Abstract
The development of sound-evoked responses in Chinchilla lanigera was studied from postnatal ages P0-1 (first 24 h) to adult. Cochlear microphonic (CMs) and compound action potentials (CAPs), representing ensemble sound-evoked activities of hair cells and auditory nerve fibers, respectively, were present as early as age P0-1. The data indicate that CM thresholds and sensitivities were generally adult-like (i.e., fall into adult ranges) at birth, but suprathreshold CM amplitudes remained below adult ranges through P28. CAP thresholds reached adult-like values between P7–P14, but the suprathreshold CAP amplitude continued to increase until ∼P28. The results confirm the auditory precociousness of the chinchilla.
Introduction
A rapid way to study the auditory periphery has been to measure sound-evoked potentials—the cochlear microphonic (CM), compound action potential (CAP), and auditory brainstem response (ABR). Although not quantitatively predictive, these responses are qualitatively predictive of the development of neuron responses in the auditory system and general behavior (Rübsamen and Lippe, 1998). Most studies on auditory development have been done in altricial species born with immature auditory systems (Rübsamen and Lippe, 1998). In contrast, in precocious species such as humans, hearing begins in utero and substantial development occurs such that the periphery is highly functional at birth with nearly adult-like sensitivity and frequency resolution (Rübsamen and Lippe, 1998). To make better laboratory comparisons to humans, a precocious animal model may be more appropriate. Chinchilla lanigera is a precocious species that hears the same frequency and sound pressure range (i.e., the audiogram) as humans (Miller, 1970) and ABR thresholds and middle ear function have been reported to be virtually adult-like already at birth (Harrison et al., 1996; Hsu et al., 2000; Pienkowski and Harrison, 2005). Although evidence suggests that chinchillas have nearly mature hearing at birth, CM and CAP development has not yet been studied. This brief report examines the development of the auditory periphery of chinchilla via measurement of CMs and CAPs.
Materials and methods
Procedures complied with the University of Colorado Animal Care and Use Committees and the National Institute of Health. Detailed methods are described in prior publications (Lupo et al., 2011). Briefly, chinchillas, bred in our facility, comprised five age groups [post-natal day (P) P0-1 (within 24 h of birth), P7, P14, P28, and adult (>P70)]. In these acute experiments, animals were anesthetized with an intramuscular (IM) dose of ketamine hydrochloride (KetaVed, 30 mg∕kg IM) and xylazine hydrochloride (TranquiVed, 5 mg∕kg IM); supplementary injections were administered to maintain an adequate level of anesthesia. Core body temperature was monitored and maintained with a heating pad (model TC 100, CWE, Inc., Ardmore, PA) at 37 °C.
Teflon-insulated silver-wire electrodes were placed on the round window [Fig. 1a] and fixed into place, and the hole partially closed (to provide middle ear venting), by the application of dental acrylic. The differential electrode was placed on the posterior musculature of the neck, the ground on one paw. Signals were amplified (×1000, DAM ISO-50, WPI, Sarasota, FL), filtered (100–20 000 Hz) and acquired at 97 656.25 Hz [Tucker-Davis Technologies (TDT) RP2.1]. The sound delivery system comprised a TDT CF1 earphone coupled by a short length of tubing to an earpiece sealed into the auditory canal [Fig. 1a]. To calibrate the earphone for each animal in situ, a 50-mm probe tube connected to a microphone (Bruel & Kjaer, type 4182, Norcross, GA) was placed within 2 mm of the tympanic membrane [Fig. 1a]. The system was calibrated from 0.1–30 kHz via a 256-tap linear-phase Finite Impulse Response filter providing a virtually flat response <30 kHz. Stimuli were presented at 97 656.25 Hz at full 24-bit resolution (TDT RX6) with sound pressure levels (SPLs) set with a TDT PA5 programmable attenuator.
Figure 1.
(Color online) (a) Illustration of experimental setup. (b) Evoked potential in response to alternating–polarity, 4 kHz stimuli presented at 40 dB SPL. CM amplitude was determined from the mean FFT amplitude (inset) at the stimulus frequency. The CAP (solid black line) was extracted by averaging the CM responses. CAP peaks N1 and P1 are shown. [(a) is adapted from Fig. 1 of (Songer and Rosowski, 2006) with permission of the authors.]
CM and CAP potentials [Fig. 1b] were measured for nine frequencies (0.25, 0.5, 1, 2, 4, 8, 12, 16, and 20 kHz) at SPLs from ∼−30 to + 90 dB. Stimuli were 10-ms duration sinusoids (2.5-ms rise∕fall, 5-ms plateau) with a 20-ms period. Each frequency and SPL was presented 50 times with the phase of every odd stimuli alternating. CM amplitude was determined by the peak of the average FFT at the stimulus frequency [Fig. 1b, inset]. CM threshold was determined as the SPL at which the CM amplitude exceeded 2SD of the mean computed for the 5–6 lowest SPLs [Fig. 2a]. CAPs were recovered by averaging the CMs [Fig. 1b]. CAP amplitudes were measured as the peak-to-peak of N1 to P1 [Fig. 1b], latency was measured from stimulus onset to N1 [Fig. 3a]. CAP thresholds were determined as the SPL that produced N1 or P1 peaks that were larger than 2SD above the mean measured at dB levels well below threshold.
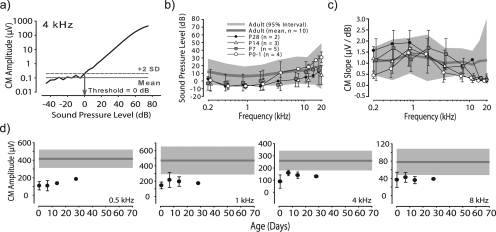
Figure 2.
(Color online) (a) CM amplitude vs SPL for a 4 kHz stimulus. The recording at 40 dB SPL for this dataset is shown in Fig. 1b. CM threshold was 0 dB SPL in this example. (b) Across-animal mean CM thresholds for five age groups, error bars indicate ±1 SD. Mean and 95% confidence interval for adults is shown by solid gray line and shaded area, respectively. (c) Across-animal mean CM slopes (μV∕dB SPL) for each age group; error bars plot ± 1 SD. Mean and 95% confidence interval for adults is shown as in (b). (d) Development of CM amplitude computed at 70 dB SPL as a function of age for four frequencies. Mean and 95% confidence interval for adults shown by solid gray and shaded area, respectively. Error bars indicate ±1 SD.
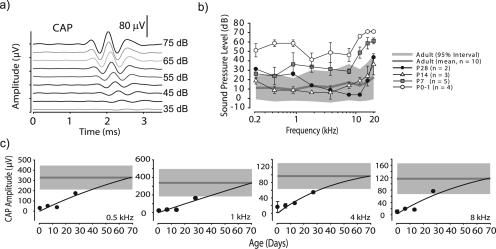
Figure 3.
(Color online) (a) Example of CAPs as a function of SPL for a 8 kHz stimuli. (b) Across-animal mean CAP thresholds for animals of five age groups, error bars indicate ± 1 SD. Mean and 95% confidence interval for adults shown as in Fig. 2b. (c) Development of CAP amplitude computed at 70 dB SPL vs age for four frequencies. Mean and 95% confidence intervals for adults are shown as in Fig. 2d. Error bars indicate ± 1 SD.
Results
Recordings were measured in chinchillas aged P0-1 (n = 4), P7 (n = 5), P14 (n = 3), P28 (n = 2), and adult (n = 10 animals). Ages represent and were chosen based on time periods of substantial growth in the physical dimensions of the head and pinnae (Jones et al., 2011) as well as from prior studies of ABRs and middle ear function (see Introduction). CM and CAP characteristics were considered “adult-like” once their values were not statistically different than adults.
Development of CM responses
Figure 2a shows CM amplitude vs SPL function for one adult animal in response to a 4 kHz stimulus. CM thresholds, amplitudes, and slopes (Δamplitude∕ΔSPL, linear part of CM-SPL functions) were computed. Across all ages and frequencies, CM amplitudes increased with SPL. Figure 2b shows across-animal mean CM thresholds for adults (gray line) along with the 95% confidence interval (shaded area). The CM threshold functions for all younger age groups, including P0-1, fell into the adult 95% confidence interval [Fig. 2b]. A non-parametric two-way analysis of variance (ANOVA) performed on the rank-order transformed data (see Akritas, 1990), with CM thresholds as the dependent variable and three frequency ranges (low: 0.25, 0.5, 1 kHz; medium: 2, 4, 8 kHz; high: 12, 16, 20 kHz) and five age groups as primary factors revealed significant main effects of age group [F(2,4) = 9.3, p < 0.0001] and frequency [F(2,4) = 27.2, p < 0.0001]; the interaction was not significant. A Sheffee post hoc analysis revealed the effect of age resulted from P28s having lower thresholds than the adults in the medium frequency range. The slopes of the CM-SPL functions for all ages generally fell into the adult 95% confidence interval [Fig. 2c]. The non-parametric two-way ANOVA on ranked data (as above) with CM slopes as the dependent variable and the three frequency ranges (as above) and five age groups as primary factors revealed significant effects of age group [F(2,4) = 2.9, p = 0.02] and frequency [F(2,4) = 24.3, p < 0.0001]; the interaction was also significant (p < 0.0001). Post hoc tests revealed that CM slopes were significantly shallower than adults for the low-frequency range at P0-1 and the high-frequency range for all ages less than P14. CM amplitudes, computed at 70 dB SPL, were plotted as a function of age [Fig. 2d] and were generally lower at P0-1 and for the lowest frequencies compared to adults. However, CM amplitudes at mid- to higher frequencies [e.g., Fig. 2d; 1, 4, and 8 kHz] fell near or within the adult ranges as early as P0-1. Overall the data indicate that CM threshold [Fig. 2b] and sensitivity [Fig. 2c] is adult-like in chinchilla at birth (P0-1), but the amplitude of the CM remains below adult ranges even at P28.
Development of CAP responses
CAPs were present at all ages, including P0-1, and all frequencies tested. However, CAP thresholds and amplitudes varied with age. Figure 3b shows across-animal mean CAP thresholds (gray line) for adults along with the 95% confidence interval (shaded area). CAP thresholds at P0-1 fell above adults for all frequencies. By P7, thresholds began to overlap the adult range for most frequencies < 8 kHz while those > 8 kHz remained elevated. CAP thresholds for P14 and P28 fell within the adult range. The non-parametric two-way ANOVA on ranked data with CAP thresholds as the dependent variable and the three frequency ranges (as above) and five age groups as factors revealed significant effects for age [F(2,4) = 55.0, p < 0.0001] and frequency [F(2,4) = 12.4, p < 0.0001]; the interaction was also significant (p < 0.0001). The Sheffee post hoc analysis revealed that CAP thresholds were significantly elevated for P0-1 for all frequencies (p < 0.0001) and P7 for the high frequency group (p < 0.0001) but thresholds were not significantly different (p > 0.05) than adults at P14 and P28. CAP amplitudes, computed at 70 dB SPL, were lower at P0-1 vs adults [Fig. 3c]. A two-parameter function, f = a*[1−exp(−b*x)], was fitted to CAP amplitude vs age data (r2 > 0.94, all frequencies). For all frequencies, amplitude increased until adult ranges were reached by ∼P28. A non-parametric two-way ANOVA on ranked data with CAP amplitudes as the dependent variable and the three frequency ranges and five age groups as factors revealed significant effects of age group [F(2,4) = 65.4, p < 0.0001] and frequency [F(2,4) = 32.6, p < 0.0001]; the interaction was not significant. A Sheffee post hoc analysis revealed that CAP amplitudes were significantly lower for all younger age groups (p < 0.0001) with the exception of P28. Finally, although CAP latencies decreased with SPL [Fig. 3a] and frequency and also generally decreased with age (not shown), latencies exhibited considerable variability within ages precluding statistical analyses. The data indicate that CAP thresholds [Fig. 3b] reach adult values in the interval from P7–P14, but the amplitudes and latencies of the CAP continue to increase and decrease, respectively, until ∼P28.
Discussion
Our results confirm the demonstrations of virtually adult-like central auditory [ABR thresholds: (Harrison et al., 1996; Hsu et al., 2000; Pienkowski and Harrison, 2005)] and middle ear function (Harrison et al., 1996; Hsu et al., 2000; Pienkowski and Harrison, 2005) at or very shortly after birth in chinchilla. Our results extend these prior outcomes by establishing that the auditory periphery, as assessed by the CM and CAP, is also quite functional already at birth.
Maturation of hair cell function—CM recordings
At P0-1, CMs exhibited adult-like sensitivities [Fig. 2c] and thresholds [Fig. 2b]. As far as potentials arising from hair cells are concerned, the cochlea has adult functionality. Middle ear function must also be relatively adult-like as well for this outcome to occur. However, there is some evidence for continual maturation of the CM as indicated by the amplitudes [Fig. 2d] not reaching adult ranges even by P28. The outer hair cells (OHCs), the main contributor of the CM potential (McGuirt et al., 1995), in the chinchilla are mature in appearance 24 h after birth (Harrison et al., 1996). Harrison et al. (1996), however, noted the presence of vestigial kinocilia on OHCs located at the middle and apical turns of the cochlea on the first postnatal day (P0-1) as an indicator of cochlear immaturity. ABR measurements by Harrison et al. (1996) revealed that thresholds for frequencies <2 kHz were significantly higher at P1 than adults, a result attributed to the immaturity of apical OHCs. Although we observed a development in some CM parameters, in conjunction with the hair cell morphological observations and ABR measurements (Harrison et al., 1996), we find that CM thresholds are adult-like by the first postnatal day (P0-1).
Maturation of auditory nerve function—CAP recordings
Although CAPs were evoked even at P0-1, thresholds were elevated re: adults [Fig. 3b]. Differences in development of CMs and CAPs indicate differences in maturation of middle-ear∕cochlear-mechanical function as well as hair cell transduction (i.e., CM) and the maturation of auditory nerve function, such as dendrite and axon distributions, myelination, and synchrony of spiking (i.e., CAP). For chinchillas P7 and older, CAP thresholds were generally adult-like for all frequencies, except >8 kHz at P7; however, thresholds were elevated for all frequencies at P0-1. While the former result is consistent with prior studies, the latter was unexpected given that ABR thresholds for some, but not all, frequencies are adult-like at P0-1 (Harrison et al., 1996; Hsu et al., 2000). One plausible explanation for the increased CAP thresholds at P0-1 could be the objective criteria we used (see Sec. 2); the methods by which ABR thresholds were determined in prior studies were not mentioned (Hsu et al., 2000; Pienkowski and Harrison, 2004) or thresholds were chosen subjectively (Harrison et al., 1996). Moreover, we calibrated the earphones in situ for each animal while this was not done in prior studies, a fact that may result in artifactually low ABR thresholds in the youngest animals (see specifically p. 924 of Hsu et al., 2000). Elevated CAP thresholds at P0-1, in conjunction with lower amplitudes for ages < P28 [Fig. 3c], likely results from immature across-neuron synchrony of spiking due to incomplete myelination of auditory nerve fibers (Rübsamen and Lippe, 1998). Overall, these data provide a baseline for studies of the development of auditory function in chinchilla models that may provide more appropriate laboratory comparisons to the human auditory system.
Acknowledgments
This work was supported by a grant from the National Organization of Hearing Research (D.J.T.) and the Basic Neuroscience Anatomy Training (BNAT) Grant No. NIH T32NS007083 (H.G.J.).
References and links
- Arkritas, M. G. (1990). “The rank transform method in some two-factor designs,” J. Am. Stat. Assoc. 85, 73–78. [Google Scholar]
- Harrison, R. V., Cullen, J. R., Takeno, S., and Mount, R. J. (1996). “The neonatal chinchilla cochlea: Morphological and functional study,” Scanning. Microsc. 10, 889–894. [PubMed] [Google Scholar]
- Hsu, G. S., Margolis, R. H., and Schachern, P. A. (2000). “Development of the middle ear in neonatal chinchillas. I. Birth to 14 days,” Acta Otolaryngol. 120, 922–932. [DOI] [PubMed] [Google Scholar]
- Jones, H. G., Koka, K., Thornton, J. L., and Tollin, D. J. (2011). “Concurrent development of the head and pinnae and the acoustical cues to sound location in a precocious species, the chinchilla (Chinchilla lanigera),” J. Assoc. Res. Otolaryngol. 12, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo, J. E., Koka, K., Thornton, J. L., and Tollin, D. J. (2011). “The effects of experimentally induced conductive hearing loss on spectral and temporal aspects of sound transmission through the ear,” Hear. Res. 272, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirt, J. P., Schmiedt, R. A., and Schulte, B. A. (1995). “Development of cochlear potentials in the neonatal gerbil,” Hear. Res. 84, 52–60. [DOI] [PubMed] [Google Scholar]
- Miller, J. D. (1970). “Audibility curve of the chinchilla,” J. Acoust. Soc. Am. 48, 513–523. [DOI] [PubMed] [Google Scholar]
- Pienkowski, M., and Harrison, R. V. (2005). “Tone frequency maps and receptive fields in the developing chinchilla auditory cortex,” J. Neurophysiol. 93, 454–466. [DOI] [PubMed] [Google Scholar]
- Rübsamen, R., and Lippe, W. (1998). “The development of cochlear function,” in Development of the Auditory System, Handbook of Auditory Research, edited by Rubel E. W., Popper A. N., and Fay R. R. (Springer-Verlag, New York: ), pp. 193–270. [Google Scholar]
- Songer, J. E., and Rosowski, J. J. (2006). “The effect of superior-canal opening on middle-ear input admittance and air-conducted stapes velocity in chinchilla,” J. Acoust. Soc. Am. 120, 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]