [DOI] [PubMed] [Google Scholar]
- Bode J., Willmitzer L., Opatz K. On the competition between protamines and histones: studies directed towards the understanding of spermiogenesis. Eur J Biochem. 1977 Jan;72(2):393–403. doi: 10.1111/j.1432-1033.1977.tb11264.x. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Dixon G. H. Sites of in vivo acetylation in trout testis histone IV. J Biol Chem. 1971 May 25;246(10):3182–3188. [PubMed] [Google Scholar]
- Candido E. P., Dixon G. H. Trout testis cells. 3. Acetylation of histones in different cell types from developing trout testis. J Biol Chem. 1972 Sep 10;247(17):5506–5510. [PubMed] [Google Scholar]
- Christensen M. E., Dixon G. H. Comparison of the high mobility group proteins and their chromatin distribution in trout testis and liver. J Biol Chem. 1981 Jul 25;256(14):7549–7556. [PubMed] [Google Scholar]
- Christensen M. E., Dixon G. H. Hyperacetylation of histone H4 correlates with the terminal, transcriptionally inactive stages of spermatogenesis in rainbow trout. Dev Biol. 1982 Oct;93(2):404–415. doi: 10.1016/0012-1606(82)90127-0. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Saunders C. A. Chemical composition of nucleosomes among domains of calf thymus chromatin differing in micrococcal nuclease accessibility and solubility properties. J Biol Chem. 1981 Dec 10;256(23):12574–12580. [PubMed] [Google Scholar]
- Gillam S., Aline R., Jr, Wylie V., Ingles C. J., Smith M. RNA synthesis and RNA polymerase activities in germ cells of developing rainbow trout testis. Biochim Biophys Acta. 1979 Dec 17;565(2):275–292. doi: 10.1016/0005-2787(79)90205-3. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Chae C-B, Irvin J. L. Acetylation of histones of rat testis. Arch Biochem Biophys. 1975 Jun;168(2):425–435. doi: 10.1016/0003-9861(75)90271-4. [DOI] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. Properties of chromatin subunits from developing trout testis. J Biol Chem. 1975 Jun 25;250(12):4643–4647. [PubMed] [Google Scholar]
- Kuehl L. Synthesis of high mobility group proteins in regenerating rat liver. J Biol Chem. 1979 Aug 10;254(15):7276–7281. [PubMed] [Google Scholar]
- Levy-Wilson B., Watson D. C., Dixon G. H. Multiacetylated forms of H4 are found in a putative transcriptionally competent chromatin fraction from trout testis. Nucleic Acids Res. 1979 Jan;6(1):259–274. doi: 10.1093/nar/6.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie A. J., Dixon G. H. Kinetics of enzymatic modification of the protamines and a proposal for their binding to chromatin. J Biol Chem. 1972 Dec 25;247(24):7962–7968. [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Developmental changes in chromosomal composition and template activity during spermatogenesis in trout testis. Dev Biol. 1969 Apr;19(4):397–414. doi: 10.1016/0012-1606(69)90050-5. [DOI] [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Transformation of trout testis chromatin. J Biol Chem. 1971 Sep 25;246(18):5799–5805. [PubMed] [Google Scholar]
- Marushige Y., Marushige K. Transformation of sperm histone during formation and maturation of rat spermatozoa. J Biol Chem. 1975 Jan 10;250(1):39–45. [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Nelson D., Covault J., Chalkley R. Segregation of rapidly acetylated histones into a chromatin fraction released from intact nuclei by the action of micrococcal nuclease. Nucleic Acids Res. 1980 Apr 25;8(8):1745–1763. doi: 10.1093/nar/8.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Wright E. B., Olins D. E. Chromatin nu bodies: isolation, subfractionation and physical characterization. Nucleic Acids Res. 1976 Dec;3(12):3271–3291. doi: 10.1093/nar/3.12.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Perry M., Chalkley R. Histone acetylation increases the solubility of chromatin and occurs sequentially over most of the chromatin. A novel model for the biological role of histone acetylation. J Biol Chem. 1982 Jul 10;257(13):7336–7347. [PubMed] [Google Scholar]
- Perry M., Chalkley R. The effect of histone hyperacetylation on the nuclease sensitivity and the solubility of chromatin. J Biol Chem. 1981 Apr 10;256(7):3313–3318. [PubMed] [Google Scholar]
- Sanders M. M. Fractionation of nucleosomes by salt elution from micrococcal nuclease-digested nuclei. J Cell Biol. 1978 Oct;79(1):97–109. doi: 10.1083/jcb.79.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. K., Marushige K. Modification of histone binding in calf thymus chromatin and in the chromatin-protamine complex by acetic anhydride. Biochemistry. 1976 May 18;15(10):2041–2046. doi: 10.1021/bi00655a003. [DOI] [PubMed] [Google Scholar]
- Wouters-Tyrou D., Martin-Ponthieu A., Sautiere P., Biserte G. Acetylation of histone H4 in chicken erythrocyte and cuttle-fish testis chromatin. FEBS Lett. 1981 Jun 15;128(2):195–200. doi: 10.1016/0014-5793(81)80079-8. [DOI] [PubMed] [Google Scholar]