Abstract
The phosphatidylinositol 3-kinase (PI3K)-dependent signaling pathway in brain of spontaneously hypertensive rats (SHR), but not Wister-Kyoto (WKY) rats, contributes to elevated mean arterial pressure (MAP). The role of PI3K in regulation of blood pressure or autonomic function in the nucleus solitary tract (NTS) is yet to be established in other AngII-dependent models of hypertension. Thus, we microinjected PI3K inhibitors, wortmannin or LY294002, into the NTS, and measured MAP, baroreflex sensitivity (BRS) for heart rate control, and heart rate variability (HRV) in mRen2.Lewis congenic and (mRen2)27 transgenic rats. Bilateral NTS microinjections of wortmannin (100 nmol/L; 50 nL) reduced MAP in (mRen2)27 and mRen2.Lewis rats (33±5 mmHg, n=7, and 32±6 mmHg, n=9, respectively) for ~90 minutes. Spectral and sequence analysis showed improvements in spontaneous BRS and HRV (50–100%) following treatment in both hypertensive strains. Injections of wortmannin into NTS of Hannover Sprague-Dawley or Lewis control rats failed to alter MAP, BRS or HRV. In mRen2.Lewis, but not control Lewis rats, LY294002 (50 µmol/L) reduced MAP and increased BRS and HRV similar to wortmannin. Thus, pharmacologic blockade of the PI3K signaling pathway in NTS reveals an important contribution to resting MAP and BRS in rats with over expression of the Ren2 gene.
Keywords: Angiotensin II, Phosphatidylinositol 3-kinase, (mRen2)27 transgenic rats, Baroreceptor Sensitivity, Nucleus of the Solitary Tract, Wortmannin
Introduction
The phosphatidylinositol 3-kinase (PI3K) has been associated historically with cellular growth, differentiation, and apoptosis.1–3 Recent studies implicate this pathway in the actions of Ang II in cells in culture and the regulation of mean arterial pressure (MAP) in spontaneously hypertensive rats (SHR). For example, bath application of Ang II activated the PI3K pathway in cultured neonatal rostral ventrolateral medullary (RVLM) neurons from SHR but not WKY.4;5 An in vivo administration of specific PI3K pathway inhibitors in the RVLM of SHR led to significant decreases in MAP, but had no effect on MAP in WKY rats.6 Taken together the above findings suggested that in hypertensive rats, a PI3K dependent pathway is up-regulated and is responsible for maintaining blood pressure, perhaps mediated by actions of Ang II, in the hypertensive rats. Because there were no effects on WKY rats, the PI3K pathway may not be active in blood pressure regulation of normotensive animals despite the fact that this pathway participates in the normal cellular responses of neurons elsewhere in the medulla. For example, neurons in the dorsomedial medulla of normotensive rats respond to leptin 7 and insulin 8 via signaling involving PI3K. Application of insulin in the nucleus tractus solitarii (NTS) suppressed baroreflex sensitivity and this was blocked by a PI3K inhibitor, independent of changes in MAP in normotensive WKY rats. It is not known, however, whether the PI3K pathway present in the NTS is involved in a regulated manner in other models of Ang II-dependent hypertension, such that this pathway becomes a requisite component in support of resting MAP. Thus, the objective of this study is to determine the effects of PI3K inhibitors in the NTS of two transgenic models of Ang II-dependent hypertension: (mRen2)27 transgenic and mRen2.Lewis congenic rats. Effects on MAP, the barorereflex sensitivity (BRS) for control of heart rate (HR), heart rate variability (HRV) and blood pressure variability (BPV) as compared with control strains of rats were determined.
Methods
Animal preparation
Experiments were performed in adult male transgenic (mRen2)27, congenic mRen2.Lewis (9–20 wk old) and age-matched HnSD obtained from the Hypertension and Vascular Research Transgenic Animal Facility at Wake Forest University School of Medicine and Lewis rats. Lewis rats were obtained from the Charles River Laboratory and acclimated for at least two weeks prior use. All experiments were carried out in accordance with the guiding principles for the care and use of animals as mandated by the American Physiological Society and were approved by the Institutional Animal Care and Use Committee. Rats were anesthetized with urethane/chloralose (750 mg and 35 mg per kg, respectively, intraperitoneally), as this anesthetic maintains parasympathetic and sympathetic components of the baroreflex, independent of resting blood pressure in these transgenic lines relative to other transgenic and normotensive animals in previous studies.9–11 The femoral artery and vein were cannulated for measurement of arterial pressure and drug injections, respectively. Animals were allowed to breathe a mixture of 65% room air and 35% oxygen. Body temperature was maintained at 37.5°C by an external heating source. Anesthetized rats were placed in a stereotaxic frame. A dorsal midline incision was made through the skin and the dorsal neck muscles retracted with sutures to visualize the foramen magnum. The medulla oblongata was exposed by incising the atlantooccipital membrane as reported.12,13
Microinjection procedures
Microinjections of either Wortmannin (100 nmol/L, 50 nL) or LY294002 (50 µmol/L, 50 nL) were made bilaterally from multi-barrel micropipettes with tip diameters of 20–50 µm. The pipettes were made from calibrated microbore capillary glass tubing. Tips were drawn on a micropipette puller (PMP-100 multibarrel puller, Micro Data Instruments). Injections (50 nl) were made bilaterally over a 30-s period with hand-held syringe as described elsewhere.13 Appropriate placement of the pipette tip within the medial NTS on each side of the brain stem was established by microinjection of L-glutamate (L-Glu, 2 nmol/L, 50 nL) and observing a transient depressor response of at least 25 mm Hg that was comparable among groups (HnSD, 43 ± 7 mm Hg, (mRen2)27 47 ± 4 mm Hg, Lewis 39 ± 4 mm Hg, mRen2.Lewis 31 ± 5 mm Hg). On this basis, injections into the medial NTS had coordinates 0.4–0.5 mm anterior and 0.5–0.6 mm lateral to calamus scriptorius and 0.4 mm below the dorsal surface of the medulla.
Mean Arterial Pressure
Arterial blood pressure and heart rate were continuously measured by a pressure transducer (DT-XX, Viggoo-Spectramed, Oxnard, CA) connected to a femoral arterial catheter. Data were recorded and digitized using a computerized acquisition system (Biopac 3.8).
Evoked and Spontaneous Baroreceptor Reflex Sensitivity for the Control of Heart Rate, Heart Rate Variability and Blood Pressure Variability
In a subset of the animals, progressive bolus injections of phenylephrine (PE) 2, 5, and 10µg/kg were made intravenously to determine the evoked bradycardic response to increases in blood pressure. The evoked BRS was then determined from the slope of the line fit to the relationship for changes in arterial pressure and changes in heart rate (expressed as the pulse interval) as previously published 11;14;15, which is a known indicator of the vagal component of the baroreflex. Evoked BRS was assessed 20 minutes before and 90 minutes following wortmannin treatment. We focused on the bradycardia evoked by increases in pressure as previous studies show no effects on the tachycardic response to decreases in pressure.9–15
In the same animals for which evoked BRS was determined, digitized MAP and HR were used for the calculation of spontaneous BRS (as LFα, HFα, Seq UP, Seq DOWN and Seq ALL), heart rate variability (HRV) as standard deviation of beat-to-beat interval (SDRR) and the root mean square of successive beat-to-beat differences in R-R interval duration (rMSSD) and blood pressure variability (BPV) as the standard deviation of the mean arterial pressure (SD-MAP) both directly before and 90 minutes after the microinjection of wortmannin or LY294002. Frequency Domain Analysis: Spontaneous BRS was calculated by the frequency-domain analysis method as in our previous work 9;16 using analysis software designed for small animals (Nevrokard SA-BRS, Medistar, Ljubljana, Slovenia). In brief, BP and HR was recorded via the data acquisition system at 1000 HZ (BIOPAC acquisition software, Santa Barbara, CA) and the systolic arterial pressure (SAP) and R to R intervals (RRI) files generated from 5 minutes of continuous recording at the specified time points were analyzed using Nevrokard SA-BRS software. Power spectral densities of SAP and RRI's oscillations were computed by Fast Fourier Transform and integrated over the specified frequency range (LF; 0.25–0.75 Hz) and (HF; 0.75–3.0 Hz). A Hanning window was applied and the spectra of SAP and RRI series, and their squared-coherence modulus, were computed if the coherence was greater than 0.5 in accordance with reported criteria.9;16 The square-root of the ratio of RRI’s and SAP powers were computed to calculate low frequency and high frequency alpha indices (LFα, HFα), which reflect the BRS.17 Sequence Method: BRS calculated by this method is based on quantification of sequences of at least three beats (n) in which systolic arterial pressure (SAP) consecutively increases (UP sequence) or decreases (DOWN sequence), which are accompanied by changes in the same direction of the RR intervals (RRI's) of the subsequent beats (n+1). In order to be included in the BRS estimate, each sequence must fulfill the following reported criteria: 16 (1) minimal RRI change 1 ms; (2) minimal SAP change 0.5 mmHg; (3) minimal number of beats, 3 in the sequence; (4) minimal correlation coefficient of 0.85. The software scans the RRI and SAP records, identifies sequences, and then calculates linear correlation between RRI and SAP for each sequence. If the correlation coefficient exceeds a pre-set critical value (0.85), the regression coefficient (slope) is calculated and accepted. The mean of all individual regression coefficients (slopes), which is a measure of sequence BRS was then calculated. Overall, three parameters were obtained by this method (Sequence UP, DOWN and ALL). Time-Domain Analysis: Heart rate variability was determined by computing the standard deviation of beat-to-beat interval (SDRR) and the root mean square of successive beat-to-beat differences in R-R interval duration (rMSSD). The standard deviation of the mean arterial pressure (SD-MAP) was used as a measure for blood pressure variability.9;16;18;19
Drugs
Drugs injected were wortmannin (100 nmol/L, Sigma-RBI), a potent, selective, irreversible PI3K inhibitor, LY294002 (50 µmol/L, Promega), and L-Glu (2 nmol/L, Sigma-RBI). The vehicle used to dissolve wortmannin and Ang II was 1% ethanol in PBS (10 mmol/L phosphate buffer and 0.9% NaCl, pH 8.0). Drug concentrations have been chosen in accordance with those of previous in vitro 5;20 and in vivo 6 studies. The effect of vehicle microinjections alone was also assessed and found to have no effect as previously published.15
Statistical Analysis
All results are expressed as mean ± SEM. Comparisons were made by 2 way ANOVA for strain and treatment. This was followed by paired or unpaired Student’s test when only two of the variables were compared and 1 way ANOVA for multiple comparisons across time. p < 0.05 was the criterion for statistical significance.
Results
Baseline Mean Arterial Pressure and Heart Rate in Transgenic (mRen2)27, HnSD, Congenic mRen2.Lewis and Lewis Rats
MAP and HR were recorded in anesthetized rats prior to PI3K inhibition. Baseline MAP was similar in (mRen2)27 versus the HnSD rats, and in mRen2.Lewis versus Lewis control rats; however, MAP was significantly higher in (mRen2)27 versus mRen2.Lewis rats. HR was similar in all groups prior to treatment (Table 1).
Table 1.
Resting values for mean arterial blood pressure and heart rate
| Strain | MAP | HR |
|---|---|---|
| HnSD (n=9) | 94±5 | 251±23 |
| (mRen2)27 (n=7) | 111±9** | 298±17 |
| Lewis (n=5) | 86±3 | 256±13 |
| mRen2.Lewis (n=9) | 85±6 | 277±14 |
Values expressed as Mean ± SEM for 5 – 9 observations in each strain. The mean arterial pressure (MAP) and heart rate (HR) under anesthesia were not different between HnSD and (mRen2)27 rats or between Lewis and mRen2.Lewis rats at baseline.
p<0.001 when comparing MAP of (mRen2)27 vs mRen2.Lewis at baseline.
Effects of Wortmannin on MAP and BRS in in Transgenic (mRen2)27, HnSD, Congenic mRen2.Lewis and Lewis Rats
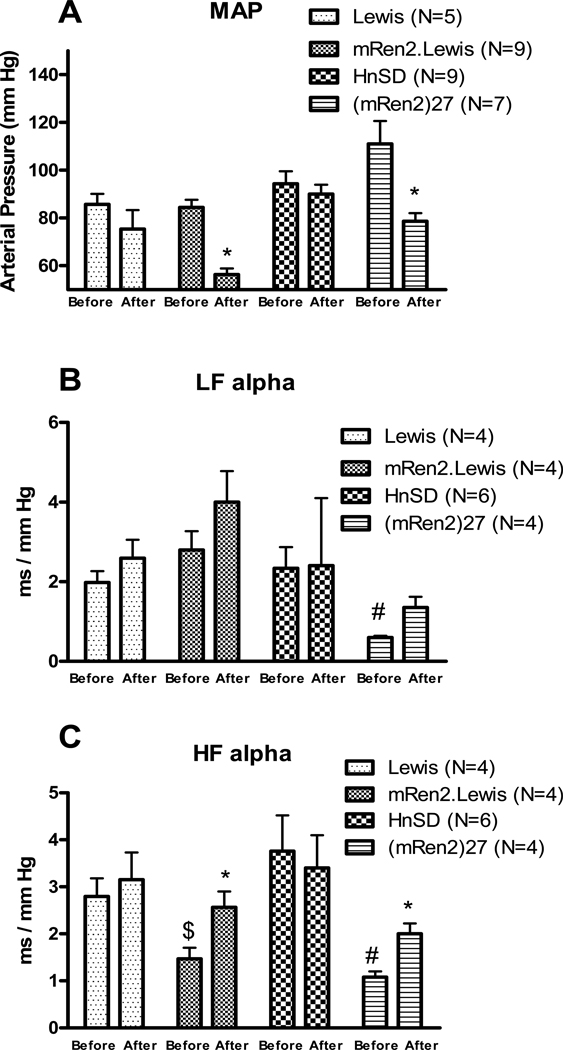
Bilateral injections of wortmannin into the NTS significantly decreased MAP in (mRen2)27 and mRen2.Lewis (Figure 1A). This depressor response persisted for ~90 minutes in mRen2.Lewis and (mRen2)27 animals but the inhibition of the PI3K failed to diminish MAP in HnSD or Lewis rats over this time frame.
Figure 1.
Effect of bilateral injection of wortmannin in the NTS on mean arterial pressure (MAP), A; spontaneous baroreflex sensitivity measured by spectral analysis, B and C. Values are mean ± SEM, * p<0.05 vs the before treatment in the same strain, # p<0.05 vs HnSD before treatment and $ p<0.05 vs Lewis before wortmannin treatment
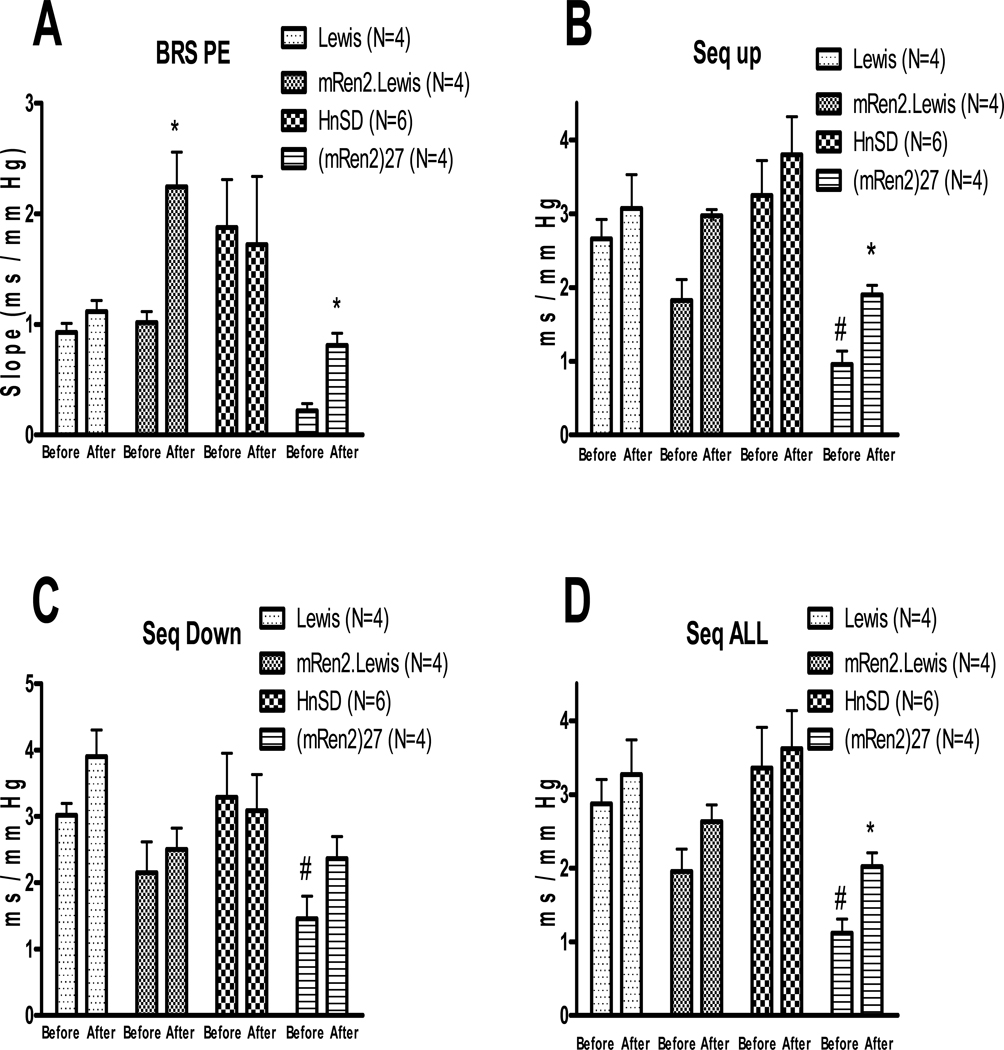
Spontaneous BRS measured by spectral analysis as LFα which measures mainly the sympathetic arc of baroreflex (Figure 1B) was lower in (mRen2)27 rats as compared with HnSD rats and did not improve with the inhibitor, wortmannin, whereas there was no difference in LFα between Lewis and mRen2.Lewis animals and no effect of the wortmannin. HFα which measures mainly the parasympathetic arc of baroreflex (Figure 1C) was lower in mRen2.Lewis and (mRen)27 rats relative to their control strains at baseline and this was significantly improved by wortmannin microinjection in the transgenic and congenic animals but not the control rats. BRS measured by either the evoked method or the sequence method was similar in mRen2.Lewis, Lewis and HnSD rats, but lower in (mRen2)27 animals relative to HnSD rats at baseline (Figure 2). The PE evoked BRS was significantly increased in (mRen2)27 and mRen2.Lewis rats following wortmannin treatment when compared to baseline values (Figure 2A). BRS measured by sequence method as Seq-UP which measures mainly the parasympathetic arc of baroreflex (Figure 2B) or Seq-ALL which measures both arms of baroreflex (Figure 2D) was significantly increased by wortmannin treatment in (mRen2)27 rats, with a similar trend in the mRen2.Lewis animals. Seq-DOWN which measures mainly the sympathetic arc of baroreflex (Figure 2C) was also lower in (mRen2)27 rats but did not significantly improve by wortmannin treatment, consistent with the spectral analysis data in Figure 1B. However, BRS measured by all 3 methods was not changed significantly in HnSD or Lewis rats following wortmannin treatment (Figure 2).
Figure 2.
Effect of bilateral injection of wortmannin in the NTS on phenylephrine-evoked BRS, A; spontaneous BRS measures, B, C and D. Values are mean ± SEM, * p<0.05 vs the before treatment in the same strain, # p<0.05 vs HnSD before wortmannin treatment.
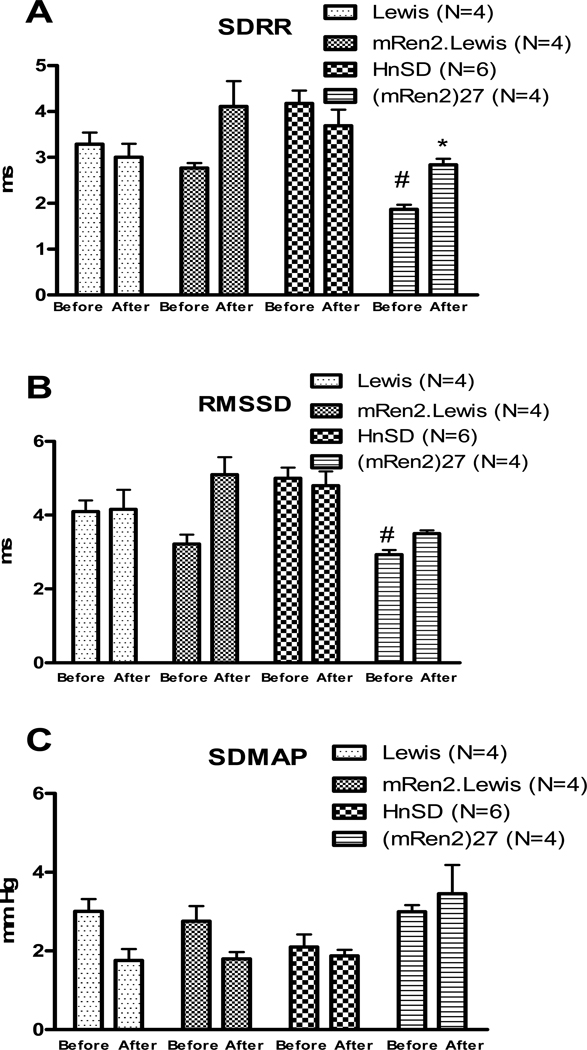
Heart rate variability measured as SDRR and rMSSD (Figure 3 A&B) was significantly lower in (mRen2)27 rats compared with the other 3 strains and increased by wortmannin treatment. Wortmannin had no effect on HRV in mRen2.Lewis, HnSD or Lewis rats (Figure 3A&B). There was no significant difference in BPV measured as SDMAP among the 4 strains and wortmannin had no effect (Figure 3C).
Figure 3.
Effect of wortmannin treatment on heart rate variability measured as standard deviation of beat-to-beat interval (SDRR), A; and the root mean square of successive beat-to-beat differences in R-R interval duration (rMSSD), B; and blood pressure variability measured as the standard deviation of the mean arterial pressure (SD-MAP), C. Values are mean ± SEM, * p<0.05 vs the before treatment in the same strain, # p<0.05 vs HnSD before wortmannin treatment.
LY294002 Effects in Lewis and Congenic mRen2.Lewis Rats
Due to the concern that wortmannin might have non-specific effects, in a separate set of Lewis and mRen2.Lewis rats we evaluated the changes in blood pressure, BRS, HRV and BPV in response to NTS injection of the PI3 kinase inhibitor 2-(4-morpholinyl)-8-phenyl-chromone (LY294002). Table 2 summarizes the effects of LY294002 treatment. Bilateral injections of LY294002 significantly decreased MAP in mRen2.Lewis rats but not in Lewis control rats, similar to what was reported for wortmannin. The BRS for control of HR measured by all 3 methods was significantly improved by LY294002 treatment. HRV measured as SDRR and rMSSD was not different between the two strains at baseline and did not change by LY294002 treatment (Table 2), which, is similar to the wortmannin data (Figure 3). BPV was higher in this set of mRen2.Lewis compared to Lewis and LY294002 treatment tended to reduce SDMAP, but this did not reach statistical significance.
Table 2.
Effect of LY 294002 treatment on mean arterial pressure and indices of baroreflex function.
| Lewis (n=3) | mRen2 Lewis (n=4) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| MAP (mmHg) | 78 ± 4.2 | 79 ± 1 | 87 ± 8.2$ | 72 ± 5.9* |
| LFα (ms/mm Hg) | 3.4 ± 0.5 | 4.0 ± 1.0 | 1.9 ± 0.2 | 1.9 ± 0.2 * |
| HFα (ms/mm Hg) | 2.5 ± 0.3 | 2.9 ± 0.3 | 1.0 ± 0.1$ | 2.0 ± 0.3 * |
| Seq UP (ms/mm Hg) | 2.5 ± 0.1 | 2.9 ± 0.4 | 1.0 ± 0.0$ | 1.9 ± 0.2* |
| Seq DOWN (ms/mm Hg) | 2.7 ± 0.2 | 3.1 ± 0.5 | 0.9 ± 0.1$ | 2.1 ± 0.2* |
| Seq ALL (ms/mm Hg) | 2.6 ± 0.1 | 2.9 ± 0.4 | 1.0 ± 0.0$ | 2.0 ± 0.2* |
| SDRR (ms) | 4.0 ± 0.5 | 3.8 ± 0.3 | 3.5 ± 0.2 | 4.4 ± 0.3 |
| rMSSD (ms) | 5.0 ± 0.4 | 5.3 ± 0.6 | 3.8 ± 0.1 | 5.6 ± 0.8 |
| SDMAP (mmHg) | 2.5 ± 0.3 | 2.0 ± 0.2 | 5.5 ± 0.7$ | 2.8 ± 0.6 |
Values are expressed as mean ± SEM. Mean arterial pressure (MAP) and baroreflex function measured by spectral analysis as low frequency alpha index (LFα), high frequency alpha index (HFα), and by sequence methods (Seq-UP, Seq-DOWN, Seq-ALL), heart rate variability measured as standard deviation of beat-to-beat interval (SDRR) and the root mean square of successive beat-to-beat differences in R-R interval duration (rMSSD), and blood pressure variability measured as the standard deviation of the mean arterial pressure (SD-MAP).
p< 0.05 versus mRen2 Lewis before LY treatment, $ p<0.05 vs Lewis before LY treatment.
Discussion
The PI3K-dependent signaling pathway in the RVLM of SHRs plays a role in maintenance of MAP under anesthesia in SHR but not WKY rats in vivo, and participates in Ang II-mediated cellular responses in SHR but not WKY neurons in vitro.4–6 Our results reveal that in the NTS of both (mRen2)27 transgenic and mRen2.Lewis congenic rats an active PI3K pathway contributes to resting MAP, despite the fact that at the time of wortmannin or LY294002 injections MAP was no longer at hypertensive levels under anesthesia. The PI3K pathway also appears to exert a negative influence on BRS for control of HR when measured as either the spontaneous BRS or in response to evoked increases in MAP in these two strains of rats, independent of either baseline MAP or BRS. In contrast, the PI3K pathway in the NTS does not appear to participate in maintenance of MAP in control HnSD or Lewis rats, nor was there evidence of PI3K tone for suppression of BRS in these animals. Thus, we confirm that the PI3K pathway is up-regulated in models of hypertension that are dependent upon a chronically overactive RAS, as previously shown for SHR, and provide evidence that these effects do not require MAP to remain at hypertensive levels at the time of study.
Ang II binding to the angiotensin type 1 receptors (AT1R) activates the PI3K signal transduction pathway for support of MAP In SHR, which are a brain RAS-dependent form of hypertension.21 Transgenic (mRen2)27 rats are characterized by overexpression of the mouse mRen2 gene in extrarenal tissues of the outbred Hannover SD rats, excessive generation of local brain tissue Ang II is a prominent feature.22 SHR and (mRen2)27 are sensitive to central RAS blockade, but there is no effect of AT1R blockade on MAP in normotensive rats.23–26 The mRen2.Lewis congenic rats are derived from the original (mRen2)27 line, through a series of backcrosses with the inbred Lewis strain.27 In spite of the varied backgrounds of the three models of hypertension and their MAP response to anesthesia, a common feature appears to be the dependence of the MAP on the PI3K pathway in brain areas controlling sympathetic nervous system outflow. In the SHR, MAP was lowered by wortmannin injections into the RVLM from an elevated baseline with respect to the WKY animals, making it difficult to determine whether the MAP lowering effect required an elevated pressure. However, we observed reductions in MAP even though resting levels of MAP were normalized in the congenic and lowered in the transgenic strain under anesthesia. The PI3K pathway was also activated in brainstem neurons in culture from neonatal SHR 5;20 and our unpublished findings suggest that the PI3K regulatory subunit (p85α) mRNA is elevated by ~50% in brain dorsal medulla of (mRen2)27 relative to HnSD rats unexposed to anesthesia (1.03 ± 0.10 vs 1.63 ± 0.25 relative gene expression, respectively; p<0.05). Thus, recruitment of the PI3K pathway as a mediator of AT1R actions appears to be an intrinsic property of the brain medulla independent of anesthesia or resting MAP at the time of study, but possibly resulting from an overactive brain RAS.
Ang II acting at the AT1R has been shown to initiate specific intracellular signaling events in the RVLM via the PI3K and MAPK.6 Both PI3K and MAPK pathway are functional in the NTS and the PI3K pathway participates in the attenuation of the baroreceptor reflex in response to NTS administration of insulin.28 Interestingly, the PI3K pathway in dorsal medulla is active in normotensive (WKY) rats in that it mediates the responses to insulin in the NTS, even though the inhibition of the pathway does not alter resting MAP.8 Hypertension associated with increased activity of the brain or peripheral RAS is accompanied by lower BRS for control of HR in response to increases in MAP.13;29–31 Ang II administration icv reduces HRV,32 and elevated Ang II at the level of the NTS attenuates BRS and HRV, effects that are mediated by the AT1R.26;33–36 Moreover, we and others show that within the NTS the effects on MAP and BRS are independently regulated.9;15;29;30 Our current studies show that blockade of the PI3K pathway in the NTS enhances BRS and improves HRV measured as a significant change in SDRR in (mRen2)27 with a similar trend in mRen2 Lewis rats in association with lowered MAP. These effects were not seen in normotensive HnSD or Lewis control rats, again suggesting that the PI3K pathway is only tonically active in animals with the hypertensive phenotype. Thus, while we cannot exclude the lower MAP as a mechanism contributing to the improvement in the BRS in the hypertensive strains, the two effects are not necessarily linked. Interestingly, the PI3K blockade-mediated improvement in BRS occurred regardless of the resting BRS. Evoked BRS was not different between Lewis and mRen2.Lewis, whereas HnSD had significantly higher BRS than the (mRen2)27 rats as previously reported. The reason for the difference in resting BRS between mRen2.Lewis and (mRen2)27 rats is not known.
The failure to sustain a hypertensive phenotype under anesthesia in the two genetic models of hypertension may be considered a concern in these studies for study of the BRS, but the finding is consistent with previous reports.31 While both the transgenic and congenic animals exhibit this same phenotype, previous studies by us and others in SHR clearly demonstrate maintenance of high resting arterial pressure under this same anesthetic.26;30 The reason for the sensitivity to anesthesia in the (mRen2)27 and mRen2.Lewis rats as opposed to SHR is not known, and blockade of the brain RAS selectively normalizes pressure in (mRen2)27 rats,23;33;37 as well as mRen2.Lewis and SHR animals.27;38 The increase in plasma Ang II in male mRen2.Lewis rats under anesthesia was much less (3 fold increase) than that seen in male Lewis rats (20-fold increase), which is only explained in part by the higher baseline levels of Ang II in the congenic rats.39;40 If there is desensitization of central pathways to the effects of Ang II in hypothalamic sites of the transgenic and congenic animals 22;39;41, then the lower level of circulating Ang II and less response to the peptide to activate or maintain brain pathways for sympathetic outflow may account for the reduction in pressure under anesthesia relative to the conscious state.37;40,41 Nonetheless, regardless of either the resting MAP or resting BRS, there were improvements in the presence of the PI3K blockade in both hypertensive strains and not in the normotensive strains. There are no data on baroreceptor reflex function in the congenic rats prior to this report. The fact that they do not exhibit an impaired baroreflex at baseline is an intriguing finding that will require further studies to define the mechanisms. However, there are other distinct differences between these models of Ang II-dependent hypertension including differences in ganglionic transmission with regards to long-term potentiation and ganglionic responses to exogenous Ang II.24
Potent PI3 kinase inhibitors such as wortmannin and LY294002 have rarely been used in vivo, so data gleaned from these studies must be interpreted cautiously. However, both wortmannin and a more specific PI3K inhibitor 2-(4-morpholinyl)-8-phenyl-chromone (LY294002) produced similar effects.4–6;20 Another caveat is that our studies with the inhibitors are short term in design and a recent report suggests that long-term interruption of the pathway with expression of a mutated form of PI3K in SHR resulted in lower MAP.42 Nonetheless, in the NTS of two genetic forms of Ang II-dependent hypertension blockade of the PI3K pathway alters acute control of MAP, BRS and HRV.
Perspective
Genetic models of hypertension, whether polygenetic such as the SHR or monogenetic such as the (mRen2)27 and mRen2.Lewis rats, exhibit a phenotype consistent with increased actions of Ang II either in the systemic circulation or in tissues including the brain, or both. While SHR express elevated AT1 receptors, the hypertensive strains used in our study functionally over express Ang II and under express Ang-(1–7),31,33 suggesting the exact mechanisms underlying the hypertension differ. In addition, investigation of differences in biochemical pathways in SHR versus WKY is limited by the potential for strain differences. The comparison of mRen2.Lewis congenic animals with the inbred Lewis control strain, as well as comparison of outbred (mRen2)27 transgenic rats with the outbred HnSD control strain show remarkably similar results with respect to the involvement of the PI3K pathway in regulation of MAP and BRS in animals exhibiting chronic hypertension and not in the control normotensive strains. Therefore, the current findings strengthen the concept that alterations in this signal transduction pathway are a crucial feature of brainstem neural cell function accompanying the hypertensive phenotype. Importantly, the current data also support the hypothesis that the central RAS not only utilizes the PI3K pathway in regulation of the SNS, but the RAS may also be involved in the regulation or recruitment of this same pathway. Since the PI3K pathway mediates actions of other hormones, such as insulin, 43 within the NTS of normotensive animals, understanding the mechanisms for differential activation of the pathway by Ang II in the hypertensive animals will be key to improved therapeutic interventions in cardiovascular as well as metabolic diseases.
Acknowledgments
Dr. Hossam A. Shaltout is currently a faculty member in the Department of Pharmacology and Toxicology, School of Pharmacy, University of Alexandria, Egypt.
Sources of funding
This research was supported by National Heart, Lung, and Blood Institute Grants F31 HL085927, P20 HL-67700 and P01 HL-51952, the National Institute of General Medical Science Grant GM64249 and the National Institute for Minority Health and Health Disparities Grant MD002303.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest / Disclosure
No conflict of interest.
Reference List
- 1.Guthridge MA, Lopez AF. Phosphotyrosine/phosphoserine binary switches: a new paradigm for the regulation of PI3K signalling and growth factor pleiotropy? Biochemical Society Transactions. 2007;35:250–252. doi: 10.1042/BST0350250. [DOI] [PubMed] [Google Scholar]
- 2.Peltier J, O'Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Developmental Neurobiology. 2007;67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- 3.Winbanks CE, Grimwood L, Gasser A, Darby IA, Hewitson TD, Becker GJ. Role of the phosphatidylinositol 3-kinase and mTOR pathways in the regulation of renal fibroblast function and differentiation. International Journal of Biochemistry & Cell Biology. 2007;39:206–219. doi: 10.1016/j.biocel.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Sun CW, Du JQ, Sumners C, Raizada MK. PI3-kinase inhibitors abolish the enhanced chronotropic effects of angiotensin II in spontaneously hypertensive rat brain neurons. Journal of Neurophysiology. 2003;90:3155–3160. doi: 10.1152/jn.00222.2003. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Dang H, Raizada M. A unique signaling pathway involving PI3 kinase in AT1 receptor (AT1R)-mediated stimulation of norepinephrine neuromodulation in SHR brain neurons. Hypertension. 1998;32:612. [Google Scholar]
- 6.Seyedabadi M, Goodchild AK, Pilowsky PM. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension. 2001;38:1087–1092. doi: 10.1161/hy1101.096054. [DOI] [PubMed] [Google Scholar]
- 7.Williams KW, Smith BN. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol. 2006;573:395–412. doi: 10.1113/jphysiol.2006.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang HN, Lu PJ, Lo WC, Lin CH, Hsiao M, Tseng CJ. In situ Akt phosphorylation in the nucleus tractus solitarii is involved in central control of blood pressure and heart rate. Circulation. 2004;110:2476–2483. doi: 10.1161/01.CIR.0000145116.75657.2D. [DOI] [PubMed] [Google Scholar]
- 9.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin Impairs Cardiovagal Baroreflex Function at the Level of the Solitary Tract Nucleus. Hypertension. 2009;54:1001–1008. doi: 10.1161/HYPERTENSIONAHA.109.138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontes MA, Baltatu O, Caligiorne SM, Campagnole-Santos MJ, Ganten D, Bader M, Santos RA. Angiotensin peptides acting at rostral ventrolateral medulla contribute to hypertension of TGR(mREN2)27 rats. Physiol Genomics. 2000;2:137–142. doi: 10.1152/physiolgenomics.2000.2.3.137. [DOI] [PubMed] [Google Scholar]
- 11.Sakima A, Averill DB, Kasper SO, Jackson L, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor reflex regulation in anesthetized transgenic rats with low glia-derived angiotensinogen. Am J Physiol Heart Circ Physiol. 2007;292:H1412–H1419. doi: 10.1152/ajpheart.00984.2006. [DOI] [PubMed] [Google Scholar]
- 12.Averill DB, Diz DI, Barnes KL, Ferrario CM. Pressor responses of angiotensin II microinjected into the dorsomedial medulla of the dog. Brain Res. 1987;414:294–300. doi: 10.1016/0006-8993(87)90009-6. [DOI] [PubMed] [Google Scholar]
- 13.Diz DI, Barnes KL, Ferrario CM. Angiotensin-Ii Injections Into the Dorsal Motor Nucleus of the Vagus Produce Hypotension and Bradycardia. Federation Proceedings. 1984;43:999. [Google Scholar]
- 14.Diz DI, Jessup JA, Westwood BM, Bosch SM, Vinsant S, Gallagher PE, Averill DB. Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla. Clin Exp Pharmacol Physiol. 2002;29:473–482. doi: 10.1046/j.1440-1681.2002.03659.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats - Role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 16.Shaltout HA, Abdel-Rahman AA. Mechanism of fatty acids induced suppression of cardiovascular reflexes in rats. J Pharmacol Exp Ther. 2005;314:1328–1337. doi: 10.1124/jpet.105.086314. [DOI] [PubMed] [Google Scholar]
- 17.Parati G, Frattola A, Di RM, Castiglioni P, Pedotti A, Mancia G. Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol. 1995;268:H1606–H1612. doi: 10.1152/ajpheart.1995.268.4.H1606. [DOI] [PubMed] [Google Scholar]
- 18.Sgoifo A, De Boer SF, Westenbroek C, Maes FW, Beldhuis H, Suzuki T, Koolhaas JM. Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am J Physiol. 1997;273:H1754–H1760. doi: 10.1152/ajpheart.1997.273.4.H1754. [DOI] [PubMed] [Google Scholar]
- 19.Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Raizada MK. Role of phosphatidylinositol 3-kinase in angiotensin II regulation of norepinephrine neuromodulation in brain neurons of the spontaneously hypertensive rat. Journal of Neuroscience. 1999;19:2413–2423. doi: 10.1523/JNEUROSCI.19-07-02413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips MI, Kimura B. Brain angiotensin in the developing spontaneously hypertensive rat. J Hypertens. 1988;6:607–612. doi: 10.1097/00004872-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Senanayake PD, Moriguchi A, Kumagai H, Ganten D, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides. 1994;15:919–926. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 23.Langheinrich M, Lee MA, Bohm M, Pinto YM, Ganten D, Paul M. The hypertensive Ren-2 transgenic rat TGR (mREN2)27 in hypertension research. Characteristics and functional aspects. Am J Hypertens. 1996;9:506–512. doi: 10.1016/0895-7061(95)00400-9. [DOI] [PubMed] [Google Scholar]
- 24.Logan EM, Diz DI, Averill DB, Ferrario CM, Ganten D, Aileru AA. Neuroplastic behavior at the ganglion level: comparative studies between (mRen2)27 and mRen2.Lewis models of hypertension. FASEB J. 2002;16:A875. [Google Scholar]
- 25.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol. 1998;275:R1611–R1619. doi: 10.1152/ajpregu.1998.275.5.R1611. [DOI] [PubMed] [Google Scholar]
- 27.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 28.Bunag RD, Krizsan-Agbas D, Itoh H. Sympathetic activation by chronic insulin treatment in conscious rats. J Pharmacol Exp Ther. 1991;259:131–138. [PubMed] [Google Scholar]
- 29.Sved AF, Schreihofer AM, Kost CK., Jr. Blood pressure regulation in baroreceptor-denervated rats. [Review] [55 refs] Clinical & Experimental Pharmacology & Physiology. 1997;24:77–82. doi: 10.1111/j.1440-1681.1997.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 30.Sved AF, Ito S, Madden CJ. Baroreflex dependent and independent roles of the caudal ventrolateral medulla in cardiovascular regulation. Brain Res Bull. 2000;51:129–133. doi: 10.1016/s0361-9230(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 31.Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol. 2008;51:542–548. doi: 10.1097/FJC.0b013e3181734a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Mevel JC, Mimassi N, Lancien F, Mabin D, Boucher JM, Blanc JJ. Heart rate variability, a target for the effects of angiotensin II in the brain of the trout Oncorhynchus mykiss. Brain Res. 2002;947:34–40. doi: 10.1016/s0006-8993(02)02903-7. [DOI] [PubMed] [Google Scholar]
- 33.Moriguchi A, Tallant EA, Matsumura K, Reilly TM, Walton H, Ganten D, Ferrario CM. Opposing actions of angiotensin-(1–7) and angiotensin II in the brain of transgenic hypertensive rats. Hypertension. 1995;25:1260–1265. doi: 10.1161/01.hyp.25.6.1260. [DOI] [PubMed] [Google Scholar]
- 34.Boscan P, Allen AM, Paton JFR. Baroreflex inhibition of cardiac sympathetic outflow is attenuated by angiotensin II in the nucleus of the solitary tract. Neuroscience. 2001;103:153–160. doi: 10.1016/s0306-4522(00)00559-5. [DOI] [PubMed] [Google Scholar]
- 35.Casto R, Phillips MI. Angiotensin-Ii Attenuates Baroreflexes at Nucleus Tractus Solitarius of Rats. American Journal of Physiology. 1986;250:R193–R198. doi: 10.1152/ajpregu.1986.250.2.R193. [DOI] [PubMed] [Google Scholar]
- 36.Paton JFR, Kasparov S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii a microinjection study in the rat. Journal of Physiology-London. 1999;521:213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriguchi A, Ferrario CM, Brosnihan KB, Ganten D, Morris M. Differential regulation of central vasopressin in transgenic rats harboring the mouse Ren-2 gene. Am J Physiol. 1994;267:R786–R791. doi: 10.1152/ajpregu.1994.267.3.R786. [DOI] [PubMed] [Google Scholar]
- 38.Morishita R, Higaki J, Nakamura Y, Aoki M, Yamada K, Moriguchi A, Rakugi H, Tomita N, Tomita S, Yu H. Effect of an antihypertensive drug on brain angiotensin II levels in renal and spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22:665–669. doi: 10.1111/j.1440-1681.1995.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 39.Diz DI, Westwood BM, Bosch SM, Ganten D, Ferrario CM. NK1 receptor antagonist blocks angiotensin II responses in renin transgenic rat medulla oblongata. Hypertension. 1998;31:473–479. doi: 10.1161/01.hyp.31.1.473. [DOI] [PubMed] [Google Scholar]
- 40.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 41.Diz DI, Falgui B, Bosch SM, Westwood BM, Kent J, Ganten D, Ferrario CM. Hypothalamic substance P release: attenuated angiotensin responses in mRen2(27) transgenic rats. Hypertension. 1997;29:510–513. doi: 10.1161/01.hyp.29.1.510. [DOI] [PubMed] [Google Scholar]
- 42.Zubcevic J, Waki H, Diez-Freire C, Gampel A, Raizada MK, Paton JFR. Chronic Blockade of Phosphatidylinositol 3-Kinase in the Nucleus Tractus Solitarii Is Prohypertensive in the Spontaneously Hypertensive Rat. Hypertension. 2009;53:97–103. doi: 10.1161/HYPERTENSIONAHA.108.122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Hayes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]