Abstract
Background
Cocaine dependence is associated with cognitive deficits and altered task-related cerebral activation in cognitive performance (see Li and Sinha, 2008, for a review). Relatively little is known whether these individuals are also impaired in regional brain activation of the default mode network (DMN). We demonstrated previously that greater activation of the default brain regions precedes errors in a stop signal task performed by healthy controls (SST, Li et al., 2007). We seek to determine whether individuals with cocaine dependence are impaired in DMN activity, specifically activity preceding error, as compared to the healthy people. We also examine the relation to years of cocaine use.
Methods
Individuals with cocaine dependence (CD, n=23) and demographics-matched healthy controls (HC, n=27) performed a SST that employed a tracking procedure to adjust the difficulty of stop trials and elicit errors approximately half of the time. Blood oxygenation level dependent (BOLD) signals of go trials preceding stop error as compared to those preceding stop success trials were extracted with generalized linear models using Statistical Parametric Mapping.
Results
HC showed activation of bilateral precuneus and posterior cingulate cortices and ventromedial prefrontal cortex (vmPFC) preceding errors during the SST. In contrast, despite indistinguishable stop signal performance, CD did not show these error predicting activations. Furthermore, the effect size of error-preceding vmPFC activation was inversely correlated with years of cocaine use.
Conclusions
These findings indicate DMN deficits and could potentially add to our understanding of the effects of chronic cocaine use on cerebral functions in cocaine dependence. Work to further clarify potential changes in functional connectivity and gray matter volume is warranted to understand the relevance of DMN to the pathology of cocaine misuse.
Keywords: attention, attentional lapse, default circuit, prefrontal, neuroimaging
1. Introduction
Past neuroimaging work has distinguished “default” activity generated during resting state from cerebral activity associated with cognitive task performance (Shulman et al., 1997; Raichle et al., 2001, 2007; Grecius et al., 2003; Grecius and Menon 2004; Vincent et al., 2007; Pfefferbaum et al., 2010). This neurological baseline seen in the resting brain can be described by the default mode network (DMN) (Raichle et al., 2001). In general, a cognitive task typically increases activation in the task-oriented network, meanwhile decreasing activation in the DMN (Raichle et al., 2001; Greicius et al., 2003). Dysfunctions of the DMN were noted in a wide variety of neurological and psychiatric conditions, including Alzheimer’s disease, attention deficit hyperactivity disorder, anxiety, and depression (Grecius et al., 2004; Rombouts et al., 2005; Wang et al., 2006; Sorg et al., 2007; Baliki et al., 2008; Uddin et al., 2008; Broyd et al., 2009; Bush 2010).
Imaging studies of cocaine dependent individuals have largely focused on task-evoked activity. In particular, numerous studies have described frontal cortical and subcortical dysfunctions in individuals with cocaine or other psychostimulant use disorders (Aron and Paulus 2007; Everitt et al., 2007; Garavan and Hester 2007; Hanlon et al., 2009, and in press; Li and Sinha 2008; Porrino et al., 2007; Wesley et al., 2011). For instance, Goldstein and colleagues utilized a sustained attention task to show that while practice is associated with altered activation in the posterior cerebral regions in healthy individuals, it is associated with changes of activation in frontal brain regions in cocaine dependent individuals (Goldstein et al., 2007). In another study, hypoactivation of the dorsal anterior cingulate cortex to an emotionally salient stimulus is associated with the current frequency of cocaine use (Goldstein et al., 2009). Our previous study of the stop signal task demonstrated decreased medial superior frontal and anterior cingulate cortical activation during inhibitory control (Li et al., 2008c) and altered thalamic processing of errors in association with loss of self control (Li et al., 2010a) in abstinent cocaine dependent individuals. These studies elicit a difference, and likely deficit, in response to cognitive tasks in the cocaine-dependent brain. On the other hand, little is known whether the DMN is also dysfunctional in these individuals. The current study addressed this question.
We built upon our previous findings from the stop signal task that areas within the DMN, including the ventromedial prefrontal cortex, posterior cingulate cortex, and precuneus, were significantly more active in go trials preceding a stop error as compared to those preceding a stop success in healthy participants, thereby marking the DMN as an inherent mechanism for “error prediction” (Li et al., 2007). A recent study which pooled data from five stop signal task experiments in healthy populations also found an increase in DMN activity prior to error commission (Congdon et al., 2010). An fMRI study of the Flanker task used independent component analysis to examine the hemodynamic response prior to an error. Results showed a decreased deactivation in the DMN combined with a decrease in task network activation up to six seconds preceding an erroneous vs. correct trial (Eichele et al., 2008). In a fourth report, the DMN is more active, or decreasingly deactivated, during error laden trials in an attention task requiring identification of a target surrounded by congruent or incongruent distracter stimuli (Weissman et al., 2006). Overall, these results indicate a dynamic range of activation in the DMN, which could influence mental state and cognitive performance. Here we seek to determine whether individuals with cocaine dependence are impaired in DMN activity as compared to the healthy people. Specifically, by contrasting go trials preceding a stop error and those preceding a stop success trial, we examined whether error-preceding activity in the DMN during the stop signal task was altered in cocaine dependent individuals and whether this altered activity was related to years of cocaine use. On the basis of our previous work, we hypothesized that cocaine dependent volunteers, as compared to healthy controls, would show less activation preceding errors in the posterior cingulate cortex/precuneus and the perigenual anterior cingulate cortex.
2. Methods
2.1. Subjects
Twenty-three abstinent individuals with cocaine dependence (CD) and 27 demographics matched healthy control (HC) volunteers were recruited from the greater New Haven area through advertisements and word-of-mouth to participate in the study (Table 1). CD volunteers met criteria for current cocaine dependence, as diagnosed by the Structured Clinical Interview for DSM-IV (SCID; First et al., 1995). Recent cocaine use was confirmed by urine toxicology screens upon admission. They were drug-free while staying in a monitored treatment unit for two weeks prior to the current fMRI study. CD volunteers were assessed with Beck Depression Inventory (BDI) at admission and once every week during their inpatient stay. The average (mean±SD) BDI scores were 17.9±10.2 at admission and 6.7±6.0 after two weeks, consistent with those reported previously for this population (Rubin et al., 2007; Falck et al., 2002; López and Becoña, 2007). They were also assessed with State-Trait Anxiety Inventory (STAI) at admission. The average STAI scores of 45.2±13.8 for trait and 39.8±12.7 for state were within the range reported previously for CD (Karlsgodt et al., 2003; Rubin et al., 2007). None of the CD volunteers had BDI or STAI scores indicating clinical depressive or anxiety disorders. All subjects were physically healthy with no major medical illnesses or current use of prescription medications. None reported having a history of head injury or neurological illness. Other exclusion criteria included a history of or current dependence on another psychoactive substance (except nicotine) and current or past history of psychotic disorders. Individuals with current depressive or anxiety symptoms requiring treatment or currently being treated for these symptoms were excluded as well. The data of 11 HC volunteers came from the cohort of our earlier study (Li et al., 2007) and the majority (18) of the HC volunteers did not undergo SCID or a urine toxicology test. The Human Investigation committee at Yale University School of Medicine approved all study procedures, and all subjects signed an informed consent prior to study participation.
Table 1.
Demographics of the subjects.
| Subject Characteristics | PCD (n=23) |
HC (n=27) |
p value | |
|---|---|---|---|---|
| age (years) | 36.2 ± 6.3 | 34.9 ± 6.5 | 0.49a | |
| women/men | 12/11 | 15/12 | 0.37b | |
| African Am. | 39.1% | 33.3% | ||
| ethnicity | Caucasian | 56.5% | 59.2% | 0.58b |
| Hispanic/other | 4.3% | 7.4% | ||
| education (years) | 11.7 ± 1.7 | 12.6± 1.6 | 0.08a | |
| avg. no. of days with cocaine use (past month) | 13.1 ± 7.4 | N/A | N/A | |
| avg. no. of yrs. with cocaine use (lifetime) | 10.8 ± 6.9 | N/A | N/A | |
Note: values are mean±standard deviation;
2-sample t test;
χ2 test.
2.2. Behavioral task
The behavioral task ran from the commercial software “Presentation” (NeuroBehavioral Systems; http://www.neurobs.com/). Visual stimuli were front projected to a screen situated in front of the scanner, and manual response via button press was recorded with a fiber-optic button box (Current Designs, Philadelphia, PA). We employed a simple reaction time task in this stop-signal paradigm (Li et al., 2006; 2009; Logan and Cowan 1984). There were two trial types: “go” and “stop,” randomly intermixed. A small dot appeared on the screen to engage attention at the beginning of a go trial. After a randomized time interval (fore-period) anywhere between 1 and 5 s, the dot turned into a circle. The subjects were instructed to quickly press a button at the “go” signal but not before. The circle vanished at button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Approximately three quarters of all trials were go trials and the remainder were stop trials. In a stop trial, an additional “X,” the “stop” signal, appeared after and replaced the go signal. The subjects were told to withhold button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed since the appearance of the stop signal. The stop signal delay (SSD), the duration of time in which the go signal remained on the screen prior to the appearance of the stop signal, started at 200 ms and varied from one stop trial to the next according to a staircase procedure: if the subject succeeded in withholding the response, the SSD increased by 67 ms; conversely, if they failed, the SSD decreased by 67 ms (Levitt 1970). There was an inter-trial-interval of 2 s. Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. Prior to the fMRI study each subject had a practice session outside the scanner. In the scanner each subject completed four 10-min runs of the task with a one to two minute break in between runs. Depending on the actual stimulus timing (trial varied in fore-period duration) and speed of response, the total number of trials varied slightly across subjects in an experiment. With the staircase procedure we anticipated that the subjects succeeded in withholding their response in approximately half of the stop trials.
2.3. Imaging protocol
We employed a 3T scanner (Siemens Trio) and a circularly-polarized head coil (with one element and no acceleration) for the current study. Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization. Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC-PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4mm and no gap. Functional, blood oxygenation level dependent (BOLD) signals were then acquired with a single-shot gradient echo echoplanar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2,000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4mm and no gap. Three hundred images were acquired in each run for a total of 4 runs.
2.4. Data analysis and statistics
Data were analyzed with Statistical Parametric Mapping (SPM5, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were realigned (motion-corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per each run from the realigned image volumes. These mean images were co-registered with the high resolution structural image and then segmented for normalization to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Ashburner and Friston 1999; Friston et al., 1995a). Finally, images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
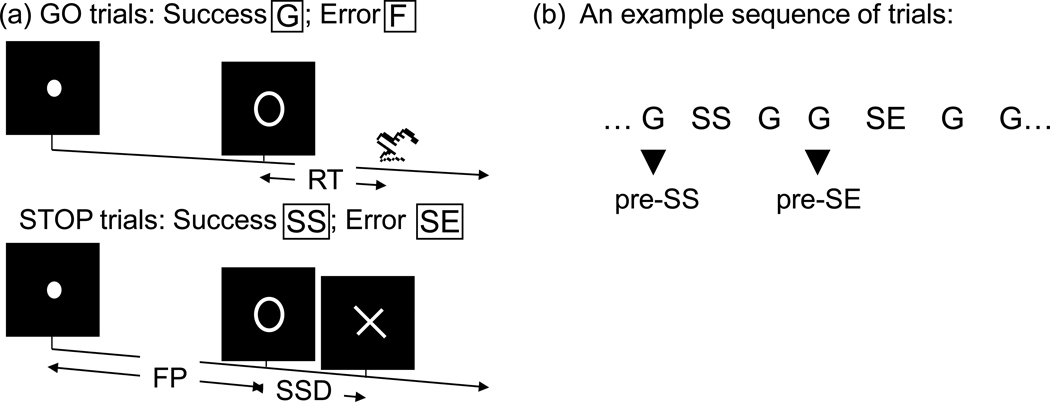
Four main types of trial outcome were first distinguished: go success (G), go error (F), stop success (SS), and stop error (SE) trial (Fig. 1a). For the evaluation of “pre-stop” processes, G trials were further divided into those that preceded a G (pre-G), SS (pre-SS), and SE (pre-SE) trial (Fig. 1b). A statistical analytical design was constructed for each individual subject using the general linear model (GLM) with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF entered as regressors in the model (Friston et al., 1995b). Realignment parameters in all 6 dimensions were also entered in the model. CD and HC volunteers did not differ in the extent (mean± standard deviation) of translational (CD: 0.35 ± 0.59 mm vs. HC: 0.32 ± 0.57 mm; p=0.376, two-sample t test) or rotational (0.16 ± 0.40° vs. 0.18 ± 0.42°; p=0.516) head motion. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts. Serial autocorrelation of the time series was corrected by a first-degree autoregressive or AR (1) model (Della-Maggiore et al., 2002; Friston et al., 2000). The GLM estimated the component of variance that could be explained by each of the regressors.
Figure 1.
(a) Stop signal paradigm. In “go” trials (75%) observers responded to the go signal (a circle) and in “stop” trials (25%) they had to withhold the response when they saw the stop signal (an X). In both trials the go signal appeared after a randomized time interval between 1 to 5 s (the fore-period or FP, uniform distribution) following the appearance of the fixation point. The stop signal followed the go signal by a time delay – the stop signal delay (SSD). The SSD was updated according to a staircase procedure, whereby it increased and decreased by 64 ms following a stop success and stop error trial, respectively. We distinguished go success (G) and go error (F), and stop success (SS) and stop error (SE) trials during the task. (b) Go successes were further distinguished by their subsequent trial; thus G trials followed by a SS, and SE trial were indicated by pre-SS, and pre-SE trials, respectively.
In the first-level analysis, we constructed the following statistical contrast for the individual subjects: pre-SE vs. pre-SS. The con or contrast (difference in β) images taken from the first-level analysis were used for the second-level group statistics (random effect analysis; Penny and Holmes 2004), including a one-sample t test each for CD and HC groups and a two-sample t test comparing CD and HC. Brain regions were identified using an atlas (Mai et al., 2003). In region of interest (ROI) analysis, we used MarsBaR (Brett et al., 2002; http://marsbar.sourceforge.net/) to derive the effect size of activity change for the ROIs in each individual subject. All voxel activations are presented in MNI coordinates.
3. Results
3.1. General behavioral performance
Table 2 shows stop signal performance. Both CD and HC participants succeeded in about half of the stop trials, indicating the successful utility of the staircase procedure in tracking subject performance. CD and HC did not differ in any aspects of the stop signal performance.
Table 2.
Stop Signal Performance.
| SSRT (ms) |
FP effect (effect size) |
Median go RT (ms) |
%go | %stop | PES (effect size) |
|
|---|---|---|---|---|---|---|
| PCD (n=23) | 200 ± 45 | 2.05 ± 1.35 | 546 ± 83 | 96.5 ± 1.5 | 51.2 ± 2.0 | 1.90 ± 1.52 |
| HC (n=27) | 196 ± 36 | 2.25 ± 1.20 | 537 ± 115 | 97.2 ± 1.7 | 50.2 ± 2.7 | 1.50 ± 1.24 |
| p value* | 0.76 | 0.582 | 0.747 | 0.15 | 0.129 | 0.309 |
Note: all values are mean ± standard deviation;
P value based on 2-tailed 2-sample t test;
CD: individuals with cocaine dependence; HC: healthy controls; SSRT: stop signal reaction time: FP: fore-period; RT: reaction time; %go: percentage of go success trials; %stop: percentage of stop success trials; PES: post-error slowing.
3.2. Neural activity preceding SE vs. SS trials
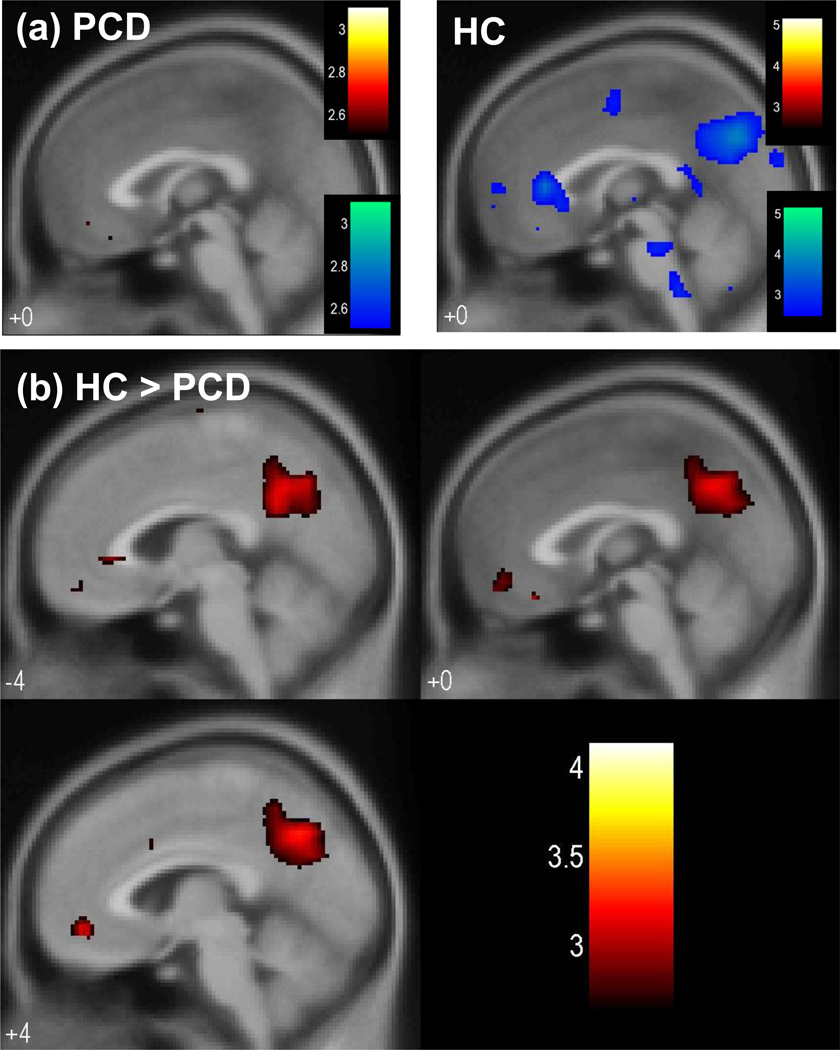
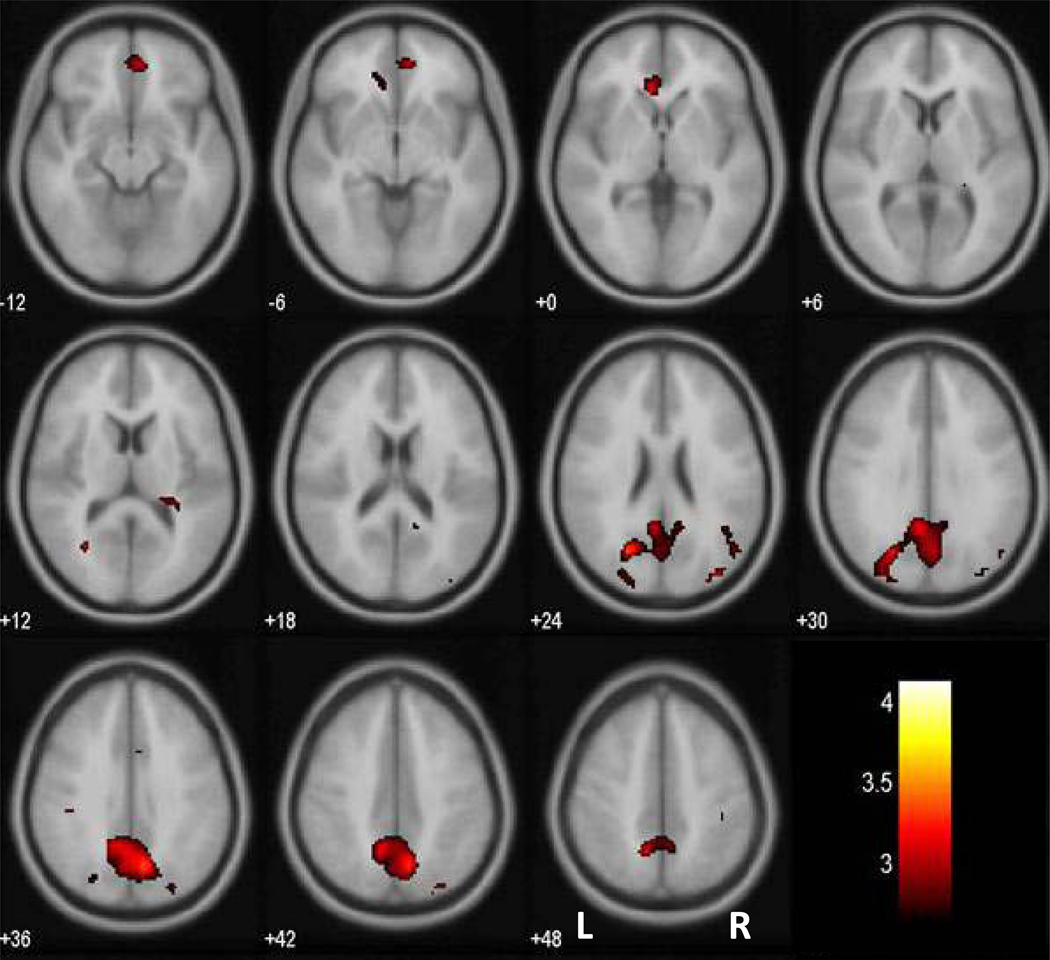
At a threshold of p<0.005, uncorrected, and 50 voxels in extent of activation, HC but not CD showed greater activation in the perigenual and subgenual anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC)/precuneus during pre-SE compared to pre-SS go trials (Fig. 2a, Table 3). Direct comparison with a two-sample t test showed greater error predicting activation (pre-SE > pre-SS) in the ventromedial prefrontal cortex and posterior cingulate cortex/precuneus (Fig. 2b, Table 3). Fig. 3 showed the two-sample t test results in axial sections of the brain. In contrast, no brain regions showed greater error-preceding activation in CD, compared to HC.
Figure 2.
(a) Brain regions showing greater activation during pre-SE than pre-SS trials (blue) in HC (right panel) but not CD (left panel) in the perigenual and subgenual anterior cingulate cortex and posterior cingulate cortex/precuneus (p<0.005, uncorrected). At the same threshold, no brain regions showed greater activation during pre-SS compared to pre-SE trials (red). T statistic for the contrast was shown for a mid-sagittal section (x=0) of a mean EPI image. (b) Two sample t-test of the contrast pre-SE > pre-SS showing greater error predicting activation in the ventromedial prefrontal cortex and posterior cingulate cortex/precuneus in HC, as compared to CD (p<0.005, uncorrected). T map of the contrast was overlaid on sagittal sections (x=−4, 0, 4) of a mean EPI image. Color bars represent voxel T value.
Table 3.
Error-preceding regional brain activations: pre-SE go trials > pre-SS go trials.
| MNI Coordinates (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Size (voxels) |
Voxel Z Value |
X | Y | Z | Side | Identified Region | |
| HC (n=27) | 703 | 4.24 | 4 | −64 | 12 | R | PCC/precuneus |
| 132 | 3.23 | −4 | 28 | −4 | L | perigenual/subgenual ACC | |
| CD (n=23) | None | ||||||
| HC > PCD | 119 | 3.59 | 0 | 32 | −20 | R/L | ventromedial PFC |
| 230 | 3.52 | −16 | −68 | 24 | L | PCC/precuneus | |
| PCD > HC | None | ||||||
Note: p<0.005, uncorrected, and 50 voxels in extent of activation;
PCC: posterior cingulate cortex; ACC: anterior cingulate cortex; PFC: prefrontal cortex.
Figure 3.
Brain regions showing greater activation during pre-SE than pre-SS trials in HC, as compared to CD (p<0.005, uncorrected). T statistic was overlaid on a mean EPI image in axial sections from z=−12 to z=48 with adjacent sections 6mm apart. Neurological orientation: Right = right. Color bars represent voxel T value.
In addition to voxel-wise whole brain activation, we also performed small volume correction based on the coordinates obtained in our earlier work (Li et al., 2007): spheres with 15 mm in radius centered on PCC/precuneus (x=−16, y=−60, z=32), perigenual ACC (x=4, y=32, z=4), and transverse frontopolar gyrus (x=4, y=52, z=24). Both the PCC/precuneus and perigenual ACC masks identified the same peaks with the former being significant at p<0.05, corrected for family-wise error of multiple comparisons.
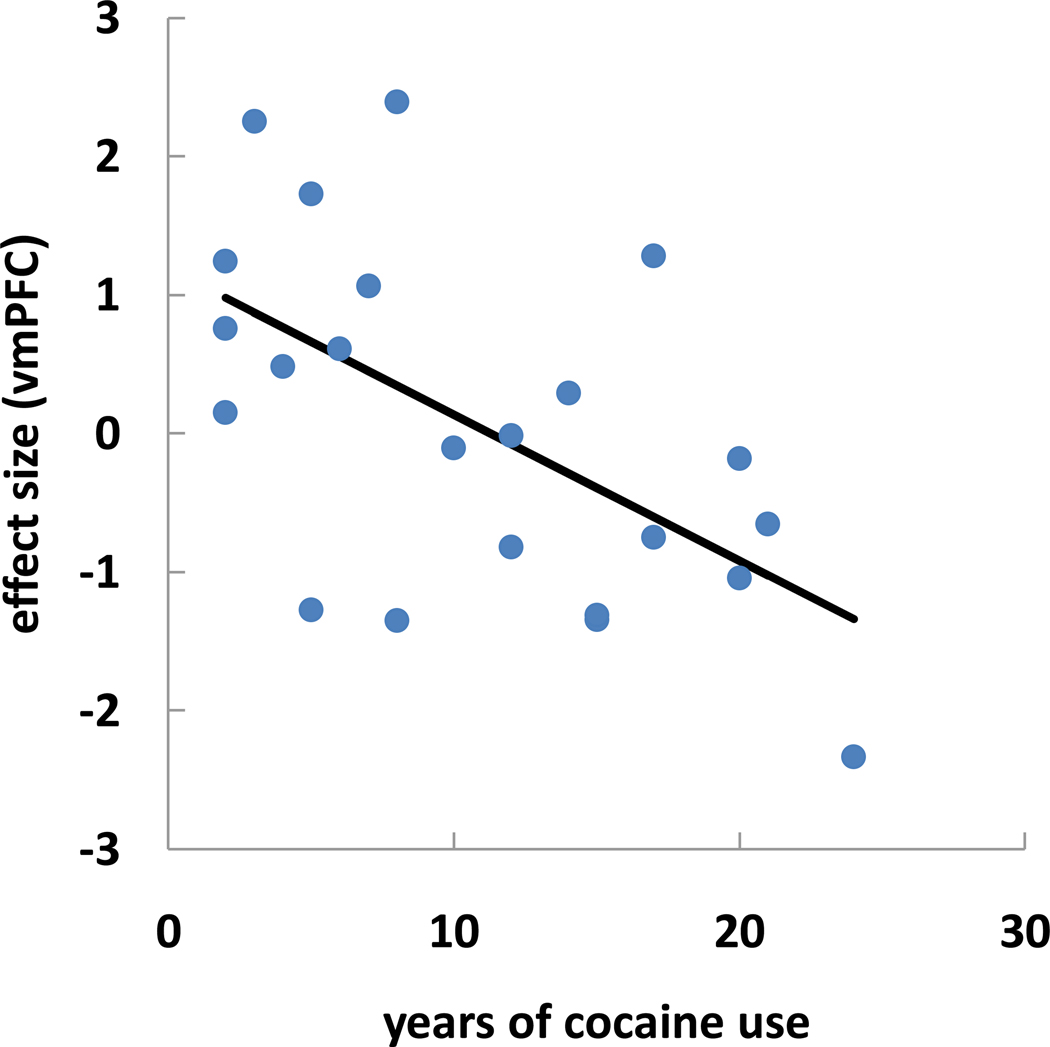
Using linear regression, we correlated the effect size of error-predicting activation of the vmPFC and posterior cingulate cortex/precuneus with years of cocaine use across the 23 CD. The results showed that the effect size of vmPFC (R2=0.332, p<0.005, Fig. 4) but not posterior cingulate cortex/precuneus (R2=0.016, p>0.5) was inversely correlated with years of cocaine use.
Figure 4.
Effect size correlation of vmPFC (pre-SE > pre-SS) is inversely correlated with years of cocaine use for 23 CD (R2=0.332, p<0.005, Pearson regression). Each dot represents the data of one subject.
4. Discussion
The main finding of this study indicates a lack of moment-to-moment DMN fluctuation in CD as compared to HC. The contrast made here is of identical go trials preceding an unanticipated stop trial with an error versus with a success. In other words, CD do not demonstrate a neural signature of impending error as seen in HC. This difference between the populations cannot be attributed to performance differences because there are none.
The contrast of pre-SE and pre-SS trials did not address any specific task outcomes such as response inhibition or post-error slowing as was the focus of our previous work (Li et al., 2006; 2008a; 2008b). Rather, both pre-SE and pre-SS are go trials, with the difference being that the pre-SE precedes a stop error and the pre-SS precedes a stop success trial. In HC, the activity of DMN can be used to differentiate between these two trial conditions. That is, in accord with many previous studies of the DMN (summarized in Congdon et al., 2010), the activity of DMN goes down when participants are more alert (less prone to errors), while at other moments the activity of the DMN goes up when they are less alert (more prone to errors). Thus, our data (current and Li et al., 2007) suggest that if a stop trial occurs at the time when the immediately preceding activity of the DMN goes down, then participants are more likely to succeed in stopping than when the activity of the DMN goes up. Our current findings indicate that this fluctuation of DMN activity does not happen in CD individuals. Whether it is a failure to deactivate or activate, the DMN of CD individuals lacks this moment to moment fluctuation in activity.
We demonstrated that this deficit in DMN was linearly correlated with the years of cocaine use in CD. Although the number of years of cocaine use is unlikely the sole indicator of the severity of cocaine dependence, this association is consistent with recent studies showing the modulation of the DMN including vmPFC functional circuitry by catecholaminergic agents (Argyelan et al., 2008; Kelly et al., 2009; Nagano-Saito et al., 2009). For instance, in Parkinson’s disease patients performing a motor sequence learning task and a simple movement task, intravenous levodopa abolished learning-related deactivation occurring in the vmPFC (Argyelan et al., 2008).
While the current results characterized dysfunctions of the DMN as a consequence of chronic cocaine use, other studies highlighted the acute effects of psychostimulants on the DMN. For instance, in Positron Emission Tomography (PET) imaging of brain glucose metabolism in healthy adults, Volkow and colleagues showed that methylphenidate, a psychostimulant with neuropharmacological properties similar to cocaine, decreases activation of the DMN during cognitive performance and that this effect is associated with improved performance in those individuals who activated the DMN during the task (Volkow et al., 2008). A more recent study showed that treatment with methylphenidate suppressed activation of the ventral ACC and posterior cingulate cortex in children with attention deficit hyperactivity disorders (Peterson et al., 2009). The authors suggested that methylphenidate may ameliorate mind-wandering by decreasing activity of the DMN. Our recent fMRI study also demonstrated that methylphenidate improved inhibitory control by decreasing vmPFC activation during stop signal inhibition in CD (Li et al., 2010b). In another study combining PET imaging and fMRI, Tomasi and colleagues showed in healthy individuals that the availability of the dopamine transporters (DAT) in the striatum is negatively correlated with the blood oxygenation level dependent signal in the precuneus – an area of the DMN – under increased attentional load in a visuospatial task (Tomasi et al., 2009). The authors suggested that stimulant medications may help improve attention and cognitive performance by blocking DAT and thereby increasing dopaminergic modulation of the DMN. Overall, these findings appeared to converge to suggest a potential impact of psychostimulants on the DMN, consistent with the current findings in cocaine dependent individuals.
We would also like to emphasize that regions within the DMN are functionally connected as part of the intrinsic activity of brain (Buckner and Vincent, 2007; Raichle and Snyder, 2007). Although the current findings suggested a deficit of certain brain regions within the DMN during an externally imposed task, they did not indicate that the pattern of intrinsic connectivity was disrupted as a result of cocaine exposure. Future work is needed to examine this issue by analyzing resting state low frequency BOLD signals in CD (Gu et al., 2010).
This study has a few important limitations. First, the majority of our HC volunteers did not undergo a structured psychiatric interview or urine toxicology test. Second, the current results did not specify a causal link between years of cocaine use and deficits of the DMN, despite a positive correlation between the two. There are many clinical factors, including history of abuse of other substances, depression, anxiety, and psychological trauma, which were not accounted for. Furthermore, given that this is a cross-sectional study, other potential variables including mood and sleep at the time of fMRI may also impact the results. An additional issue is that the current findings were obtained with a relatively liberal threshold despite a moderate sample size. Finally, studies have reported changes in cerebral gray matter volumes in cocaine dependence (Lim et al., 2008; Sim et al., 2007; but see also Narayana et al., in press). Although it is unclear how changes in gray matter volume and functional activity are associated, this issue deserves attention in future studies. Taken together, the current results should be considered preliminary and require replication in the future with greater concessions made to the limitations presented.
In conclusion, we show in the current study that cocaine dependence is associated with dysfunction of the default mode network of the cerebral cortex. This finding complements previous work on task-related deficits in cocaine dependence.
Acknowledgements
This study was supported by NIH grants R01DA023248 (Li), K12DA000167 (Rounsaville), K02DA026990 (Li), CTSA Grant UL1 RR024139 (Robert Sherwin), P50-DA16556 (Sinha). The study also receives partial support from the State of Connecticut. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Drug Abuse, National Center for Research Resources or the National Institutes of Health. We thank the medical and nursing staff at the Clinical Neuroscience Research Unit, Connecticut Mental Health Center, and Connecticut Department of Mental Health and Addiction Services for their inpatient care.
Role of Funding Source
The NIH and the State of Connecticut had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Sarah R. Bednarski collected and analyzed the data, and wrote the manuscript. Kwang-Ik Hong and Sheng Zhang analyzed the data. Chiang-shan R. Li designed the study, analyzed and interpreted the data, and wrote the manuscript. Rajita Sinha and Bruce J. Rounsaville participated in the interpretation of the data and writing of the manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Argyelan M, Carbon M, Ghilardi MF, Feigin A, Mattis P, Tang C, Dhawan V, Eidelberg D. Dopaminergic suppression of brain deactivation responses during sequence learning. J. Neurosci. 2008;28:10687–10695. doi: 10.1523/JNEUROSCI.2933-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102 Suppl. 1:33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum. Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-P. Region of interest analysis using an SPM toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6; Sendai, Japan. 2002. [Google Scholar]
- Broyd SJ, Demanuele C, Debenr S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;22:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Chau W, Peres-Neto PR, McIntosh AR. An empirical comparison of SPM preprocessing parameters to the analysis of fMRI data. Neuroimage. 2002;17:19–28. doi: 10.1006/nimg.2002.1113. [DOI] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, Yves von Cramon D, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc. Natl. Acad. Sci. USA. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann. NY Acad. Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Falck RS, Wang J, Carlson RG, Eddy M, Siegal HA. The prevalence and correlates of depressive symptomatology among a community sample of crack-cocaine smokers. J. Psychoactive Drugs. 2002;34:281–288. doi: 10.1080/02791072.2002.10399964. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Rounsaville B. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part II: multi-site test-retest reliability study. J. Pers. Disord. 1995;9:92–104. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Polone J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum. Brain Mapp. 1995a;2:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J. To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12:196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol. Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc. Natl. Acad. Sci. USA. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. Neuroimage. 2007;35:194–206. doi: 10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cogn. Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Grecius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug Alcohol Depend. 2009;102:88–94. doi: 10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ. The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend. 115:240–243. doi: 10.1016/j.drugalcdep.2010.11.008. in press. Epub ahead of print: 2010 Dec. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karslgodt KH, Lukas SE, Elman I. Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. Am. J. Drug Alcohol Abuse. 2003;29:539–551. doi: 10.1081/ada-120023457. [DOI] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J5 Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 1970;49:467–477. [PubMed] [Google Scholar]
- Li CS, Huang C, Constable T, Sinha R. Imaging response inhibition in a stop signal task – neural correlates independent of signal monitoring and post-response processing. J. Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Bergquist KL, Sinha R. Greater activation of the “Default” brain circuitry predicts stop signal errors. NeuroImage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci. Biobehav. Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J. Cogn. Neurosci. 2008a;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Chao HH, Sinha R, Paliwal P, Constable RT, Zhang S, Lee TW. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience. 2008b;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008c;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Chao HH-A, Lee TW. The neural correlates of speeded compared to delayed responses in a stop signal task: an indirect analogue of risk taking and association with an anxiety trait. Cereb. Cortex. 2009;19:839–848. doi: 10.1093/cercor/bhn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Luo X, Sinha R, Rounsaville BJ, Carroll KM, Malison RT, Ding YS, Zhang S, Ide JS. Increased error-related thalamic activity during early compared to late cocaine abstinence. Drug Alcohol Depend. 2010a;109:181–189. doi: 10.1016/j.drugalcdep.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JLK, Rounsaville BJ, Ding YS, Malison RT. Biological markers of the effects of methylphenidate on improving inhibitory control in cocaine dependent patients. Proc. Natl. Acad. Sci. USA. 2010b;107:14455–14459. doi: 10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol. Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- López A, Becoña E. Depression and cocaine dependence. Psychol. Rep. 2007;100:520–524. doi: 10.2466/pr0.100.2.520-524. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Asheuer JK. Atlas of the Human Brain. Second Edition. New York, NY: Academic Press; 2003. [Google Scholar]
- Nagano-Saito A, Liu J, Doyon J, Dagher A. Dopamine modulates default mode network deactivation in elderly individuals during the Tower of London task. Neurosci. Lett. 2009;458:1–5. doi: 10.1016/j.neulet.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes AP. Random-effects analysis. In: Frackowiak RSJ, editor. Human Brain Function. San Diego: Elsevier; 2004. pp. 843–850. [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am. J. Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Müller-Oehring E, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cereb. Cortex. 2010;21:233–244. doi: 10.1093/cercor/bhq090. Epub ahead of print, 2010 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum. Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E, Aharonovich E, Bisaga A, Levin FR, Raby WN, Nunes EV. Early abstinence in cocaine dependence: influence of comorbid depression. Am. J. Addict. 2007;16:283–290. doi: 10.1080/10550490701389880. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RI, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cognit. Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Mühlau M, Calhoun VD, Eichele T, Läer L, Drezga A, Förstl H, Kurz A, Zimmer C, Wohlschläger AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum. Brain Mapp. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Telang F, Wang GJ, Chang L, Ernst T, Fowler JS. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4:e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Bisqal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Retrosen J, Adler LA, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J. Neurosci. Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]