Abstract
PURPOSE
To obtain estimates of time to recruit the study sample, retention, facility-based class attendance and home practice for a study of yoga in breast cancer survivors, and its efficacy on fatigue, quality of life (QOL), and weight change.
METHODS
Sixty-three post-treatment stage 0–III borderline overweight and obese (body mass index ≥ 24 kg/m2) breast cancer survivors were randomly assigned to a 6-month, facility- and home-based viniyoga intervention (n = 32) or a waitlist control group (n = 31). The yoga goal was 5 practices per week. Primary outcome measures were changes in self-reported QOL, fatigue, and weight from baseline to 6 months. Secondary outcomes included changes in waist and hip circumference.
RESULTS
It took 12 months to complete recruitment. Participants attended a mean of 19.6 classes and practiced at home a mean of 55.8 times during the 6-month period. At follow-up, 90% of participants completed questionnaires and 87% completed anthropometric measurements. QOL and fatigue improved to a greater extent among women in the yoga group relative to women in the control group, although no differences were statistically significant. Waist circumference decreased 3.1 cm (95% CI: −5.7, −0.4) more among women in the yoga compared with the control group, with no differences in weight change.
CONCLUSIONS
This study provides important information regarding recruitment, retention, and practice levels achieved during a 6-month, intensive yoga intervention in overweight and obese breast cancer survivors. Yoga may help decrease waist circumference and improve quality of life; future studies are needed to confirm these results.
Keywords: yoga, breast cancer, quality of life, weight
INTRODUCTION
Breast cancer is the most common cancer among women in the United States [2]. Five-year relative survival is 89% [2], indicating that most breast cancer patients today can expect to live many years after their diagnosis. However, breast cancer survivors may experience sequelae years after their diagnosis [30, 38], including fatigue, pain, fear of recurrence, and reduced quality of life. Weight gain and obesity are also common problems after treatment and increase the risk of recurrence, cardiovascular disease, and diabetes [10, 28].
Higher levels of aerobic physical activity (PA) have been associated with reduced risks of breast cancer-specific and all-cause mortality [19–20, 22]. However, PA levels are generally low among breast cancer survivors and many women decrease their PA following diagnosis [25]. Identifying physical activities that are enjoyable and safe for breast cancer survivors is therefore a priority.
Yoga, a comprehensive system of practices for health and well-being has recently been investigated as a possible adjunct therapy in cancer patients and survivors [5, 11, 15–16, 32]. Yoga includes physical postures, conscious breathing and meditation [42]. For breast cancer survivors, yoga is an appealing intervention because it is an accessible, low-risk activity that requires little equipment and may confer numerous physical and psychological benefits. Studies in individuals with and without a history of cancer suggest that yoga interventions of 8–12 weeks may improve quality of life (QOL), cardiovascular endurance, and sleep, and reduce feelings of tension, depression, anger, and stress [15–16].
At the time this study was conceived, data from several observational and experimental studies suggested that yoga, either alone, or as part of a more comprehensive lifestyle program including dietary changes, may also promote weight loss [4, 26–27, 41, 44] or attenuate weight gain [24]. All but one of the experimental studies were conducted in India among healthy adolescents or men with heart disease or hypertension and most also included a residential component at a retreat center or school. To our knowledge, only one randomized trial of yoga in breast cancer survivors (conducted in Canada) included weight as an outcome [16]. Following a 7-week yoga intervention, there was no statistically significant difference in weight change between the yoga group (n=20) and the control group (n=18) [16]. However, this study's ability to detect differences was limited by the short study duration and the small sample size. A longer, more intensive intervention may be needed to see weight loss. Behavioral treatment for weight loss is recommended to last for at least 6 months [34] and longer programs lead to greater weight loss than shorter programs [37].
More information is needed about how best to conduct a study to rigorously evaluate the extent to which a longer (e.g., 6 month), intensive yoga program may ameliorate long-term breast cancer sequelae in American women. Thus, the purpose of this randomized controlled pilot trial was to generate data on a 6-month yoga intervention in breast cancer survivors in regards to: time to recruit the sample, operational aspects of delivering the yoga intervention (location, timing of classes, difficulty), acceptable yoga exercises, maximizing attendance and home practice, retention for follow-up measures, and preliminary estimates of the intervention's effects on fatigue, QOL, and weight.
METHODS
Participants
Eligibility criteria included age between 21 and 75 years, completion of breast cancer treatment (Stage 0–III) at least 3 months prior (with the possible exception of ongoing hormonal therapies such as tamoxifen or aromatase inhibitors), and a body mass index (BMI) ≥ 24 kg/m2 (or ≥23 kg/m2 if of Asian descent). Exclusion criteria included a myocardial infarction or stroke in the previous 6 months, diabetes, current yoga practice, pregnancy or plans to become pregnant, and other factors that might lead to poor retention and yoga practice, which included plans to leave the study area during the follow-up period or any contraindications to practicing yoga.
Participants were recruited via oncologist referrals, community-based advertising, public service announcements, website, and direct mailings to women who had expressed an interest in Fred Hutchinson Cancer Research Center (FHCRC) studies. The recruitment goal was 60 women. It was estimated that 60 women would provide sufficient power to detect 3.2–3.4% difference from baseline to 6 months in weight, 15–20% in QOL and 40–45% in fatigue scores between the two groups. At the time of study development, it was acknowledged that these were optimistic effect sizes, particularly for QOL and fatigue. However, the sample size was thought to be sufficient to guide the investigators in piloting various aspects of the trial and determining an appropriate sample size for a future full-scale trial.
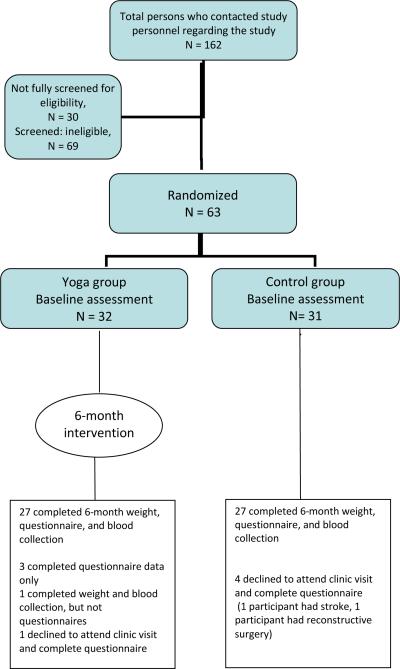
One hundred and sixty-two women contacted study personnel regarding the study (Figure 1), of whom 132 were screened for eligibility, while 30 were not fully screened, primarily because they contacted study personnel after the recruitment goal had been reached. Primary reasons for ineligibility were BMI< 24 kg/m2 (n=32), failure to agree to meet study requirements related to yoga practice (n=8), already practicing yoga (n=5), diabetes (n=5), and unwillingness to be randomized (n=4). A total of 63 women were eventually randomized.
Figure 1.
Flow diagram of participant recruitment, enrollment, and retention
Procedures
The protocol for this study was approved by the FHCRC Institutional Review Board and all women provided written, informed consent to participate. All study procedures and classes took place at the FHCRC. Eligibility was assessed in stages, first through a brief interest survey, followed by an information session and clinic visit for weight assessment. Eligible women were block randomized to the intervention or a wait-list control group on age (3 strata: 21–49, 50–69, 70–75 years), stage (2 strata: 0/I and II/III), and BMI (2 strata: 24–29.9 kg/m2 and ≥30 kg/m2) to assure comparability in the two groups. No monetary compensation was provided for participation. Outcome data were collected via self-administered questionnaires and a one-hour clinic visit at baseline and 6-months follow-up. To reduce participant burden and permit assessments among all study participants, satisfaction was assessed (among intervention and wait-list control participants) via anonymous internet-administered surveys after completing the 6-month yoga intervention.
Yoga Intervention
The yoga intervention was based on viniyoga, a Hatha therapeutic style of yoga that involves physical stretches and poses, breath control, and meditation. The yoga intervention was developed for use with overweight and obese breast cancer survivors without prior yoga experience by two certified yoga instructors with more than 10 years of experience teaching yoga to cancer patients, survivors, and those with chronic illnesses. A manual with detailed class guidelines was developed to standardize delivery of the intervention.
Participants were given a goal of practicing five times per week, including at least one 75-minute facility-based class. For physical activity in general, and particularly for interventions aimed at weight loss, five days of activity per week is often prescribed [36]. We hypothesized that benefits would most likely be accrued by making yoga a near-daily habit. To accommodate different schedules, three facility-based classes were offered each week. Women were permitted and encouraged to attend two or three facility-based classes if they desired; the remainder of their weekly practices (i.e., two [if they attended 3 classes] to four [if they attended 1 class] sessions were to be completed at home).
Each yoga practice opened with 5–10 minutes of centering exercises to promote relaxation and internal focus, followed by 50–60 minutes of seated and standing poses, and closed with 10–15 minutes of guided relaxation, breathing exercises, and meditation. Key poses included cobra (bhujangasana), sunbird (chakravakasana), lunges, warrior variations, bridge, forward bends, triangle, twists, and corpse (savasana). To aid with poses, all participants received a yoga mat and strap; blankets, blocks, and chairs were available in class. To aid with home practice, participants were given a DVD, CD, and booklets of four home practices lasting 20–30 minutes each that were designed specifically for the study. Enrollment into the study was conducted continuously. Thus, as occurs in community-based yoga classes, yoga experience varied among participants. No dietary advice was given.
Class attendance and home yoga practice were encouraged among those not meeting the study practice goals by email and/or telephone counseling. Motivational interviewing techniques were used to encourage class attendance and home yoga practice. These included discussing barriers to class attendance and home practice, identifying potential solutions to the barriers, and setting class attendance and home practice goals.
Wait-list control group
Participants in the wait-list control group were asked to not begin yoga and were not contacted again until it was time to schedule their 6-month follow-up assessment. After their 6-month follow-up assessment, they were offered facility-based yoga classes for 6 months and given home practice materials.
Measures
Quality of life
To assess QOL, we used the Functional Assessment of Cancer Therapy-General (FACT-G), a generic, well-validated measure of health-related QOL [13–14, 45]. The 27-item FACT-G is composed of four subscales (physical well-being, social well-being, emotional well-being, and functional well-being). The breast cancer module (FACIT-B+4) consists of thirteen additional items [6]. Fatigue, including limitations in daily activity and energy level, was assessed by the 13-item Fatigue Scale (FACIT-F) developed specifically for the cancer population [12]. Prior studies have demonstrated that the scales are reliable (r=0.90) with a high internal consistency (alpha=0.94) [6, 13–14].
Physical activity
Information on physical activity (PA) was collected using a self-administered version of the Modifiable Activity Questionnaire (22). We inquired about usual frequency, duration, and number of months of recreational activities performed during the previous 12 (baseline) or 6 (6-month follow-up questionnaire) months. Twenty-nine activities were listed (e.g., walking at a moderate pace, bicycling, jogging, and tai chi) and space for activities not listed was also included.
From duration and frequency, we calculated average MET-hours per week as a measure of usual PA after assigning each activity a metabolic equivalent task (MET) code [1]. METs range from 0.9 (sleeping) to 18 (running at 10.9 mph). To assess how the yoga intervention affected non-yoga PA, we calculated the average MET-hours per week both including and excluding yoga MET-hours per week.
Anthropometric measures
Height was measured to the nearest centimeter at the baseline clinic visit using a stadiometer. Weight, waist, and hip circumference were measured in a dressing gown with undergarments at the baseline and 6-month follow-up visits. Standard procedures were used to measure hip and waist circumference and duplicate measures were averaged [33]. BMI was calculated as kg/m2.
Class attendance and home practice
Attendance to facility-based classes was directly monitored and tracked through attendance sheets. Home practice was assessed by practice logs that were returned to instructors each week. We calculated the total number of classes and home practices for each woman.
Knowledge of yoga
Knowledge of yoga was assessed by a single question which asked respondents to rate, on a scale of 1 (“no knowledge”) to 5 (“a lot of knowledge”).
Feasibility
Feasibility was assessed by time to recruit the sample, retention, frequency of class attendance and home yoga practice, adverse events, and participant's satisfaction.
Statistical analyses
Chi square and t-tests were used to compare baseline characteristics between yoga and control group participants. We used an intent-to-treat approach. Those who did not provide follow-up values (see Figure 1) were not included in analyses. We used linear regression to estimate changes in outcomes between groups, adjusted for baseline values. As a secondary analysis, we assessed changes in outcomes by number of facility-based practices (tertiles: 1–13, 14–23, and ≥24 classes) and a sum of facility- and home-based practices (tertiles: 5–43, 44–104, and ≥105 sessions).
RESULTS
Feasibility measures
Approximately 5 subjects were recruited per month between May 2007 and April 2008. Retention of participants for follow-up measures was high; 90% completed questionnaires for the primary outcomes and 87% attended the clinic visit so that anthropometric measures could be assessed (Figure 1).
Women attended an average of 19.6 facility-based classes (range: 1–61; median: 20.5) and practiced at home an average of 55.8 times (range: 2–102; median: 62) during the 6-month intervention (Table 2).
Table 2.
Distribution of facility-based and home yoga practice among women in the yoga intervention arm (n=32)
| Number of sessions | N | % | Mean (SD) |
|---|---|---|---|
| Facility-based classes | |||
| Overall | 32 | 100.0 | 19.6 (13.0) |
| 1–13 | 11 | 34.4 | 6.5 (4.0) |
| 14–23 | 11 | 34.4 | 20.1 (2.5) |
| 24–61 | 10 | 31.3 | 33.5 (11.3) |
| Home practice | |||
| Overall | 32 | 100.0 | 55.8 (32.5) |
| 2–32 | 10 | 31.3 | 16.1 (10.2) |
| 33–78 | 11 | 34.4 | 57.5 (17.4) |
| 79–102 | 11 | 34.4 | 90.1 (7.9) |
| Facility and home practice | |||
| Overall | 32 | 100.0 | 75.3 (42.8) |
| 5–43 | 10 | 31.3 | 23 (13.2) |
| 44–104 | 11 | 34.4 | 79.3 (22.4) |
| 105–155 | 11 | 34.4 | 119 (13.1) |
Participation goal was one facility-based class and four home yoga sessions per week, for 26 weeks. To meet the participation goal, participants could substitute facility-based classes for home yoga practice sessions. Three facility-based classes were offered each week.
The yoga intervention was well received among the 32 (50.8% of total) women who completed anonymous internet-based satisfaction surveys at 6-months follow-up. On a scale of 1 to 10, with 10 being the most positive, the mean scores were 9.2 for the home practice DVD, 8.3 for enjoyment, and 9.1 and 9.3, respectively, for instructor's ability to lead classes and teach to the participant's ability. Qualitative assessments were also very positive. There were no significant injuries or adverse events.
Participant characteristics at baseline
The mean age was 60 years and the majority of participants were white, college graduates, married, with an income ≥$60,000 (Table 1). About 44% of women were diagnosed with in situ breast cancer, 27% with Stage I, and 39% with Stage II or III. The mean PA of 13 MET-hours per week was equivalent to approximately 4 hours of moderate-paced walking per week, similar to what has been observed in a cohort of breast cancer survivors using a similar assessment tool [21]. Knowledge of yoga was moderately low (mean score = 2.2 on a scale of 1 (“no knowledge”) to 5 (“a great deal of knowledge”) and did not differ between the two groups.
Table 1.
Demographic, medical, physical activity and yoga knowledge characteristics of study participants†
| Characteristic | Yoga group (N=32) | Control group (N=31) | p |
|---|---|---|---|
| % or Mean (SD) | % or Mean (SD) | ||
| Age, years | 0.91 | ||
| 33–49 | 6.3 | 9.7 | |
| 50–59 | 40.6 | 45.2 | |
| 60–69 | 40.6 | 35.5 | |
| 70–74 | 12.5 | 9.7 | |
| Mean (SD) | 60.6 (7.1) | 58.2 (8.8) | 0.25 |
| Race/ethnicity | 0.72 | ||
| White, non-Hispanic | 93.7 | 93.5 | |
| African American | 3.1 | 6.5 | |
| Other | 3.2 | 0.0 | |
| Education | 0.81 | ||
| High school graduate | 6.3 | 3.2 | |
| Some college | 25.0 | 29.0 | |
| College graduate | 28.1 | 35.5 | |
| Professional degree | 40.6 | 32.3 | |
| Marital status | 0.55 | ||
| Married or living as married | 46.9 | 51.6 | |
| Widowed | 12.5 | 3.3 | |
| Divorced/separated | 25.0 | 29.0 | |
| Never married | 15.6 | 16.1 | |
| Income ($) | 0.06 | ||
| <40,000 | 21.9 | 0 | |
| 40,000–<60,000 | 18.8 | 22.6 | |
| 60,000–<80,000 | 37.5 | 29.0 | |
| ≥80,000 | 18.8 | 38.7 | |
| Missing | 3.1 | 9.7 | |
| Stage of disease | 0.47 | ||
| In situ | 43.8 | 45.2 | |
| Stage I | 21.9 | 32.3 | |
| Stage II | 31.3 | 16.1 | |
| Stage III | 3.1 | 6.5 | |
| Time since diagnosis, years | 0.35 | ||
| <2 | 16.7 | 32.3 | |
| 2–<5 | 30.0 | 19.4 | |
| 5–<8 | 31.3 | 19.4 | |
| ≥ 8 | 25.0 | 29.0 | |
| Mean (range) | 6.0 (0.6, 18.1) | 6.5 (0.5, 22.9) | 0.72 |
| Physical activity (MET-hrs/wk) | 0.10 | ||
| 0–5.8 | 21.9 | 45.2 | |
| 5.9–15.7 | 43.8 | 22.6 | |
| ≥ 15.8 | 34.4 | 32.3 | |
| Mean (SD) | 14.6 (11.6) | 11.7 (12.2) | 0.33 |
| Knowledge of yoga‡, Mean (SD) | 2.2 (1.1) | 2.3 (0.8) | 0.52 |
Percentages may sum to more than 100.0 due to rounding.
Knowledge of yoga scale: 1 (“no knowledge”) to 5 (“a great deal of knowledge”)
Efficacy estimates
There were no statistically significant differences between the two groups for any of the QOL, fatigue, anthropometric, and PA measures at baseline (Table 3). Six-month changes in QOL and fatigue did not differ between the groups, although the mean differences suggested benefits for the yoga group. Waist circumference decreased 3.1 cm (95% CI: −5.7, −0.4) more among women in the yoga group compared with the control group, with no between-group differences in weight, BMI, hip circumference, or non-yoga PA.
Table 3.
Mean baseline and 6-month follow-up values for quality of life, anthropometric, and physical activity measures
| Outcome measures | Yoga group, Mean (SD)a | Control group, Mean (SD)a | 6-month Δ (95% CI) between groups | ||
|---|---|---|---|---|---|
| Baselineb | 6-month follow-up | Baselineb | 6-month follow-up | ||
| Quality of life (QOL) c | |||||
| Overall QOL: FACT-G | 89.0 (9.4) | 90.3 (11.0) | 87.8 (14.2) | 87.7 (15.0) | +1.6 (−2.6, +5.7) |
| Breast cancer subscale | 25.5 (4.1) | 26.8 (4.2) | 25.4 (5.2) | 25.2 (5.5) | +1.5 (−0.3, +3.3) |
| Physical well-being | 24.7 (2.3) | 25.4 (1.7) | 24.2 (3.9) | 24.3 (4.4) | +0.6 (−0.5, +1.8) |
| Functional well-being | 22.7 (3.3) | 22.6 (3.9) | 21.6 (5.1) | 21.7 (4.7) | +0.1 (−1.6, +1.8) |
| Emotional well-being | 19.8 (2.8) | 20.3 (4.0) | 20.3 (2.6) | 20.8 (3.1) | −0.1 (−2.1, +1.1) |
| Social/ family well-being | 21.7 (5.1) | 22.1 (5.0) | 21.7 (5.4) | 20.9 (6.0) | +1.2 (−0.8, +3.1) |
| Fatigue | |||||
| FACIT-Fatigue | 43.1 (5.8) | 45.0 (5.3) | 43.2 (8.5) | 43.1 (10.3) | +1.9 (−1.0, +4.9) |
| Anthropometric measures | |||||
| Weight (kg) | 80.4 (12.0) | 81.1 (13.6) | 81.3 (13.6) | 81.3 (14.3) | +0.8 (−0.9, +2.5) |
| Body mass index (kg/m2) | 29.3 (3.7) | 29.5 (4.1) | 29.5 (4.3) | 29.5 (4.7) | +0.2 (−0.4, +0.8) |
| Waist circumference (cm) | 94.4 (7.2) | 93.1 (8.5) | 91.1 (8.9) | 92.7 (10.5) | −3.1 (−5.7, −0.4) |
| Hip circumference (cm) | 113.0 (9.1) | 113.0 (10.1) | 112.7 (8.4) | 113.9 (10.3) | −1.2 (−3.4, +1.0) |
| Physical activity (MET-hours per week) | |||||
| Total | 15.1 (11.7) | 19.2 (19.1) | 12.4 (12.8) | 12.1 (13.6) | +4.4 (−1.7, +10.5) |
| Total, excluding yoga | 15.0 (11.6) | 16.9 (18.9) | 12.4 (11.8) | 12.0 (13.6) | +2.3 (−3.8, +8.4) |
Abbreviations: Cl, confidence interval; FACT-G, Functional Assessment of Cancer Therapy-General; FACIT, Functional Assessment of Chronic Illness Therapy; MET, metabolic equivalent task; QOL, quality of life; SD, standard deviation.
Intervention group: n=30 for QOL and n=28 for anthropometric measures; Control group: n=27 for QOL and for anthropometric measures
All p>0.3 between groups for baseline measures.
Higher scores indicate better functioning for all FACT/FACIT measures.
QOL improved and fatigue decreased more among women who attended a greater number of facility-based classes (Table 4). For fatigue and breast cancer-related QOL, those who attended at least 24 classes had 4.2- and 3.5-point improvements (p<0.05) compared with the control group, with a statistically significant trend of increasing improvement for breast cancer-related QOL (p=0.006). However, there was no suggestion of greater reductions in anthropometric measures among women who attended more classes. When we stratified on the combination of facility-and home-based yoga sessions (effectively giving more weight to the home-based practice, as a greater proportion of the weekly yoga sessions were performed at home), results were generally similar (data not presented). However, the greatest improvements in fatigue were observed in women in the 2nd tertile who practiced a mean of 3 times per week (4.8, 95% CI +0.9, +8.8). In addition, there was a statistically significant trend (p=0.048) of increasing BMI with increasing facility- and home-based yoga sessions, though this trend was no longer statistically significant after adjusting for age. Similar to the results for facility-based classes only, women in the first tertile of facility- and home-based sessions experienced the greatest reduction in waist circumference (−7.3 cm, 95% CI −11.6, −3.0).
Table 4.
Mean baseline and 6-month follow-up values for outcome measures, stratified by number of facility-based classes
| Outcome and adherence subgroup | Baseline | 6-month follow-up | 6-month Δ (95% CI) relative to controls | Pb | |
|---|---|---|---|---|---|
| N | Mean (SD) | ||||
| Quality of life measures | |||||
| Overall QOL: FACT-G | 0.30 | ||||
| Controls | 27 | 87.8 (14.2) | 87.7 (14.9) | Ref | |
| 1–13 | 9 | 85.4 (6.8) | 86.5 (10.4) | +1.0 (−5.1, +7.0) | |
| 14–23 | 11 | 92.3 (10.3) | 91.9 (11.1) | +0.2 (−5.4, +5.9) | |
| 24–61 | 10 | 88.7 (9.9) | 92.0 (11.7) | +3.5 (−2.3, +9.3) | |
| FACIT-Breast cancer subscale | 0.006 | ||||
| Controls | 27 | 25.4 (5.2) | 25.2 (5.5) | Ref | |
| 1–13 | 9 | 25.1 (1.8) | 24.4 (3.6) | −0.6 (−3.1, +1.9) | |
| 14–23 | 11 | 26.9 (4.8) | 27.7 (4.5) | +1.4 (−1.0, +3.7) | |
| 24–61 | 10 | 24.3 (4.8) | 27.9 (3.8) | +3.5 (+1.1, +6.0) | |
| Fatigue | 0.10 | ||||
| FACIT-Fatigue | |||||
| Controls | 27 | 43.2 (8.5) | 43.1 (10.3) | Ref | |
| 1–13 | 9 | 41.3 (7.5) | 43.2 (5.8) | +1.6 (−2.6, +5.8) | |
| 14–23 | 11 | 45.3 (5.0) | 45.1 (5.0) | +0.2 (−3.8, +4.1) | |
| 24–61 | 10 | 42.3 (4.6) | 46.6 (5.1) | +4.2 (+0.1, +8.2) | |
| Anthropometric measures | |||||
| Weight (kg) | 0.34 | ||||
| Controls | 27 | 81.3 (13.6) | 81.3 (14.3) | Ref | |
| 1–13 | 7 | 80.6 (7.6) | 81.6 (11.6) | +1.0 (−1.6, +3.7) | |
| 14–23 | 11 | 75.5 (9.3) | 75.3 (9.2) | +0.2 (−2.1, +2.5) | |
| 24–61 | 10 | 85.6 (15.3) | 87.2 (17.0) | +1.3 (−1.1, +3.6) | |
| Body mass index (kg/m2) | 0.41 | ||||
| Controls | 27 | 29.5 (4.3) | 29.5 (4.7) | Ref | |
| 1–13 | 7 | 30.1 (2.7) | 30.5 (3.6) | +0.3 (−0.7, +1.2) | |
| 14–23 | 11 | 28.0 (2.8) | 28.0 (2.7) | 0.0 (−0.8, +0.8) | |
| 24–61 | 10 | 30.0 (4.9) | 30.5 (5.3) | +0.4 (−0.4, +1.3) | |
| Waist circumference (cm) | 0.12 | ||||
| Controls | 27 | 91.1 (8.9) | 92.7 (10.5) | Ref | |
| 1–13 | 7 | 100.1 (7.5) | 96.8 (11.5) | −5.4 (−9.8, −1.0) | |
| 14–23 | 11 | 91.9 (5.5) | 91.3 (7.0) | −2.2 (−5.7, +1.2) | |
| 24–61 | 10 | 93.3 (7.1) | 92.3 (7.6) | −2.7 (−6.3, +0.9) | |
| Hip circumference (cm) | 0.27 | ||||
| Controls | 27 | 112.7 (8.4) | 113.9 (10.3) | Ref | |
| 1–13 | 7 | 112.3 (9.3) | 112.9 (10.0) | −0.6 (−4.2, +3.0) | |
| 14–23 | 11 | 109.9 (7.3) | 109.4 (8.9) | −1.4 (−4.5, +1.6) | |
| 24–61 | 10 | 116.8 (10.1) | 116.9 (11.0) | −1.4 (−4.6, +1.8) | |
| Non-yoga physical activity (MET-hrs per wk) | 0.64 | ||||
| Controls | 27 | 12.4 (12.8) | 12.0 (13.6) | Ref | |
| 1–13 | 9 | 14.9 (8.8) | 17.7 (7.2) | +3.2 (−5.6, +12.0) | |
| 14–23 | 11 | 13.4 (12.1) | 11.7 (13.7) | −1.2 (−9.4, +6.9) | |
| 24–61 | 10 | 16.9 (14.0) | 21.9 (28.9) | +5.5 (−3.0, +14.0) | |
Abbreviations: CI, confidence interval; FACT-G, Functional Assessment of Cancer Therapy-General; FACIT, Functional Assessment of Chronic Illness Therapy; MET, metabolic equivalent task; QOL, quality of life; SD, standard deviation.
P value for trend test of baseline-to-6-month differences across groups (control, low, medium, high), controlling for baseline value of outcome measures.
DISCUSSION
This pilot study provides important information regarding recruitment, retention, and frequency of yoga practice that were achieved among breast cancer survivors in a 6-month intervention. The mean “dose” of yoga was high -- nearly 3 sessions per week for 26 weeks. Follow-up measures were obtained on all but approximately 10% of participants. Assessments of the program indicated that it was well-received and safe. Together, these findings suggest that a larger study with a similar design could be conducted to definitively assess associations with the outcomes.
Recruitment in clinical trials is often time-consuming and expensive. Use of a cancer registry to identify potentially eligible participants might have led to meeting the recruitment goal more quickly and including a greater proportion of women with invasive cancer who were diagnosed more recently [35]. However, the cost required to access information from the cancer registry was too high for this pilot trial. Researchers who choose to use methods similar to the ones employed in the current study (e.g., postings, newsletters, and public service announcements), may find that, like our study, they enroll a relatively large proportion of participants with in situ cancer who are long-term survivors.
Results from this study suggest that yoga practice may improve fatigue and QOL and decrease waist circumference. Although not entirely consistent, these benefits appeared to be greater among women who attended more facility-based classes. One notable exception was the greater reduction in waist circumference among women who attended a mean of 6.5 classes over 26 weeks (the lowest tertile). The mean waist circumference among women in this group (100.1 cm) was greater than in the other groups (91.9 and 93.3 cm); changes may have been easier to detect in this group. Nevertheless, estimates of changes in waist circumference were similar in the other groups, though confidence intervals for the 2nd and 3rd tertiles included the null value. Non-yoga PA did not decrease among women in the yoga group, suggesting that women in the yoga group did not substitute yoga for other activities, but simply added yoga to their daily routines. In addition, there was no evidence of an increase in yoga practice among control women, indicating no crossover.
A major challenge in synthesizing results from prior studies is the heterogeneity in terms of the populations studied (e.g., cancer stage, timing of study in relation to time since diagnosis and treatment, and ethnicity of study participants), type and intensity of yoga (e.g., Iyengar, restorative, or vigorous), duration and intensity of the intervention, comparison group (e.g., none, waitlist, or attention), and statistical methods. Unlike an intervention involving walking, for example, the term “yoga” typically comprises a multi-modal intervention involving physical poses, meditation, and breathing techniques, but each school of yoga emphasizes different components and uses different techniques. Consequently, it would not be surprising if outcomes varied depending on these factors.
Taking these differences into consideration, there is a growing body of evidence suggesting benefits of yoga in terms of fatigue and QOL for cancer survivors. In a 12-week yoga trial, QOL improved significantly (as measured by the FACT-G, effect size = .29, p<0.05) in breast cancer survivors (n=71) diagnosed within the previous 5 years who were not on chemotherapy [32]. Likewise, in a pilot randomized trial (n=38) of longer-term survivors (mean time since diagnosis = 4.7 years), a 7-week yoga program was associated with improvements in global QOL (p<0.01) [16]. Trials of yoga and interventions including a yoga component, in breast and other cancer survivors have also observed similar benefits [5, 9, 11, 17].
There are a number of mechanisms by which yoga may confer benefits on QOL and fatigue. Studies have demonstrated that yoga produces a relaxation response, which encompasses an integrated set of changes that includes increased breath volume and decreased heart rate [43]. Yoga also places an emphasis on accepting one's moment-to-moment experience. Learning such acceptance may reduce psychological distress and restore normal cortisol rhythms through an interaction between the autonomic nervous system and the endocrine system [18]. Such changes may result in concomitant effects not only on perceptions of fatigue, but also on inflammatory cytokines underlying fatigue [31] and global QOL.
Mean weight losses in the prior studies ranged from 2.5 to 5.2 kg for interventions lasting 14 weeks to 1 year [4, 26–27, 44], no measures of body composition were reported in these studies. Unlike the prior studies, the current study did not include any meals or dietary advice, and this difference may partly explain the apparent lack of effect. Yoga, without a dietary intervention, may be insufficient to result in clinically important weight loss.
However, while we did not observe weight loss, waist circumference did decrease, suggesting that there may have been a gain of lean muscle mass and loss of fat. Increasing evidence suggests that exercise can reduce abdominal obesity, even with minimal or no weight loss [40]. Waist circumference is a measure of subcutaneous and intraabdominal fat mass and provides an independent prediction of risk over and above that of BMI [34]. Waist circumference is strongly associated with cardiometabolic risk [23] and all-cause mortality [39]. The waist circumference reduction observed in the current study (approximately 3%) is similar to that of other exercise-only trials [3, 29]. Changes of the magnitude observed in this study have been associated with reductions in cardiometabolic markers including c-reactive protein, oxidative stress, and insulin resistance, and thus may represent an important potential benefit of yoga [7–8].
As a pilot with limited funding, the study had several limitations. The study was not powered to detect relatively small, but potentially clinically important effects. Second, the wait-list control group did not control for the increased attention and group support yoga participants may have received. It is possible that differences between groups were in part due to these nonspecific aspects. Third, relatively high QOL and moderate levels of baseline PA (i.e., non-sedentary) may have potentially attenuated effect estimates. Furthermore, generalizability to women with characteristics different than those in the current study (e.g., Caucasian, well-educated, longer-term survivors, with lower stage disease) may be limited. In addition, although retention was high for our primary outcomes, it was relatively poor for the internet-delivered satisfaction surveys; satisfaction in the non-responders may have been worse than in the responders. Lastly, due to the pilot nature of the study and limited funding, we were unable to blind assessors to group assignment. Although we conducted measurements (e.g., waist circumference) using a standardized protocol, the potential for bias cannot be completely eliminated.
In summary, this pilot study provides important information regarding the feasibility of conducting an intensive and long-term yoga intervention in a population of breast cancer survivors. It also suggests that clinically important improvements in QOL, fatigue, breast cancer symptoms, and waist circumference may be achievable, even in a group of women, the majority of whom had been diagnosed 5 or more years prior. Future studies can help to confirm and refine estimates of these and other outcomes.
ACKNOWLEDGMENTS
This material is based upon work supported in part by R25CA094880 and the Transdisciplinary Research in Energetics in Cancer (NCI 1U54 CA116847). The contents do not represent the views of the Department of Veterans Affairs or the United States Government. We gratefully acknowledge the study participants; Laura Yon Brooks and Christy Fisher, for assistance designing the yoga protocol and teaching classes; Ann Enomoto, Lisa Yeager, and Linda Heuertz, for teaching classes.
Footnotes
This study has been presented in part at the TREC-FHCRC-NCI Conference on Energy Balance, Cancer Prognosis, and survivorship in Seattle, WA in October 2009.
The trial was registered at clinicaltrials.gov (identifier: NCT00476203).
Conflict of Interest Statement None declared.
References
- 1.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr., Schmitz KH, Emplaincourt PO, Jacobs DR, Jr., Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Medicine and science in sports and exercise. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society [Accessed 24 March 2010];Cancer Facts & Figures 2009. 2009 http://www.cancer.org/downloads/STT/500809web.pdf.
- 3.Arsenault BJ, Cote M, Cartier A, Lemieux I, Despres JP, Ross R, Earnest CP, Blair SN, Church TS. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207:530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. doi:S0021-9150(09)00404-3 [pii] 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bera TK, Rajapurkar MV. Body composition, cardiovascular endurance and anaerobic power of yogic practitioner. Indian J Physiol Pharmacol. 1993;37:225–228. [PubMed] [Google Scholar]
- 5.Bower JE, Woolery A, Sternlieb B, Garet D. Yoga for cancer patients and survivors. Cancer Control. 2005;12:165–171. doi: 10.1177/107327480501200304. [DOI] [PubMed] [Google Scholar]
- 6.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 7.Campbell PT, Campbell KL, Wener MH, Wood BL, Potter JD, McTiernan A, Ulrich CM. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Medicine and science in sports and exercise. 2009;41:1533–1539. doi: 10.1249/MSS.0b013e31819c7feb. doi:10.1249/MSS.0b013e31819c7feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, Ulrich CM. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Medicine and science in sports and exercise. 2010;42:1448–1453. doi: 10.1249/MSS.0b013e3181cfc908. doi:10.1249/MSS.0b013e3181cfc908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson LE, Speca M, Patel KD, Faris P. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Carmichael AR. Obesity and prognosis of breast cancer. Obes Rev. 2006;7:333–340. doi: 10.1111/j.1467-789X.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 11.Carson JW, Carson KM, Porter LS, Keefe FJ, Shaw H, Miller JM. Yoga for women with metastatic breast cancer: results from a pilot study. J Pain Symptom Manage. 2007;33:331–341. doi: 10.1016/j.jpainsymman.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. doi:S0885392402005298 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 14.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 15.Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 16.Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psychooncology. 2006;15:891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 17.Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, Levine EA. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18:360–368. doi: 10.1002/pon.1503. doi:10.1002/pon.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harinath K, Malhotra AS, Pal K, Prasad R, Kumar R, Kain TC, Rai L, Sawhney RC. Effects of Hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J Altern Complement Med. 2004;10:261–268. doi: 10.1089/107555304323062257. [DOI] [PubMed] [Google Scholar]
- 19.Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 20.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 21.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, Ballard-Barbash R. Physical activity levels among breast cancer survivors. Medicine and science in sports and exercise. 2004;36:1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, Baumgartner RN, Baumgartner KB, Bernstein L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. doi:26/24/3958 [pii] 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30:1647–1652. doi: 10.2337/dc07-9921. doi:dc07-9921 [pii] 10.2337/dc07-9921. [DOI] [PubMed] [Google Scholar]
- 24.Kristal AR, Littman AJ, Benitez D, White E. Yoga practice is associated with attenuated weight gain in healthy, middle-aged men and women. Altern Ther Health Med. 2005;11:28–33. [PubMed] [Google Scholar]
- 25.Littman AJ, Tang MT, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. J Cancer Surviv. 2010 doi: 10.1007/s11764-009-0113-2. doi:10.1007/s11764-009-0113-2. [DOI] [PubMed] [Google Scholar]
- 26.Mahajan AS, Reddy KS, Sachdeva U. Lipid profile of coronary risk subjects following yogic lifestyle intervention. Indian Heart J. 1999;51:37–40. [PubMed] [Google Scholar]
- 27.Manchanda SC, Narang R, Reddy KS, Sachdeva U, Prabhakaran D, Dharmanand S, Rajani M, Bijlani R. Retardation of coronary atherosclerosis with yoga lifestyle intervention. J Assoc Physicians India. 2000;48:687–694. [PubMed] [Google Scholar]
- 28.McTiernan A. Obesity and cancer: the risks, science, and potential management strategies. Oncology (Williston Park) 2005;19:871–881. discussion 881–872, 885–876. [PubMed] [Google Scholar]
- 29.McTiernan A, Sorensen B, Irwin ML, Morgan A, Yasui Y, Rudolph RE, Surawicz C, Lampe JW, Lampe PD, Ayub K, Potter JD. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring) 2007;15:1496–1512. doi: 10.1038/oby.2007.178. doi:15/6/1496 [pii] 10.1038/oby.2007.178. [DOI] [PubMed] [Google Scholar]
- 30.Mehnert A, Koch U. Psychological comorbidity and health-related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register-based sample of long-term breast cancer survivors. J Psychosom Res. 2008;64:383–391. doi: 10.1016/j.jpsychores.2007.12.005. doi:S0022-3999(07)00487-4 [pii] 10.1016/j.jpsychores.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. doi:26/6/971 [pii] 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moadel AB, Shah C, Wylie-Rosett J, Harris MS, Patel SR, Hall CB, Sparano JA. Randomized Controlled Trial of Yoga Among a Multiethnic Sample of Breast Cancer Patients: Effects on Quality of Life. J Clin Oncol. 2007 doi: 10.1200/JCO.2006.06.6027. [DOI] [PubMed] [Google Scholar]
- 33.National Center for Health Statistics [Accessed March 30];National Health and Nutrition Examination Survey: body composition procesures manual. http://www.cdc.gov/nchs/data/nhanes/bc.pdf.
- 34.National Institutes of Health. National Heart L. Blood Institute. NHLBI Obesity Education Initiative. North American Association for the Study of Obesity [Accessed January 16];The practical guide: Identification, evaluation, and treatment of overweight and obesity in adults. 2000 http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf.
- 35.Pakilit AT, Kahn BA, Petersen L, Abraham LS, Greendale GA, Ganz PA. Making effective use of tumor registries for cancer survivorship research. Cancer. 2001;92:1305–1314. doi: 10.1002/1097-0142(20010901)92:5<1305::aid-cncr1452>3.0.co;2-m. doi:10.1002/1097-0142(20010901)92:5<1305::AID-CNCR1452>3.0.CO;2-M [pii] [DOI] [PubMed] [Google Scholar]
- 36.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Jama. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 37.Perri MG, Nezu AM, Patti ET, McCann KL. Effect of length of treatment on weight loss. J Consult Clin Psychol. 1989;57:450–452. [PubMed] [Google Scholar]
- 38.Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, Moller S, Eriksen J, Sjogren P. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain. 2009;13:478–485. doi: 10.1016/j.ejpain.2008.05.015. doi:S1090-3801(08)00130-4 [pii] 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. doi:359/20/2105 [pii] 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 40.Ross R, Janiszewski PM. Is weight loss the optimal target for obesity-related cardiovascular disease risk reduction? Can J Cardiol. 2008;24(Suppl D):25D–31D. doi: 10.1016/s0828-282x(08)71046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt T, Wijga A, Von Zur Muhlen A, Brabant G, Wagner TO. Changes in cardiovascular risk factors and hormones during a comprehensive residential three month kriya yoga training and vegetarian nutrition. Acta Physiol Scand Suppl. 1997;640:158–162. [PubMed] [Google Scholar]
- 42.Shiffmann E. The spirit and practice of moving in stillness. Simon and Schuster; New York: 1996. [Google Scholar]
- 43.Vempati RP, Telles S. Yoga-based guided relaxation reduces sympathetic activity judged from baseline levels. Psychol Rep. 2002;90:487–494. doi: 10.2466/pr0.2002.90.2.487. [DOI] [PubMed] [Google Scholar]
- 44.Yang K. A review of yoga programs for four leading risk factors of chronic diseases. Evid Based Complement Alternat Med. 2007;4:487–491. doi: 10.1093/ecam/nem154. doi:10.1093/ecam/nem154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]