Abstract
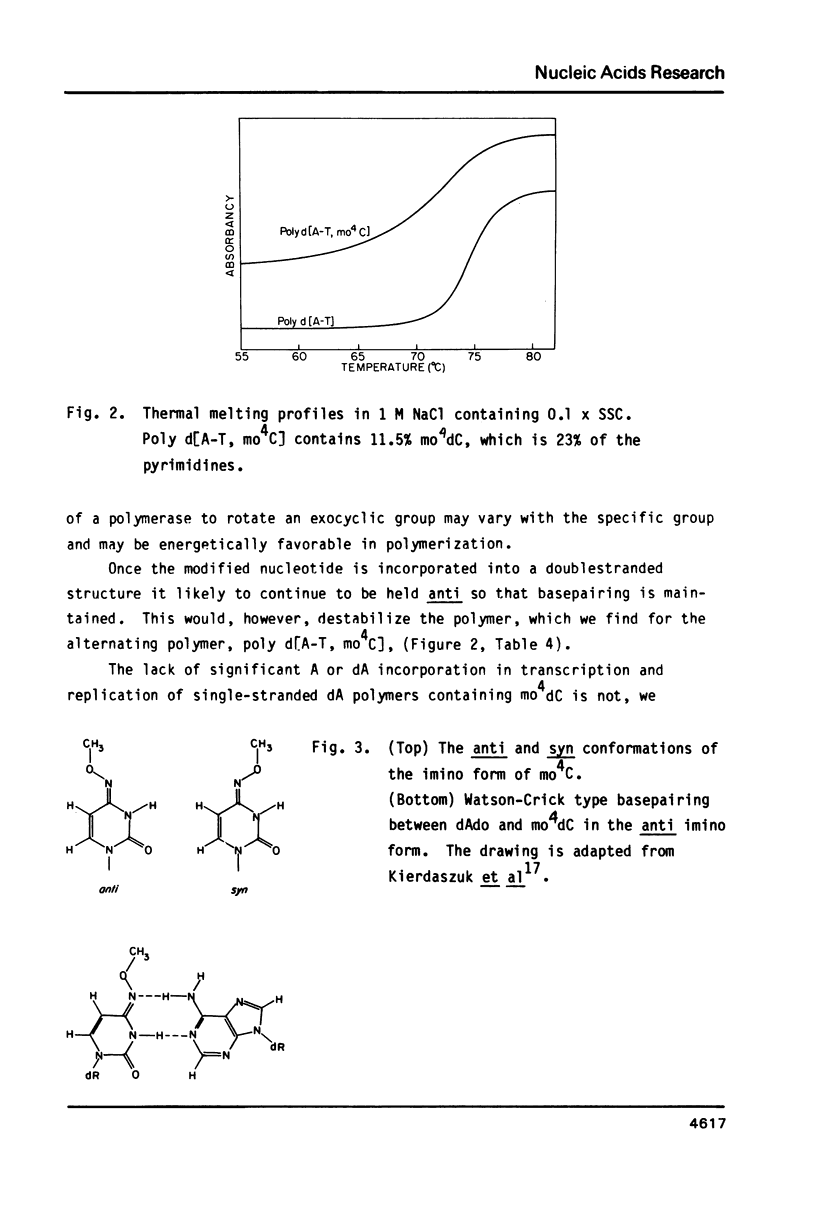
N4- Methoxydeoxycytidine triphosphate ( mo4dCTP ) substitutes for dTTP in poly d[A-T] synthesis with E. coli DNA polymerase I (Pol I). In parallel experiments using as template-primer, poly d[G-C], no incorporation of [14C] mo4dC was detected. This indicates that this deoxy derivative acts as the imino tautomer, as previously found for the riboderivative . Nearest neighbor analysis of transcripts of poly d[A-T] containing mo4dC shows that the derivative substitutes for only one base. In replication, singlestranded mo4dC -containing polymers gave little misincorporation, including that of dATP which can hydrogen-bond to mo4dC in the imino form, if the methoxy group is anti to the N-3. It is therefore assumed that the methoxy group is constrained anti in a polymer such as d[A-T], but can be in the syn form in singlestranded polymers and not recognized by DNA polymerase. mo4dC destabilizes the poly d[A-T] helix, as indicated by a lowered and less cooperative melting. Steric factors such as adjacent base displacement were invoked for similar findings with the doublestranded r( U61 , mo4C39 ) X r(A).
Full text
PDF

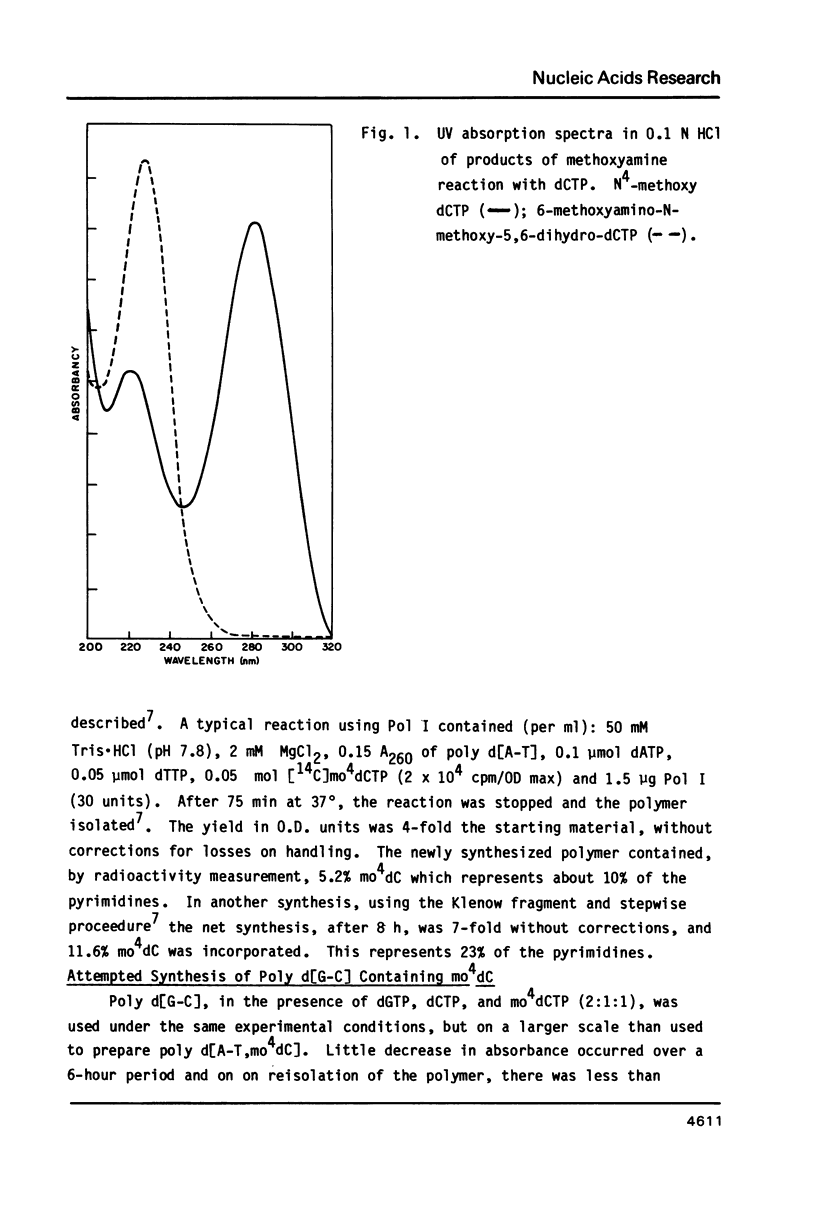


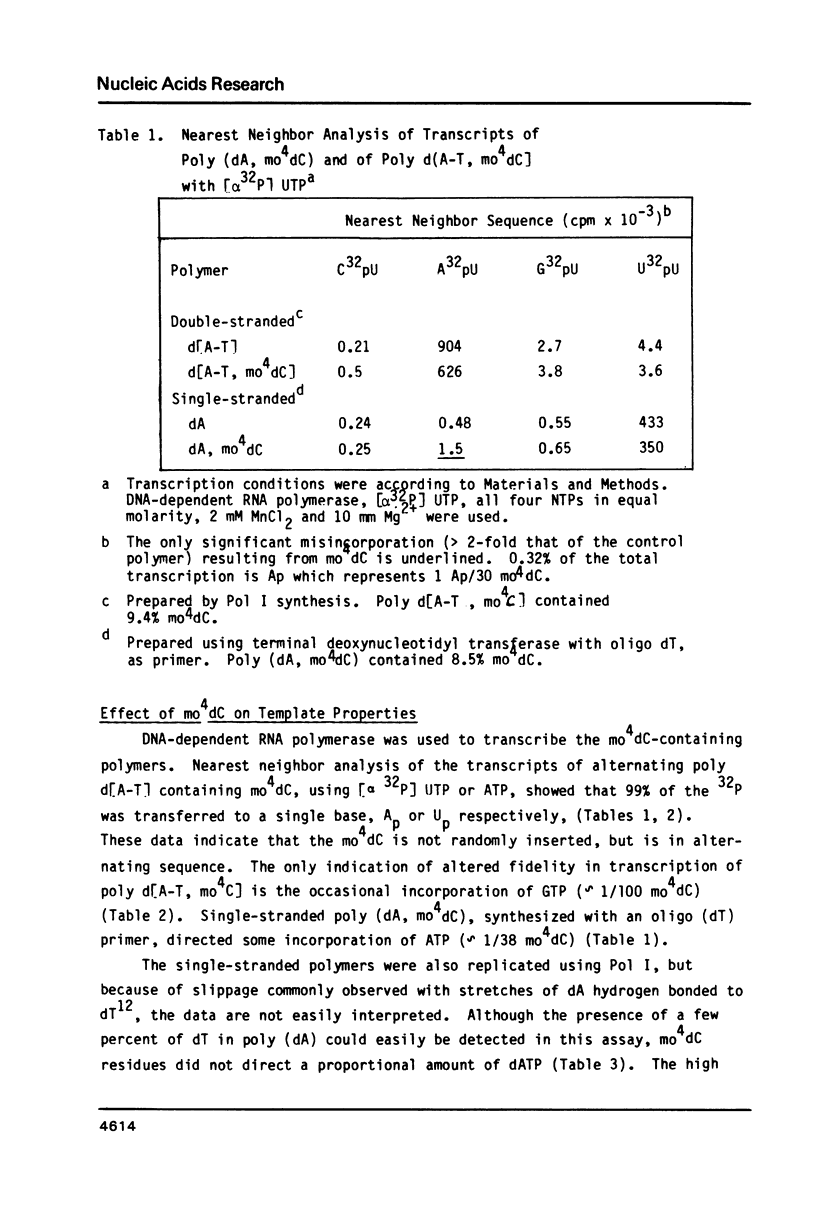
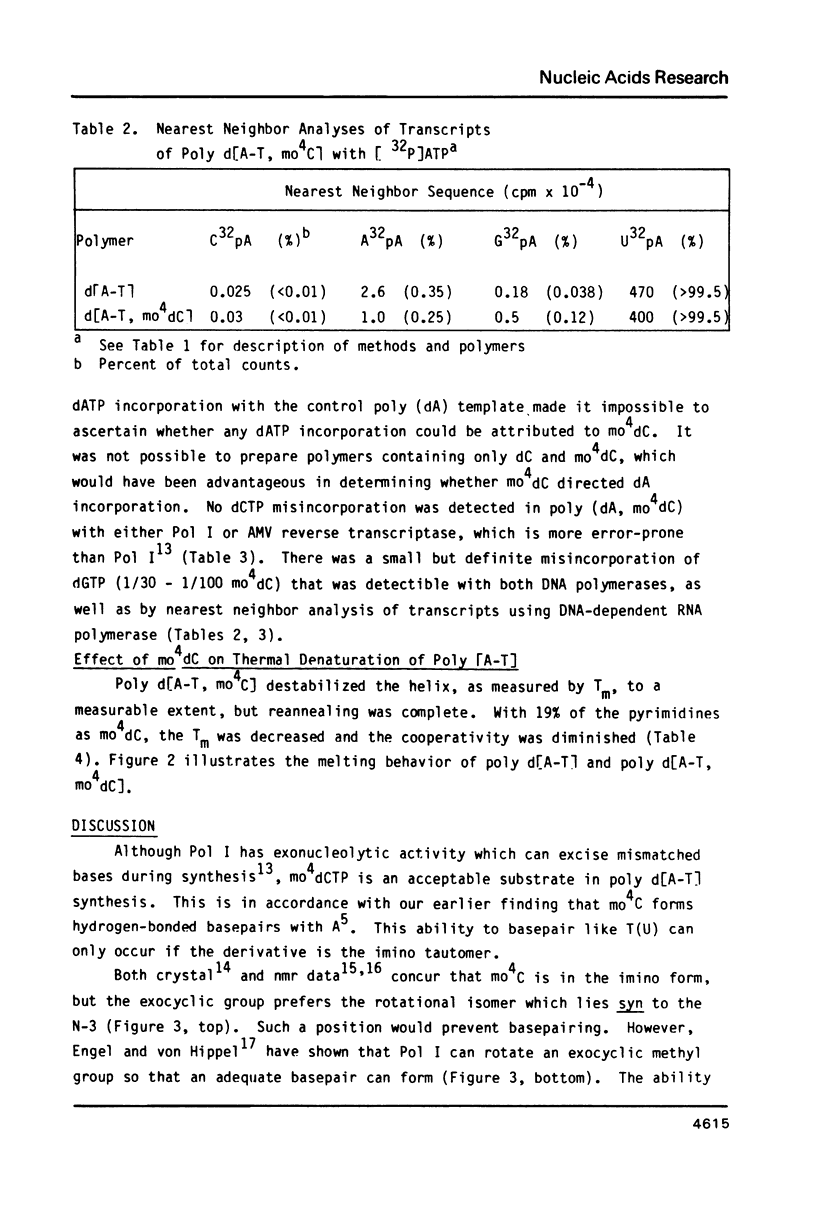
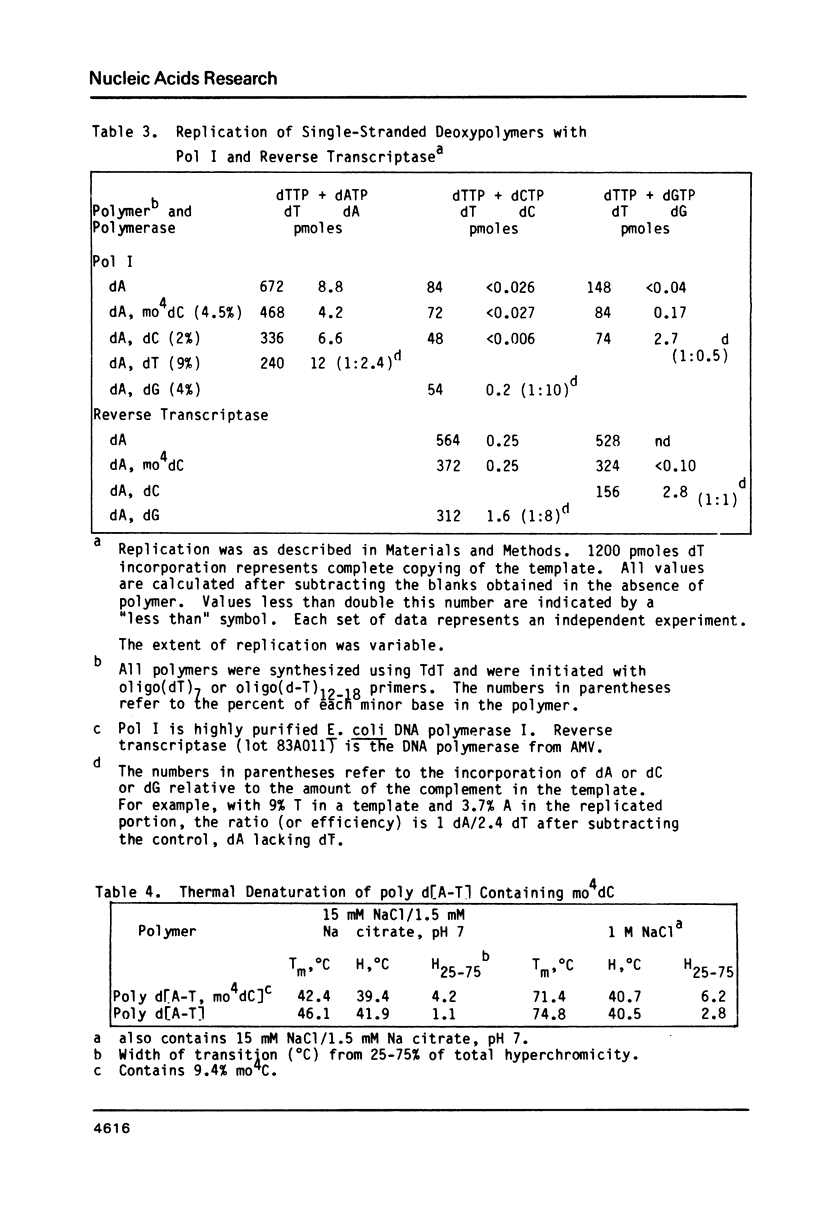


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J., Mehta J. R., Ludlum D. B. Synthesis of 8-14C-labeled O6-methyldeoxyguanosine and its deoxynucleotide copolymers. Biochemistry. 1980 Feb 19;19(4):643–647. doi: 10.1021/bi00545a006. [DOI] [PubMed] [Google Scholar]
- Agarwal S. S., Dube D. K., Loeb L. A. On the fidelity of DNA replication. Accuracy of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Jan 10;254(1):101–106. [PubMed] [Google Scholar]
- Birnbaum G. I., Kulikowski T., Shugar D. Conformation of exocyclic amino groups in purines and pyrimidines: crystal structure and conformation of 1-methyl-N4-hydroxycytosine hydrochloride. Can J Biochem. 1979 Apr;57(4):308–313. doi: 10.1139/o79-039. [DOI] [PubMed] [Google Scholar]
- Budowsky E. I., Sverdlov E. D., Shibaeva R. P., Monastyrskaya G. S., Kochetkov N. K. Mechanism of the mutagenic action of hydroxylamine. 3. Reaction of hydroxylamine and O-methylhydroxylamine with the cytosine nucleus. Biochim Biophys Acta. 1971 Aug 26;246(2):300–319. [PubMed] [Google Scholar]
- Budowsky E. I., Sverdlov E. D., Spasokukotskaya T. N. Mechanism of the mutagenic action of hydroxylamine. VII. Functional activity and specificity of cytidine triphosphate modified with hydroxylamine and O-methylhydroxylamine. Biochim Biophys Acta. 1972 Dec 6;287(2):195–210. doi: 10.1016/0005-2787(72)90370-x. [DOI] [PubMed] [Google Scholar]
- Budowsky E. I. The mechanism of the mutagenic action of hydroxylamines. Prog Nucleic Acid Res Mol Biol. 1976;16:125–188. doi: 10.1016/s0079-6603(08)60757-6. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Cassani G. R., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. VII. Replication of homopolymers. J Biol Chem. 1972 Dec 10;247(23):7718–7723. [PubMed] [Google Scholar]
- Engel J. D., von Hippel P. H. Effects of methylation on the stability of nucleic acid conformations. Studies at the polymer level. J Biol Chem. 1978 Feb 10;253(3):927–934. [PubMed] [Google Scholar]
- Flavell R. A., Sabo D. L., Bandle E. F., Weissmann C. Site-directed mutagenesis: generation of an extracistronic mutation in bacteriophage Q beta RNA. J Mol Biol. 1974 Oct 25;89(2):255–272. doi: 10.1016/0022-2836(74)90517-8. [DOI] [PubMed] [Google Scholar]
- Kierdaszuk B., Stolarski R., Shugar D. Hydroxylamine mutagenesis: observation of inverted Watson-Crick base-pairing between N4-methoxycytosine and adenine with the aid of natural-abundance high-resolution 15N NMR spectroscopy. Eur J Biochem. 1983 Feb 15;130(3):559–564. doi: 10.1111/j.1432-1033.1983.tb07186.x. [DOI] [PubMed] [Google Scholar]
- Kuśmierek J. T., Singer B. Chloroacetaldehyde-treated ribo- and deoxyribopolynucleotides. 2. Errors in transcription by different polymerases resulting from ethenocytosine and its hydrated intermediate. Biochemistry. 1982 Oct 26;21(22):5723–5728. doi: 10.1021/bi00265a051. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Ornstein R. L., Fresco J. R. Correlation of Tm and sequence of DNA duplexes with delta H computed by an improved empirical potential method. Biopolymers. 1983 Aug;22(8):1979–2000. doi: 10.1002/bip.360220811. [DOI] [PubMed] [Google Scholar]
- Sakurai M., Tazawa I., Inoue Y. Simple model to account for the deoxy-versus ribodimer stacking quotient data. Estimation of apparent and intrinsic equilibrium quotients for intramolecular stacking association of purine deoxy- and ribodinucleoside monophosphates. J Mol Biol. 1983 Feb 5;163(4):683–686. doi: 10.1016/0022-2836(83)90119-5. [DOI] [PubMed] [Google Scholar]
- Singer B., Kröger M. Isolation of polyribonucleotides using cellulose thin-layer plates. Anal Biochem. 1978 Oct 15;90(2):590–595. doi: 10.1016/0003-2697(78)90152-5. [DOI] [PubMed] [Google Scholar]
- Singer B., Spengler S. Ambiguity and transcriptional errors as a result of modification of exocyclic amino groups of cytidine, guanosine, and adenosine. Biochemistry. 1981 Mar 3;20(5):1127–1132. doi: 10.1021/bi00508a013. [DOI] [PubMed] [Google Scholar]
- Singer B., Sági J., Kuśmierek J. T. Escherichia coli polymerase I can use O2-methyldeoxythymidine or O4-methyldeoxythymidine in place of deoxythymidine in primed poly(dA-dT).poly(dA-dT) synthesis. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4884–4888. doi: 10.1073/pnas.80.16.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler S., Singer B. Effect of tautomeric shift on mutation: N4-methoxycytidine forms hydrogen bonds with adenosine in polymers. Biochemistry. 1981 Dec 8;20(25):7290–7294. doi: 10.1021/bi00528a037. [DOI] [PubMed] [Google Scholar]


