Abstract
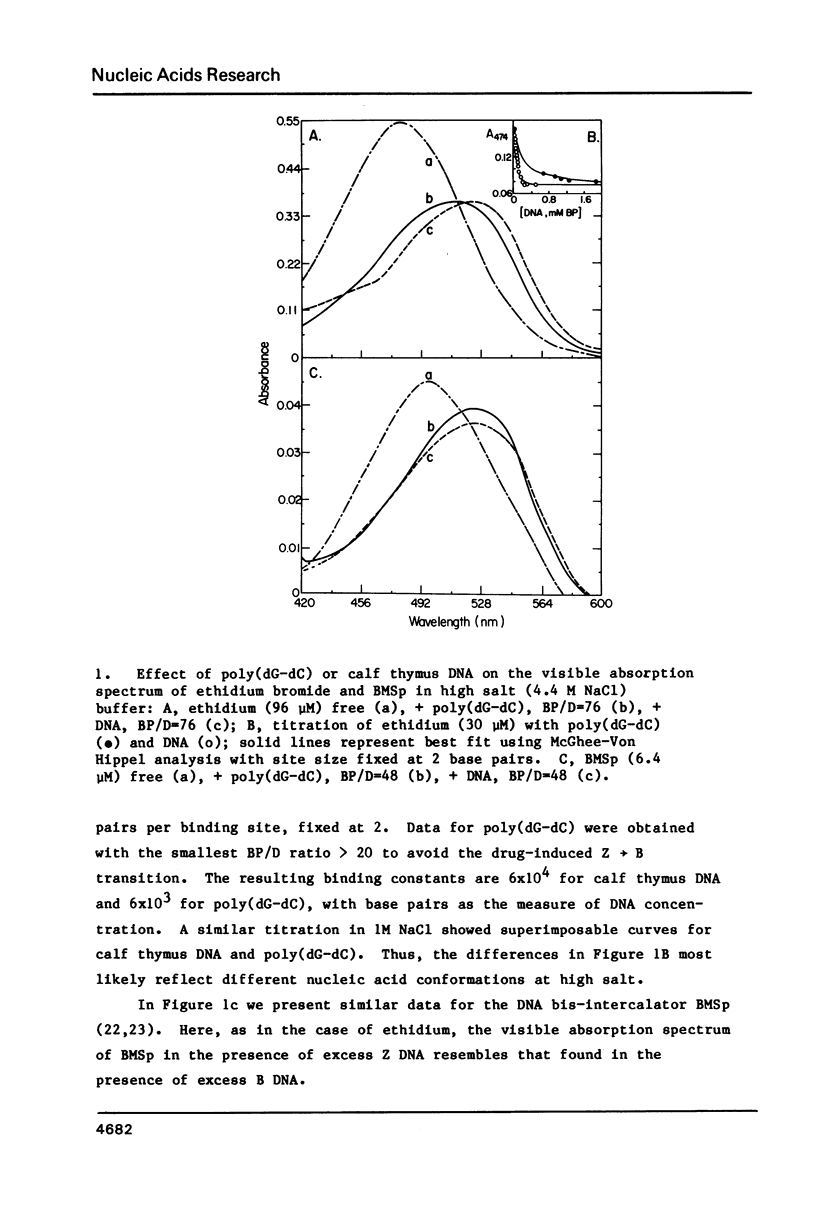
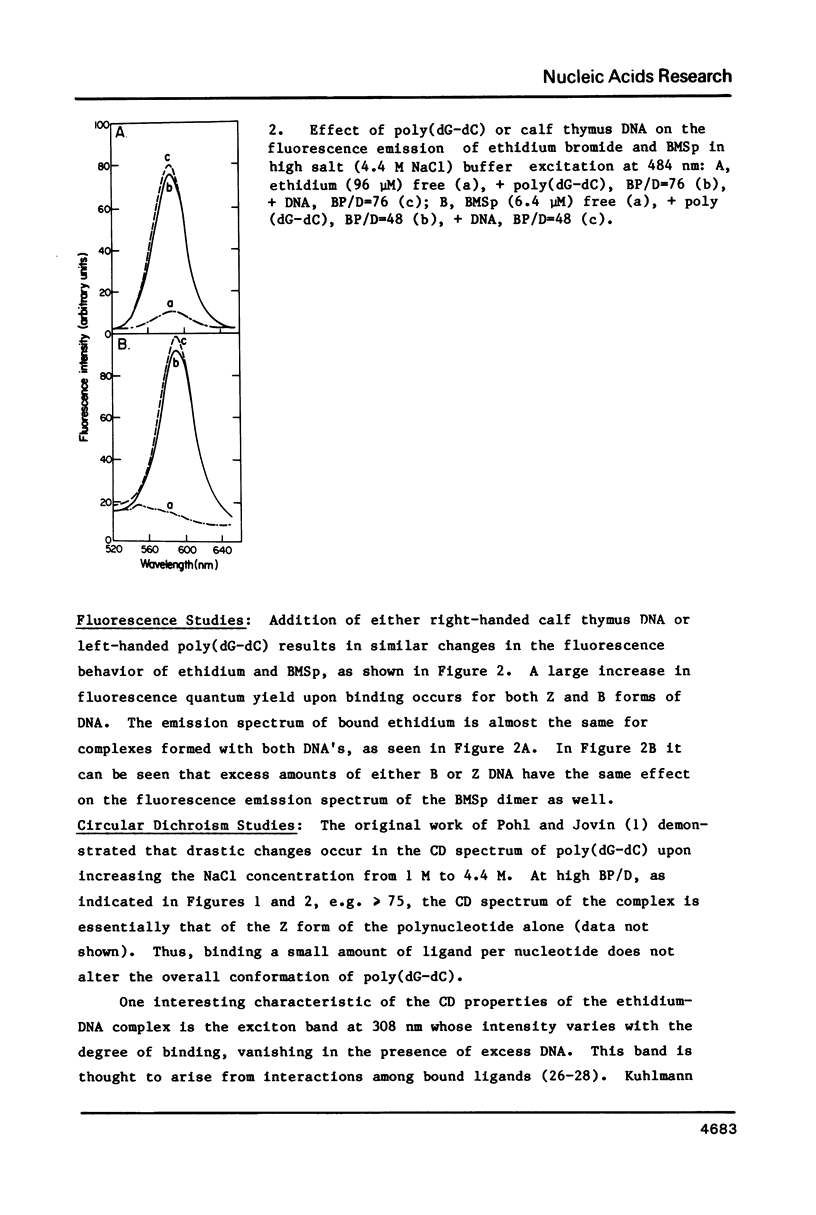
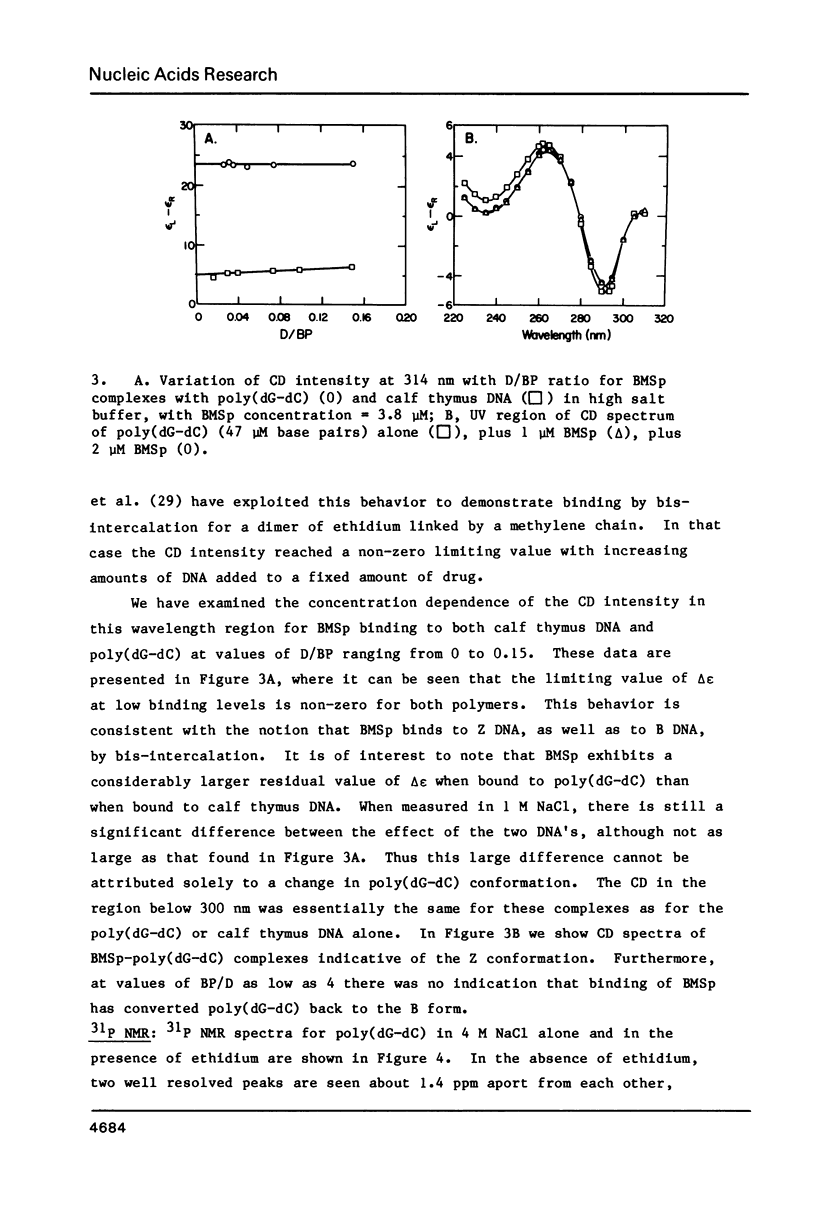
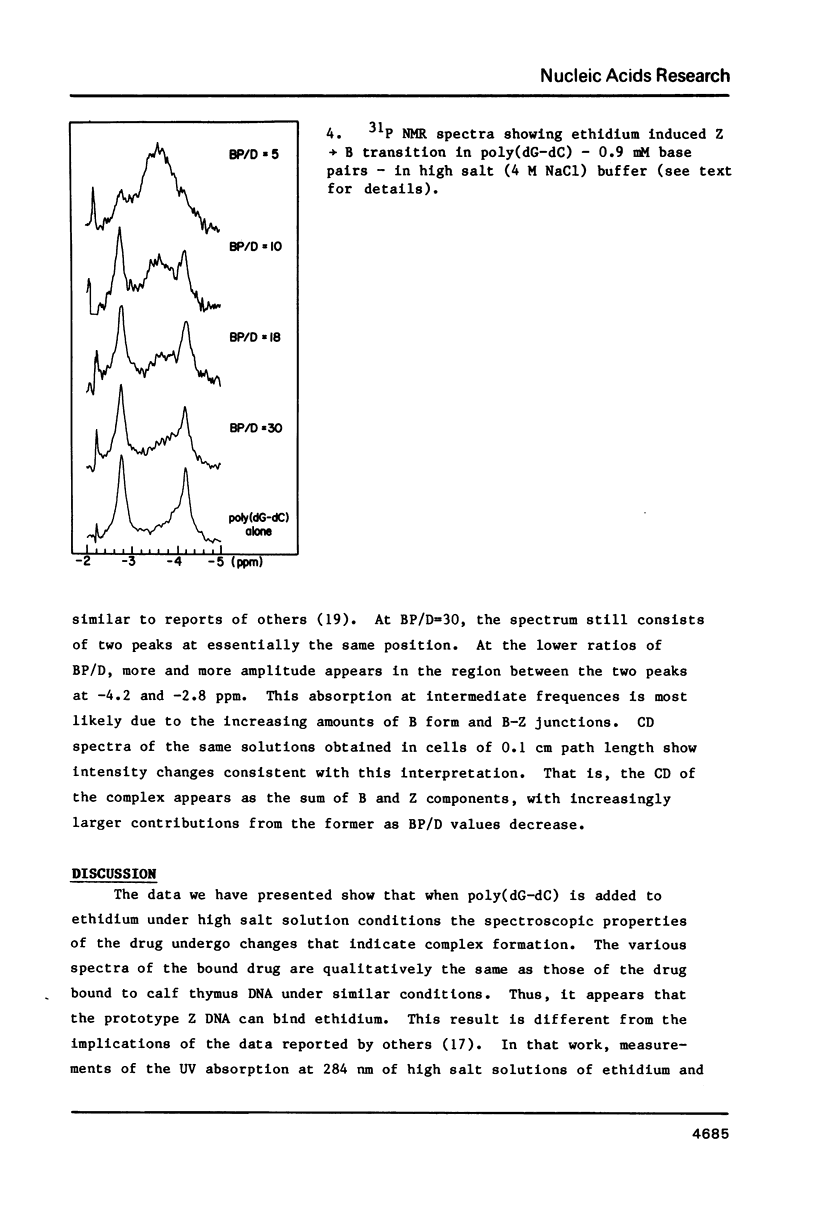
The interaction of ethidium bromide, a DNA intercalating drug, and bis( methidium )spermine, a DNA bis-intercalating compound, with the left-handed Z form of poly(dG-dC) has been studied in 4.4 M NaCl. Spectrophotometric analysis using absorption, fluorescence and circular dichroism indicates that the complex formed between ethidium and Z DNA resembles very closely that formed with B DNA. This suggests that ethidium binds to Z DNA by intercalation. 31P NMR spectra are presented showing both the conversion of the Z form to the B form with increasing amounts of drug and the typical Z form spectrum at low binding densities. Data are also presented which show that the bifunctional intercalator bis( methidium )spermine binds to Z DNA in a manner similar to its binding to B DNA, i.e., by bis-intercalation. These results are important for our understanding the behavior of Z DNA and its biological significance.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktipis S., Kindelis A. Optical properties of the deoxyribonucleic acid-ethidium bromide complex. Effect of salt. Biochemistry. 1973 Mar 13;12(6):1213–1221. doi: 10.1021/bi00730a031. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish D. G., Peacocke A. R. The circular dichroism in the ultraviolet of aminoacridines and ethidium bromide bound to DNA. Biopolymers. 1971 Oct;10(10):1853–1863. doi: 10.1002/bip.360101008. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Gupta G., Dhingra M. M., Sarma R. H. Left-handed intercalated DNA double helix: rendezvous of ethidium and actinomycin D in the Z-helical conformation space. J Biomol Struct Dyn. 1983 Oct;1(1):97–113. doi: 10.1080/07391102.1983.10507428. [DOI] [PubMed] [Google Scholar]
- Houssier C., Hardy B., Fredericq E. Interaction of ethidium bromide with DNA. Optical and electrooptical study. Biopolymers. 1974 Jun;13(6):1141–1160. doi: 10.1002/bip.1974.360130607. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Lanier A. C., Keel R. A., Wilson W. D. The effect of ionic strength on DNA-ligand unwinding angles for acridine and quinoline derivatives. Nucleic Acids Res. 1980 Apr 11;8(7):1613–1624. doi: 10.1093/nar/8.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann K. F., Mosher C. W., Hammen R. F. Induced circular dichroism as an indicator of double intercalation. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1172–1179. doi: 10.1016/0006-291x(80)90410-6. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemeunier F., Derbin C., Malfoy B., Leng M., Taillandier E. Identification of left-handed Z-DNA by indirect immunofluorescence in polytene chromosomes of Chironomus thummi thummi. Exp Cell Res. 1982 Oct;141(2):508–513. doi: 10.1016/0014-4827(82)90245-2. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Rousseau N., Leng M. Interaction between antibodies to Z-form deoxyribonucleic acid and double-stranded polynucleotides. Biochemistry. 1982 Oct 26;21(22):5463–5467. doi: 10.1021/bi00265a013. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- McIntosh L. P., Grieger I., Eckstein F., Zarling D. A., van de Sande J. H., Jovin T. M. Left-handed helical conformation of poly[d(A-m5C).d(G-T)]. Nature. 1983 Jul 7;304(5921):83–86. doi: 10.1038/304083a0. [DOI] [PubMed] [Google Scholar]
- Mirau P. A., Kearns D. R. The effect of intercalating drugs on the kinetics of the B to Z transition of poly(dG-dC). Nucleic Acids Res. 1983 Mar 25;11(6):1931–1941. doi: 10.1093/nar/11.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenegg G., Celio M. R., Malfoy B., Leng M., Kuenzle C. C. Z-DNA immunoreactivity in rat tissues. Nature. 1983 Jun 9;303(5917):540–543. doi: 10.1038/303540a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J., 3rd, Kearns D. R. Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry. 1977 Aug 9;16(16):3647–3654. doi: 10.1021/bi00635a022. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Ramstein J., Leng M. Salt-dependent dynamic structure of poly(dG-dC) x poly(dG-dC). Nature. 1980 Nov 27;288(5789):413–414. doi: 10.1038/288413a0. [DOI] [PubMed] [Google Scholar]
- Rio P., Leng M. Preferential binding of the chemical carcinogen N-hydroxy-2-aminofluorene to B-DNA as compared to Z-DNA. Nucleic Acids Res. 1983 Jul 25;11(14):4947–4956. doi: 10.1093/nar/11.14.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Chain flexibility and hydrodynamics of the B and Z forms of poly(dG-dC).poly(dG-dC). Nucleic Acids Res. 1983 Mar 25;11(6):1919–1930. doi: 10.1093/nar/11.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlíckovă M., Kypr J., Stokrová S., Sponar J. A Z-like form of poly(dA-dC).poly(dG-dT) in solution? Nucleic Acids Res. 1982 Feb 11;10(3):1071–1080. doi: 10.1093/nar/10.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Jones R. L. Interaction of actinomycin D, ethidium, quinacrine, daunorubicin, and tetralysine with DNA: 31P NMR chemical shift and relaxation investigation. Nucleic Acids Res. 1982 Feb 25;10(4):1399–1410. doi: 10.1093/nar/10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Tymen S., Marck C., Guschlbauer W. Conformational transitions of poly(dA-dC).poly(dG-dT) induced by high salt or in ethanolic solution. Nucleic Acids Res. 1982 Feb 11;10(3):1081–1091. doi: 10.1093/nar/10.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


