Abstract
Purpose: The purpose of this study was to investigate whether or not a stem effect removal technique is necessary when performing Ir-192 HDR brachytherapy in vivo dosimetry using a scintillation detector.
Methods: A red-green-blue photodiode connected to a multichannel electrometer was used to detect the light emitted from a plastic scintillation detector (PSD) during irradiation with an Ir-192 HDR brachytherapy source. Accuracy in dose measurement was compared with and without the use of stem effect removal techniques. Monochromatic and polychromatic filtration techniques were studied. An in-house template was built for accurate positioning of catheters in which the source and the PSD were inserted. Dose distribution was measured up to 5 cm from source to detector in the radial and longitudinal directions.
Results: The authors found the stem effect to be particularly important when the source was close to the optical fiber guide and far from the scintillation component of the detector. It can account for up to (72±3)% of the signal under clinically relevant conditions. The polychromatic filtration outperformed the monochromatic filtration as well as the absence of filtration in regard to dose measurement accuracy.
Conclusions: It is necessary to implement a stem effect removal technique when building a PSD for in vivo dosimetry during Ir-192 HDR brachytherapy. The PSD that the authors have developed for this study would be suitable for such an application.
Keywords: stem effect, Cerenkov, plastic scintillation detectors, in vivo dosimetry, HDR
INTRODUCTION
There is an increased interest in performing real-time in vivo dosimetry during Ir-192 high dose rate (HDR) brachytherapy.1, 2, 3, 4, 5, 6, 7, 8, 9 The small size, near water equivalence, energy independence (>100 keV), dose linearity, dose rate independence, fast response, and temperature independence10, 11, 12 make plastic scintillation detectors (PSDs) an attractive choice for this purpose. Advantages of PSDs were demonstrated extensively for megavoltage external beams10, 11, 12, 13 and it is well known for high energy beams that it is necessary to remove the stem effect light: The light induced by radiation in the optical fiber guiding the scintillation light toward the photodetector.14, 15, 16, 17, 18
For Ir-192 HDR brachytherapy, the need for a stem effect removal technique is not so obvious. Previous studies4, 9 revealed that it is not necessary to implement a stem effect removal technique when building PSDs for in vivo dosimetry during Ir-192 HDR brachytherapy. However, most of the energy spectrum of an Ir-192 source is above the threshold for Cerenkov. Cerenkov radiation is emitted in a material whenever a charged particle passes through a medium with a velocity higher than that of light in that medium. In the case of polymethyl methacrylate (PMMA), the material that comprises many optical fibers, the Cerenkov threshold corresponds to an energy of about 178 keV. The photon energy spectrum for Ir-192 is between 0.136 and 1.06 MeV with an average energy of 380 keV, leading to the expectation of a substantial production of Cerenkov radiation in the optical fiber light guide of the PSD. Andersen et al.19 reported results supporting the latter statement, showing situations where the stem effect account for up to 30% of the total signal when irradiating an Al2O3 scintillator coupled to a PMMA fiber with an Ir-192 source used for pulsed dose rate (PDR) brachytherapy.
The purpose of our study was to investigate whether or not a stem effect removal technique is necessary when performing Ir-192 HDR brachytherapy in vivo dosimetry using a PSD. The PSDs we developed allowed for the implementation of monochromatic and polychromatic stem effect removal techniques. Comparison between dose measurements obtained with these different techniques as well as without any stem effect removal technique was performed.
MATERIALS AND METHODS
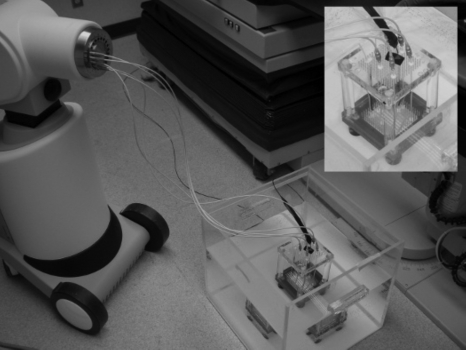
A Nucletron HDR Ir-192 brachytherapy system (Nucletron B.V., Veenendaal, The Netherlands) was used for all measurements conducted in this study. Irradiation was delivered using a microSelectron V2 Ir-192 afterloader, with a source of 0.90 mm in diameter and 4.95 mm in length connected to a stainless steel cable. Source was traveling inside plastic catheters inserted in an in-house developed positioning template placed inside a water tank (see Fig. 1). A PSD was also inserted inside a catheter reserved for dosimetry purposes. The detection volume of the PSD was composed of a 1 mm diameter×2 mm long BCF-60 green scintillating fiber (Saint-Gobain Crystals and Detectors, Paris, France). The scintillating fiber was coupled to a light tight 8 m long×1 mm diameter PMMA optical fiber (Eska Premier GH-4001, Mitsubishi Rayon Co., Ltd., Tokyo, Japan), which was then coupled to a photodetector outside the treatment room. The photodetector used in this study was a red-green-blue (RGB) photodiode (Mazet MCSiAT). To prevent electric perturbation from the environment as well as light leakage, the photodiode was enclosed in an aluminum box connected to the ground. Two of the three color outputs from the RGB photodiode were used throughout the experiments—the blue and the green—in order to allow for the implementation of both the monochromatic and the polychromatic stem effect removal techniques. The photodiode outputs were triax connected to a double input channel electrometer (SuperMax, Standard Imaging, Madison, WI) with a computer controlling the electrometer and collecting data from it via an RS-232 cable.
Figure 1.
Experimental setup and positioning template system (embedded). Catheters were connected to transfer tubes, which were connected to the afterloader. The PSD (with the black jacket on the picture) was connected to the RGB photodiode outside the treatment room.
Different stem effect removal approaches were compared in this study. Table 1 shows the dose equation proper to each technique. The approach without any filtration technique was mimicked by the summation of the blue and green outputs. The monochromatic filtration technique was first introduced by de Boer et al.16 and was used by Andersen et al. in their study.19 This technique confines the acquired light to a specific spectral band: The one in which the scintillation light is dominant (in this study, the green one), thus ensuring an increase in signal to stem effect ratio. The polychromatic technique was first introduced by Fontbonne et al.13 and is now extensively used for higher energy dosimetry.18, 20, 21, 22 It is based on the assumption that light emission spectra from scintillation and stem effect light are different. It then uses the light signals collected in two different spectral bands to obtain, following a proper calibration, the dose deposited at the PSD’s position.
Table 1.
Dose calculation for the different stem effect removal approaches. In this study, M1 corresponds to the charge recorded from the green output and M2 corresponds to the charge from the blue output.
| Method | Dose equations |
|---|---|
| No filtration | D=a(M1+M2) (1) |
| Monochromatic filtration | D=aM1 (2) |
| Polychromatic filtration | D=aM1+bM2 (3) |
In order to calibrate the PSD for the different techniques, measurements were performed in two known-dose irradiation conditions. The first two methods depicted in Table 1 require only one known-dose calibration point as there is only one calibration factor to determine. In contrast, the polychromatic approach requires at least two known-dose conditions to solve for the two unknown calibration factors in the equation. Using the Monte Carlo (MC) calculated data from Daskalov et al.23—computed for the same Ir-192 source model—for the dose at the point of measurement, calibration factors were obtained following the equations in Table 1.
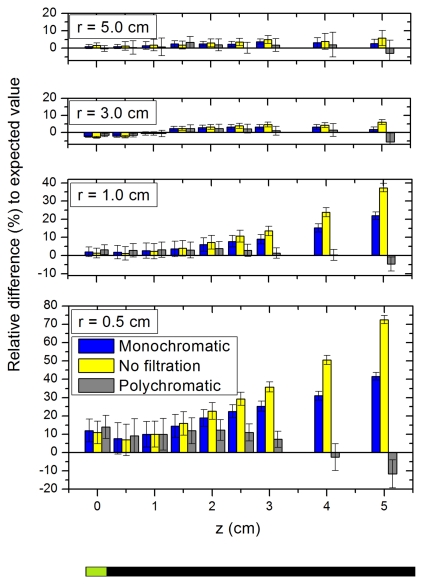
The efficiency of each technique to remove the stem effect and lead to accurate dosimetry was investigated. Definition of the z axis is presented at the bottom of Fig. 2, where the active component of the PSD is located at z=0 cm. r is simply the radial distance from the center of the cylindrical scintillating component. In order to decrease the source-to-scintillator positioning uncertainty in the z axis and ensure an accurate comparison of measured to expected values, we matched the dose distributions along the z axis obtained from measurement with the MC data from Daskalov et al.23 The positioning uncertainty in the z direction was evaluated to be 0.4 mm. The use of the template allowed achieving a positioning uncertainty of 0.2 mm along the radial axis. The experiment consisted of measuring dose using the PSD at different source-to-scintillator positions. Dose delivery catheters were placed at radial distances (r) of 0.5, 1.0, 3.0, and 5.0 cm from the catheter where the PSD was inserted. The source was moved in each catheter for z positions ranging from −5.0 to 5.0 cm by 0.5 cm steps. The dose was then calculated for each source-to-scintillator position with each of the three different approaches and compared to the expected values from Daskalov et al.23 Five measurements were taken for each position. The mean and standard deviation were calculated. The standard deviation is a combination, in quadrature, of the measurements uncertainty and the effect on dose comparison from the uncertainty in positioning.
Figure 2.
Relative difference (in %) of the doses obtained using the different stem effect removal approaches to the expected dose as a function of z for r=0.5, 1.0, 3.0, and 5.0 cm. Error bars were calculated from addition in quadrature between the standard deviation of measurements and the effect on dose from positioning accuracy.
RESULTS
Dose measurements were performed for each source positions described above. The relative differences to the expected values are shown in Fig. 2 with their uncertainty for the different radial positions (r=0.5, 1.0, 3.0, and 5.0 cm). Only positive z positions are presented in Fig. 2 for clarity purposes. As it was expected, when the source was far from the optical fiber guide (z<0), the results were consistent among the techniques and with the reference data within measurements uncertainty. We can see in Fig. 2 that the stem effect is particularly important when the source is close to the fiber and far from the scintillator. The polychromatic filtration technique was shown to outperform both the no filtration and the monochromatic techniques. In the highest stem effect contribution condition (r=0.5 cm, z=5.0 cm), the relative difference to the expected value was equal to (72±3)% for no filtration, (41±3)% with the monochromatic filtration, and (−12±8)% with the polychromatic technique. According to our results and associated error bars, the need for stem effect correction is not as necessary for radial positions greater than 3 cm.
DISCUSSION AND CONCLUSION
Our study showed that it is necessary to implement a stem effect removal technique in order to perform accurate in vivo dosimetry during Ir-192 HDR brachytherapy treatments. The polychromatic filtration technique yielded the best results. The use of a second color channel (blue) allowed for the subtraction of the stem effect component from the signal of interest beside than a simple optimization of the scintillation to stem effect ratio as it is in the case for the monochromatic technique. The necessity for a stem effect removal technique under certain irradiation conditions is supported by the results from Andersen et al.19 using a different type of scintillation detector. Using an Al2O3 radioluminescence∕optically stimulated luminescence detector for in vivo dosimetry under a Ir-192 PDR brachytherapy source, they reported up to a 30% influence from the stem effect on the signal and concluded that it was relevant to account for the stem effect component. Their study was already implementing a monochromatic filtration technique in their detector. That result is in good agreement with our measured worst-case difference of (41±3)% when using the monochromatic filtration technique.
As stated before, another group of researchers stated that it was not necessary to account for the stem effect to achieve accurate in vivo dosimetry using PSDs during Ir-192 HDR brachytherapy.4, 9 The results from Cartwright et al.9 look at first sight in contradiction with what we showed here. They reported an average stem effect contribution of 3% at r=3.5 cm with a 4 mm scintillator and no filtration technique was implemented in their study. However, they reported a dependence of the stem effect with the radial source-to-detector distance of 1∕r0.83, which would then lead to an average stem effect contribution of 15.1% for a radial distance of 0.5 cm. The average value of the data we obtained without filtration at r=0.5 cm was about a 27% difference to the expected values attributed to the stem effect. As the scintillator we used in our study was half the length of the one used by Cartwright et al. (2 mm vs 4 mm), we would expect the stem effect contribution in our study being higher. Thus, once compared on a similar footing, all the results seem to converge toward significant stem effect for conditions explored in this work. However, because of the particularly large source-to-scintillator radial positions r=3.5 cm selected by Cartwright et al. to state their conclusion, the clinical importance of stem effect removal could be misleading.
When considering the different possible source-to-scintillator positions, we showed that it is necessary to implement a stem effect removal technique when building a PSD for dosimetry during Ir-192 HDR brachytherapy, especially for dwell positions close to the optical fiber (r<3 cm) and far from the scintillating component (z>+3 cm). Such conditions are seen on regular basis in most brachytherapy applications. Even if there exist some source-to-scintillator positions where the stem effect could be neglected, taking into account the stem effect should ensure a better versatility of the detector and should prevent possible measurement errors when performing Ir-192 HDR brachytherapy dose verification using scintillation detectors. In light of our results, the PSD we developed together with the polychromatic filtration technique would be suitable for Ir-192 HDR brachytherapy dosimetry applications.
ACKNOWLEDGMENTS
This work was supported, in part, by the National Cancer Institute of Canada (NCIC) (Grant No. 017133), by the NSERC Discovery (Grant No. 262105), and by the National Cancer Institute (NCI) (Grant No. 1R01CA120198-01A2) F.T.-P. was supported by the NSERC (BESC-D and BESC-SEEMS) and FQRNT.
References
- Kirov A. S., Williamson J. F., Meigooni A. S., and Zhu Y., “TLD, diode and Monte Carlo dosimetry of an 192Ir source for high dose-rate brachytherapy,” Phys. Med. Biol. 40, 2015–2036 (1995). 10.1088/0031-9155/40/12/002 [DOI] [PubMed] [Google Scholar]
- Kinhikar R. A., Sharma P. K., Tambe C. M., and Deshpande D. D., “Dosimetric evaluation of a new OneDose MOSFET for Ir-192 energy,” Phys. Med. Biol. 51, 1261–1268 (2006). 10.1088/0031-9155/51/5/015 [DOI] [PubMed] [Google Scholar]
- Zilio V. O., Joneja O. P., Popowski Y., Rosenfeld A., and Chawla R., “Absolute depth-dose-rate measurements for an 192Ir HDR brachytherapy source in water using MOSFET detectors,” Med. Phys. 33, 1532–1539 (2006). 10.1118/1.2198168 [DOI] [PubMed] [Google Scholar]
- Lambert J., McKenzie D. R., Law S., Elsey J., and Suchowerska N., “A plastic scintillation dosimeter for high dose rate brachytherapy,” Phys. Med. Biol. 51, 5505–5516 (2006). 10.1088/0031-9155/51/21/008 [DOI] [PubMed] [Google Scholar]
- Das R., Toye W., Kron T., Williams S., and Duchesne G., “Thermoluminescence dosimetry for in-vivo verification of high dose rate brachytherapy for prostate cancer,” Australas. Phys. Eng. Sci. Med. 30, 178–184 (2007). 10.1007/BF03178424 [DOI] [PubMed] [Google Scholar]
- Lambert J., Nakano T., Law S., Elsey J., McKenzie D. R., and Suchowerska N., “In vivo dosimeters for HDR brachytherapy: A comparison of a diamond detector, MOSFET, TLD, and scintillation detector,” Med. Phys. 34, 1759–1765 (2007). 10.1118/1.2727248 [DOI] [PubMed] [Google Scholar]
- Fagerstrom J. M., Micka J. A., and DeWerd L. A., “Response of an implantable MOSFET dosimeter to 192Ir HDR radiation,” Med. Phys. 35, 5729–5737 (2008). 10.1118/1.3013574 [DOI] [PubMed] [Google Scholar]
- Andersen C. E., Nielsen S. K., Greilich S., Helt-Hansen J., Lindegaard J. C., and Tanderup K., “Characterization of fiber-optic coupled Al2O3:C luminescence dosimetry system for online in vivo dose verification during 192Ir brachytherapy,” Med. Phys. 36, 708–718 (2009). 10.1118/1.3063006 [DOI] [PubMed] [Google Scholar]
- Cartwright L. E., Suchowerska N., Yin Y., Lambert J., Haque M., and McKenzie D. R., “Dose mapping of the rectal wall during brachytherapy with an array of scintillation dosimeters,” Med. Phys. 37, 2247–2255 (2010). 10.1118/1.3397446 [DOI] [PubMed] [Google Scholar]
- Beddar A. S., Mackie T. R., and Attix F. H., “Water-equivalent plastic scintillation detectors for high-energy beam dosimetry: I. Physical characteristics and theoretical considerations,” Phys. Med. Biol. 37, 1883–1900 (1992). 10.1088/0031-9155/37/10/006 [DOI] [PubMed] [Google Scholar]
- Beddar A. S., Mackie T. R., and Attix F. H., “Water-equivalent plastic scintillation detectors for high-energy beam dosimetry: II. Properties and measurements,” Phys. Med. Biol. 37, 1901–1913 (1992). 10.1088/0031-9155/37/10/007 [DOI] [PubMed] [Google Scholar]
- Beddar A. S., “Plastic scintillation dosimetry and its application to radiotherapy,” Radiat. Meas. 41, S124–S133 (2007). 10.1016/j.radmeas.2007.01.002 [DOI] [Google Scholar]
- Fontbonne J. M., Iltis G., Ban G., Battala A., Vernhes J. C., Tillier J., Bellaize N., LeBrun C., Tamain B., Mercier K., and Motin J. C., “Scintillating fiber dosimeter for radiation therapy accelerator,” IEEE Trans. Nucl. Sci. 49, 2223–2227 (2002). 10.1109/TNS.2002.803680 [DOI] [Google Scholar]
- Jelley J. V., “Cerenkov radiation and its applications,” Br. J. Appl. Phys. 6, 227 (1955). 10.1088/0508-3443/6/7/301 [DOI] [Google Scholar]
- Beddar A. S., Mackie T. R., and Attix F. H., “Cerenkov light generated in optical fibres and other light pipes irradiated by electron beams,” Phys. Med. Biol. 37, 925–935 (1992). 10.1088/0031-9155/37/4/007 [DOI] [Google Scholar]
- de Boer S. F., Beddar A. S., and Rawlinson J. A., “Optical filtering and spectral measurements of radiation-induced light in plastic scintillation dosimetry,” Phys. Med. Biol. 38, 945–958 (1993). 10.1088/0031-9155/38/7/005 [DOI] [Google Scholar]
- Clift M. A., Johnston P. N., and Webb D. V., “A temporal method of avoiding the Cerenkov radiation generated in organic scintillator dosimeters by pulsed mega-voltage electron and photon beams,” Phys. Med. Biol. 47, 1421–1433 (2002). 10.1088/0031-9155/47/8/313 [DOI] [PubMed] [Google Scholar]
- Archambault L., Beddar A. S., Gingras L., Roy R., and Beaulieu L., “Measurement accuracy and Cerenkov removal for high performance, high spatial resolution scintillation dosimetry,” Med. Phys. 33, 128–135 (2006). 10.1118/1.2138010 [DOI] [PubMed] [Google Scholar]
- Andersen C. E., Nielsen S. K., Lindegaard J. C., and Tanderup K., “Time-resolved in vivo luminescence dosimetry for online error detection in pulsed dose-rate brachytherapy,” Med. Phys. 36, 5033–5043 (2009). 10.1118/1.3238102 [DOI] [PubMed] [Google Scholar]
- Frelin A. -M., Fontbonne J. M., Ban G., Colin J., Labalme M., Battala A., Isambert A., Vela A., and Leroux T., “Spectral discrimination of Cerenkov radiation in scintillating dosimeters,” Med. Phys. 32, 3000–3006 (2005). 10.1118/1.2008487 [DOI] [PubMed] [Google Scholar]
- Archambault L., Briere T. M., Ponisch F., Beaulieu L., Kuban D. A., Lee A., and Beddar S., “Toward a real-time in vivo dosimetry system using plastic scintillation detectors,” Int. J. Radiat. Oncol., Biol., Phys. 78, 280–287 (2010). 10.1016/j.ijrobp.2009.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D., Tailor R., Archambault L., Wang L., Therriault-Proulx F., and Beddar A. S., “Measuring output factors of small fields formed by collimator jaws and multileaf collimator using plastic scintillation detectors,” Med. Phys. 37, 5541–5549 (2010). 10.1118/1.3488981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalov G. M., Loffler E., and Williamson J. F., “Monte-Carlo-aided dosimetry of a new high dose-rate brachytherapy source,” Med. Phys. 25, 2200–2208 (1998). 10.1118/1.598418 [DOI] [PubMed] [Google Scholar]