Abstract
The keratinocytes of the skin are unique in being not only the primary source of vitamin D for the body, but in possessing the enzymatic machinery to metabolize vitamin D to its active metabolite 1,25(OH)2D. Furthermore, these cells also express the vitamin D receptor (VDR) that enables them to respond to the 1,25(OH)2D they produce. Numerous functions of the skin are regulated by 1,25(OH)2D and/or its receptor. These include inhibition of proliferation, stimulation of differentiation including formation of the permeability barrier, promotion of innate immunity, and promotion of the hair follicle cycle. Regulation of these actions is exerted by a number of different coregulators including the coactivators DRIP and SRC, the cosuppressor hairless (Hr), and β-catenin. This review will examine the regulation of vitamin D production and metabolism in the skin, and explore the various functions regulated by 1,25(OH)2D and its receptor.
Keywords: CYP27B1, differentiation, skin cancer, innate immunity
1 Introduction
The epidermis is the major source of vitamin D for the body. However, the keratinocytes within the epidermis are further capable of metabolizing the vitamin D to its active metabolite, 1,25(OH)2D. 1,25(OH)2D, acting through the vitamin D receptor (VDR), regulates epidermal proliferation in the basal layer (stratum basale) and promotes the sequential differentiation of keratinocytes as they form the upper layers of the epidermis. Loss of VDR or loss of the capacity to produce 1,25(OH)2D (CYP27B1 mutations/deletion) disrupts differentiation of the epidermis and results in hyperproliferation of the basal layers. The keratinocytes lining the outer layer of the hair follicle (the outer root sheath or ORS) also possess VDR. Loss of VDR function either by inactivating mutations or bioengineered deletions leads to loss of hair follicle cycling and alopecia. In this case, it is less obvious that the VDR requires 1,25(OH)2D for its activity in that deletion of CYP27B1 does not produce alopecia. VDR also functions as a tumor suppressor, a function seen in other epithelial tissues such as the colon, breast and prostate. As for hair follicle cycling, the role of 1,25(OH)2D in this tumor suppressor function is not clear. The specificity of VDR action within the skin for the different functions it regulates is attributed at least in part to the different coregulators that modulate its genomic actions. In the proliferating keratinocytes of the epidermis and hair follicle, the DRIP complex (vitamin D receptor interacting protein complex) also known as Mediator is the dominant coregulator. In the more differentiated keratinocytes of the epidermis, the SRC (steroid receptor coactivator) complexes (SRC 2 and 3) dominate VDR function. In the hair follicle, the coregulator hairless (Hr) plays an important role. For 1,25(OH)2D regulated VDR actions, Hr acts as a cosuppressor. But its interaction with VDR in regulating hair follicle cycling, a 1,25(OH)2D independent action of VDR, is less clear. In this review we will examine the production of vitamin D and its subsequent metabolism to 1,25(OH)2D, then review the different actions of 1,25(OH)2D and its receptor in the skin, emphasizing the many roles vitamin D signaling plays in regulating epidermal proliferation and differentiation, hair follicle cycling, and tumorigenesis.
2 Vitamin D Production and Metabolism in the Skin
2.1 Vitamin D3 production
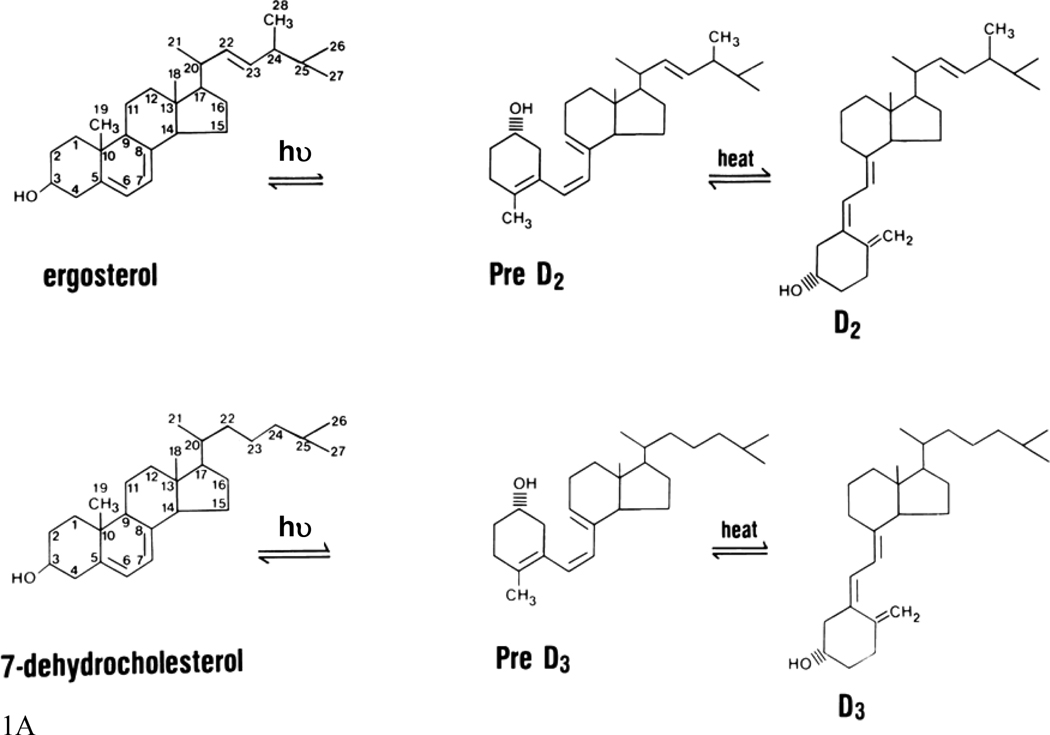
Vitamin D3 is produced from 7-dehydrocholesterol (7-DHC) (figure 1). Although irradiation of 7-DHC was known to produce pre-D3 (which subsequently undergoes a temperature rearrangement of the triene structure to form D3), lumisterol, and tachysterol, the physiologic regulation of this pathway was not well understood until the studies of Holick and colleagues (Holick, et al. 1979, Holick, et al. 1980, Holick, et al. 1981). They demonstrated that the formation of pre-D3 under the influence of solar or UVB irradiation (maximal effective wavelength between 280–320) is relatively rapid and reaches a maximum within hours. UV irradiation further converts pre-D3 to lumisterol and tachysterol. Both the degree of epidermal pigmentation and the intensity of exposure correlate with the time required to achieve this maximal concentration of pre-D3, but do not alter the maximal level achieved. Although pre-D3 levels reach a maximum level, the biologically inactive lumisterol accumulates with continued UV exposure. Tachysterol is also formed, but like pre-D3, does not accumulate with extended UV exposure. The formation of lumisterol is reversible and can be converted back to pre-D3 as pre-D3 levels fall. At 0°C, no D3 is formed; however, at 37°C pre-D3 is rapidly converted to D3. Prolonged exposure to sunlight would not produce toxic amounts of D3 because of the photoconversion of pre-D3 to lumisterol and tachysterol as well as the photoconversion of D3 itself to suprasterols I and II and 5,6 transvitamin D3 (Webb, et al. 1989). Thus, stimulation of epidermal D3 production is a safe way to provide D3 to the body.
Figure 1. The production of vitamin D and its subsequent metabolism.
A. Vitamin D production. Sunlight (the ultraviolet B component) breaks the B ring of the precursor sterol to form pre- D2 or pre-D3 from ergosterol or 7-dehydrocholesterol, respectively. In a temperature dependent step the A ring is rotated around the C5 to C6 double bond so that the 3β-hydroxyl group is positioned below the plane of the A ring to form D2 and D3.
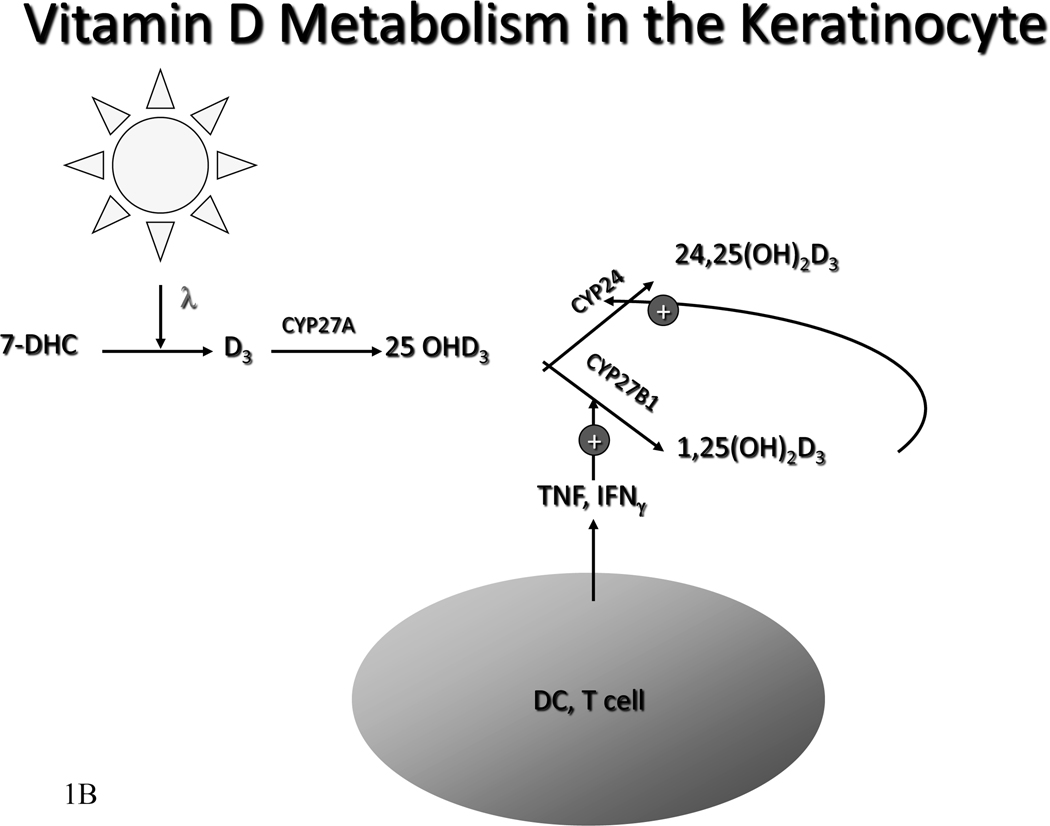
B. Metabolism of Vitamin D in the keratinoocyte. In the rest of the body the liver converts vitamin D to 25OHD. The kidney converts 25OHD to 25OHD and 1,25(OH)2D. Regulation of CYP27B1 in the kidney is exerted by calcium, phosphorus, parathyroid hormone, FGF23, and 1,25(OH)2D itself. However, in the keratinocyte, vitamin D can be directly metabolized to 25OHD and further metabolized to 25OHD and 1,25(OH)2D. Cytokines such as TNF and IFN-γ are the principal regulators of CYP27B1 in the keratinocyte.
Melanin in the epidermis, by absorbing UV irradiation, can reduce the effectiveness of sunlight in producing D3 in the skin. This may be one important reason for the lower 25OHD levels in dark skinned individuals living in temperate latitudes (Bell, et al. 1985). Sunlight exposure increases melanin production, and so provides another mechanism by which excess D3 production can be prevented. The intensity of UV irradiation is also important for effective D3 production. The seasonal variation of 25OHD levels can be quite pronounced with higher levels during the summer months and lower levels during the winter in areas furthest from the equator. In Edmonton, Canada (52°N), for example, very little D3 is produced in exposed skin from mid-October to mid-April; Boston (42 °N) has a somewhat longer period for effective D3 production; whereas in Los Angeles (34 °N) and San Juan (18 °N) the skin is able to produce D3 all year long (Webb, et al. 1988). Peak D3 production occurs around noon, with a larger portion of the day being capable of producing D3 in the skin during the summer than other times of the year. Clothing (Matsuoka, et al. 1992) and sunscreens (Matsuoka, et al. 1987) effectively prevent D3 production in the covered areas. This is one likely explanation for the observation that the Bedouins in the Middle East, who totally cover their bodies with clothing, are more prone to develop rickets and osteomalacia than the Israeli Jews with comparable sunlight exposure.
2.2 Metabolism of D3 to its biologically active products
Keratinocytes are not only capable of producing D3 but of metabolizing D3 via the vitamin D-25 hydroxylase (CYP27A) and 25OHD-1α-hydroxylase (CYP27B1) to its active metabolite 1,25(OH)2D3 (Bikle, et al. 1986a, Bikle, et al. 1986b, Lehmann, et al. 2001, Matsumoto, et al. 1991, Fu et al. 1997). Keratinocytes are the only cells in the body containing the entire pathway (Figure 1). Most of the circulating 1,25(OH)2D3 is produced by the kidney. However, the expression of CYP27B1 is higher in the keratinocyte than in any other cell including the cells of the proximal renal tubule. Presumably the 1,25(OH)2D3 produced in the skin is used for autocrine or paracrine purposes.
Parathyroid hormone (PTH) exerts a modest stimulation of 1,25(OH)2D production by keratinocytes. However, this involves a different mechanism than that resulting in stimulation of 1,25(OH)2D3 production by PTH in the kidney. The keratinocyte does not have a classic PTH receptor coupled to adenylate cyclase. Furthermore, these effects of PTH are not reproduced by cAMP or its membrane-permeable derivatives, suggesting that the actions of PTH may be operating through a mechanism independent of cAMP (Bikle, et al. 1986a). The effects of PTH are maximal after a 4-hr incubation of cells with these agents before adding substrate (25OHD3); that is, the effects are not immediate. In renal cells PTH exerts a more acute stimulation of 1,25(OH)2D3 production (Rasmussen, et al. 1972), and cAMP appears to play a second messenger role (Rost, et al. 1981). The mechanism by which PTH stimulates 1,25(OH)2D3 production in the keratinocyte remains unclear.
1,25(OH)2D3 negatively regulates its own levels within the keratinocyte. This negative feedback loop is similar to that observed in the kidney, but it differs from that seen in the macrophage, which lacks this feedback loop. In the keratinocyte, this feedback inhibition is not mediated by an effect on 1,25(OH)2D3 production but is due solely to induction of 25OHD 24-hydroxylase (CYP24A) that converts 25OHD3 and 1,25(OH)2D3 to 24,25(OH)2D3 and 1,24,25(OH)3D3, respectively (Xie et al. 2002b), and then on to further catabolic products. The exquisite responsiveness of CYP24A to 1,25(OH)2D3 in keratinocytes may explain why so little 1,25(OH)2D3 appears to enter the circulation from the skin when renal production of 1,25(OH)2D3 is intact.
The expression and activity of CYP27B1 changes with differentiation (Pillai, et al. 1988a). Enzymatic activity is greatest in the undifferentiated cells. Growing the cells in 0.1 mM calcium, which retards differentiation (Pillai, et al. 1988b), permits the cells to maintain higher activity than when they are grown in 1.2 mM calcium (Bikle, et al. 1989), although acute changes in calcium have little effect on 1,25(OH)2D3 production (Bikle, et al. 1986a). These observations in vitro are consistent with the finding that CYP27B1 expression is highest in the stratum basale of the epidermis in vivo (Zehnder, et al. 2001).
Both tumor necrosis factor-α (TNFα) and interferon-γ (IFNγ) stimulate 1,25(OH)2D3 production by keratinocytes (Bikle, et al. 1989, 1991b, Morhenn and Wood 1988, Pillai, et al. 1989) (figure 1). These cytokines are stimulated by ultraviolet light (Trefzer, et al. 1993) and barrier disruption (Wood, et al. 1994) and contribute to the increased CYP27B1 expression following such environmental stimuli (Loser and Beissert 2009, Schauber, et al. 2007).
3 Vitamin D regulation of epidermal proliferation and differentiation
3.1 The differentiation process
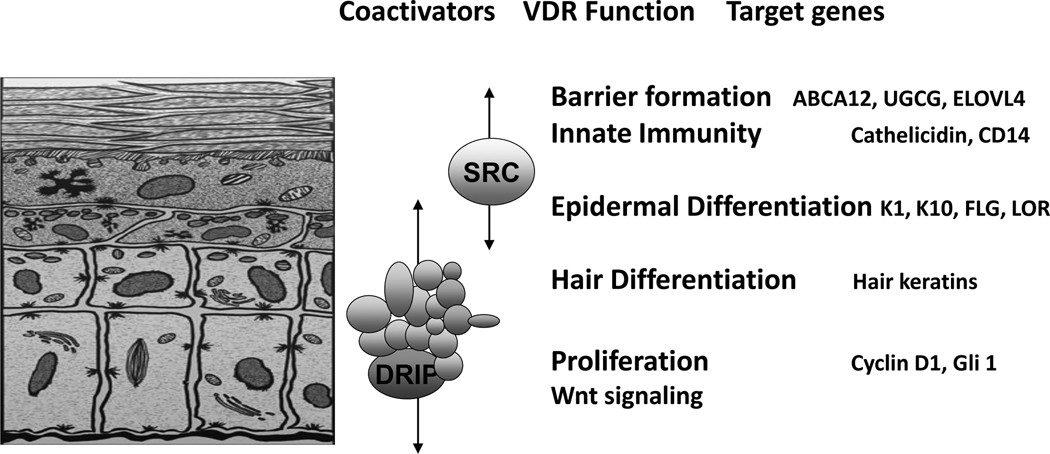
The epidermis is composed of four layers of keratinocytes at different stages of differentiation (figure 2). The basal layer (stratum basale) rests on the basal lamina separating the dermis and epidermis. These cells proliferate, providing the cells for the upper differentiating layers. They are large, columnar cells forming intercellular attachments with adjacent cells through desmosomes. An asymmetric distribution of integrins on their lateral and basal surface may also regulate their attachment to the basal lamina and adjacent cells (Guo, et al. 1991, Marchisio, et al. 1991, Peltonen, et al. 1989). They contain an extensive keratin network comprising principally keratins K5 (58 kDa) and K14 (50 kDa) (Moll, et al. 1982). As these cells migrate outward from this basal layer, they acquire the characteristics of a fully differentiated corneocyte, which is eventually sloughed off.
Figure 2. The different layers of the epidermis, and the functions within those layers regulated by VDR and its coactivators.
The basal layer of the epidermis (stratum basale) contains the stem cells that through proliferation provide the cells for the upper layers. As the keratinocytes leave the basal layer differentiation takes place with K1, K10, involucrin, and transglutaminase being expressed in the stratum spinosum, filaggrin and loricrin being expressed in the stratum granulosum. Lamellar bodies forming in the stratum granulosum inject their lipid content into the intercellular spaces between the stratum granulosum and stratum corneum to provide the water proofing for the permeability barrier. DRIP205 is most highly expressed in the stratum basale and spinosum where it participates with VDR in regulating proliferation in partnership with the wnt/β-catenin signaling pathway. Cyclin D1 and Gli 1 (a transcriptional factor for the hedgehog pathway) are regulated by VDR, DRIP205, and β-catenin. SRC3 on the other hand is found in highest concentration in the stratum granulosum where it participates with VDR in the regulation of terminal differentiation. SRC3 is critical for lipid processing and lamellar body formation required for formation of the permeability barrier as well as 1,25(OH)2D induction of cathelicidin and CD14 required for innate immunity.
The layer above the basal cells is the spinous layer (stratum spinosum). These cells initiate the production of the keratins K1 and K10, which are the keratins characteristic of the more differentiated layers of the epidermis (Eichner, et al. 1986). Cornified envelope precursors such as involucrin (Warhol, et al. 1985) also appear in the spinous layer as does the enzyme transglutaminase, responsible for the ε–(γ-glutamyl) lysine cross-linking of these substrates into the insoluble cornified envelope (Thacher and Rice 1985). The keratinocyte contains both the soluble (tissue, TG-C, or type II) and membrane-bound (particulate, TG-K, or type I) forms of transglutaminase. It is the membrane-bound form that correlates with differentiation and is thought to be responsible for the formation of the cornified envelope (Thacher and Rice 1985). The granular layer (stratum granulosum), lying above the spinous layer, is characterized by electron-dense keratohyalin granules. These are of two types (Steven, et al. 1990). The larger of the two granules contains profilaggrin, the precursor of filaggrin, a protein thought to facilitate the aggregation of keratin filaments (Dale, et al. 1985). The smaller granule contains loricrin, a major component of the cornified envelope (Mehrel, et al. 1990). The granular layer also contains lamellar bodies, lipid-filled structures that fuse with the plasma membrane, divesting their contents into the extracellular space where the lipid contributes to the permeability barrier of skin (Elias, et al. 1988). As the cells pass from the granular layer to the cornified layer (stratum corneum), they undergo destruction of their organelles with further maturation of the cornified envelope into an insoluble, highly resistant structure surrounding the keratin-filaggrin complex and linked to the extracellular lipid milieu (Hohl 1990). The outer layer of the epidermis provides not only a barrier to water loss (permeability barrier) but a barrier to invasion by infectious organisms via its expression of the innate immune system. In particular disruption of the barrier triggers the induction of defensins such as cathelicidin that provide the initial defense in killing such organisms (Hennings and Holbrook 1983). Calcium forms a steep gradient within the epidermis, with highest concentration in the stratum granulosum (Menon, et al. 1985). Disruption of the permeability barrier by removing the stratum corneum or extracting its lipids leads to a loss of this calcium gradient (Mauro, et al. 1998) resulting in increased lamellar body secretion but reduced expression of the genes for loricrin, profilaggrin, and involucrin (Elias, et al. 2002).
3.2 Role of calcium and 1,25(OH)2D
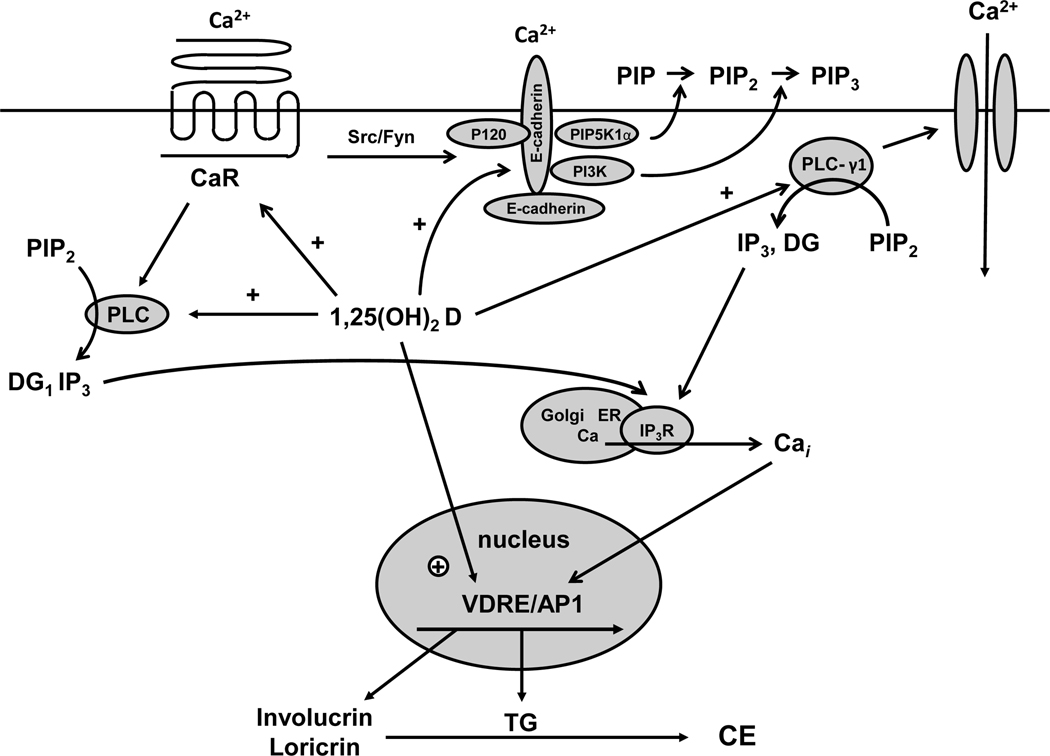
Both calcium and 1,25(OH)2D play important and interacting roles in regulating the differentiation process (figure 3). 1,25(OH)2D increases the expression of involucrin, transglutaminase, loricrin, and filaggrin and increases cornified envelope formation (Bikle, et al. 1991a, Bikle and Pillai 1993, Hawker, et al. 2007, Hosomi, et al. 1983, McLane, et al. 1990, Pillai and Bikle 1991, Smith, et al. 1986) while inhibiting proliferation. These actions are due at least in part to the ability of 1,25(OH)2D to increase intracellular calcium levels achieved by induction of the calcium receptor (CaR) (Ratnam, et al. 1999) and the phospholipases C (PLC) (Pillai et al. 1995) that are critical for the ability of calcium to stimulate keratinocyte differentiation (Tu, et al. 2001, Xie and Bikle 1999, 2001). The antiproliferative effects are accompanied by a reduction in the mRNA levels for c-myc (Matsumoto, et al. 1990) and cyclin D1 and an increase in the cell cycle inhibitors p21cip and p27kip. In addition, 1,25(OH)2D and its receptor regulate the processing of the long chain glycosylceramides that are critical for permeability barrier formation (Oda, et al. 2009) and induce the receptors, toll like receptor 2 (TLR2) and its coreceptor CD14, that initiate the innate immune response in skin (Schauber, et al. 2007). Activation of these receptors leads to the induction of CYP27B1, which in turn induces cathelicidin resulting in the killing of invasive organisms(Schauber, et al. 2006, Schauber, et al. 2007). Mice lacking the VDR or the enzyme (CYP27B1) producing its ligand 1,25(OH)2D show defective epidermal differentiation manifesting as reduced levels of involucrin and loricrin and loss of keratohyalin granules(Bikle, et al. 2006, Xie, et al. 2002a), decreased lipid content of the lamellar bodies leading to a defective permeability barrier (Oda, et al. 2009), and a defective response of the innate immune system to wounding (Schauber, et al. 2007).
Figure 3. Calcium and 1,25(OH)2D interactions in the regulation of keratinocyte differentiation.
Critical steps in calcium induced keratinocyte differentiation involve activation of the calcium sensing receptor (CaR) and formation of the E-cadherin complex at the membrane. This complex includes a number of catenins and phospholipid modifying enzymes (PI3K, PIP5K1α) that are critical for subsequent differentiation events. In particular the activation of phospholipase C-γ1 (PLC-γ1), the enzyme largely responsible for maintaining increased levels of intracellular calcium (Cai) through its effects on both plasma membrane calcium channels and in the production of IP3 from PIP2 for stimulation of calcium release from intracellular stores, requires the formation of the E-cadherin complex. Hydrolysis of PIP2 by PLC-γ1 also leads to diacylglycerol production (DG) and activation of protein kinases C, that also play an important role in keratinocyte differentiation. Within hours of the calcium switch keratinocytes change from making the basal keratins K5 and K14 to making keratins K1 and K10 followed, subsequently, by increased levels of profilaggrin, involucrin and loricrin. Loricrin, involucrin and other proteins are cross linked into the insoluble cornified envelope by the calcium sensitive, membrane bound form of transglutaminase, which like involucrin and loricrin increases within 24 hours after the calcium switch. The induction of these proteins represents a genomic action (likely indirect) of calcium as indicated by a calcium induced increase in mRNA levels and transcription rates. The CaR by regulating phospholipase C (PLC) activity controls the production of inositol tris phosphate (IP3) and diacyl glycerol (DG). PLC-β is activated directly by CaR via a G protein coupled mechanism, whereas PLC-γ1 is activated by phosphatidyl inositol tris phosphate (PIP3), levels that are maintained in the membrane by phosphatidyl inositol 3 kinase (PI3K) bound to the E-cadherin complex. CaR regulates E-cadherin complex formation through src/fyn tyrosine kinases that phosphorylate the catenins and PI3K essential for their binding to E-cadherin. Extracellular calcium (Cao) activates these processes through the CaR and by stabilizing E-cadherin membrane localization. 1,25(OH)2D modulates calcium regulated differentiation at several steps. First, 1,25(OH)2D increases CaR expression, thus making the cell more responsive to calcium. Second, 1,25(OH)2D induces all the PLCs, as does calcium, again increasing the responsiveness of the cell to calcium. Third, the VDR is required for calcium induced formation of the E-cadherin complex in the membrane, in part through induction of E-cadherin as well as CaR. Finally, 1,25(OH)2D induces the transcription of genes such as involucrin and transglutaminase and possibly the other differentiation markers in addition to PLC and CaR.
4 Role of VDR coactivators in epidermal proliferation and differentiation
4.1 VDR coactivator overview
The process of epidermal differentiation is sequential. 1,25(OH)2D and VDR regulate all steps from the control of proliferation in the SB, to the regulation of K1, K10, involucrin, and transglutaminase production in the SS, to the regulation of loricrin and filaggrin production in the SG, to the synthesis of lipids required for the permeability barrier in the SC, and to the development of the innate immune system(Bikle and Pillai 1993, Hawker, et al. 2007, Schauber, et al. 2007). How does this occur? Although CYP27B1 and VDR are found in highest concentration in the SB, they are both distributed throughout the epidermis(Milde, et al. 1991, Stumpf, et al. 1984, Zehnder, et al. 2001), so this does not provide an obvious explanation for the sequential induction of genes involved in the differentiation process. However, VDR requires the binding of coactivators to stimulate transcription. The two major coactivator complexes in the epidermis are DRIP (Mediator) and SRC (McKenna, et al. 1999, Oda, et al. 2003). We (Oda, et al. 2003) observed that in proliferating keratinocytes DRIP was the major coactivator complex binding to VDR, whereas the SRC complex dominated VDR binding in differentiated keratinocytes(Oda, et al. 2003, Oda, et al. 2007). These results are consistent with our finding that in the epidermis DRIP205 (Med1) is expressed in highest concentration in the SB and SS, whereas SRC3 is expressed in highest concentration in the SG(Oda, et al. 2007, Schauber, et al. 2008). The DRIP complex is anchored to VDR via DRIP205 (Rachez, et al. 1999, Rachez, et al. 2000). The SRC complex is anchored to VDR with one of three homologous proteins, SRC1, 2, and 3(Leo and Chen 2000), but only SRC2 and 3 are found in keratinocytes(Oda, et al. 2003). These coactivator complexes interact with the C terminal (AF2) domain of VDR following ligand binding via LxxLL motifs (NR boxes). They do not bind to VDR at the same time, competing as they do for the same region of the VDR(Rachez, et al. 2000). The SRC coactivators have 3 NR boxes, whereas DRIP205 has 2. Our recent studies (Teichert, et al. 2009) indicate that VDR binds most strongly to the 2nd and 3rd NR box of SRC1 and 2, the 3rd NR box of SRC3, and the 2nd NR box of DRIP205. Different nuclear hormone receptors differ in the affinity for the different NR boxes of the different coactivators suggesting some degree of specificity (Acevedo, et al. 2004). SRC recruits CREB-binding protein (CBP) or P300 and other histone acetyl transferases (HATs) and methyltranferases (MeTs) to the VDR resulting in a multisubunit complex(Christakos, et al. 2003). The HAT and MeT activity of the SRC complex is thought to destabilize the interaction between DNA and the histone core, enabling transcription to occur. The DRIP complex does not have HAT or MeT activity but functions, at least in part, through recruitment of RNA polymerase II to the transcription start site(Rachez, et al. 2000, Rachez and Freedman 2000). Some studies suggest that the order of coactivator binding to its nuclear hormone receptor is sequential with different kinetics, generally with SRC binding preceding and being required for DRIP binding(Acevedo, et al. 2004). Other studies indicate that the specificity of coactivator binding to VDR depends on the gene being regulated(Carvallo, et al. 2008, Issa, et al. 2002), the ligand being evaluated(Bouillon, et al. 2005), and the cellular context(Maeda, et al. 2002, Peleg, et al. 2003). Our data, summarized below, indicate that the sequential action of 1,25(OH)2D and its receptor on keratinocyte differentiation is due to the differential expression and distribution of these coactivators according to the differentiation status of the cell coupled with the selectivity of genes regulated by VDR for one or the other of the coactivator complexes.
4.2 Coactivator regulation of VDR function in the skin
DRIP205 is expressed in proliferating keratinocytes, and its expression decreases with differentiation, as the expression of SRC3 is increased(Oda, et al. 2003). Knockdown of DRIP205 using siRNA results in increased keratinocyte proliferation, similar to that seen by knocking down VDR itself, but knockdown of SRC3 does not show such an effect (Oda, et al. 2007). Inhibition of DRIP205 binding to VDR in proliferating keratinocytes blocks VDR transcriptional activity with a VDRE reporter construct, but such inhibition is not seen in differentiated keratinocytes(Oda, et al. 2003). However, this inhibition of transcriptional activity turns out to be gene specific. For example, knockdown of DRIP205 using siRNA methodology has a greater impact on keratins 1, 10, and involucrin expression than does knockdown of SRC3, although depletion of both coactivators profoundly reduces loricrin and filaggrin expression(Hawker, et al. 2007). On the other hand SRC3 knockdown, not DRIP205 knockdown, reduces glucosylceramide production and lamellar body formation similar to that of VDR knockdown(Oda, et al. 2009), and prevents 1,25(OH)2D induced cathelicidin expression(Schauber, et al. 2008). Thus, our hypothesis is that SRC facilitates the ability of VDR to regulate the more differentiated functions of the keratinocyte, whereas DRIP facilitates the ability of VDR to regulate proliferation and early keratinocyte differentiation, although some overlap in coactivator function is observed.
5 Role of VDR in hair follicle cycling
5.1 The hair follicle cycle
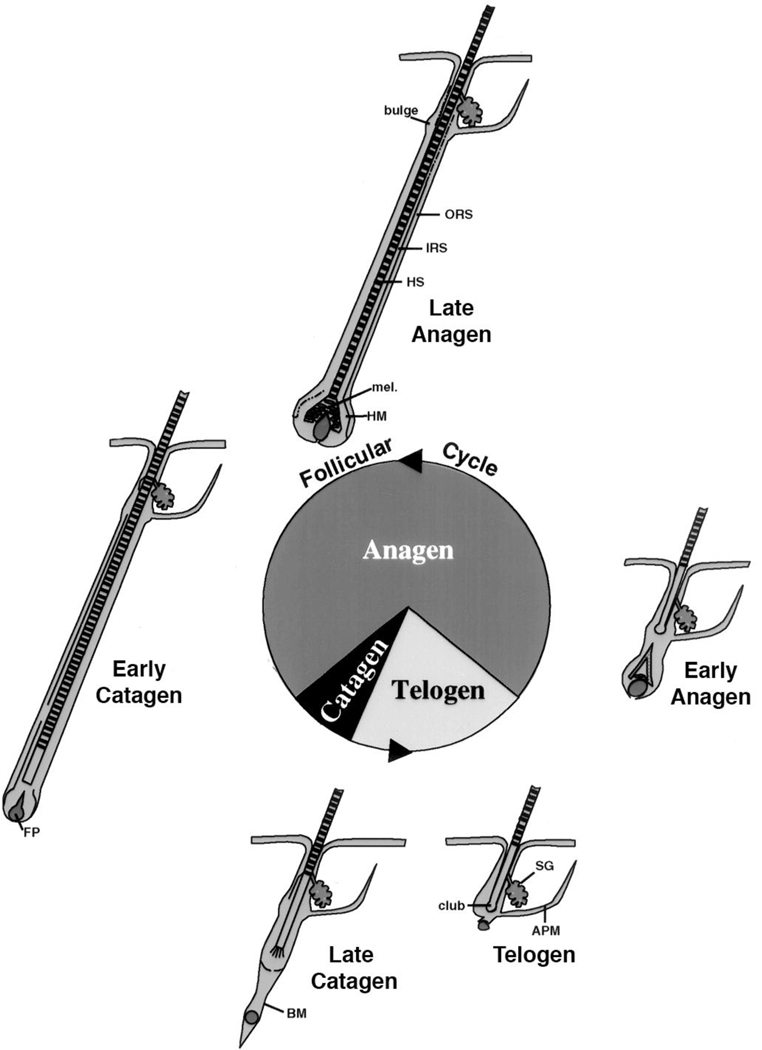
The hair follicle cycle is divided into three main stages: anagen, catagen, and telogen (figure 4). Anagen is the stage of hair follicle growth; catagen is the stage of regression; telogen is the resting stage. Only the proximal or dermal portion of the hair follicle cycles; the distal or epidermal portion does not. The duration of these stages in a given species varies from location to location on the body and between genders. Furthermore, there are two types of cycles: developmental and postnatal. The developmental cycle is initiated during embryogenesis. The follicle develops from specific regions of the epidermis called placodes. The follicle is induced to grow by its interaction with a collection of specialized mesenchymal cells in the dermis called the dermal papilla. Wnt signaling (β-catenin) appears to be necessary to maintain the ability of the dermal papilla to stimulate hair follicle growth (Kishimoto, et al. 2000, Shimizu and Morgan 2004). Following the developmental cycle, which leads to the initial coat of hair, the follicle undergoes repetitive cycling until senescence. The length of the hair is dependent on the duration of anagen. During this stage the follicle grows through the dermis into the subcutaneous tissue. As the follicle develops different cell layers appear. The outer root sheath (ORS) is a direct extension of the stratum basale, and separates the hair follicle from the surrounding connective tissue sheath (CTS). From outside in are found the companion layer, the three layers of the inner root sheath (IRS)--Henle’s layer, Huxley’s layer, cuticle of the IRS—and the hair shaft itself including the cuticle of the shaft, shaft cortex, and shaft medulla. Stem cells in the bulge are capable of generating all cells in the hair follicle and epidermis (Morris, et al. 2004). The keratins produced by the cells of the IRS and hair shaft differ from those expressed by epidermal keratinocytes (Langbein, et al. 2003). Of particular interest is these hair keratins have β-catenin/lef1 binding sites in their promoters that regulate their expression (Zhou, et al. 1995). Following anagen, the follicle enters catagen during which massive apoptosis occurs primarily in the cells of the proximal follicle (the dermal portion), and the hair shaft produced during anagen is generally shed. At the end of catagen the follicle enters telogen, the resting phase. Duration of telogen is highly variable. A new cycle then begins with anagen. The juxtaposition of the dermal papilla to the bulge is critical for this process to begin, and it is associated with increased proliferation of stem cells in the bulge with migration of cells from the bulge into the hair bulb to restart the growth of the hair follicle. The regulatory elements that control the transition from one stage to the next are not well understood.
Figure 4. Hair follicle cycling.
The initial developmental phase of hair follicle development terminates with catagen and the first telogen, after which repetitive cycles of anagen (the growth phase), catagen (the regression phase), and telogen (the resting phase) occur throughout the life span of the animal. In general the hair follicle spends most of its time in anagen. But cycle duration varies according to location, gender, age, species. The bulge is the source of stem cells for the regenerating hair follicle, responding to signals from the dermal or follicular papilla (FP). Although VDR is not required for the developmental phase of the hair follicle, it is essential for subsequent hair follicle cycling. ORS: outer root sheath, IRS: inner root sheath, HS: hair shaft, mel: melanin for the hair shaft, HM: hair matrix, BM: basement membrane, SG: sebaceous gland, APM: arrector pili muscle. Figure adapted from figure 1 in KS Stenn and R Paus, Physiol Rev:81:449–494, 2001.
5.2 Role of VDR in hair follicle cycling
Alopecia is a well-known part of the phenotype of many patients with mutations in their VDR (Hochberg, et al. 1985, Marx, et al. 1986), a syndrome known as hereditary vitamin D resistant rickets (HVDRR). Vitamin D deficiency per se or CYP27B1 mutations are not associated with alopecia, indicating that the regulation of hair follicle cycling requires VDR but not its principal ligand 1,25(OH)2D. VDR null mice develop their first coat of hair normally, but reinitiation of anagen following the first cycle or after depilation is impaired (Sakai and Demay 2000). Reconstitution of the VDR to the VDR null mouse skin using a keratinocyte specific promoter reverses the defect in hair growth without reversing the metabolic defects of skeletal growth retardation, hypocalcemia, and rickets otherwise associated with the VDR null condition (Chen, et al. 2001, Kong, et al. 2002). On the other hand, correction of the metabolic abnormalities with a high calcium diet prevents the rickets and hyperparathyroidism but does not prevent the alopecia (Li, et al. 1998). Furthermore, it is the lack of VDR in the keratinocyte as opposed to the dermal papilla that is critical. Dermal papilla cells obtained from either VDR null or wildtype mice can initially induce hair growth in a hair reconstitution assay when mixed with epidermal keratinocytes obtained from wildtype or VDR null mice, but if the hair grown with keratinocytes from VDR null mice is then depilated, anagen will not be reinitiated regardless of the source of dermal papilla cells (Sakai and Demay 2000).
5.3 Role of hairless (Hr) in the regulation of hair follicle cycling and its interactions with VDR
Hr mutations in both mice (Panteleyev, et al. 1999) and humans (Ahmad, et al. 1998, Miller, et al. 2001) result in phenocopies of the VDR null mouse and some human VDR mutations, respectively, with regard to the morphologic changes observed in hair follicle cycling. The dissociation of the dermal papilla from the hair bulb by the end of catagen is thought to account for the failure to initiate the subsequent anagen in both Hr mutant and VDR null mice (Bikle, et al. 2006, Panteleyev, et al. 1999). The distal (epidermal) portion of the hair follicle including the sebaceous gland as well as the interfollicular epidermis is less impacted (Bikle, et al. 2006, Panteleyev, et al. 1999, Xie, et al. 2002). We have found VDR and Hr in the nuclei of keratinocytes in the stratum basale and ORS (Bikle, et al. 2006). However, we found little or no VDR in the IRS and hair bulb or cells of the dermal papilla and CTS, whereas we did find Hr in those locations (Bikle, et al. 2006). As will be discussed subsequently, deletion of the transcriptional domain of β-catenin results in a similar phenotype (DasGupta, et al. 2002, Huelsken, et al. 2001).
Hr has characteristics of a coregulator in that it resides in the nucleus; its structure contains a nuclear localization signal, a putative zinc finger, and three LXXLL motifs (Djabali, et al. 2001) like that found in coactivators that interact with nuclear hormone receptors such as VDR as well as ΦXXΦΦ motifs (Φ=hydrophobic amino acid) similar to regions in corepressors like SMRT and NCoR responsible for the binding of these corepressors to nuclear hormone receptors. In the brain, Hr has been suggested as a corepressor of the thyroid receptor (THRb) in that Hr can bind to THRb and inhibit its transcriptional activity (Thompson and Bottcher 1997). However, Hr does not appear to regulate thyroid hormone action in the keratinocyte (Engelhard and Christiano 2004). Rather, VDR appears to be the target (Xie, et al. 2006). Hsieh et al. (Hsieh, et al. 2003) demonstrated that Hr could bind to VDR in COS cells. They noted that Hr bound to VDR in the same region predicted for corepressor binding, and different from the C-terminal region to which coactivators bind. The region of Hr responsible for VDR binding contains one LXXLL motif, but also a ΦXXΦΦ motif. However, only mutations in the ΦXXΦΦ motif altered binding to VDR (Hsieh, et al. 2003). Furthermore, when we tested both motifs separately for their binding to VDR, the ΦXXΦΦ motif had the higher affinity regardless of the presence or absence of 1,25(OH)2D (Teichert, et al. 2009). We have shown that the endogenous VDR binds to endogenous Hr in keratinocytes (Xie, et al. 2006). Binding of Hr to VDR inhibited 1,25(OH)2D stimulation of a CYP24A1 (24-hydroxylase) promoter construct containing the vitamin D response element (VDRE) of this vitamin D target gene. Overexpression of Hr blocks the ability of 1,25(OH)2D to induce differentiation markers in keratinocytes, whereas inhibition of Hr expression enhances the stimulation by 1,25(OH)2D of these markers (Xie, et al. 2006). The Hr null animal demonstrates upregulation of differentiation markers in the epidermis (Zarach, et al. 2004) consistent with a corepressor role for Hr in vitamin D regulated epidermal differentiation. Antibodies to Hr enhance the binding of VDR to VDREs in vitamin D target genes in gel retardation assays (Xie, et al. 2006) suggesting that Hr binding to VDR blocks its binding to VDREs. 1,25(OH)2D displaces Hr from VDREs as it recruits the coactivators DRIP205 and SRC3 to these same VDREs (Xie, et al. 2006). Thus at least for 1,25(OH)2D stimulated actions of VDR, Hr is a cosuppressor.
6 Role of β-catenin as VDR coregulator
6.1 Wnt/β-catenin signaling pathway
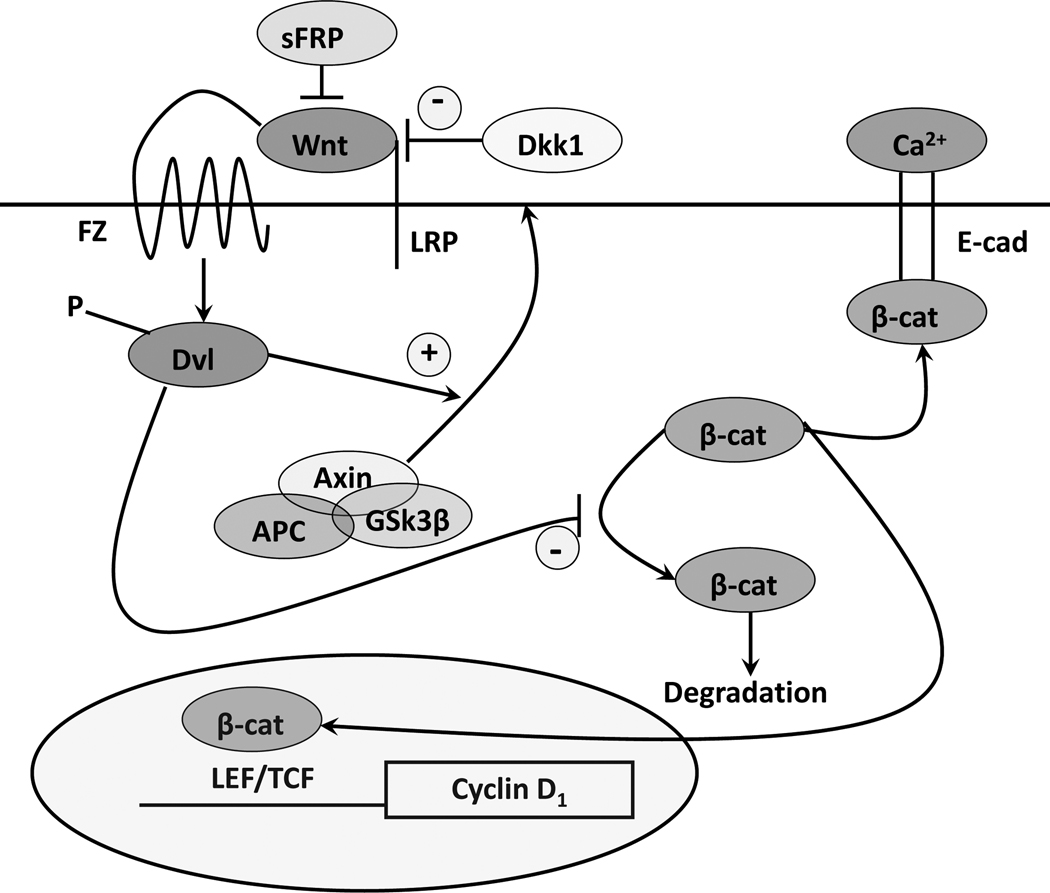
Wnt signaling via activation of β-catenin has a complex role in VDR actions (figure 5). Wnt ligands bind to their seven-transmembrane Frizzled receptors and an LRP5 or LRP6 co-receptor leading to phosphorylation of disheveled (Dvl) resulting in disruption of the axin/APC complex and inhibition of the kinase activity of glycogen synthase kinase-3β (GSK-3β)), which otherwise phosphorylates the serine(s) within exon 3 of β-catenin facilitating its degradation by the E3 ubiquitin ligase. Thus wnt signaling increases the availability of β-catenin in the cytoplasm, which can then bind to transcription factors of the T-cell factor (TCF) and lymphoid enhancer factor (LEF) families to promote expression of genes such as cyclin D1 and c-myc (He, et al. 1998) important for proliferation. β-catenin also forms part of the adherens junction complex with E-cadherin where it may play an important role in keratinocyte differentiation (Xie and Bikle 2007). Tyrosine phosphorylation of β-catenin, as occurs after calcium administration to keratinocytes, promotes the binding of β-catenin and other catenins to the adherens junction complex (Bienz 2005, Xie and Bikle 2007) making it less available for transcriptional activity. Cells differ markedly in the components of the β-catenin signaling pathway utilized. This is well illustrated in the keratinocytes of the hair follicle and interfollicular epidermis (DasGupta, et al. 2002, Huelsken, et al. 2001). LEF1 is the dominant transcription partner for β-catenin in the dermal portion of the hair follicle, which has little E-cadherin. The epidermal keratinocyte, on the other hand, has little LEF1 but a lot of E-cadherin especially in the differentiated layers(Stenn and Paus 2001). Over expression and/or activating mutations in the β-catenin pathway lead to skin tumors, in this case pilomatricomas or trichofolliculomas (hair follicle tumors) (Chan, et al. 1999, Gat, et al. 1998, Xia, et al. 2006) indicative of the hyperproliferative response to β-catenin in these cells. Activating mutations of specific serines within exon 3, or deletion of exon 3, block phosphorylation of β–catenin by GSK-3β, phosphorylation which otherwise leads to its proteosomal degradation. As a result β-catenin levels increase in the nucleus where its transcriptional activity is exerted in association with members of the LEF/TCF family of transcription factors.
Figure 5. The canonical wnt signaling pathway.
Wnts bind to their frizzled receptors (FZ) and coreceptors LRP in the membrane. This binding can be blocked by dickkopf (Dkk) or soluble frizzled related proteins (sFRP). Activation of FZ by wnt results in phosphorylation of disheveled (Dvl), which induces the disruption of the axin/APC/GSK-3β complex and recruitment of axin to the membrane. This complex when active phosphorylates β-catenin, leading to its proteosomal degradation. However, following wnt stimulation β-catenin is no longer degraded and can enter the nucleus where in combination with members of the LEF/TCF family can induce expression of its target genes such as cyclin D1. β-catenin also binds to the E-cadherin complex in the plasma membrane, binding which promotes differentiation. 1,25(OH)2D/VDR interact with wnt signaling at multiple points. 1,25(OH)2D/VDR binds β-catenin reducing its transcriptional activity. 1,25(OH)2D/VDR promotes the formation of the E-cadherin/catenin complex in the membrane also serving to reduce translocation of β-catenin into the nucleus.
6.2 β-catenin interactions with VDR
In colon cancer cells VDR has been shown to bind to β-catenin, and reduce its transcriptional activity in a ligand dependent fashion(Palmer, et al. 2001). Furthermore, in these cells 1,25(OH)2D has been shown to increase E-cadherin expression, such that β-catenin is redistributed from the nucleus to the plasma membrane where it forms a complex with E-cadherin and other catenins at adherens junctions (Shah, et al. 2003). However, the suppression of β-catenin signaling by 1,25(OH)2D does not necessarily require E-cadherin (Shah, et al. 2006). Rather β-catenin binds to VDR in its AF-2 domain, binding that enhances the ability of 1,25(OH)2D to activate the transcriptional activity of the VDR (Shah, et al. 2006) but blocks the transcriptional activity of β-catenin. Mutations in the AF-2 domain of VDR that block coactivator binding do not necessarily block β-catenin binding (Shah, et al. 2006). Whether β-catenin binding alters DRIP205 or SRC3 binding to this same region has not been determined. Palmer et al. (Palmer, et al. 2008) evaluated the interaction between VDR and β-catenin in transcriptional regulation in keratinocytes, and identified putative response elements for VDR and β-catenin/LEF in a number of genes. These interactions were either positive or negative, depending on the gene being evaluated. The hypothesis put forward is that genes in which the interaction is positive (ie. stimulated transcription) benefit from β-catenin acting as a coactivator for VDR on VDREs, whereas in situations where the interaction is negative (ie. suppression of transcription) VDR prevents β-catenin from binding to TCF/LEF required for transcription of those genes. We (Y Oda and D Bikle, unpublished) have found in keratinocytes that knockdown of VDR reduces E-cadherin expression and formation of the β-catenin/E-cadherin membrane complex resulting in increased β-catenin transcriptional activity, whereas 1,25(OH)2D administration has the opposite effect. This was associated with increased (with VDR and DRIP205 knockdown) or decreased (with 1,25(OH)2D administration) keratinocyte proliferation and cyclin D1 expression, respectively. Other studies suggest that VDR potentiates, not inhibits, β-catenin transcriptional activity. Cianferotti et al. (Cianferotti, et al. 2007) found a reduction in proliferation of keratinocytes in the dermal portion of the hair follicle (below the bulge) in VDR null mice, and no stimulation of proliferation when β-catenin was overexpressed in these cells in contrast to the stimulation of proliferation in control animals. Thus VDR/β-catenin interactions can be positive or negative, depending on the gene/cell/function being evaluated.
7 Conclusions
The skin is unique in being not only the source of vitamin D for the body but in being capable of producing and responding to the active metabolite of vitamin D, 1,25(OH)2D. 1,25(OH)2D and its receptor have many roles in the skin. Some of these roles-- induction of genes required for differentiation, suppression of genes involved with proliferation-- appear to require both 1,25(OH)2D and VDR and synergize with the actions of calcium. Other roles such as the regulation of hair follicle cycling require VDR but do not require 1,25(OH)2D. Different coactivator complexes including DRIP and SRC modulate the actions of VDR, and the choice of coactivator complex in many cases is gene specific. Regulation of proliferation is dependent on DRIP, whereas more differentiated functions including innate immunity and permeability barrier formation are SRC dependent. Hr is a coregulator with profound actions in hair follicle cycling. Although Hr blocks 1,25(OH)2D regulated VDR functions, its role in VDR regulated hair follicle cycling is less clear. β-catenin interactions with VDR function can enhance VDR induction of some genes, whereas VDR can suppress β-catenin induction of other genes. Many genes in the skin contain both putative VDREs and LEF/TCF sites. At this point we are a long ways away from understanding how 1,25(OH)2D and VDR regulate all the various functions in the skin that they impact. But what we have learned indicates that the skin is a fertile area for understanding the mechanisms by which vitamin D signaling regulates so many different physiologic processes.
Acknowledgements
The author acknowledges the administrative support of Teresa Tong, the scientific contributions of Zhongjian Xie, Yuko Oda, Arnaud Teichert, Sreekumar Pillai, Chialing Tu, Dean Ng, and Anita Ratnam, and the financial support of grants from the Veterans Administration, American Institute for Cancer Research (98A079), and the National Institutes for Health ((RO1 AR050023,PO1 AR39448).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Acevedo ML, Lee KC, Stender JD, et al. Selective recognition of distinct classes of coactivators by a ligand-inducible activation domain. Mol Cell. 2004;13:725–738. doi: 10.1016/s1097-2765(04)00121-2. [DOI] [PubMed] [Google Scholar]
- Ahmad W, Faiyaz ul Haque M, Brancolini V, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998;279:720–724. doi: 10.1126/science.279.5351.720. [DOI] [PubMed] [Google Scholar]
- Bell NH, Greene A, Epstein S, et al. Evidence for alteration of the vitamin D-endocrine system in blacks. Journal of Clinical Investigation. 1985;76:470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15:R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Nemanic MK, Gee E, et al. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986a;78:557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Nemanic MK, Whitney JO, et al. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986b;25:1545–1548. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Pillai S, Gee E, et al. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–660. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Pillai S, Gee E. Squamous carcinoma cell lines produce 1,25 dihydroxyvitamin D, but fail to respond to its prodifferentiating effect. J Invest Dermatol. 1991a;97:435–441. doi: 10.1111/1523-1747.ep12481267. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Pillai S, Gee E, et al. Tumor necrosis factor-alpha regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology. 1991b;129:33–38. doi: 10.1210/endo-129-1-33. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocrine Review. 1993;14:3–19. doi: 10.1210/edrv-14-1-3. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Elalieh H, Chang S, et al. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340–353. doi: 10.1002/jcp.20578. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Verlinden L, Eelen G, et al. Mechanisms for the selective action of Vitamin D analogs. J Steroid Biochem Mol Biol. 2005;97:21–30. doi: 10.1016/j.jsbmb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Carvallo L, Henriquez B, Paredes R, et al. 1,25-dihydroxy vitamin D3-enhanced expression of the osteocalcin gene involves increased promoter occupancy of basal transcription regulators and gradual recruitment of the 1,25-dihydroxy vitamin D3 receptor-SRC-1 coactivator complex. J Cell Physiol. 2008;214:740–749. doi: 10.1002/jcp.21267. [DOI] [PubMed] [Google Scholar]
- Chan EF, Gat U, McNiff JM, et al. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- Chen CH, Sakai Y, Demay MB. Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology. 2001;142:5386–5389. doi: 10.1210/endo.142.12.8650. [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Liu Y, et al. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- Cianferotti L, Cox M, Skorija K, et al. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci U S A. 2007;104:9428–9433. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale BA, Resing KA, Lonsdale-Eccles JD. Filaggrin: a keratin filament associated protein. Ann N Y Acad Sci. 1985;455:330–342. doi: 10.1111/j.1749-6632.1985.tb50420.x. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Rhee H, Fuchs E. A developmental conundrum: a stabilized form of beta-catenin lacking the transcriptional activation domain triggers features of hair cell fate in epidermal cells and epidermal cell fate in hair follicle cells. J Cell Biol. 2002;158:331–344. doi: 10.1083/jcb.200204134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djabali K, Aita VM, Christiano AM. Hairless is translocated to the nucleus via a novel bipartite nuclear localization signal and is associated with the nuclear matrix. J Cell Sci. 2001;114:367–376. doi: 10.1242/jcs.114.2.367. [DOI] [PubMed] [Google Scholar]
- Eichner R, Sun TT, Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986;102:1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Menon GK, Grayson S, et al. Membrane structural alterations in murine stratum corneum: relationship to the localization of polar lipids and phospholipases. J Invest Dermatol. 1988;91:3–10. doi: 10.1111/1523-1747.ep12463279. [DOI] [PubMed] [Google Scholar]
- Elias PM, Ahn SK, Denda M, et al. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J Invest Dermatol. 2002;119:1128–1136. doi: 10.1046/j.1523-1747.2002.19512.x. [DOI] [PubMed] [Google Scholar]
- Engelhard A, Christiano AM. The hairless promoter is differentially regulated by thyroid hormone in keratinocytes and neuroblastoma cells. Exp Dermatol. 2004;13:257–264. doi: 10.1111/j.0906-6705.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- Fu GK, Lin D, Zhang MY, et al. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, et al. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Guo M, Kim LT, Akiyama SK, et al. Altered processing of integrin receptors during keratinocyte activation. Exp Cell Res. 1991;195:315–322. doi: 10.1016/0014-4827(91)90379-9. [DOI] [PubMed] [Google Scholar]
- Hawker NP, Pennypacker SD, Chang SM, et al. Regulation of Human Epidermal Keratinocyte Differentiation by the Vitamin D Receptor and its Coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol. 2007;127:874. doi: 10.1038/sj.jid.5700624. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hennings H, Holbrook KA. Calcium regulation of cell-cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp Cell Res. 1983;143:127–142. doi: 10.1016/0014-4827(83)90115-5. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Gilhar A, Haim S, et al. Calcitriol-resistant rickets with alopecia. Arch Dermatol. 1985;121:646–647. [PubMed] [Google Scholar]
- Hohl D. Cornified cell envelope. Dermatologica. 1990;180:201–211. doi: 10.1159/000248031. [DOI] [PubMed] [Google Scholar]
- Holick MF, Richtand NM, McNeill SC, et al. Isolation and identification of previtamin D3 from the skin of exposed to ultraviolet irradiation. Biochemistry. 1979;18:1003–1008. doi: 10.1021/bi00573a011. [DOI] [PubMed] [Google Scholar]
- Holick MF, McLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- Holick MF, McLaughlin JA, Clark MB, et al. Factors that influence the cutaneous photosynthesis of previtamin D3. Science. 1981;211:590–593. [Google Scholar]
- Hosomi J, Hosoi J, Abe E, et al. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha,25-dihydroxyvitamin D3. Endocrinology. 1983;113:1950–1957. doi: 10.1210/endo-113-6-1950. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Sisk JM, Jurutka PW, et al. Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem. 2003;278:38665–38674. doi: 10.1074/jbc.M304886200. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, et al. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Issa LL, Leong GM, Sutherland RL, et al. Vitamin D Analogue-Specific Recruitment of Vitamin D Receptor Coactivators. Journal of Bone and Mineral Research. 2002;17:879–890. doi: 10.1359/jbmr.2002.17.5.879. [DOI] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Kong J, Li XJ, Gavin D, et al. Targeted expression of human vitamin d receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol. 2002;118:631–638. doi: 10.1046/j.1523-1747.2002.01727.x. [DOI] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Praetzel S, et al. K6irs1, K6irs2, K6irs3, and K6irs4 represent the inner-root-sheath-specific type II epithelial keratins of the human hair follicle. J Invest Dermatol. 2003;120:512–522. doi: 10.1046/j.1523-1747.2003.12087.x. [DOI] [PubMed] [Google Scholar]
- Lehmann B, Genehr T, Knuschke P, et al. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001;117:1179–1185. doi: 10.1046/j.0022-202x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Genes. 2000;245:1–11. doi: 10.1016/s0378-1119(00)00024-x. [DOI] [PubMed] [Google Scholar]
- Li YC, Amling M, Pirro AE, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- Loser K, Beissert S. Regulation of cutaneous immunity by the environment: an important role for UV irradiation and vitamin D. Int Immunopharmacol. 2009;9:587–589. doi: 10.1016/j.intimp.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Rachez C, Hawel IL, et al. Polyamines Modulate the Interaction between Nuclear Receptors and Vitamin D Receptor-Interacting Protein 205. Mol Endocrinol. 2002;16:1502–1510. doi: 10.1210/mend.16.7.0883. [DOI] [PubMed] [Google Scholar]
- Marchisio PC, Bondanza S, Cremona O, et al. Polarized expression of integrin receptors (alpha 6 beta 4, alpha 2 beta 1, alpha 3 beta 1, and alpha v beta 5) and their relationship with the cytoskeleton and basement membrane matrix in cultured human keratinocytes. J Cell Biol. 1991;112:761–773. doi: 10.1083/jcb.112.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SJ, Bliziotes MM, Nanes M. Analysis of the relation between alopecia and resistance to 1,25-dihydroxyvitamin D. Clin Endocrinol (Oxf) 1986;25:373–381. doi: 10.1111/j.1365-2265.1986.tb01703.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Hashimoto K, Nishida Y, et al. Growth-inhibitory effects of 1,25-dihydroxyvitamin D3 on normal human keratinocytes cultured in serum-free medium. Biochem Biophys Res Commun. 1990;166:916–923. doi: 10.1016/0006-291x(90)90898-w. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Azuma Y, Kiyoki M, et al. Involvement of endogenously produced 1,25-dihydroxyvitamin D-3 in the growth and differentiation of human keratinocytes. Biochim Biophys Acta. 1991;1092:311–318. doi: 10.1016/s0167-4889(97)90006-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. Journal of Clinical Endocrinology and Metabolism. 1987;64:1165–1168. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- Matsuoka LY, Wortsman J, Dannenberg MJ, et al. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol Metab. 1992;75:1099–1103. doi: 10.1210/jcem.75.4.1328275. [DOI] [PubMed] [Google Scholar]
- Mauro T, Bench G, Sidderas-Haddad E, et al. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. Journal of Investigative Dermatology. 1998;111:1198–1201. doi: 10.1046/j.1523-1747.1998.00421.x. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- McLane JA, Katz M, Abdelkader N. Effect of 1,25-dihydroxyvitamin D3 on human keratinocytes grown under different culture conditions. In Vitro Cell Dev Biol. 1990;26:379–387. doi: 10.1007/BF02623829. [DOI] [PubMed] [Google Scholar]
- Mehrel T, Hohl D, Rothnagel JA, et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. Journal of Investigative Dermatology. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- Milde P, Hauser U, Simon T, et al. Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol. 1991;97:230–239. doi: 10.1111/1523-1747.ep12480255. [DOI] [PubMed] [Google Scholar]
- Miller J, Djabali K, Chen T, et al. Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol. 2001;117:612–617. doi: 10.1046/j.0022-202x.2001.01438.x. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Morhenn VB, Wood GS. Gamma interferon-induced expression of class II major histocompatibility complex antigens by human keratinocytes. Effects of conditions of culture. Ann N Y Acad Sci. 1988;548:321–330. doi: 10.1111/j.1749-6632.1988.tb18820.x. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Oda Y, Sihlbom C, Chalkley RJ, et al. Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Mol Endocrinol. 2003;17:2329–2339. doi: 10.1210/me.2003-0063. [DOI] [PubMed] [Google Scholar]
- Oda Y, Ishikawa MH, Hawker NP, et al. Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol. 2007;103:776–780. doi: 10.1016/j.jsbmb.2006.12.069. [DOI] [PubMed] [Google Scholar]
- Oda Y, Uchida Y, Moradian S, et al. Vitamin D receptor and coactivators SRC 2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol. 2009;129:1367–1378. doi: 10.1038/jid.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer HG, Anjos-Afonso F, Carmeliet G, et al. The Vitamin D Receptor Is a Wnt Effector that Controls Hair Follicle Differentiation and Specifies Tumor Type in Adult Epidermis. PLoS ONE. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleyev AA, Botchkareva NV, Sundberg JP, et al. The role of the hairless (hr) gene in the regulation of hair follicle catagen transformation. Am J Pathol. 1999;155:159–171. doi: 10.1016/S0002-9440(10)65110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Ismail A, Uskokovic M, et al. Evidence for tissue- and cell-type selective activation of the vitamin D receptor by Ro-26-9228, a noncalcemic analog of vitamin D3. J Cell Biochem. 2003;88:267–273. doi: 10.1002/jcb.10344. [DOI] [PubMed] [Google Scholar]
- Peltonen J, Larjava H, Jaakkola S, et al. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989;84:1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Bikle DD, Su M-J, Ratnam A, Abe J. 1,25 dihydroxyvitaminD upregulates the phosphatidyl inositol signalling pathway in human keratinocytes by increasing phospholipase C levels. J Clin Invest. 1995;96:602–609. doi: 10.1172/JCI118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Bikle DD, Elias PM. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J Biol Chem. 1988a;263:5390–5395. [PubMed] [Google Scholar]
- Pillai S, Bikle DD, Hincenbergs M, et al. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J Cell Physiol. 1988b;134:229–237. doi: 10.1002/jcp.1041340208. [DOI] [PubMed] [Google Scholar]
- Pillai S, Bikle DD, Eessalu TE, et al. Binding and biological effects of tumor necrosis factor alpha on cultured human neonatal foreskin keratinocytes. J Clin Invest. 1989;83:816–821. doi: 10.1172/JCI113963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Bikle DD. Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol. 1991;146:94–100. doi: 10.1002/jcp.1041460113. [DOI] [PubMed] [Google Scholar]
- Rachez C, Lemon BD, Suldan Z, et al. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- Rachez C, Gamble M, Chang CP, et al. The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol Cell Biol. 2000;20:2718–2726. doi: 10.1128/mcb.20.8.2718-2726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene. 2000;246:9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Wong M, Bikle D, et al. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972;51:2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam AV, Bikle DD, Cho JK. 1,25 dihydroxyvitamin D3 enhances the calcium response of keratinocytes. Journal of Cellular Physiology. 1999;178:188–196. doi: 10.1002/(SICI)1097-4652(199902)178:2<188::AID-JCP8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rost CR, Bikle DD, Kaplan RA. In vitro stimulation of 25-hydroxycholecalciferol 1 alpha-hydroxylation by parathyroid hormone in chick kidney slices: evidence for a role for adenosine 3',5'-monophosphate. Endocrinology. 1981;108:1002–1006. doi: 10.1210/endo-108-3-1002. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Demay MB. Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology. 2000;141:2043–2049. doi: 10.1210/endo.141.6.7515. [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Yamasaki K, et al. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Oda Y, Buchau AS, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- Shah S, Hecht A, Pestell R, et al. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278:48137–48145. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- Shah S, Islam MN, Dakshanamurthy S, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Morgan BA. Wnt signaling through the beta-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J Invest Dermatol. 2004;122:239–245. doi: 10.1046/j.0022-202X.2004.22224.x. [DOI] [PubMed] [Google Scholar]
- Smith EL, Walworth NC, Holick MF. Effect of 1 alpha,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986;86:709–714. doi: 10.1111/1523-1747.ep12276343. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Steven AC, Bisher ME, Roop DR, et al. Biosynthetic pathways of filaggrin and loricrin--two major proteins expressed by terminally differentiated epidermal keratinocytes. Journal of Structural Biology. 1990;104:150–162. doi: 10.1016/1047-8477(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Clark SA, Sar M, et al. Topographical and developmental studies on target sites of 1,25 (OH)2 vitamin D3 in skin. Cell Tissue Res. 1984;238:489–496. doi: 10.1007/BF00219863. [DOI] [PubMed] [Google Scholar]
- Teichert A, Arnold LA, Otieno S, et al. Quantification of the vitamin D receptor-coregulator interaction. Biochemistry. 2009;48:1454–1461. doi: 10.1021/bi801874n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher SM, Rice RH. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985;40:685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- Thompson CC, Bottcher MC. The product of a thyroid hormone-responsive gene interacts with thyroid hormone receptors. Proc Natl Acad Sci U S A. 1997;94:8527–8532. doi: 10.1073/pnas.94.16.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trefzer U, Brockhaus M, Lotscher H, et al. The 55-kD tumor necrosis factor receptor on human keratinocytes is regulated by tumor necrosis factor-alpha and by ultraviolet B radiation. J Clin Invest. 1993;92:462–470. doi: 10.1172/JCI116589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor Is Required for calcium- induced differentiation in human keratinocytes. J Biol Chem. 2001;276:41079–41085. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- Warhol MJ, Roth J, Lucocq JM, et al. Immuno-ultrastructural localization of involucrin in squamous epithelium and cultured keratinocytes. J Histochem Cytochem. 1985;33:141–149. doi: 10.1177/33.2.2578499. [DOI] [PubMed] [Google Scholar]
- Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. Journal of Clinical Endocrinology and Metabolism. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. Journal of Clinical Endocrinology and Metabolism. 1989;68:882–887. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- Wood LC, Elias PM, Sequeira-Martin SM, et al. Occlusion lowers cytokine mRNA levels in essential fatty acid-deficient and normal mouse epidermis, but not after acute barrier disruption. J Invest Dermatol. 1994;103:834–838. doi: 10.1111/1523-1747.ep12413597. [DOI] [PubMed] [Google Scholar]
- Xia J, Urabe K, Moroi Y, et al. beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 2006;41:67–75. doi: 10.1016/j.jdermsci.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bikle DD. Phospholipase C-gamma1 is required for calcium-induced keratinocyte differentiation. J Biol Chem. 1999;274:20421–20424. doi: 10.1074/jbc.274.29.20421. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bikle DD. Inhibition of 1,25-Dihydroxyvitamin-D-Induced Keratinocyte Differentiation by Blocking the Expression of Phospholipase C-gamma1. J Invest Dermatol. 2001;117:1250–1254. doi: 10.1046/j.0022-202x.2001.01526.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002a;118:11–16. doi: 10.1046/j.1523-1747.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Munson SJ, Huang N, Portale AA, Miller WJ, Bikle DD. The mechanism of 1,25 dihydroxyvitamin D3 auto-regulation in keratinocytes. J Biol Chem. 2002b;277:36987–36990. doi: 10.1074/jbc.M201404200. [DOI] [PubMed] [Google Scholar]
- Xie Z, Chang S, Oda Y, et al. Hairless suppresses vitamin D receptor transactivation in human keratinocytes. Endocrinology. 2006;147:314–323. doi: 10.1210/en.2005-1111. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- Zarach JM, Beaudoin GM, 3rd, Coulombe PA, et al. The co-repressor hairless has a role in epithelial cell differentiation in the skin. Development. 2004;131:4189–4200. doi: 10.1242/dev.01303. [DOI] [PubMed] [Google Scholar]
- Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- Zhou P, Byrne C, Jacobs J, et al. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 1995;9:700–713. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]