Abstract
Over the course of the past decade, contradictory claims have been made regarding the neural bases of deductive reasoning. Researchers have been puzzled by apparent inconsistencies in the literature. Some have even questioned the effectiveness of the methodology used to study the neural bases of deductive reasoning. However, the idea that neuroimaging findings are inconsistent is not based on any quantitative evidence. Here, we report the results of a quantitative meta-analysis of 28 neuroimaging studies of deductive reasoning published between 1997 and 2010, combining 382 participants. Consistent areas of activations across studies were identified using the multilevel kernel density analysis method. We found that results from neuroimaging studies are more consistent than what has been previously assumed. Overall, studies consistently report activations in specific regions of a left fronto-parietal system, as well as in the left Basal Ganglia. This brain system can be decomposed into three subsystems that are specific to particular types of deductive arguments: relational, categorical, and propositional. These dissociations explain inconstancies in the literature. However, they are incompatible with the notion that deductive reasoning is supported by a single cognitive system relying either on visuospatial or rule-based mechanisms. Our findings provide critical insight into the cognitive organization of deductive reasoning and need to be accounted for by cognitive theories.
INTRODUCTION
Deductive reasoning is the process of drawing conclusions that are guaranteed to follow from given premises. Perhaps because deductions are an essential element of cognitive development (Nunes et al., 2007; Halberda, 2003, 2006; Markovits, Schleifer, & Fortier, 1989) and human thinking (Stanovich & West, 2000), the study of deductive reasoning has been central to the reasoning literature for over 50 years (Evans, 2005). In particular, much emphasis has been placed on identifying the mental representations that underlie standard deductive tasks, such as relational arguments in (1), categorical arguments in (2), and propositional arguments in (3).
-
A is to the left of B.
B is to the left of C.
Therefore, A is to the left of C.
-
All As are Bs.
All Bs are Cs.
Therefore, all As are Cs.
-
If there is an A, then there is a B.
There is an A.
Therefore, there is a B.
Psychological theories have often been interpreted as providing mutually exclusive hypotheses regarding the nature of the mental representations that support deductive reasoning (Johnson-Laird, 1999). For example, researchers have been divided on whether deductive arguments such as those above rely on visuospatial or rule-based mechanisms. On the one hand, proponents of the Mental Model Theory (MMT) have argued that deductive reasoning is a nonverbal process that involves the construction and manipulation of a spatial representation of the problem premises (Johnson-Laird, 1983, 2001). On the other hand, advocates of the formal rule approach (FRA) assume that deductions call upon the retrieval and application of rules to a propositional representation of the problem premises (Braine & O’Brien, 1998; Rips, 1994). Overall, testing between these two kinds of models has allowed significant progress to be made in the field. However, evidence for the involvement of both visuospatial and rule-based mechanisms can be found in the cognitive literature (Johnson-Laird, 2001; Braine & O’Brien, 1998), and the exact nature of the mental representations underlying deductive reasoning remains debated.
Just as for other cognitive domains in which competing theories make divergent predictions about underlying mental representations (Henson, 2005), the emergence of neuroimaging techniques two decades ago held the promise of using information about the neural correlates of deductive reasoning to inform the debate between the MMT and the FRA. For example, in the first series of experiments that investigated the neural bases of deductive reasoning (Goel & Dolan, 2001; Goel, Buchel, Frith, & Dolan, 2000; Goel, Gold, Kapur, & Houle, 1997, 1998), Goel and colleagues made a neurological hypothesis that still serves as framework for neuroimaging studies of reasoning (Prado, Van Der Henst, & Noveck, 2010; Reverberi et al., 2007; Knauff, Mulack, Kassubek, Salih, & Greenlee, 2002). They posited that if deductive reasoning relies on visuospatial mechanisms (as claimed by the MMT), then the brain regions involved in visuospatial processing should be activated during deductive tasks. In other words, one should observe reasoning-related activations in parietal and/or occipital regions of the brain that are known to be engaged in tasks with a visuospatial component (Sack, 2009; Kosslyn & Thompson, 2003). However, if deductive reasoning is a rule-based process (as claimed by the FRA), then the brain regions that are involved in rule-based syntax processing should be preferentially engaged during deductive tasks. In this case, one might expect to measure enhanced activity in the left inferior frontal gyrus (IFG), a brain region that has been claimed to be critical for rule-governed grammar processing in natural language (Grodzinsky & Santi, 2008; Ullman, 2006; Friederici & Kotz, 2003). Such predictions are based on the idea that enhanced activity in the same brain region under two different conditions (e.g., deductive reasoning tasks and visuospatial tasks) implies a common cognitive function (e.g., visuospatial processing), a frequent assumption in neuroimaging studies (but see Poldrack, 2006, for a critique of this logic).
Since the seminal studies by Goel et al. (1997, 1998), a growing body of data has been collected on the neural bases of deductive reasoning. To date, however, there is no consensus on whether these data support the MMT or the FRA (Goel, 2007). Indeed, there seems to be enough variability in the location, extent, and strength of brain activations across studies that deductive reasoning has been alternatively associated with activations in visuospatial regions (Knauff et al., 2002; Goel & Dolan, 2001), linguistic/syntactic regions (Reverberi et al., 2007, 2010), or neither of those (Monti, Parsons, & Osherson, 2009; Kroger, Nystrom, Cohen, & Johnson-Laird, 2008; Monti, Osherson, Martinez, & Parsons, 2007). Two explanations have been advanced to explain this apparent lack of consistency. On the one hand, some have suggested that studies vary substantially in the efficiency of their experimental designs, materials, and statistical analyses. For example, some studies might have missed or incorrectly identified the critical processes involved in deduction because they used non-adequate baseline conditions, relied on overly simple arguments, exposed the subjects to too much training, or did not appropriately model brain activity (Reverberi et al., 2007, 2010; Monti et al., 2007, 2009). This line of argumentation does not consider that inconsistencies in deductive reasoning studies are meaningful per se but rather that they reflect flaws in the experimental design or analysis method. On the other hand, it has also been proposed that differences in the patterns of brain activity across studies may indicate meaningful differences that are not necessarily accounted for by the main psychological theories of reasoning (Prado, Noveck, & Van Der Henst, 2010; Reverberi et al., 2010; Goel, 2007). For example, studies diverge in the type of deductive argument (i.e., relational, categorical, propositional) that reasoners have to evaluate. Both the MMT and the FRA have often been interpreted as universal theories because they can account for virtually all types of reasoning (Johnson-Laird, 1999; Rips, 1994; Hagert, 1984). However, reasoners may be able to use a broad range of strategies to solve reasoning problems (Roberts & Newton, 2005), and some proponents of the FRA have acknowledged that certain types of arguments might preferentially trigger certain strategies (Braine & O’Brien, 1998, p.194). This is consistent with two recent neuroimaging studies that showed differences in the neural correlates of (1) relational and propositional arguments (Prado, Van Der Henst et al., 2010) and (2) categorical and propositional arguments (Reverberi et al., 2010). However, the extent to which this hypothesis can account for variability in the location of brain activations across studies remains to be tested.
The present study constitutes the first quantitative, coordinate-based meta-analysis of neuroimaging studies of deductive reasoning. It makes use of the multilevel kernel density analysis (MKDA) method (Wager, Lindquist, Nichols, Kober, & Van Snellenberg, 2009) to analyze data from 28 studies conducted between 1997 and 2010, thus combining 382 participants. This study has two empirical objectives. The first one is to quantify the extent to which the patterns of brain activity associated with deductive reasoning are consistent across studies and tasks. Although some have argued that such patterns are largely inconsistent (Monti et al., 2007), this claim is not based on any quantitative evidence and this variability may be more apparent than real. Specifically, the present meta-analysis statistically evaluates the consistency of the patterns of brain activity associated with deductive reasoning over and above variations in experimental factors such as differences in baseline condition, materials, practice, analysis method, and argument type. The second aim is to test whether some of the variability in neural activations can be accounted for by a fundamental difference between studies, that is, the type of deductive argument that participants are asked to evaluate. In other words, the present meta-analysis tests the specificity of certain brain regions for certain types of argument over and above differences in other experimental factors. Our study affords a unique glimpse into more than 10 years of neuroimaging research on deductive reasoning and the implications of our findings for cognitive theories of reasoning are discussed.
METHODS
Study Selection
A recent qualitative review of the neuroimaging literature conducted by Goel (2007) served as the starting point for our meta-analysis. All of the studies included in this review were considered in the present meta-analysis as long as they reported the Montreal Neurological Institute (MNI) or Talairach coordinates of the activation peaks in a contrast of a reasoning condition versus a baseline condition (e.g., Goel & Dolan, 2003, was not included because the authors do not report the results of such a contrast). However, because Goel’s review only included studies published up to April 2007, we searched for additional neuroimaging studies of deductive reasoning published from May 2007 to September 2010. This search was conducted in the PubMed and ScienceDirect databases.
Overall, 28 published, peer-reviewed fMRI and PET studies on the neural substrates of deductive reasoning were included in the present meta-analysis. Much like Goel’s review article, studies were dissociated by type of argument (relational, categorical, and propositional). However, whereas the aforementioned review was qualitative, the present meta-analysis used a quantitative approach. It is important to note that the studies included differ in aspects other than the type of deductive argument employed. For example, some categorical reasoning studies make use of arguments with an abstract content (e.g., All As are Bs, All Bs are Cs, therefore All As are Cs), whereas others employ arguments with a concrete semantic content (e.g., All poodles are pets, All pets have names, therefore All poodles have names). Similarly, relational reasoning studies have used both linguistic (e.g., Tom is taller than Bill, Bill is taller than John, therefore Tom is taller than John) and nonlinguistic (e.g., A > B, B > C, therefore A > C) materials. Finally, propositional reasoning studies report activations associated with different kinds of inferences, such as modus ponens (i.e., If P then Q; P; therefore Q), modus tollens (i.e., If P then Q; not Q; therefore not P), and disjunction elimination (i.e., P or Q; not P; therefore Q). Studies also differ in factors such as imaging technique (e.g., PET vs. fMRI), experimental design (e.g., block vs. event-related), input modality (e.g., visual vs. auditory), period analyzed (e.g., second premise vs. conclusion), and amount of practice given. Although we acknowledge that these factors might lead to differences in the neural bases of deductive reasoning across studies, there were insufficient studies to examine each separately. Moreover, one of the main goals of the present meta-analysis was to examine the consistency of the patterns of brain activity associated with deductive reasoning over and above variations in the aforementioned factors.
Studies were included in the present meta-analysis based on three main requirements: (1) the studies’ participants were healthy adults, (2) the studies reported MNI or Talairach coordinates for a contrast of a deductive reasoning condition versus a baseline condition, and (3) the studies reported activation coordinates for the whole brain. Many studies reported activation coordinates for a contrast of reasoning versus baseline where the baseline was an unrelated task (e.g., sentence comprehension task, matching task; Goel et al., 1997), a reasoning argument in which the premises could not be integrated (e.g., If P then Q; R; therefore R; Reverberi et al., 2007), or a lower-level baseline (e.g., fixation cross; Knauff, Fangmeier, Ruff, & Johnson-Laird, 2003). Other studies designated a simple reasoning condition as their baseline condition, thus reporting a contrast of complex argument versus simple argument (Monti et al., 2007; Prado & Noveck, 2007). Both of these types of studies were included. From each study, contrasts corresponding most closely to a comparison between a reasoning condition and a baseline condition were selected. The studies included in the meta-analysis, together with their respective contrasts, are listed in Table 1.
Table 1.
Studies and Contrasts Included in the Meta-analysis
| Study | Type of Argument | Scanning Method | Stimuli Modality | n | No. of Coord. | Contrasts Included |
|---|---|---|---|---|---|---|
| Goel et al., 1997 | Categorical | PET | Visual, linguistic | 10 | 3 | 1. Deduction versus baseline |
| Goel et al., 1998 | Categorical | PET | Visual, linguistic | 12 | 4 | 1. Syllogism versus baseline |
| Goel et al., 1998 | Relational | PET | Visual, linguistic | 12 | 8 | 1. Spatial relational versus baseline |
| 2. Nonspatial relational versus baseline | ||||||
| Osherson et al., 1998 | Categorical | PET | Visual, linguistic | 10 | 8 | 1. Logic versus meaning |
| Goel et al., 2000 | Categorical | fMRI | Visual, linguistic | 11 | 31 | 1. Main effect of reasoning |
| 2. Content versus preparation | ||||||
| 3. No-content versus preparation | ||||||
| Houde et al., 2000 | Propositional | PET | Visual, nonlinguistic | 8 | 19 | 1. Posttest versus pretest |
| Parsons & Osherson, 2001 | Propositional | PET | Visual, linguistic | 10 | 24 | 1. Deduction versus probabilistic reasoning |
| Goel & Dolan, 2001 | Relational | fMRI | Visual, linguistic | 14 | 36 | 1. Main effect of reasoning |
| 2. Concrete reasoning versus concrete baseline | ||||||
| 3. Abstract reasoning versus abstract baseline | ||||||
| Acuna et al., 2002 | Relational | fMRI | Visual, nonlinguistic | 15 | 30 | 1. Transitive inference task versus height comparison |
| 2. Transitive inference task versus passive visual task | ||||||
| Knauff et al., 2002a | Propositional and relational | fMRI | Auditory, linguistic | 12 | 18 | 1. Relational versus baseline or conditional reasoning versus baseline |
| Knauff et al., 2003 | Relational | fMRI | Auditory, linguistic | 12 | 26 | 1. Visuospatial versus rest interval |
| 2. Visual versus rest interval | ||||||
| 3. Spatial versus rest interval | ||||||
| 4. Control versus rest interval | ||||||
| 5. All inferences versus rest interval | ||||||
| Goel & Dolan, 2004 | Categorical | fMRI | Visual, linguistic | 16 | 25 | 1. Main effect of reasoning |
| 2. Deductive reasoning versus baseline | ||||||
| Goel, Makale, & Grafman, 2004 | Relational | fMRI | Visual linguistic | 14 | 19 | 1. Familiar environment reasoning versus familiar environment baseline |
| 2.Unfamiliar environment reasoning versus unfamiliar environment baseline | ||||||
| Heckers, Zalesak, Weiss, Ditman, & Titone, 2004 | Relational | fMRI | Visual, nonlinguistic | 16 | 17 | 1. Inference-by-sequence interaction |
| 2. Simple effects of transitive inference | ||||||
| 3. Transitive inference network | ||||||
| Noveck et al., 2004 | Propositional | fMRI | Visual, linguistic | 16 | 10 | 1. Modus Ponens versus baseline |
| 2. Modus tollens versus baseline | ||||||
| Canessa et al., 2005 | Propositional | fMRI | Visual, linguistic | 12 | 41 | 1. Descriptive reasoning versus baseline |
| 2. Social exchange reasoning versus baseline | ||||||
| Fangmeier et al., 2006 | Relational | fMRI | Visual, nonlinguistic | 12 | 24 | Reasoning problems: |
| 1. Premise processing phase | ||||||
| 2. Premise integration phase | ||||||
| 3. Reasoning validation phase | ||||||
| Monti et al., 2007 Experiment 1 | Propositional | fMRI | Visual, linguistic | 10 | 42 | 1. Complex versus simple deductions (block and pseudoword trials) |
| 2. Complex versus simple deductions (block trials) | ||||||
| 3. Complex versus simple deductions (pseudoword trials) | ||||||
| Monti et al., 2007 Experiment 2 | Propositional | fMRI | Visual, linguistic | 12 | 35 | 1. Complex versus simple deductions (house and face trials) |
| 2. Complex versus simple deductions (house trials) | ||||||
| 3. Complex versus simple deductions (face trials) | ||||||
| Reverberi et al., 2007 | Propositional | fMRI | Visual, linguistic | 14 | 8 | 1. (Integrable versus nonintegrable)conditional and (Integrable versus nonintegrable)disjunctive and [(Integrable versus nonintegrable) disjunctive versus (Integrable versus nonintegrable)conditional |
| Prado & Noveck, 2007 | Propositional | fMRI | Visual, linguistic | 20 | 16 | 1. Verification task (2-mismatch versus 1-mismatch versus 0-mismatch) |
| 2. Falsification task (2-mismatch versus 1-mismatch versus 0-mismatch) | ||||||
| Kroger et al., 2008 | Categorical | fMRI | Visual, linguistic | 16 | 24 | Logic problems versus math problems: |
| 1. Type of problem | ||||||
| 2. Level of difficulty | ||||||
| 3. Type × Difficulty interaction | ||||||
| Rodriguez-Moreno & Hirsch, 2009 | Categorical | fMRI | Visual and auditory, linguistic | 11 | 14 | 1. Premise 2: Reasoning versus control |
| 2. Conclusion: Reasoning versus control | ||||||
| Fangmeier & Knauff, 2009 | Relational | fMRI | Auditory, nonlinguistic | 12 | 18 | Reasoning problems: |
| 1. Premise processing phase | ||||||
| 2. Premise integration phase | ||||||
| 3. Reasoning validation phase | ||||||
| Goel, Stollstorff, Nakic, Knutson, & Grafman, 2009 | Relational | fMRI | Visual linguistic | 17 | 10 | 1. Main effect of reasoning (reasoning versus baseline) |
| Monti et al., 2009 | Propositional | fMRI | Visual, linguistic | 15 | 26 | 1. Inference versus grammar for logic arguments |
| Reverberi et al., 2010 | Categorical | fMRI | Visual, linguistic | 26 | 9 | 1. Integration effect for syllogistic problems |
| Reverberi et al., 2010 | Propositional | fMRI | Visual, linguistic | 26 | 4 | 1. Integration effect for conditional problems |
| Prado, Van Der Henst, et al., 2010 | Relational | fMRI | Visual, linguistic | 13 | 12 | 1. Relational syllogism (integration effect) |
| Prado, Van Der Henst, et al., 2010 | Propositional | fMRI | Visual, linguistic | 13 | 12 | 1. Modus tollens (integration effect) |
| Wendelken & Bunge, 2010 | Relational | fMRI | Visual, nonlinguistic | 16 | 11 | 1. Transitive inference (inference versus direct) |
| 2. Relational encoding (specific relations versus general relations) |
n = number of subjects; No. of Coord. = number of coordinates.
This article did not make it possible to report coordinates separately for propositional and relational reasoning. Coordinates from this article were, thus, only included in the overall density analysis.
MKDA
To uncover the brain regions that are consistently activated across reasoning studies, we employed the MKDA method (Wager et al., 2009). This density analysis method has been successfully used in other meta-analyses of brain imaging studies (Wang, Conder, Blitzer, & Shinkareva, 2010; Kober et al., 2008). Its statistical indicator is the probability of activation of a given voxel in the brain with the null hypothesis being that all of the reported activation coordinates are randomly distributed through the gray matter of the brain. Therefore, a significant result indicates that more reported activation coordinates lie near the specified voxel than would be expected by chance. The MKDA technique also allows for nested analysis of data: Multiple activation coordinates are nested within a contrast, and multiple contrasts are nested within a study. This method precludes the results being driven by studies that report a large number of activation peaks or have a large number of contrasts. Additionally, MKDA allows for weighting of studies with respect to their sample size and effect (fixed vs. random). Specifically, studies with a large number of participants and random effects designs are given more weight than studies with fewer participants or fixed effects designs.
The analyses were performed in Matlab 2009 with the MKDA tool package developed by Wager et al. (2009; psych.colorado.edu/~tor/). Peaks from each study were convolved with a spherical smoothing kernel with a radius of 10 mm. The MKDA statistic at each voxel, P, represents the proportion of contrasts that contained activated voxels within 10 mm of the specified voxel:
Each study was weighted by the number of participants (N) and the type of analysis (δ). c is the index factor, ranging from 1 to the number of comparison maps I. A δ value (weight) of 1.00 is assigned to studies that use random effect analysis, whereas studies that use fixed effects analysis are assigned a δ value of 0.75 (Kober et al., 2008). A Monte Carlo simulation with 5000 iterations was used to determine the threshold for statistical significance, that is, the proportion that exceeds the whole-brain maximum in 95% of the Monte Carlo maps. Correction for multiple comparisons was performed using the family-wise error rate at p < .05.
RESULTS
In what follows, we first present the results of the overall density analysis identifying the regions that are the most consistently reported in neuroimaging studies of deductive reasoning across all types of deductive arguments. We then report the results of density analyses performed separately for each type of deductive arguments, together with direct contrasts comparing each argument with one another. All analyses are conducted at a voxelwise significance threshold of p < .05, FWE corrected across the whole brain.
All Deductive Arguments
Despite the apparent variability in the location of activated peaks across studies (Figure 1A), the density analysis performed on the entire corpus of neuroimaging studies revealed that several brain regions were consistently activated across studies (see Figure 1B and Table 2). Significant clusters of activation were located in the left IFG, left medial frontal gyrus (MeFG), bilateral middle frontal gyrus (MFG), bilateral precentral gyrus (PG), bilateral posterior parietal cortex (PPC), and left Basal Ganglia (BG).
Figure 1.
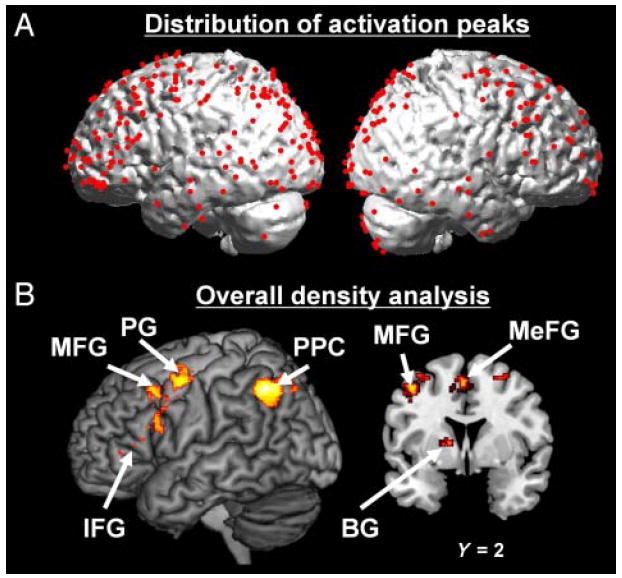
Overall density analysis. (A) Location of the activation peaks from the 28 studies included in the meta-analysis. (B) MKDA map representing the regions most consistently activated in neuroimaging studies of deductive reasoning (irrespective of the type of deductive argument). Peaks and activations are overlaid on 3-D renderings and slices of the MNI-normalized anatomical brain.
Table 2.
Regions Consistently Activated across All Neuroimaging Studies of Deductive Reasoning
| Anatomical Location | ~BA | Talairach Coordinates
|
Volume (mm3) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Main Effect of Reasoning | |||||
| L. IFG | 46 | −45 | 35 | 10 | 24 |
| L. putamen | −16 | 1 | 11 | 120 | |
| L. IFG | 46 | −44 | 26 | 13 | 8 |
| L. IFG | 45 | −49 | 22 | 16 | 16 |
| L. IFG | 9 | −48 | 10 | 24 | 344 |
| L. IFG | 9 | −46 | 15 | 23 | 16 |
| L. IFG | 9 | −44 | 5 | 33 | 96 |
| L. angular gyrus | 39 | −37 | −59 | 38 | 3904 |
| L. angular gyrus | 39 | −39 | −57 | 36 | 3240 |
| L. precuneus | 7 | −24 | −71 | 40 | 664 |
| L. middle frontal gyrus | 9 | −44 | 12 | 35 | 8 |
| L. middle frontal gyrus | 8 | −42 | 10 | 42 | 408 |
| R. middle frontal gyrus | 9 | 39 | 10 | 40 | 72 |
| L. precentral gyrus | 6 | −42 | −3 | 39 | 8 |
| R. precuneus | 7 | 21 | −69 | 38 | 32 |
| L. precentral gyrus | 6 | −39 | −6 | 50 | 1136 |
| L. precentral gyrus | 6 | −40 | −6 | 48 | 776 |
| L. precentral gyrus | 6 | −33 | −8 | 53 | 280 |
| L. middle frontal gyrus | 6 | −35 | −5 | 55 | 80 |
| L. MeFG | 6 | −5 | 3 | 51 | 1904 |
| L. middle frontal gyrus | 6 | −44 | 4 | 45 | 8 |
| R. precuneus | 7 | 20 | −69 | 41 | 8 |
| R. superior parietal lobule | 7 | 24 | −64 | 42 | 56 |
| L. precentral gyrus | 4 | −33 | −14 | 49 | 8 |
| L. precentral gyrus | 4 | −33 | −14 | 53 | 8 |
| R. middle frontal gyrus | 6 | 24 | −9 | 58 | 456 |
L. = left; R. = right; ~BA = approximate Brodmann’s area.
Relational Arguments
We found that studies employing relational arguments display consistent activation in three brain regions: the right MFG, left MeFG, and bilateral PPC (see Figure 2A and Table 3). Direct contrasts between studies revealed that only the bilateral PPC and right MFG are both (1) more consistently associated with relational arguments than categorical arguments (Figure 3A) and (2) more consistently associated with relational arguments than propositional arguments (Figure 3B). Indeed, a contrast of relational arguments versus categorical arguments showed activation in bilateral PPC (BA 39; left: x = −33, y = −61, z = 36; right: x = 22, y = −62, z = 40) and right MFG (BA 6; x = 22, y = −5, z = 60). A contrast of relational arguments versus propositional arguments also showed activation in bilateral PPC (BA 7; x = −24, y = −69, z = 41; x = 20, y = −69, z = 41) and right MFG (BA 6; x = 22, y = −7, z = 56). It is important to note that, of the 11 studies included in the meta-analysis, 5 used nonlinguistic materials as stimuli (see Table 1). To ensure that the activation of the PPC observed in our meta-analysis was not driven by these three studies, we conducted the same density analyses without these five studies. The bilateral PPC was still significantly activated even when these studies were eliminated.
Figure 2.
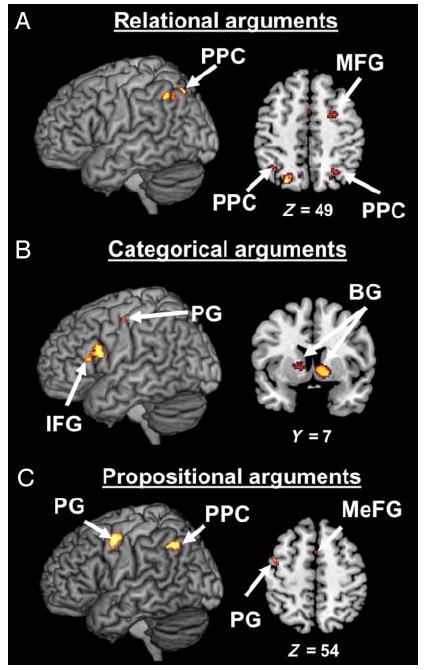
Density analyses performed separately for studies employing relational, categorical, and propositional arguments. (A) MKDA map representing the regions most consistently activated in neuroimaging studies employing relational arguments. (B) MKDA map representing the regions most consistently activated in neuroimaging studies employing categorical arguments. (C) MKDA map representing the regions most consistently activated in neuroimaging studies employing propositional arguments. Activations are overlaid on 3-D renderings and slices of the MNI-normalized anatomical brain.
Table 3.
Regions Consistently Activated in Studies Employing Relational Arguments, Categorical Arguments, and Propositional Arguments
| Anatomical Location | ~BA | Talairach Coordinates
|
Volume (mm3) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Relational Arguments | |||||
| R. middle frontal gyrus | 6 | 22 | −7 | 56 | 1184 |
| L. MeFG | 6 | −2 | −1 | 53 | 144 |
| L. intraparietal sulcus | 40 | −37 | −56 | 38 | 304 |
| L. angular gyrus | 39 | −33 | −61 | 38 | 152 |
| R. superior parietal lobule | 7 | 24 | −62 | 40 | 120 |
| L. precuneus | 7 | −24 | −69 | 41 | 360 |
| R. precuneus | 7 | 20 | −69 | 41 | 8 |
| L. precuneus | 7 | −9 | −67 | 41 | 8 |
| Categorical Arguments | |||||
| L. IFG | 9/44 | −47 | 12 | 23 | 936 |
| L. precentral gyrus | 4 | −39 | −15 | 47 | 88 |
| R. caudate head | 8 | 6 | 3 | 1296 | |
| L. putamen | −16 | 7 | 7 | 40 | |
| L. putamen | −12 | 0 | 9 | 104 | |
| Propositional Arguments | |||||
| L. angular gyrus | 39 | −37 | −57 | 34 | 992 |
| L. precentral gyrus | 6 | −42 | −6 | 46 | 904 |
| L. MeFG | 6 | −2 | 1 | 53 | 8 |
L. = left; R. = right; ~BA = approximate Brodmann’s area.
Figure 3.
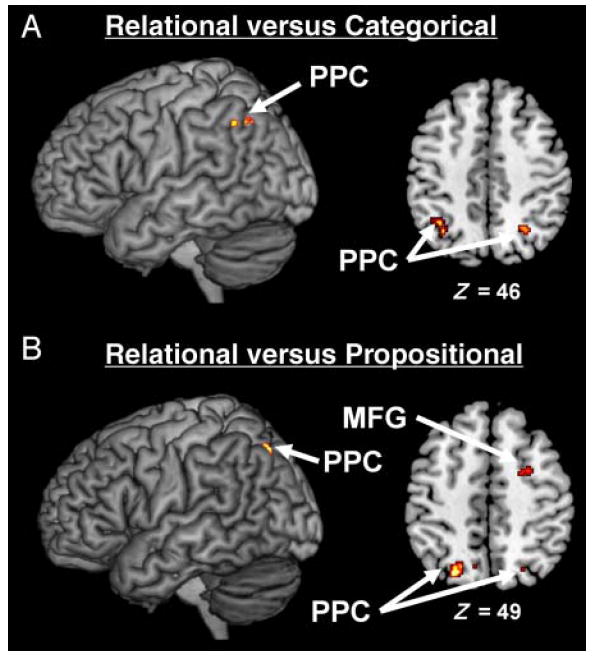
Density analyses for the contrasts of relational arguments versus categorical arguments and relational arguments versus propositional arguments. (A) MKDA map representing the regions more consistently activated in studies that employ relational arguments than in studies that employ categorical arguments. (B) MKDA map representing the regions more consistently activated in studies that employ relational arguments than in studies that employ propositional arguments. Activations are overlaid on 3-D renderings and slices of the MNI-normalized anatomical brain.
Categorical Arguments
When only studies employing categorical arguments are included in the density analysis, three brain regions are found to be consistently activated in the literature: left IFG, left PG, and bilateral BG (see Figure 2B and Table 3). However, direct contrasts between studies revealed that only the left IFG and bilateral BG are both (1) more consistently associated with categorical arguments than relational arguments (Figure 4A) and (2) more consistently associated with categorical arguments than propositional arguments (Figure 4B). Indeed, the contrast of categorical arguments versus relational arguments revealed activation of the left IFG (BA 9/44; x = −48, y = 10, z = 24), bilateral BG (left: x = −16, y = 7, z = 7; right: x = 8, y = 10, z = 2), and left PG (BA 4; x = −41, y = −15, z = 47). The contrast of categorical arguments versus propositional arguments revealed activation of the left IFG (BA 9/44; x = −46, y = 10, z = 22) and bilateral BG (left: x = 12, y = 6, z = 1; right: x = −21, y = 11, z = 7).
Figure 4.
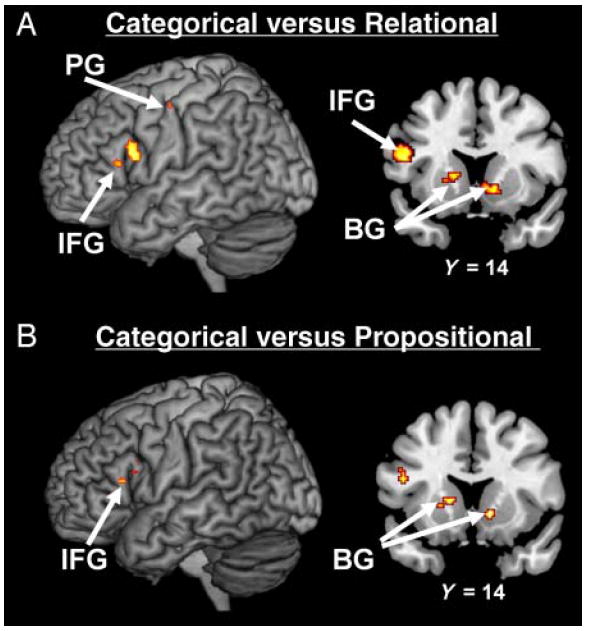
Density analyses for the contrasts of categorical arguments versus relational arguments and categorical arguments versus propositional arguments. (A) MKDA map representing the regions more consistently activated in studies that employ categorical arguments than in studies that employ relational arguments. (B) MKDA map representing the regions more consistently activated in studies that employ categorical arguments than in studies that employ propositional arguments. Activations are overlaid on 3-D renderings and slices of the MNI-normalized anatomical brain.
Propositional Arguments
A density analysis conducted on studies employing propositional arguments revealed activation of the left PPC, left PG, and MeFG (see Figure 2C and Table 3). Direct contrasts between studies revealed that only the left PG is both (1) more consistently associated with propositional arguments than categorical arguments (Figure 5A) and (2) more consistently associated with propositional arguments than relational arguments (Figure 5B). A contrast between studies employing propositional arguments versus studies employing categorical arguments revealed activations in the left PPC (BA 39; x = −39, y = −59, z = 32) and left PG (BA 6; x = −46, y = −4, z = 45). A contrast between studies employing propositional arguments versus studies employing relational arguments only revealed activation in the left PG (BA 6; x = −44, y = −4, z = 46).
Figure 5.
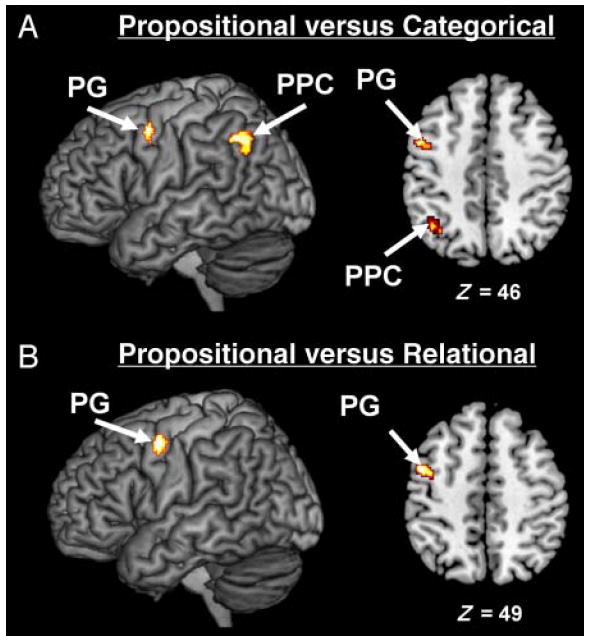
Density analyses for the contrasts of propositional arguments versus categorical arguments and propositional arguments versus relational arguments. (A) MKDA map representing the regions more consistently activated in studies that employ propositional arguments than in studies that employ categorical arguments. (B) MKDA map representing the regions more consistently activated in studies that employ propositional arguments than in studies that employ relational arguments. Activations are overlaid on 3-D renderings and slices of the MNI-normalized anatomical brain.
DISCUSSION
Using the MKDA method (Wager et al., 2009), the present meta-analysis combines data from 28 functional neuroimaging studies to uncover the brain regions that are consistently activated during deductive reasoning. It has been argued that neuroimaging studies of deductive reasoning have generated mostly inconsistent results that are somewhat difficult to interpret (Monti et al., 2007). Although this has led some to question the methodology used to investigate the neural bases of deduction (Monti et al., 2007; Reverberi et al., 2007), this claim is only based on a qualitative survey of the literature. The present quantitative meta-analysis demonstrates that the results gathered from the neuroimaging literature are far more consistent than what has been assumed. Over and above differences in type of deductive argument and methodology used, deductive reasoning studies consistently report activation in a mostly left-lateralized brain system, which includes the left lateral (IFG, MFG, PG) and medial (MeFG) frontal cortices, the left parietal cortex (PPC), and the left BG. We further show that this left hemisphere brain system can be broken down into several subsystems that are specific to particular types of deductive arguments, for example, PPC for relational arguments, IFG for categorical arguments, and PG for propositional arguments. Finally, we demonstrate that two other cortical regions located in the right hemisphere (i.e., PPC and MFG) are also engaged in deductive reasoning, but only consistently in studies employing relational arguments as materials. Overall, our results provide evidence that the brain system that underlies deductive reasoning is dependent upon the type of deductive argument. We argue that these findings provide critical insight into the cognitive organization of deductive reasoning and need to be accounted for by cognitive theories.
Deductive Reasoning Mainly Engages a Left-lateralized Fronto-parieto BG Brain System
Across all types of deductive arguments, the meta-analysis reveals that five left-lateralized (IFG, MFG, PG, PPC, and BG) and one medial (MeFG) brain regions are consistently activated in deductive reasoning studies. Patient studies have long supported a left hemisphere dominance for reasoning, whether the brain lesions are in the Prefrontal Cortex (Goel et al., 2007), temporal cortex (Langdon & Warrington, 2000; Read, 1981), or widespread throughout the entire hemisphere (Golding, 1981). For example, Goel et al. showed that patients with damage to the left Prefrontal Cortex are less accurate in evaluating the validity of determinate arguments than normal controls and patients with damage to the right Prefrontal Cortex. The present meta-analysis indicates that the neuroimaging literature is in keeping with this longstanding neuropsychological literature. As noted elsewhere (Goel, 2007), the involvement of the left hemisphere in reasoning is broadly consistent with Gazzaniga’s “left brain interpreter” hypothesis (Roser & Gazzaniga, 2006). According to this hypothesis, the general role of the left hemisphere is to construct a coherent representation of reality by generating hypotheses about missing information in the environment (Roser & Gazzaniga, 2006). For example, when confronted with the premises of a deductive argument, the left hemisphere might recognize its logical structure and generate a hypothesis regarding its conclusion (Goel, 2007). Although this provides a relatively parsimonious explanation of the involvement of left brain regions in deductive reasoning, there are undoubtedly intrahemi-spheric differences in the functions supported by these regions. In fact, our meta-analysis revealed that this left hemisphere brain system could be broken down into different subsystems that are specific to different types of deductive arguments. Below, we describe these subsystems (and additional systems in the right hemisphere), discuss the potential role of each region in deductive reasoning, and interpret the relevance of these findings for cognitive theories of reasoning.
Relational Arguments Are Associated with Activations in Bilateral PPC and Right MFG
We found that studies that use relational arguments consistently show activations in the bilateral PPC and right MFG. Furthermore, relational arguments are more consistently associated with activations in these regions than propositional or categorical arguments (although the right MFG cluster was only reliable in the contrast of relational vs. propositional arguments). The parietal cortex is a functionally heterogeneous structure (Culham & Kanwisher, 2001), but there is a consensus that the PPC is predominantly involved in spatial cognition (Marshall & Fink, 2001; Colby & Goldberg, 1999). Specifically, although bilateral activations of the PPC are often observed in neuroimaging studies of visuospatial tasks, there is right hemisphere dominance for visuospatial processing. Indeed, TMS studies show that disruptions of the right PPC, but not the left, are associated with deficits in visuospatial cognition (Muri et al., 2002; Sack, Hubl, et al., 2002) and visuospatial imagery (Sack, Camprodon, Pascual-Leone, & Goebel, 2005; Sack, Sperling, et al., 2002). The lack of left IFG activation and the consistent involvement of the right PPC in tasks utilizing relational arguments are not easily accounted for by the FRA. Rather, this finding seems to lend support for the MMT, which posits that deductive reasoning is a nonverbal process that requires a visuospatial representation of the premises (Johnson-Laird, 2001).
The visuospatial nature of relational reasoning might be explained by the relative ease with which linear orderings can be mapped onto a single, analogical dimension. For example, the premises John is older than Tom and Tom is older than Bill can be easily mapped onto a linear continuum that represents the characters’ ages (i.e., John–Tom–Bill). Consistent with this hypothesis, studies have shown that the number of premises that need to be considered to evaluate the conclusion of a relational argument is inversely related to the difficulty of the problem. For example, given the problem A is larger than B, B is larger than C, C is larger than D, and D is larger than E, participants take longer to evaluate the conclusion B is larger than C than the conclusion B is larger than D (Prado, Van der Henst, & Noveck, 2008; Potts, 1972, 1974). This “distance” effect is consistent with the claim that participants construct an integrated representation of the problem premises (i.e., A–B–C–D–E): Two items that are close on this representation (e.g., BC) are less easily distinguishable than two items that are further away (e.g., BD). We have recently found that items at the beginning of a relational ordering (e.g., A, B) are automatically associated with the left side of space, whereas items at the end of the ordering (e.g., D, E) are automatically associated with the right side of space (Prado et al., 2008). This further supports the idea that the mental representations that underlie relational arguments are strongly visuospatial. The results of the present meta-analysis, associating relational arguments with the PPC, are consistent with this behavioral research.
Categorical Arguments Are Associated with Activation of the Left IFG and Left BG
Unlike relational reasoning, categorical reasoning was not found consistently linked to the right PPC across studies. Rather, the meta-analysis associates two other brain regions with categorical reasoning: the left IFG (BA 9/44) and the left BG (putamen and caudate nucleus). We show that these regions are consistently activated in studies that use categorical arguments and that their engagement is more often observed for categorical arguments than relational or propositional arguments. A large body of lesion and neuroimaging studies suggests that the left IFG supports rule-governed syntax processing in natural language (Grodzinsky & Santi, 2008; Ullman, 2006; Friederici & Kotz, 2003). Furthermore, this region is often found to be coactivated with the BG in studies that investigate grammar processing (Friederici, 2002; Moro et al., 2001; Embick, Marantz, Miyashita, O’Neil, & Sakai, 2000; Ni et al., 2000) and musical syntax (Tillmann, Janata, & Bharucha, 2003; Maess, Koelsch, Gunter, & Friederici, 2001). The consistent involvement of these regions in studies that use categorical arguments (and the lack of right PPC engagement) seems more consistent with the idea that reasoning is a linguistic/syntactic process than with the claim that it is a visuospatial process. In other words, this finding seems more consistent with the FRA than the MMT.
Why would categorical arguments involve a different type of mental representation than relational arguments? It is possible that categorical arguments are more difficult to represent with visuospatial models than relational arguments. Unlike relational premises, categorical premises contain items that represent sets of objects rather than single elements. Such items cannot be mapped onto a single analogical dimension (e.g., age for the relational premise John is older than Tom) and would, thus, require more elaborate visuospatial representations. Furthermore, as noted by Favrel and Barrouillet, categorical premises are often ambiguous because they can be compatible with different mental models. For example, the premise All As are Bs might be represented with a model in which As are identical to Bs or with a model in which As are included in Bs. Overall, it might be more difficult to construct a visuospatial representation of a categorical premise than a visuospatial representation of a relational premise. Most reasoners might, thus, choose to rely on a propositional representation of the premises when faced with a categorical argument. This hypothesis is consistent with behavioral research. Unlike relational arguments, the number of premises that need to be considered to evaluate the conclusion of a categorical argument is positively related to the difficulty of the problem. For example, given the problem All As are Bs, All Bs are Cs, All Cs are Ds, and All Ds are Es, participants take longer to evaluate the conclusion All Bs are Ds than the conclusion All Bs are Cs (Favrel & Barrouillet, 2000; Barrouillet, 1996). This “reverse” distance effect is inconsistent with the idea that participants form a unified spatial representation of the premises. It rather suggests that they apply sequential rules of inference to an atomic representation of the premises (i.e., the more premises reasoners have to consider, the more rules they have to apply; Favrel & Barrouillet, 2000). Our present finding that categorical arguments are consistently associated with the activation of regions involved in rule-based processing in natural language (i.e., the left IFG and BG) is consistent with this claim.
Propositional Arguments Are Associated with Activation of the Left PPC, Left PG, and MeFG
Three brain regions are associated with propositional arguments in our meta-analysis: the left PPC, the left PG, and MeFG. However, only the left PG is more consistently found in studies employing propositional arguments than in studies employing categorical or relational argument. It is difficult to determine whether the engagement of these three regions in propositional reasoning supports the MMT or the FRA. First, as mentioned in the previous section, one might argue that activation of the PPC is broadly consistent with the MMT, especially in the absence of left IFG activation as is the case here. However, the PPC cluster associated with propositional arguments is left-lateralized and centered around the angular gyrus, a region that has been linked to verbal (although not syntactic) processing (Booth, Coch, Fischer, & Dawson, 2007; Fiez & Petersen, 1998). Second, the MeFG has been implicated in the maintenance of abstract rules in memory (Bunge, Kahn, Wallis, Miller, & Wagner, 2003) and activations along the medial wall of the frontal cortex have been tentatively linked to the FRA in previous studies (Monti et al., 2007, 2009). Third, the engagement of the left PG might reflect some combination of attentional and motor processes that are involved in the reasoning tasks (Acuna, Eliassen, Donoghue, & Sanes, 2002) but does not seem to speak to the debate between the MMT and FRA per se. Overall, there seems to be some heterogeneity in the neural processes that are engaged across propositional reasoning studies. This might be explained by the heterogeneity of the propositional reasoning tasks themselves. For example, neuroimaging studies have used arguments that contained conditional propositions (Prado, Van Der Henst, et al., 2010; Reverberi et al., 2007, 2010; Prado & Noveck, 2007; Noveck, Goel, & Smith, 2004; Houde et al., 2000), disjunctive propositions (Reverberi et al., 2007), or a combination of both (Monti et al., 2007, 2009). Even within conditional reasoning tasks, studies vary in the type of arguments participants are presented with: Some studies employ the modus ponens form (If P than Q; P; therefore Q; Reverberi et al., 2007; Noveck et al., 2004), whereas others focus on the more difficult modus tollens form (If P than Q; not Q; therefore not P; Prado, Van Der Henst, et al., 2010; Reverberi et al., 2010; Monti et al., 2007; Noveck et al., 2004). Although both the FRA and the MMT can account for virtually all forms of propositional reasoning (Braine & O’Brien, 1998; Rips, 1994; Johnson-Laird, Byrne, & Schaeken, 1992), it is possible that some propositional arguments preferentially involve syntactic processes, whereas others are more strongly associated with visuospatial processes. For example, a previous study has shown that modus ponens is associated with the left PPC, whereas modus tollens engages the left IFG (Noveck et al., 2004). Unfortunately, there are not enough studies in the literature to allow us to investigate different forms of propositional reasoning in our meta-analysis. Nonetheless, our study shows that propositional arguments are consistently associated with both parietal (left PPC) and frontal (left PG and MeFG) regions. Such a pattern could be consistent with both the FRA (i.e., activation of the MeFG) and the MMT (i.e., activation of the left PPC). Future studies are needed to determine whether the contribution of these regions can be teased apart, thus providing a clearer picture on the role of rule-based and visuospatial processes in propositional reasoning.
Deductive Reasoning Relies on a Fractionated Neural System
It is important to note that most of our interpretations so far rely on the idea that a cognitive process (e.g., visuospatial processing) can be inferred from activation in a particular brain region (e.g., the right PPC). This logic, based on the idea that brain organization is modular to some extent, can provide useful insights into the cognitive processes that are involved in a given task (Poldrack, 2006). However, the logic is undermined by the fact that there is rarely a one-to-one mapping between a brain region and a cognitive function. For example, because the left PPC has been linked to both spatial and non-spatial processes (Husain & Nachev, 2007), it is difficult to know whether activation of this region provides evidence for one or the other of these processes. For this reason, our interpretations of the patterns of brain activation observed in this study (as is the case for many neuroimaging studies) are limited in that they cannot provide definitive evidence for either visuospatial or syntactic processing in deductive reasoning.
However, our findings allow us to make a much stronger conclusion that is highly relevant to cognitive theories of reasoning: There is consistent evidence in the neuroimaging literature that deductive reasoning does not rely on a unitary brain system. Rather, our meta-analysis demonstrates clear dissociations between the neural representations of relational, categorical, and propositional arguments. This is inconsistent with any cognitive theory that would posit that the same cognitive mechanism underlies these three forms of reasoning, such as the MMT (Johnson-Laird, 1999) or a parsimonious interpretation of the FRA (Rips, 1994; Hagert, 1984). Instead, the neuroimaging literature is consistent with the notion that visuospatial representations and rules of inference are both available to reasoners. The engagement of one or the other of these mechanisms is likely to depend upon intraindividual as well as interindividual factors (Roberts & Newton, 2005). Our meta-analysis suggests that the type of deductive argument is a critical intraindividual factor. Importantly, evidence for such a claim is not unique to neuroimaging studies but can be found in the cognitive literature as well. For example, studies have found that relational reasoning performance is affected by taxing the visuospatial working memory resources (Vandierendonck & De Vooght, 1997). In contrast, propositional reasoning performance is correlated with measures of verbal, but not visuospatial, working memory (Handley, Capon, Copp, & Harper, 2002). This suggests that relational and propositional reasoning rely on visuospatial and linguistic mechanisms, respectively. Furthermore, participants differ in the strategies they use to make deductions (Roberts & Newton, 2005). For example, when evaluating categorical arguments, some participants report using spatial strategies whereas others indicate using verbal strategies (Bacon, Handley, & Newstead, 2005; Ford, 1995). In line with this observation, the engagement of the PPC in deductive tasks has been found to depend upon interindividual differences in visuospatial skills (Fangmeier, Knauff, Ruff, & Sloutsky, 2006; Ruff, Knauff, Fangmeier, & Spreer, 2003). Overall, there is evidence in the cognitive literature that deductive behavior is not easily explained by a parsimonious mechanism involving either rule-based or visuospatial processes (Roberts & Newton, 2005). The neuroimaging literature is in line with such an observation.
Relationship of the Present Work to Previous Accounts on the Neural Bases of Deductive Reasoning
The present study constitutes the first quantitative meta-analysis of neuroimaging studies of deductive reasoning. However, there have been some previous attempts to review and interpret the findings from this literature, sometimes with conflicting conclusions. For example, although both Goel (2007) and Monti et al. (2007) noted a high degree of variability in the location of the activated regions across studies, these authors differ substantially in their interpretation of these discrepancies. On the one hand, Goel concludes that there might be no unitary neural system for reasoning, but instead “a fractionated system that is dynamically configured in response to certain task and environmental cues” (p. 440). On the other hand, Monti et al. (2007, 2009) defend the idea that, despite the large variability in neural activation across studies, there are two “core” regions of deduction: the left rostro-lateral pFC (RLPFC; BA 10) and the medial superior frontal gyrus (MeFG; BA 8). Our study reveals that (1) the brain network for reasoning depends upon the type of deductive task and (2) neither the left RLPFC nor the MeFG is consistently activated across studies. These results seem more consistent with Goel’s account than with Monti et al.’s account. It is interesting to note that Monti et al. demonstrate the engagement of the left RLPFC and MeFG in two relatively complex propositional tasks. In these tasks, participants had to evaluate arguments in which disjunctions or conjunctions were embedded in conditional sentences (e.g., If the block is either red or square then it is not large; The block is not large; therefore The block is not red). Such arguments are likely to require the joint consideration of multiple logical relations (e.g., the conditional relation If (p or q) then r; not r; therefore not (p or q) and the disjunction not (p or q); therefore not p). Studies have found that the left RLPFC is particularly activated when distinct mental representations have to be integrated and considered simultaneously (Ramnani & Owen, 2004; Christoff et al., 2001). Rather than constituting a generic system for deductive reasoning, the left RLPFC might thus be specifically engaged when several logical relations have to be considered at the same time. Future studies might further investigate this possibility.
Conclusion
Our meta-analysis shows that deductive reasoning is subserved by several neural systems located in both cortical (frontal and parietal cortices) and subcortical (BG) structures. We demonstrate that these systems are highly sensitive of the type of deductive argument processed: bilateral PPC and right MFG for relational arguments, left IFG and BG for categorical arguments, and left PG for propositional arguments. This is inconsistent with the idea that deductive reasoning is a unitary cognitive mechanism that relies either on visuospatial or rule-based representations (Johnson-Laird, 1999). Instead, our findings suggest that reasoners can make use of both kinds of representations depending on the type of argument they are presented with. Our meta-analysis indicates that the neural regions that underlie deductive reasoning are sensitive to the type of argument, but their engagement is likely to be modulated by other factors as well. As Goel (2007) pointed out, neuroimaging studies have also shown that the neural bases of deduction depend upon factors such as semantic content of the premises, absence/ presence of conflicting information in the argument, or degree of certainty of the conclusion. In addition to such intraindividual factors, brain imaging research also suggests that there are some interindividual differences in the degree to which visuospatial or rule-based representations are recruited. In summary, more than a decade of neuroimaging research suggests that it may be time to move beyond the question of whether deductive reasoning is a visuospatial or rule-based process because both representations are likely to be available to reasoners. Future behavioral and neuroimaging studies might instead focus on understanding how these representations are selected and how this selection is influenced by interindividual and intraindividual factors.
Acknowledgments
This research was supported by a grant from the National Institute of Child Health and Human Development (HD059177) to James R. Booth.
References
- Acuna B, Eliassen J, Donoghue J, Sanes J. Frontal and parietal lobe activation during transitive inference in humans. Cerebral Cortex. 2002;12:1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- Bacon A, Handley SJ, Newstead SE. Verbal and spatial strategies in reasoning. In: Roberts MJ, Newton EJ, editors. Methods of thought: Individual differences in reasoning strategies. Hove, UK: Psychology Press; 2005. pp. 80–105. [Google Scholar]
- Barrouillet P. Transitive inferences from set inclusion relations and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;6:1408–1422. [Google Scholar]
- Booth J, Coch D, Fischer K, Dawson G. Brain bases of learning and development of language and reading. Human behavior, learning and the developing brain. 2007:279–300. [Google Scholar]
- Braine M, O’Brien D. Mental logic 1998 [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of Neurophysiology. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Canessa N, Gorini A, Cappa SF, Piattelli-Palmarini M, Danna M, Fazio F, et al. The effect of social content on deductive reasoning: An fMRI study. Human Brain Mapping. 2005;26:30–43. doi: 10.1002/hbm.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annual Review of Neuroscience. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Embick D, Marantz A, Miyashita Y, O’Neil W, Sakai KL. A syntactic specialization for Broca’s area. Proceedings of the National Academy of Sciences, USA. 2000;97:6150–6154. doi: 10.1073/pnas.100098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JSBT. Deductive reasoning. In: Holyoak KJ, Morrison RJ, editors. The Cambridge handbook of thinking and reasoning. New York: Cambridge University Press; 2005. pp. 169–184. [Google Scholar]
- Fangmeier T, Knauff M. Neural correlates of acoustic reasoning. Brain Research. 2009;1249:181–190. doi: 10.1016/j.brainres.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Fangmeier T, Knauff M, Ruff CC, Sloutsky V. FMRI evidence for a three-stage model of deductive reasoning. Journal of Cognitive Neuroscience. 2006;18:320–334. doi: 10.1162/089892906775990651. [DOI] [PubMed] [Google Scholar]
- Favrel J, Barrouillet P. On the relation between representations constructed from text comprehension and transitive inference production. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:187–203. doi: 10.1037//0278-7393.26.1.187. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences, USA. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. Two modes of mental representation and problem solution in syllogistic reasoning. Cognition. 1995;54:1–71. doi: 10.1016/0010-0277(94)00625-u. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA. The brain basis of syntactic processes: Functional imaging and lesion studies. Neuroimage. 2003;20(Suppl. 1):S8–S17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Goel V. Anatomy of deductive reasoning. Trends in Cognitive Sciences. 2007;11:435–441. doi: 10.1016/j.tics.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Goel V, Buchel C, Frith C, Dolan RJ. Dissociation of mechanisms underlying syllogistic reasoning. Neuroimage. 2000;12:504–514. doi: 10.1006/nimg.2000.0636. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Functional neuroanatomy of three-term relational reasoning. Neuropsychologia. 2001;39:901–909. doi: 10.1016/s0028-3932(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Explaining modulation of reasoning by belief. Cognition. 2003;87:B11–B22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Differential involvement of left prefrontal cortex in inductive and deductive reasoning. Cognition. 2004;93:B109–B121. doi: 10.1016/j.cognition.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. The seats of reason? An imaging study of deductive and inductive reasoning. NeuroReport. 1997;8:1305–1310. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. Neuroanatomical correlates of human reasoning. Journal of Cognitive Neuroscience. 1998;10:293–302. doi: 10.1162/089892998562744. [DOI] [PubMed] [Google Scholar]
- Goel V, Makale M, Grafman J. The hippocampal system mediates logical reasoning about familiar spatial environments. Journal of Cognitive Neuroscience. 2004;16:654–664. doi: 10.1162/089892904323057362. [DOI] [PubMed] [Google Scholar]
- Goel V, Stollstorff M, Nakic M, Knutson K, Grafman J. A role for right ventrolateral prefrontal cortex in reasoning about indeterminate relations. Neuropsychologia. 2009;47:2790–2797. doi: 10.1016/j.neuropsychologia.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Tierney M, Sheesley L, Bartolo A, Vartanian O, Grafman J. Hemispheric specialization in human prefrontal cortex for resolving certain and uncertain inferences. Cerebral Cortex. 2007;17:2245–2250. doi: 10.1093/cercor/bhl132. [DOI] [PubMed] [Google Scholar]
- Golding E. The effect of unilateral brain lesion on reasoning. Cortex. 1981;17:31–40. doi: 10.1016/s0010-9452(81)80004-4. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Santi A. The battle for Broca’s region. Trends in Cognitive Sciences. 2008;12:474–480. doi: 10.1016/j.tics.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Hagert G. Modeling mental models: Experiments in cognitive modeling spatial reasoning. In: O’Shea T, editor. Advances in artificial intelligence. Amsterdam: North-Holland; 1984. pp. 389–398. [Google Scholar]
- Halberda J. The development of a word-learning strategy. Cognition. 2003;87:B23–B34. doi: 10.1016/s0010-0277(02)00186-5. [DOI] [PubMed] [Google Scholar]
- Halberda J. Is this a dax which I see before me? Use of the logical argument disjunctive syllogism supports word-learning in children and adults. Cognitive Psychology. 2006;53:310–344. doi: 10.1016/j.cogpsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Handley SJ, Capon A, Copp C, Harper C. Conditional reasoning and the Tower of Hanoi: The role of spatial and verbal working memory. British Journal of Psychology. 2002;93:501–518. doi: 10.1348/000712602761381376. [DOI] [PubMed] [Google Scholar]
- Heckers S, Zalesak M, Weiss A, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Henson R. What can functional neuroimaging tell the experimental psychologist? Quarterly Journal of Experimental Psychology A. 2005;58:193–233. doi: 10.1080/02724980443000502. [DOI] [PubMed] [Google Scholar]
- Houde O, Zago L, Mellet E, Moutier S, Pineau A, Mazoyer B, et al. Shifting from the perceptual brain to the logical brain: The neural impact of cognitive inhibition training. Journal of Cognitive Neuroscience. 2000;12:721–728. doi: 10.1162/089892900562525. [DOI] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends in Cognitive Sciences. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Laird PN. Mental models: Towards a cognitive science of language, inference, and consciousness. Cambridge, MA: Harvard University Press; 1983. [Google Scholar]
- Johnson-Laird PN. Formal rules verses mental models in reasoning. In: Sternberg RJ, editor. The nature of cognition. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- Johnson-Laird PN. Mental models and deduction. Trends in Cognitive Sciences. 2001;5:432–442. doi: 10.1016/s1364-6613(00)01751-4. [DOI] [PubMed] [Google Scholar]
- Johnson-Laird PN, Byrne RM, Schaeken W. Propositional reasoning by model. Psychological Review. 1992;99:418–439. doi: 10.1037/0033-295x.99.3.418. [DOI] [PubMed] [Google Scholar]
- Knauff M, Fangmeier T, Ruff CC, Johnson-Laird PN. Reasoning, models, and images: Behavioral measures and cortical activity. Journal of Cognitive Neuroscience. 2003;15:559–573. doi: 10.1162/089892903321662949. [DOI] [PubMed] [Google Scholar]
- Knauff M, Mulack T, Kassubek J, Salih HR, Greenlee MW. Spatial imagery in deductive reasoning: A functional MRI study. Brain Research, Cognitive Brain Research. 2002;13:203–212. doi: 10.1016/s0926-6410(01)00116-1. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL. When is early visual cortex activated during visual mental imagery? Psychological Bulletin. 2003;129:723–746. doi: 10.1037/0033-2909.129.5.723. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Nystrom LE, Cohen JD, Johnson-Laird PN. Distinct neural substrates for deductive and mathematical processing. Brain Research. 2008;1243:86–103. doi: 10.1016/j.brainres.2008.07.128. [DOI] [PubMed] [Google Scholar]
- Langdon D, Warrington EK. The role of the left hemisphere in verbal and spatial reasoning tasks. Cortex. 2000;36:691–702. doi: 10.1016/s0010-9452(08)70546-x. [DOI] [PubMed] [Google Scholar]
- Maess B, Koelsch S, Gunter TC, Friederici AD. Musical syntax is processed in Broca’s area: An MEG study. Nature Neuroscience. 2001;4:540–545. doi: 10.1038/87502. [DOI] [PubMed] [Google Scholar]
- Markovits H, Schleifer M, Fortier L. Development of elementary deductive reasoning in young children. Developmental Psychology. 1989;25:787–793. [Google Scholar]
- Marshall JC, Fink GR. Spatial cognition: Where we were and where we are. Neuroimage. 2001;14:S2–S7. doi: 10.1006/nimg.2001.0834. [DOI] [PubMed] [Google Scholar]
- Monti MM, Osherson DN, Martinez MJ, Parsons LM. Functional neuroanatomy of deductive inference: A language-independent distributed network. Neuroimage. 2007;37:1005–1016. doi: 10.1016/j.neuroimage.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Monti MM, Parsons LM, Osherson DN. The boundaries of language and thought in deductive inference. Proceedings of the National Academy of Sciences, USA. 2009;106:12554–12559. doi: 10.1073/pnas.0902422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro A, Tettamanti M, Perani D, Donati C, Cappa SF, Fazio F. Syntax and the brain: Disentangling grammar by selective anomalies. Neuroimage. 2001;13:110–118. doi: 10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- Muri RM, Buhler R, Heinemann D, Mosimann UP, Felblinger J, Schlaepfer TE, et al. Hemispheric asymmetry in visuospatial attention assessed with transcranial magnetic stimulation. Experimental Brain Research. 2002;143:426–430. doi: 10.1007/s00221-002-1009-9. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, et al. An event-related neuroimaging study distinguishing form and content in sentence processing. Journal of Cognitive Neuroscience. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Noveck IA, Goel V, Smith KW. The neural basis of conditional reasoning with arbitrary content. Cortex. 2004;40:613–622. doi: 10.1016/s0010-9452(08)70157-6. [DOI] [PubMed] [Google Scholar]
- Nunes T, Bryant P, Evans D, Bell D, Gardner S, Gardner A, et al. The contribution of logical reasoning to the learning of mathematics in primary school. British Journal of Developmental Psychology. 2007;25:147–166. [Google Scholar]
- Osherson D, Perani D, Cappa S, Schnur T, Grassi F, Fazio F. Distinct brain loci in deductive versus probabilistic reasoning. Neuropsychologia. 1998;36:369–376. doi: 10.1016/s0028-3932(97)00099-7. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Osherson DN. New evidence for distinct right and left brain systems for deductive versus probabilistic reasoning. Cerebral Cortex. 2001;11:954–965. doi: 10.1093/cercor/11.10.954. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Potts G. Information processing stragies used in the encoding of linear ordering. Journal of Verbal Learning and Verbal Behavior. 1972;11:727–740. [Google Scholar]
- Potts G. Storing and retrieving information about ordered relationship. Journal of Experimental Psychology: General. 1974;103:431–439. [Google Scholar]
- Prado J, Noveck IA. Overcoming perceptual features in logical reasoning: A parametric functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2007;19:642–657. doi: 10.1162/jocn.2007.19.4.642. [DOI] [PubMed] [Google Scholar]
- Prado J, Noveck IA, Van Der Henst J-B. Overlapping and distinct neural representations of numbers and verbal transitive series. Cerebral Cortex. 2010;20:720–729. doi: 10.1093/cercor/bhp137. [DOI] [PubMed] [Google Scholar]
- Prado J, Van der Henst J-B, Noveck IA. Spatial associations in relational reasoning: Evidence for a SNARC-like effect. Quarterly Journal of Experimental Psychology (Colchester) 2008;61:1143–1150. doi: 10.1080/17470210801954777. [DOI] [PubMed] [Google Scholar]
- Prado J, Van Der Henst J-B, Noveck IA. Recomposing a fragmented literature: How conditional and relational arguments engage different neural systems for deductive reasoning. Neuroimage. 2010;51:1213–1221. doi: 10.1016/j.neuroimage.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Read DE. Solving deductive-reasoning problems after unilateral temporal lobectomy. Brain and Language. 1981;12:116–127. doi: 10.1016/0093-934x(81)90008-0. [DOI] [PubMed] [Google Scholar]
- Reverberi C, Cherubini P, Frackowiak RSJ, Caltagirone C, Paulesu E, Macaluso E. Conditional and syllogistic deductive tasks dissociate functionally during premise integration. Human Brain Mapping. 2010 doi: 10.1002/hbm.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverberi C, Cherubini P, Rapisarda A, Rigamonti E, Caltagirone C, Frackowiak RSJ, et al. Neural basis of generation of conclusions in elementary deduction. Neuroimage. 2007;38:752–762. doi: 10.1016/j.neuroimage.2007.07.060. [DOI] [PubMed] [Google Scholar]
- Rips L. The psychology of proof. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Roberts MJ, Newton EJ. Methods of thought: Individual differences in reasoning strategies. Hove, UK: Psychology Press; 2005. [Google Scholar]
- Rodriguez-Moreno D, Hirsch J. The dynamics of deductive reasoning: An fMRI investigation. Neuropsychologia. 2009;47:949–961. doi: 10.1016/j.neuropsychologia.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Roser ME, Gazzaniga MS. The interpreter in human psychology. In: Preuss TM, Kaas JH, editors. The evolution of primate nervous systems. Oxford, UK: Academic Press; 2006. pp. 503–508. [Google Scholar]
- Ruff CC, Knauff M, Fangmeier T, Spreer J. Reasoning and working memory: Common and distinct neuronal processes. Neuropsychologia. 2003;41:1241–1253. doi: 10.1016/s0028-3932(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Sack AT. Parietal cortex and spatial cognition. Behavioural Brain Research. 2009;202:153–161. doi: 10.1016/j.bbr.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Sack AT, Camprodon JA, Pascual-Leone A, Goebel R. The dynamics of interhemispheric compensatory processes in mental imagery. Science. 2005;308:702–704. doi: 10.1126/science.1107784. [DOI] [PubMed] [Google Scholar]
- Sack AT, Hubl D, Prvulovic D, Formisano E, Jandl M, Zanella FE, et al. The experimental combination of rTMS and fMRI reveals the functional relevance of parietal cortex for visuospatial functions. Brain Research, Cognitive Brain Research. 2002;13:85–93. doi: 10.1016/s0926-6410(01)00087-8. [DOI] [PubMed] [Google Scholar]
- Sack AT, Sperling JM, Prvulovic D, Formisano E, Goebel R, Di Salle F, et al. Tracking the mind’s image in the brain: II. Transcranial magnetic stimulation reveals parietal asymmetry in visuospatial imagery. Neuron. 2002;35:195–204. doi: 10.1016/s0896-6273(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Stanovich K, West R. Individual differences in reasoning: Implications for the rationality debate? Behavioral and Brain Sciences. 2000;23:645–665. doi: 10.1017/s0140525x00003435. discussion 665-726. [DOI] [PubMed] [Google Scholar]
- Tillmann B, Janata P, Bharucha JJ. Activation of the inferior frontal cortex in musical priming. Brain Research, Cognitive Brain Research. 2003;16:145–161. doi: 10.1016/s0926-6410(02)00245-8. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Is Broca’s area part of a basal ganglia thalamocortical circuit? Cortex. 2006;42:480–485. doi: 10.1016/s0010-9452(08)70382-4. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A, De Vooght G. Working memory constraints on linear reasoning with spatial and temporal contents. Quarterly Journal of Experimental Psychology A. 1997;50:803–820. doi: 10.1080/713755735. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage. 2009;45(1 Suppl):S210–S221. doi: 10.1016/j.neuroimage.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: A meta-analysis of neuroimaging studies. Human Brain Mapping. 2010;31:1459–1468. doi: 10.1002/hbm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA. Transitive inference: Distinct contributions of rostrolateral prefrontal cortex and the hippocampus. Journal of Cognitive Neuroscience. 2010;22:837–847. doi: 10.1162/jocn.2009.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]


