SUMMARY
Purpose
To investigate the characteristics of intracranial ictal high frequency oscillations (HFOs).
Methods
Among neocortical epilepsy patients who underwent intracranial monitoring and surgery, we studied patients with well-defined, unifocal seizure onsets characterized by discrete HFOs (≥70 Hz). Patients with multifocal or bilateral independent seizure onsets, EEG acquired at <1,000 Hz sampling rate and non-resective surgery were excluded. Based on a prospectively-defined protocol, we defined the seizure onset zone (SOZ) presurgically to include only those channels with HFOs that showed subsequent sustained evolution (HFOs+ev channels) but not the channels that lacked evolution (HFOs-ev channels). We then resected the SOZ as defined above, 1 cm of the surrounding cortex and immediate spread area, modified by the presence of eloquent cortex in the vicinity. For purposes of this study, we also defined the SOZ based on the conventional frequency activity (CFA: <70 Hz) at seizure onset although that information was not considered for preoperative determination of the surgical boundary. We investigated the temporal and spatial characteristics of the ictal HFOs post-hoc by visual and spectral methods, and also compared them to the seizure onset defined by the CFA.
Key Findings
Out of 14 consecutive neocortical epilepsy patients, six patients met the inclusion criteria. MRI was normal or showed heterotopia. All had subdural electrodes, with additional intracerebral depth electrodes in some. Electrode coverage was extensive (median 94 channels), including limited contralateral coverage. Seizure onsets were lobar or multilobar. Resections were performed per protocol except in two patients where complete resection of the SOZ could not be done due to overlap with speech area. Histology was abnormal in all patients. Postoperative outcome was class I/II (n=5, 83%) or class III over a mean follow-up of 27 months. Post-hoc analysis of 15 representative seizures showed that the ictal HFOs were widespread at seizure onset but evolved subsequently with different characteristics. In contrast to HFOs-ev, the HFOs+ev were significantly higher in peak frequency (97.1 versus 89.1 Hz, p=0.001), more robust (nearly 2-fold higher peak power, p<0.0001), and spatially restricted [mean 12.2 versus 22.4 channels; odds ratio (OR) 0.51, 95% confidence interval (CI) 0.42–0.62; p<0.0001]. The seizure onset defined by HFOs+ev was earlier (by an average of 0.41 sec), and occurred in a significantly different and smaller distribution (OR 0.27, 95% CI 0.21–0.34, p<0.0001), than the seizure onset defined by the CFA. As intended, the HFOs+ev channels were 10 times more likely to have been resected than the HFOs-ev channels (OR 9.7, 95% CI 5–17, p<0.0001).
Significance
Our study demonstrates the widespread occurrence of ictal HFOs at seizure onset, outlines a practical method to localize the SOZ based on their restricted pattern of evolution, and highlights the differences between the SOZs defined by HFOs and CFA. We show that smaller resections, restricted mainly to the HFOs channels with evolution, can lead to favorable seizure outcome. Our findings support the notion of widespread epileptic networks underlying neocortical epilepsy.
Keywords: Epilepsy surgery, High frequency oscillations, Intracranial EEG, HFOs, Seizure
In the last two decades, the association between high frequency oscillations (HFOs) and epileptogenicity has become increasingly evident. From their initial description in animals using microwire recordings (Buzsaki et al., 1992), the HFOs have now been successfully recorded in human epileptic patients (Bragin et al., 1999a, Jirsch et al., 2006, Ochi et al., 2007, Worrell et al., 2008, Khosravani et al., 2009, Modur and Scherg, 2009). To date, most of the published literature on HFOs has focused on interictal HFOs, broadly classified as ripples (80–250 Hz) and fast ripples (250–500 Hz) (Bragin et al., 1999a). It has been suggested that the interictal HFOs localize the epileptogenic focus (Jirsch et al., 2006), generate seizures (Bragin et al., 1999b, Bragin et al., 2000, Staba et al., 2002, Timofeev and Steriade, 2004, Worrell et al., 2008), correlate with surgical outcome (Jacobs et al., 2010, Wu et al., 2010), and provide markers for epileptogenicity (Jacobs et al., 2009) and epileptic disease activity (Zijlmans et al., 2009).
In contrast to the interictal HFOs, only a limited number of studies have explored the ictal HFOs (i.e., the HFOs occurring at seizure onset). Although the frequency cut-off for ictal HFOs is not clearly defined, we found a 60 Hz cut-off to be useful in practice (Rodin et al., 2009). Traditionally, the seizure onset zone (SOZ) is defined based on conventional frequency activity (CFA: 1–70 Hz), consisting of low voltage beta or gamma activity, rhythmic sinusoidal activity, or rhythmic or semi-rhythmic spikes (Schiller et al., 1998, Lee et al., 2000). However, several studies have convincingly demonstrated the occurrence of HFOs at neocortical seizure onset, suggesting their advantage in a more accurate localization of the seizure focus (Fisher et al., 1992, Alarcon et al., 1995, Worrell et al., 2004, Ochi et al., 2007, Modur and Scherg, 2009). The neocortical seizures, unlike seizures of mesial temporal onset, can arise anywhere in the vast expanse of the cerebral cortex, necessitating extensive (often bilateral) intracranial coverage. While the earlier studies were limited to a maximum of 16 contacts to evaluate the ictal HFOs (Allen et al., 1992, Fisher et al., 1992, Alarcon et al., 1995), the later studies were limited by either the narrow bandwidth (200 Hz sampling rate) of recording (Worrell et al., 2004) or the use of a combination of electrodes (depth and epidural) with different morphological characteristics (Jirsch et al., 2006). Thus, the methodological differences and limitations preclude definitive conclusions regarding the spatial characteristics of HFOs, and therefore, their utility in seizure localization. However, a few recent studies have described the ictal HFOs in neocortical epilepsy using extensive intracranial coverage (Akiyama et al., 2005, Ochi et al., 2007, Modur and Scherg, 2009). We reported a patient with MRI-negative frontal lobe epilepsy in whom the ictal HFOs occurred in a widespread distribution at seizure onset, evolved in a more restricted fashion, and localized the SOZ to a much smaller area than the CFA (Modur and Scherg, 2009). In the present study, we aimed to extend and confirm our findings by investigating the ictal HFOs in a larger group of neocortical epilepsy patients who underwent resective surgery. Specifically, we analyzed the EEG data post-hoc using visual and spectral methods to evaluate the frequency and spatial characteristics of the ictal HFOs. We also compared the characteristics of the SOZ defined by the ictal HFOs to the SOZ defined by the CFA although the latter was not considered for determining the surgical boundary in our cohort.
METHODS
Patient population
This study was conducted at the University of Louisville Comprehensive Epilepsy Center when the authors (PNM and TWV) were employed there. We evaluated our patients based on a standard presurgical protocol, and decided the need, type of electrodes and extent of coverage for intracranial monitoring at a multidisciplinary conference. Based on a prospectively-defined protocol, we considered HFOs as ≥70 Hz activity to reflect the fact that such activity would not be faithfully recorded at the commonly used sampling rate of 200 Hz, and that a higher sampling rate is required to record such activity. We retrospectively identified the patients with neocortical epilepsy who underwent intracranial monitoring followed by surgery. Among them, the inclusion criteria for the study were: 1) well-defined, unifocal intracranial seizure onset preceding objective clinical change on video; 2) seizure onset characterized by discrete HFOs (≥70 Hz activity, ≥400 ms duration). The exclusion criteria were: 1) patients with multifocal or bilateral independent seizure onsets; 2) recordings acquired at <1,000 Hz sampling rate; 3) non-resective surgery. Informed consent was obtained from all the patients. The patients were personally followed by the authors (PNM, TWV), the EEGs were interpreted by the same epileptologist (PNM), and the resections were performed by the same surgeon (TWV).
Intracranial recordings
The EEGs were acquired on a 128-channel NK 1100 system (Nihon-Kohden America, Foothill Ranch, CA, USA), with 1,000 Hz sampling rate, 2 second time constant and 200 MΩ input impedance, allowing visualization of 0.08–300 Hz activity. All patients had implantation of subdural grids and strips (contact diameter 2.3 mm, inter-contact distance 10 mm); in some patients, additional intracerebral depth electrodes (4–8 contacts per electrode, contact diameter 1.1 mm, inter-contact distance 10 mm) were implanted as well. The electrodes were made of platinum, had the same characteristics across all patients, and obtained from the same manufacturer (Ad-Tech Medical, Racine, WI, USA). We performed extensive implantation ipsilateral to the suspected seizure onset hemisphere, as determined from the non-invasive studies. When lateralization was doubtful, we implanted limited additional subdural strips over the contralateral hemisphere to rule out seizure onset from that side. Electrodes showing persistent artifact due to poor contact, line noise, movement or muscle contraction (e.g., due to jaw clenching, neck contraction) that could be mistaken for rhythmic ictal activity were excluded for the purposes of interpretation.
Presurgical determination of seizure onset
We marked the clinical seizure onset at the occurrence of earliest observable behavioral change on the video. We defined the electrical seizure onset as the earliest occurrence of rhythmic or semi-rhythmic sinusoidal activity or repetitive spikes that clearly evolved in frequency and morphology. In all patients, we determined the SOZ by analyzing each seizure systematically as follows. First, we determined the SOZ based on CFA by displaying all the recorded channels using a bipolar montage to minimize reference contamination, 1.6–70 Hz bandpass/60 Hz notch filter and 10-second/page window; initially, we marked the point of occurrence of clear-cut rhythmic activity indicative of seizure spread; from that point, we reviewed the tracing backwards to identify the channels showing the earliest occurrence of discrete <70 Hz rhythmic activity (indicated by the marker in Fig. 1). Second, we determined the tentative SOZ based on HFOs by searching for their presence around the time of occurrence of conventional seizure onset; we used an expanded time scale of 2-second/page window (2,000 samples) and a 53–300 Hz bandpass/60 Hz notch filter, the tools and filter settings being constrained by the capabilities of the vendor’s software; we reviewed the tracing and visually identified all the channels showing the occurrence of the earliest fast rhythmic discrete oscillations indicative of HFOs (Fig. 2, panel B), clearly distinct from the preictal baseline (Fig. 2, panel A). Finally, we localized the definitive SOZ to those channels with HFOs that showed subsequent evolution (HFOs+ev channels) as opposed to those that did not (HFOs-ev channels), as illustrated in Fig. 2, panels C and D. As described previously (Modur and Scherg, 2009), we defined the HFOs+ev channels when the following criteria were satisfied: 1) presence of ≥70 Hz activity at seizure onset, followed by its sustained evolution into slower frequency activity (<70 Hz) in the same channels, occurring along with the progression of the clinical seizure; and 2) the channels showing the initial HFOs had to be electrically active (with either HFOs or slower frequencies) at the time of occurrence of the first behavioral change. In other words, if a given channel showed HFOs at the presumed electrical onset but that activity disappeared before the initial behavioral change, then that channel was not considered part of seizure onset. The time of occurrence of HFOs defined the temporal seizure onset. The HFOs+ev channels defined the spatial extent of the SOZ; the HFOs-ev channels were not considered part of the SOZ. When multiple seizures were evaluated from the same patient, if a given channel was designated as HFOs+ev in one seizure and HFOs-ev in another, then that channel’s final designation was HFOs+ev. If detailed visual inspection using the above method failed to reveal the HFOs but showed <70 Hz activity only, then the SOZs were defined by the CFA, and such seizures were not considered for the purposes of this study. Except for the window and filter settings, our method of determining the SOZ in the presence of HFOs was intuitively similar to the traditional methods of seizure localization based on the CFA.
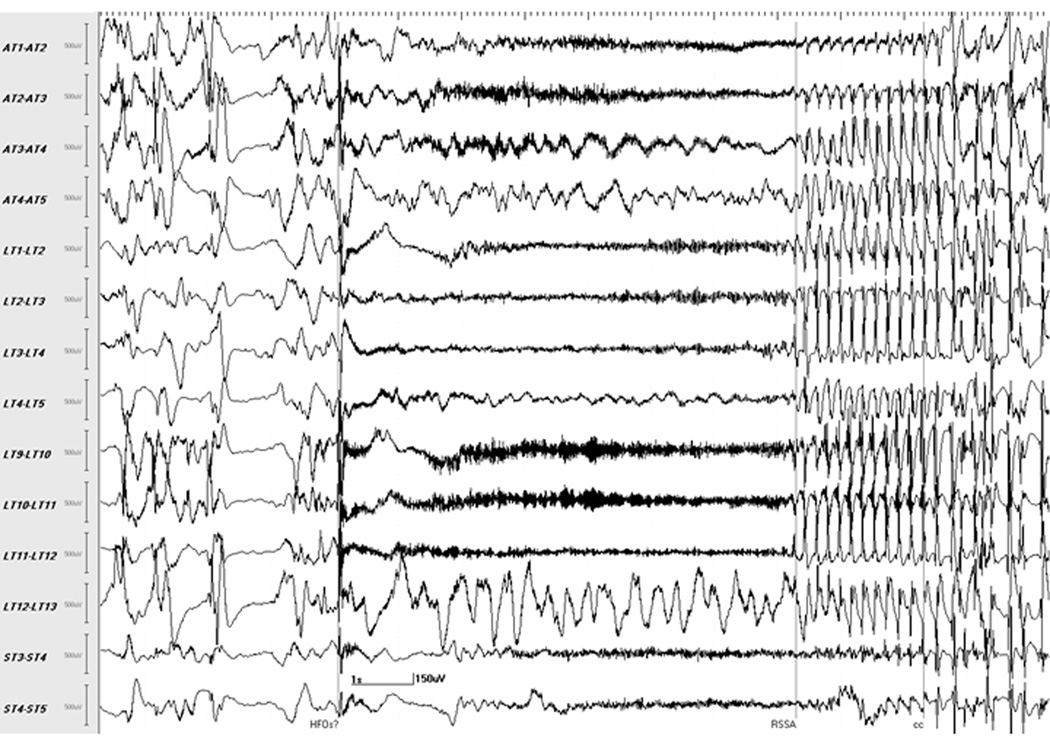
Fig. 1.
Localization of seizure onset using conventional setting. Selected channels of subdural recording of seizure 3 from patient F, visualized at 10 sec/page, 1.6–70 Hz bandpass/60 Hz notch filter, shows the point of occurrence of readily identifiable rhythmic activity indicating seizure spread (marker: RSSA). Reviewing backwards from this point reveals the seizure onset occurring a few seconds earlier, consisting of a sharp transient in multiple channels followed by slow waves with superimposed fast activity suggestive of high frequency oscillations (marker: HFOs?). At this filter setting, HFOs are seen as thick, dark waveforms initially in the LT (9–10, 10–11 and 11–12) and AT (2–3 and 3–4) channels with subsequent involvement of ST (3–4 and 4–5), AT (1–2) and LT (1–2, 2–3 and 3–4) channels. Earliest clinical change in this seizure (marker: cc), consisting of repetitive eye blinks, occurred several seconds after the electrical onset.
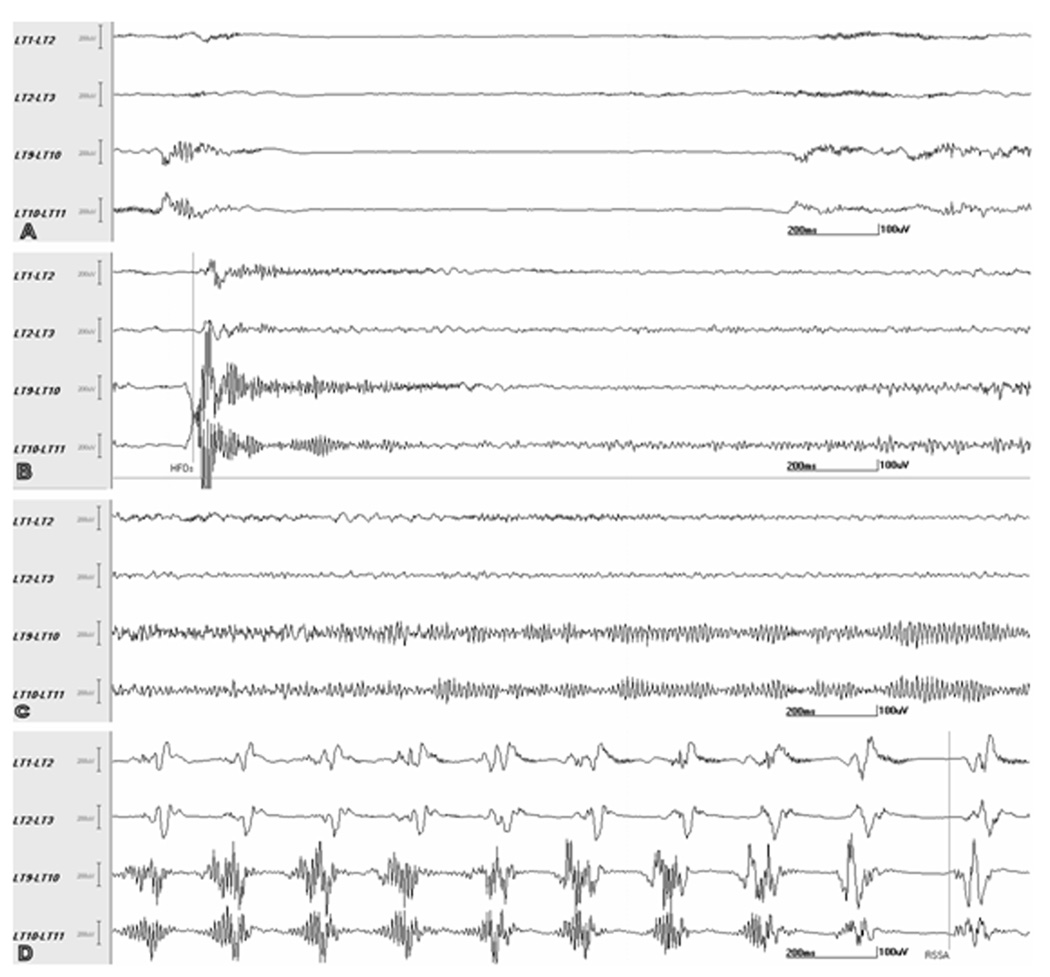
Fig. 2.
Localization of seizure onset based on high frequency oscillations (HFOs). Selected adjacent channels of subdural recording of seizure 3 from patient F (same as Fig. 1), visualized at 2 sec/page, 53–300 Hz bandpass/60 Hz notch filter. The panels A, B and C are consecutive 2-sec epochs each whereas panel D is a 2-sec epoch which starts 4 sec after the end of epoch C. The preictal baseline (A) shows interictal HFOs superimposed on a background of relative attenuation. The seizure onset (B) shows ictal HFOs (marker: HFOs), which appear to involve all the 4 channels. However, the subsequent seizure evolution (C) shows that the ictal HFOs continue to evolve prominently in LT9-10 and LT10-11 (HFOs+ev channels) but not in LT1-2 and LT2-3 (HFOs-ev channels). Further evolution of the seizure (D) shows the transition of HFOs+ev channels into repetitive slower frequency spikes (marker: RSSA); at this time, repetitive spikes have already appeared in the HFOs-ev channels indicating spread of seizure activity to those channels. Although the ictal HFOs evolved in 2/4 channels, the seizure spread to involve all four channels as evidenced by the spikes. Earliest clinical change (i.e., repetitive eye blinks in this seizure) occurred about 2 sec after the end of epoch D (not shown). See text for details.
Presurgical determination of the resection boundary
We defined the final SOZ for each patient by constructing the union of the HFOs+ev channels from all the seizures, to account for subtle variations in seizure onset channels across multiple seizures from the patient. Based on our protocol, we defined the boundary for surgical resection by the contiguous region consisting of the SOZ, 1 cm of the cortex surrounding the SOZ and the immediate spread area (defined by the channels involved during the first 2 seconds after onset). The boundaries were modified, if necessary, by the presence of eloquent cortex in the vicinity as determined by functional stimulation. We did not resect the HFOs+ev channels that were non-contiguous with the primary SOZ, or the HFOs-ev channels. We evaluated the interictal data, and defined the channels with interictal HFOs but did not resect them unless they overlapped with the previously defined surgical boundary. The resection margins were confirmed before and after surgery, and any modifications to the planned resection during surgery were noted.
Post-hoc review and analysis
Prior to surgery, the SOZ and the surgical boundary were determined solely by visual analysis as described above, and the resections were performed based on that data. However, for purposes of this study, we supplemented our post-hoc analysis with spectral methods (BESA software, version 5.3, MEGIS Software GmbH, Graefelfing, Germany), to identify the ictal HFOs that may have been missed by visual inspection, and to quantify their frequency and spatial extent. Our post-hoc analysis was essentially similar to our presurgical protocol of determining the seizure onset except for a few variations as noted below. We looked for the presence of ictal HFOs visually using a 50–300 Hz bandpass/60 Hz notch filter and a 2-second/page time scale (2,000 samples), and identified the HFOs+ev channels by a combination of visual and spectral methods as follows. First, we marked a 2-second window at the occurrence of the earliest HFOs such that it accounted for the channels showing immediate spread. Second, within this 2-second window, we identified the HFOs by calculating fast Fourier transform (FFT)-derived power spectra for the 50–300 Hz range (at 2.08 Hz resolution) over 400-ms epochs. This is illustrated in Fig. 3 which shows the selected epoch on the left and the corresponding power spectra on the right along with the HFOs+ev channels (highlighted). The frequency range and the epoch length for the FFT transform were the same across all seizures and all patients, and were chosen to approximate as closely as possible the criteria that we used to define the HFOs preoperatively. Third, we selected those channels with HFOs exhibiting ≥70 Hz as the peak (dominant) frequency among all the analyzed epochs. We relied on the actual value of the frequency as calculated by the software program instead of searching for the graphically displayed peaks since the latter could be subject to the gain setting of the display. Fourth, among these channels with HFOs (≥70 Hz), we selected only the ones which contained power at or above the median power of all the channels with HFOs and identified them as the HFOs+ev channels; the channels with HFOs that did not meet the power criterion were not considered further for determining the seizure onset. We chose the median power as the criterion to define the relevant HFOs since it allows comparison of power within a given seizure in contrast to a fixed cut-off value which tends to be more arbitrary as the power could vary widely between seizures within the same patient and between patients. Using the same method, we then identified all the HFOs-ev channels. We did not specifically investigate the interictal HFOs in this study.
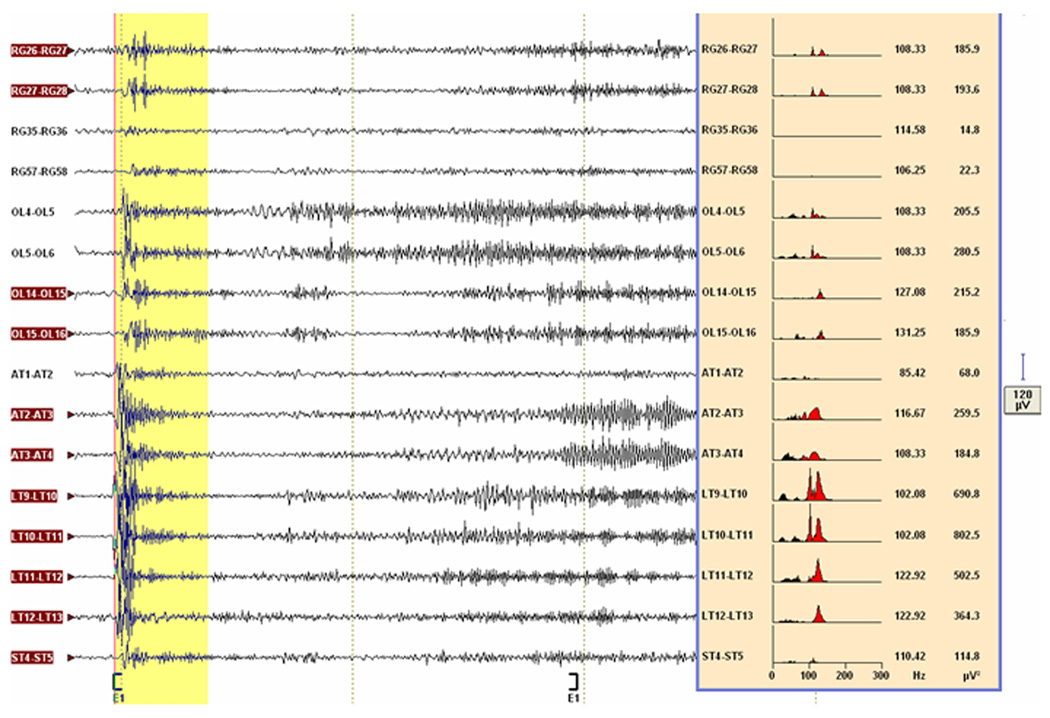
Fig. 3.
Seizure localization by post-hoc spectral analysis. Selected channels of the subdural recording of seizure 1 from patient F, visualized with 50–300 Hz bandpass/60 Hz notch filter, shows the high frequency oscillations (HFOs) on the left side of the figure. Note that the block E1 enclosed by the square brackets is 2 sec long. A 400-ms epoch (highlighted in yellow) is placed at the beginning of this block. The fast Fourier transform-derived power spectra over this epoch (≥70 Hz in red, <70 Hz in black), the dominant frequencies (Hz) and power (µV2) are shown at the right side of the figure. Although all the depicted channels could be considered as potential ictal HFOs channels because of dominant frequencies ≥70 Hz, the three channels (RG35-36, RG57-58 and AT1-2) would not qualify as ictal HFOs channels because of their power being <median power. The channels with subsequent evolution of the ictal HFOs into slower frequency activity (i.e., HFOs+ev channels) are highlighted. See text for details.
For each patient, we extracted the relevant demographic, imaging, EEG, histology and outcome data. We performed analysis for the whole group and for each individual patient. After logarithmic transformation of the measurements to adjust for the skewness in the data, we constructed a linear mixed model with random effects and the type of ictal HFOs as an indicator effect to assess the frequency and power differences between the HFOs+ev and HFOs-ev channels. We compared the spatial distribution of HFOs+ev versus HFOs-ev channels using chi-square or Fisher’s exact test. We compared the time delay between the seizure onsets defined by ictal HFOs and CFA using a linear mixed model. We compared the differences in the spatial distribution of the SOZs defined by the ictal HFOs and CFA using McNemar’s test. Using odds ratio (OR) and 95% confidence interval (CI), we evaluated the likelihood of the channels exhibiting different activities (HFOs+ev, HFOs-ev and CFA), as determined by post-hoc analysis, having been actually resected. Statistical significance was declared for a p-value <0.05. Statistical analyses were performed by the author (SZ) using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Among 14 consecutive patients with neocortical epilepsy who underwent intracranial monitoring and surgery, 8 were excluded because of: multifocal or bilateral independent seizure onsets (n=2), inadequate sampling rate to assess HFOs (n=2), lack of ictal HFOs meeting the frequency and duration criteria (n=3), and non-resective surgery (n=1). The latter patient, who did not have resective surgery but underwent multiple subpial transections only, has already been reported (Modur and Scherg, 2009). Thus, 6 patients (3 females), aged 19–32 years, with an 18-year mean duration of epilepsy, were included in the study (Table 1). MRI was normal (n=4) or showed heterotopia (n=2). Seizure onsets were lobar (frontal, n=2; temporal, n=1) or multilobar (n=3, parieto-occipital, parieto-temporal or temporo-occipital). Surgical resections were performed per protocol with the following exceptions: in patients A and D, complete resection of the SOZ could not be performed due to overlap with speech area; in patient B, additional resection of the medial temporal structures was performed although the seizure originated in the temporal neocortex. Small areas of heterotopia in patients C and E, in the occipital and anterior temporal regions respectively, outside the main SOZs were not resected. Histology was abnormal in all patients. The postoperative seizure outcome was Engel class I/II in 5/6 patients, and class III in the other over a mean follow-up period of 27 months (range 20–38 months). We analyzed 15 representative seizures including simple partial, complex partial and secondary generalized tonic-clonic seizures (supplemental Table 1). The median number of recorded channels was 94 (range 70–107 channels), covering the different lobar areas of neocortex. In 4/6 patients, the implantations were bilateral, with limited subdural strips implanted contralateral to the suspected seizure onset side. In addition to the subdural electrodes, intracerebral depth electrodes were implanted in 3/6 patients.
Table 1.
Patient data
| Patient | Age/Sex | Epilepsy duration (years) |
MRI | Sites of intracranial recording |
Intracranial seizure onset |
Pathology | Follow-up (months) |
Engel outcome |
|---|---|---|---|---|---|---|---|---|
| A | 32/M | 30 | Normal | Left: F-P convexity, PF, MF, LT. Right*: MF, LF |
Left frontal | Gliosis, heterotopic neurons | 38 | Class III |
| B | 26/F | 22 | Normal | Right: F-P convexity, OF, MF, LT, MT, ST, insula. Left*:MF,LF |
Right inferior and lateral temporal |
Focal dysgenesis | 30 | Class II |
| C | 19/F | 6 | Right parietal focal cortical dysplasia and nodular heterotopia |
Right: P-O convexity, LT, ST,MO |
Right parieto- occipital |
Focal dysgenesis, subcortical heterotopia, gliosis |
25 | Class I |
| D | 20/M | 17 | Normal | Left: F-P convexity, PF, MF, LT. Right*: MF, LF |
Left frontal | Gliosis, heterotopic neurons | 24 | Class II |
| E | 24/F | 19 | Right temporo-parietal nodular heterotopia |
Right: F-P convexity, PF, LT, ST, MT. Left*: LF |
Right parieto- temporal |
Subcortical heterotopia, gliosis | 22 | Class I |
| F | 19/M | 12 | Normal | Right: F-P convexity, LT, ST, LP, LO, MO |
Right posterior temporal-occipital |
Gliosis, heterotopic neurons | 20 | Class I |
Limited coverage with subdural strips.
F-P, fronto-parietal; P-O, parieto-occipital; PF, prefrontal; MF, medial frontal; LF, lateral frontal; OF, orbitofrontal; LP, lateral parietal; LT, lateral temporal; MT, mesial temporal; ST, subtemporal; LO, lateral occipital; MO, medial occipital.
Characteristics of ictal HFOs
The observed peak frequencies of ictal HFOs varied widely above the 70 Hz cutoff, reaching a maximum of 185 Hz. However, the peak frequency in the HFOs+ev channels (97.1 Hz, 95% CI: 93–101 Hz) was higher than the HFOs-ev channels (89.1 Hz, 95% CI: 86–93 Hz) by about 9% (p=0.001). The observed peak power of ictal HFOs also showed wide variation (range 3–3837 µV), with the peak power of HFOs+ev being about 2-fold higher than that of HFOs-ev (p<0.0001). Although the number of depth electrodes was much smaller than the subdural electrodes, there were no appreciable differences in the characteristics of the recorded ictal HFOs between the two types of electrodes. The ictal HFOs occurred in a widespread distribution, involving a mean of 34.9 ± 1.9 channels. However, the spatial extent of HFOs+ev channels was smaller than that of HFOs-ev channels (12.2 ± 2.4 vs. 22.4 ± 2.2 channels). This difference was statistically significant (OR 0.51, 95% CI 0.42–0.62, p<0.0001), suggesting that the spatial extent of the HFOs+ev was about one-half of the HFOs-ev. In each patient, at least one large cluster of HFOs+ev channels was identified, constituting the SOZ. This is illustrated in Fig. 4 which shows the location of the subdural and depth electrodes in patient E along with the clusters of the HFOs+ev and HFO-ev contacts that were resected. Occasionally, HFOs+ev occurred in smaller clusters, discontinuous from the larger SOZ. Although the majority of the HFOs-ev channels were located adjacent to the HFOs+ev channels, in 4 patients, clusters of HFOs-ev channels were found at sites remote from the HFOs+ev channels, including the hemisphere contralateral to the seizure onset. Further analysis among individual patients yielded consistent results in 5/6 patients where the spatial extent of HFOs+ev was smaller than HFOs-ev; the exception was patient D, in whom the distribution of HFOs+ev was slightly (3%) larger than the distribution of HFOs-ev but this finding was not statistically significant.
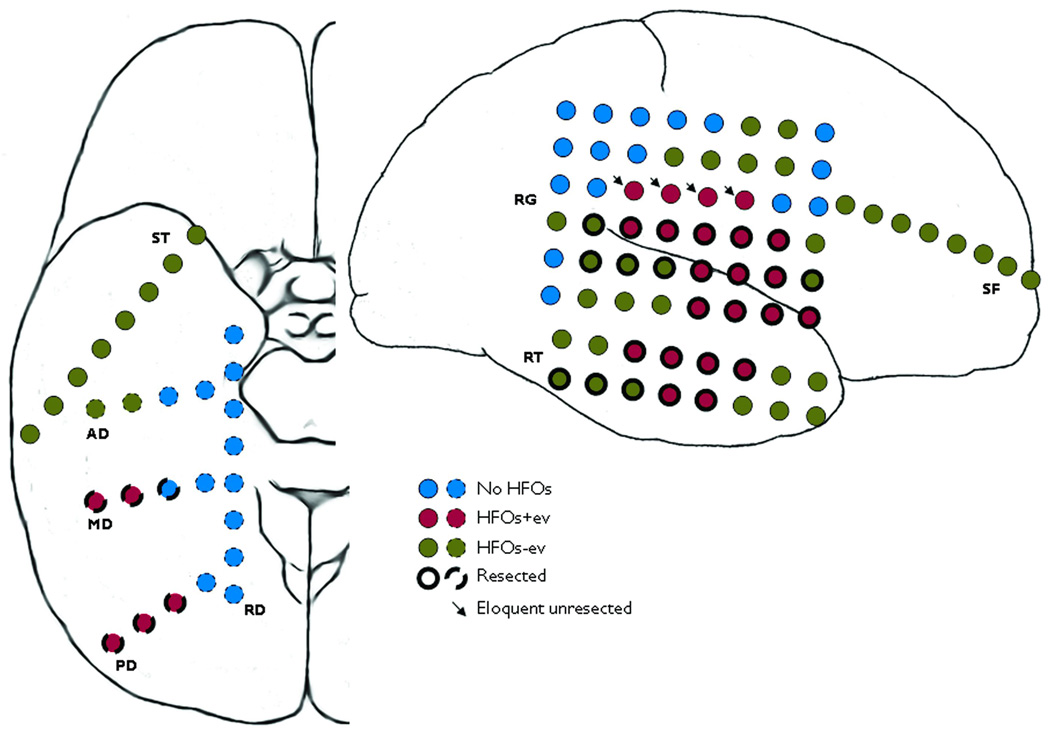
Fig. 4.
Spatial distribution of ictal high frequency oscillations (HFOs). Implanted electrodes over the right subtemporal and right hemispheric convexity in patient E are shown. RG, RT, SF and ST are subdural electrodes whereas RD, AD, MD and PD are intracerebral depth electrodes. Contacts containing the ictal HFOs with evolution (HFOs+ev) and without evolution (HFOs-ev) are indicated along with those resected. The contacts of the three HFOs+ev channels overlapping with the eloquent sensorimotor cortex that were not resected are also shown (arrows). See text for details.
Comparison of Ictal HFOs and CFA
The CFA at seizure onset was <40 Hz, in the beta or low gamma range. Temporally, the seizure onset defined by the ictal HFOs preceded the onset defined by the CFA by an average of 0.41 ± 0.05 sec (95% CI 0.50–0.32 sec). Spatially, the CFA was widespread at seizure onset, involving an average of 28.9 ± 4.4 channels. However, the spatial extent of the SOZ defined by the HFOs+ev channels was significantly different and smaller than the SOZ defined by the CFA (OR 0.27, 95% 0.21–0.34, p<0.0001, McNemar’s test). Although the HFOs+ev and CFA channels were spatially non-overlapping, they were not completely mutually exclusive. The same findings were further replicated in each individual patient although not statistically significant in patient F. Similarly, the spatial extent of HFOs-ev was also significantly different and smaller than the CFA (OR 0.75, 95% 0.63–0.88, p<0.001) but these findings were further replicated in only 3 patients.
Ictal HFOs, resection and outcome
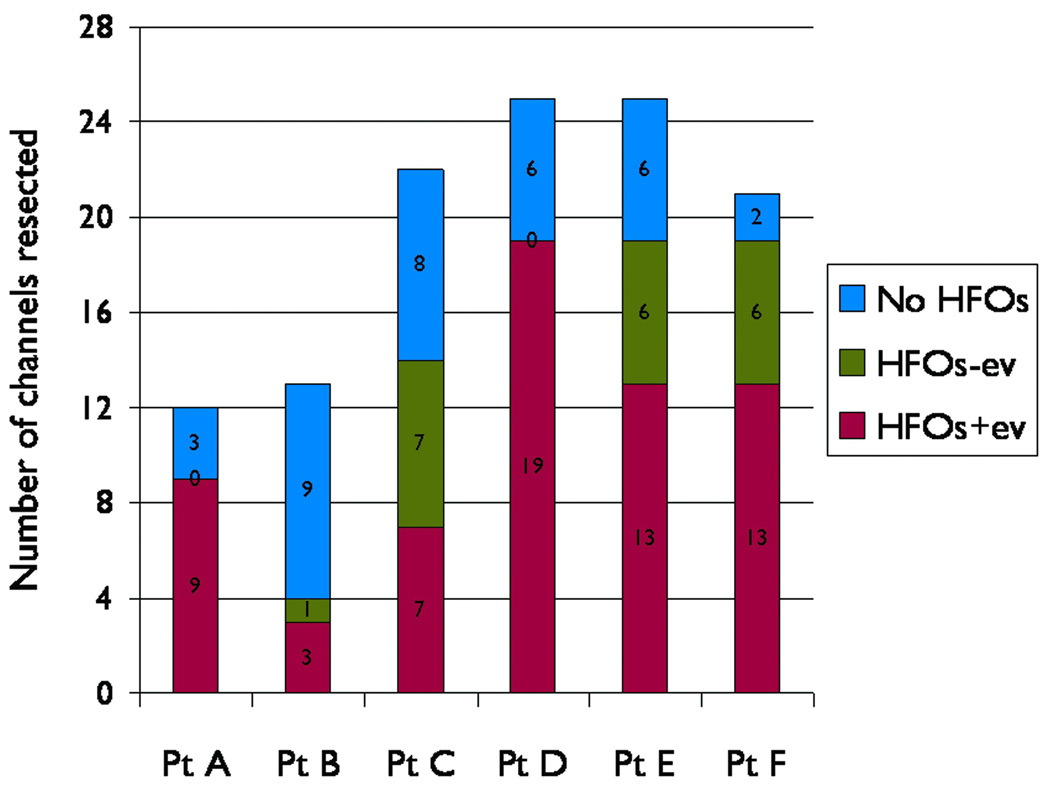
The HFOs+ev channels were nearly 10-fold more likely to have been resected than the HFOs-ev channels (OR 9.7, 95% CI 5–17, p<0.0001). Four patients had resection of variable numbers of HFOs-ev channels due to adjacency to the SOZ while two had none of the HFOs-ev channels resected. The likelihood of the presence or absence of HFOs in the resected channels in each patient is illustrated in Fig. 5. The details regarding the resected neocortical areas, the number of channels with HFOs and CFA that were resected, and the reasons for not resecting are summarized in supplemental Table 2. In retrospect, these results were broadly consistent with our presurgical protocol of restricting the surgical resections mainly to the HFOs+ev channels with two exceptions: patients A and D underwent only partial resection of the HFOs+ev channels in the left frontal lobe because of overlap with the Broca’s area. With this strategy, the overall seizure outcome was class I/II in 5 patients (83%), and class III in one (patient A).
Fig. 5.
Likelihood of ictal high frequency oscillations (HFOs) in resected channels. The total number of resected channels is shown for each patient, broken down in terms of channels without HFOs, channels with ictal HFOs with subsequent evolution (HFOs+ev), and channels with ictal HFOs but without evolution (HFOs-ev). Resections in patients A and D did not include any of the HFOs-ev channels.
DISCUSSION
We investigated the characteristics of intracranial ictal HFOs recorded from extensive (often bilateral) brain regions in 6 consecutive patients with neocortical epilepsy who underwent surgical resections after their SOZs were localized based on HFOs by a prospectively-defined presurgical protocol. The main findings were: 1) ictal HFOs (≥70 Hz) occurred across all seizure types as a widespread activity at seizure onset but evolved subsequently in a restricted manner; 2) the seizure onset defined by the ictal HFOs with evolution (HFOs+ev) was earlier, and occurred in a significantly different and smaller distribution, than the seizure onset defined by the <40 Hz CFA; 3) majority of the patients achieved favorable seizure outcome after resections that included mainly the HFOs+ev channels. The implications of ictal HFOs with respect to seizure localization and surgical resection are discussed below.
Seizure localization based on ictal HFOs
Using appropriate acquisition and display methods, we have demonstrated the occurrence of ictal HFOs in the range of ripple oscillations at the onset of neocortical seizures. These ictal HFOs are distinct from the interictal HFOs, and are along the lines of those described by us (Modur and Scherg, 2009) and others (Fisher et al., 1992, Jirsch et al., 2006, Ochi et al., 2007). The frequency of the observed ictal HFOs is much lower than the frequencies reported by other investigators using depth electrodes (Jirsch et al., 2006), and this may be due to the differences in electrode size, type and location (Worrell et al., 2008). More importantly, the ictal HFOs occurred as a widespread activity in multiple channels (sometimes contralateral to the side of seizure onset). Despite widespread onset, the ictal HFOs evolved subsequently in a more robust and restricted manner in only 50% of the channels (HFOs+ev) but not the rest (HFOs-ev). Furthermore, compared to the seizure onset defined by the CFA, the HFOs+ev defined an earlier onset temporally, and a much smaller, somewhat non-overlapping distribution spatially. These findings extend our previous observations (Modur and Zhang, 2009, Modur and Scherg, 2009), and are consistent with those reported recently by Wu et al., who found that the interictal HFOs (fast ripples) recorded during intraoperative electrocorticography were spatially restricted and occurred in a different distribution compared to the conventional spikes (Wu et al., 2010). To our knowledge, the current study is the first to describe the above characteristics with respect to the ictal HFOs and CFA.
Surgical resection based on ictal HFOs
Based on our post-hoc analysis, the HFOs+ev channels were nearly 10-fold more likely to have been resected than the HFOs-ev channels. This surgical strategy led to a class I/II outcome in 5/6 patients (83%) over a mean follow-up period of over 2 years, which is comparable to that of a recent meta-analysis in neocortical epilepsy (Engel et al., 2003). The class III outcome in patient A could have been related to undersampling, but we feel that it was most likely due to incomplete resection of the SOZ (as defined by HFOs+ev) because of its overlap with the language area. Although one can speculate on the seizure outcome that would have occurred if the resections had been based on the SOZ defined by all of the ictal HFOs or the CFA, it is clearly evident that such results would have come at the cost of resecting twice the amount of tissue. Considering this, our results suggest that smaller targeted resections, mainly confined to the HFOs+ev channels, can produce favorable results even in the context of widespread occurrence of ictal HFOs at seizure onset or the equally widespread SOZs defined by the CFA. We observed similar postoperative outcome after multiple subpial transections in a single patient (Modur and Scherg, 2009). Our finding is consistent with the prevailing notion that both ictal HFOs (Fisher et al., 1992, Alarcon et al., 1995, Ochi et al., 2007) and interictal HFOs (Jacobs et al., 2010, Wu et al., 2010) underlie seizure genesis, and that resection of the tissue containing HFOs results in favorable outcome. Along the same lines, one can also argue that the location of ictal HFOs may be the most appropriate site for implanting closed-loop neurostimulation devices in patients considered unsuitable for resective surgery (Kossoff et al., 2004, Smith et al., 2010).
The exact mechanisms underlying the generation of HFOs and their relationship to seizure genesis remain unclear (Rampp and Stefan, 2006). It is believed that the ripple oscillations represent synchronization of inhibitory postsynaptic potentials (Ylinen et al., 1995), whereas the fast ripples represent hypersynchronous bursts of action potentials generated by small clusters of pathologically connected neurons (Bragin et al., 2000). It has been proposed that the inhibitory networks synchronize the cortical activity at the beginning of a seizure when the principal neurons are quiescent resulting in the fast activity; after a few seconds, excessive excitatory activation, mediated by local increase in extracellular potassium, results in the focal seizure discharge generated by irregular firing and rhythmic bursting of the principal neurons (de Curtis and Gnatkovsky, 2009). In the context of these studies, the widespread occurrence of ictal HFOs in contiguous and non-contiguous regions in both lesional and nonlesional epilepsy suggests the existence of large neocortical epileptic networks. We speculate that the ictal HFOs and CFA represent activity in different neuronal populations near the “seizure focus” at seizure onset, and that HFOs+ev and HFOs-ev may represent different clusters, with the HFOs+ev being more intimately involved in seizure genesis and propagation. Since the HFOs are brief and felt to be generated in close proximity to the seizure focus, it is likely that they will be missed by inappropriate location of the recording electrodes. Conversely, depending on the extent of the underlying epileptic network, one may find widespread ictal HFOs if extensive intracranial coverage is employed, as demonstrated in this study.
The small sample size in our study limits definitive conclusions regarding seizure outcome. The results cannot be extrapolated to seizures of mesial temporal onset since such patients were not studied. We evaluated the temporal seizure evolution based on traditional visual analysis in the time domain familiar to clinicians but a more quantitative approach using time-frequency analysis, as described elsewhere (Modur and Scherg, 2009), could have been supplementary. Nevertheless, our study outlines a method to localize the seizure onset based on the evolution of ictal HFOs, highlights the differences between the seizure onsets defined by HFOs and CFA, demonstrates the benefits of surgical resection limited to the HFOs channels with evolution, and supports the idea for widespread epileptic networks underlying neocortical epilepsy. Future studies should be directed to clarify the inter-seizure and inter-patient variability of ictal HFOs, address the pathophysiologic basis of the ictal HFOs and CFA, and examine the value of ictal HFOs and CFA as predictors of seizure outcome in larger cohorts.
Supplementary Material
Table S1: Intracranial seizure analysis
Table S2: Analysis of type of electrical activity in the surgically resected channels
Acknowledgements
This study was supported in part by NIH CTSA Grant UL1 RR024982. We thank Dr. Michael Scherg and his staff at MEGIS Software GmbH for their continued support with the BESA software during the project.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosure of Conflict of Interest
None of the authors has any conflicts of interest to disclose.
References
- Akiyama T, Otsubo H, Ochi A, Ishiguro T, Kadokura G, Ramachandrannair R, Weiss SK, Rutka JT, Carter Snead O., 3rd. Focal cortical high-frequency oscillations trigger epileptic spasms: confirmation by digital video subdural EEG. Clin Neurophysiol. 2005;116:2819–2825. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Alarcon G, Binnie CD, Elwes RD, Polkey CE. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. 1995;94:326–337. doi: 10.1016/0013-4694(94)00286-t. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. 1992;82:155–159. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999a;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100--500 Hz) in human epileptic brain and in kainic acid--treated rats with chronic seizures. 1999b;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41 Suppl 6:S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. Reevaluating the mechanisms of focal ictogenesis: The role of low-voltage fast activity. Epilepsia. 2009;50:2514–2525. doi: 10.1111/j.1528-1167.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B Quality Standards Subcommittee of the American Academy of N, American Epilepsy S, American Association of Neurological S. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Chatillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Mehrotra N, Rigby M, Hader WJ, Pinnegar CR, Pillay N, Wiebe S, Federico P. Spatial localization and time-dependant changes of electrographic high frequency oscillations in human temporal lobe epilepsy. Epilepsia. 2009;50:605–616. doi: 10.1111/j.1528-1167.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, Spencer DD, Bergey GK. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45:1560–1567. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Modur P, Zhang S. Comparison of high frequency oscillations and conventional freqeuncy activity at seizure onset. Epilepsia. 2009;50 Suppl. 11:27–28. Abstract. [Google Scholar]
- Modur PN, Scherg M. Intracranial broadband EEG analysis and surgical outcome: Case report. Clin Neurophysiol. 2009;120:1220–1224. doi: 10.1016/j.clinph.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, Akizuki Y, Akiyama T, Imai K, Rutka JT, Snead OC., 3rd Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Rampp S, Stefan H. Fast activity as a surrogate marker of epileptic network function? Clin Neurophysiol. 2006;117:2111–2117. doi: 10.1016/j.clinph.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Rodin E, Constantino T, Rampp S, Modur P. Seizure Onset Determination. 2009;26:1–12. doi: 10.1097/WNP.0b013e3181969017. [DOI] [PubMed] [Google Scholar]
- Schiller Y, Cascino GD, Busacker NE, Sharbrough FW. Characterization and comparison of local onset and remote propagated electrographic seizures recorded with intracranial electrodes. 1998;39:380–388. doi: 10.1111/j.1528-1157.1998.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Smith JR, Fountas KN, Murro AM, Park YD, Jenkins PD, Morrell M, Esteller R, Greene D. Closed-loop stimulation in the control of focal epilepsy of insular origin. Stereotact Funct Neurosurg. 2010;88:281–287. doi: 10.1159/000316760. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Intracranial seizure analysis
Table S2: Analysis of type of electrical activity in the surgically resected channels