Abstract
Intensive chemotherapy regimens are not feasible in many adults with mantle cell lymphoma (MCL). We sought to build upon our previous experience with a non-intensive regimen, modified R-hyperCVAD chemotherapy (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone) with maintenance rituximab (MR), by the incorporation of bortezomib (VcR-CVAD) and the extension of MR beyond 2 years. Patients with previously untreated MCL received VcR-CVAD chemotherapy every 21 days for 6 cycles. Patients achieving at least a partial response to induction chemotherapy received rituximab consolidation (375 mg/m2 × 4 weekly doses) and MR (375 mg/m2 every 12 weeks × 20 doses). The primary end points were overall and complete response (CR), and secondary endpoints were progression-free (PFS) and overall survival (OS). Thirty patients were enrolled, with a median age of 61 years. All patients had advanced stage disease, and 60% had medium/high MCL International Prognostic Index risk factors. A CR or unconfirmed CR was achieved in 77% of patients. After a median follow-up of 42 months, the 3-year PFS and OS were 63% and 86%, respectively. The observed 3-year PFS and OS with VcR-CVAD in MCL were comparable to reported outcomes with more intensive regimens. A cooperative group trial (E1405) is attempting to replicate these promising results.
Keywords: bortezomib, chemotherapy, mantle cell lymphoma, non-Hodgkin lymphoma, rituximab
Introduction
Mantle cell lymphoma (MCL) is a biologically and clinically unique form of non-Hodgkin lymphoma (NHL), which has been shown to have the worst 5 year overall survival (OS) of any lymphoma subtype (The Non-Hodgkin’s Lymphoma Classification Project, 1997). However, outcomes appear to be improving, with a recent study suggesting the median OS has increased from 3 to 5 years in the modern era.(Herrmann, et al 2009) Several studies using intensive regimens in younger MCL patients have demonstrated excellent long-term outcomes, with 5-year progression-free survival (PFS) of 50–60% and 5-year OS of 65–75%.(Damon, et al 2009, Delarue, et al 2008, Geisler, et al 2008, Romaguera, et al 2005) Whether intensive strategies improve the OS relative to non-intensive strategies is unclear; one randomized clinical trial showed improved OS using an intensive strategy while one observational study revealed similarly excellent outcomes using non-intensive strategies.(Dreyling, et al 2008, Martin, et al 2008)
Due to age considerations and co-morbidities, intensive treatment strategies can only be administered to about 50% of newly diagnosed MCL patients. In an effort to find an upfront treatment strategy that could be administered to all MCL patients, in 2000 we developed a regimen called “modified R-hyperCVAD with maintenance rituximab” (MR). Patients received “part A” of conventional R-hyperCVAD chemotherapy (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone), minus the day 11 vincristine and mid-cycle dexamethasone, in 28 day intervals for 6 cycles. No cytarabine or methotrexate was administered. Following completion of the modified R-hyperCVAD induction, patients received MR administered as 4 weekly doses every 6 months for 2 years. The complete response (CR) rate to induction was 64% and the 3-year PFS was 50%.(Kahl, et al 2006) Similar outcomes were observed for older and younger patients, and this regimen is listed as an option for older patients in the National Comprehensive Cancer Network (NCCN) guidelines.(NCCN 2008) With a median follow up of 62 months, the 5-year OS was 62% and the median OS was 70 months (unpublished data), which is comparable to some U.S. studies using more intense regimens.(Damon, et al 2009, Fayad, et al 2007)
In the present study, we sought to improve the CR rate after induction therapy with the introduction of bortezomib, an agent with significant activity in relapsed MCL.(Fisher, et al 2006, Goy, et al 2005, O’Connor, et al 2005) Given the effect of proteasome inhibition on several intracellular pathways important to MCL biology, including proliferation, apoptosis, and nuclear factor kappa B (NFκB) signalling, we hypothesized bortezomib may potentiate cytotoxic chemotherapy.(Mitsiades, et al 2003, Paoluzzi and O’Connor 2006) Additionally, we hypothesized that a more prolonged MR strategy would lead to more durable remissions.
Patients and methods
Patients
Patients were eligible for this trial if they were older than 18 years of age with histologically confirmed MCL. The diagnosis was established by the local pathologist and confirmed by the study team reference pathologist. Prior treatment was exclusionary with the exception of 1 cycle of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or CHOP-like chemotherapy. Patients were required to have measurable or evaluable disease and have a baseline Eastern Cooperative Oncology Group (ECOG) performance status of no greater than 2. Minimal laboratory requirements included a neutrophil count ≥ 1.5 × 109/l, platelet count ≥ 100 × 109/l, serum creatinine ≤ 176.8 μmol/l, serum bilirubin ≤ 34.2.μmol/l, and aspartate transaminase ≤ 2.5 times the laboratory upper limit of normal. Low blood counts were not exclusionary if related to splenomegaly or disease replacement of bone marrow. Patients were excluded if they were known to have central nervous system involvement by lymphoma, an active second malignancy requiring radiation or chemotherapy treatments, human immunodeficiency virus infection, chronic or active hepatitis B infection, or New York Heart Association (NYHA) class III or IV heart failure.
The study was approved by the Human Subjects Committee at the University of Wisconsin and by the Institutional Review Board at each participating Wisconsin Oncology Network (WON) institution. All patients signed an informed consent document describing the investigational nature of the proposed treatments.
Treatment
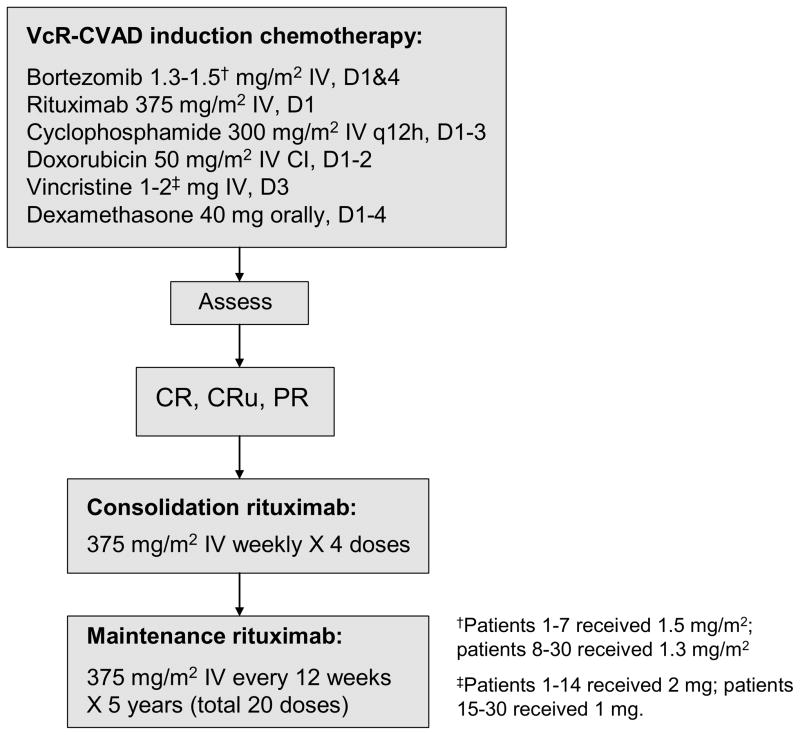
Protocol therapy consisted of 6 cycles of induction chemotherapy (VcR-CVAD regimen) every 21 days. Patients achieving at least a partial response to induction therapy were then eligible to proceed with consolidation and maintenance therapy with rituximab (Figure 1). VcR-CVAD induction chemotherapy (final version) consisted of rituximab 375 mg/m2 intravenously (IV) on day 1, bortezomib 1.3 mg/m2 IV on days 1 and 4, cyclophosphamide 300 mg/m2 IV every 12 h on days 1–3 (total of 6 doses), doxorubicin 50 mg/m2 IV continuous infusion days 1–2 (total dose over 48 h equal to 50 mg/m2), vincristine 1 mg IV day 3, and dexamethasone 40 mg orally on days 1–4 of each 21-day cycle. Due to an excessive rate of painful peripheral neuropathy (PPN), two protocol modifications were required during the trial. The first 7 patients received bortezomib at 1.5 mg/m2 IV days 1 and 4 and vincristine 2 mg IV day 3. The next 7 patients received bortezomib at 1.3 mg/m2 on days 1 and 4 and vincristine 2 mg IV day 3. The remaining 16 patients received bortezomib 1.3 mg/m2 on days 1 and 4 and vincristine 1 mg IV on day 3.
Figure 1.
VcR-CVAD treatment regimen.
Patients received growth factor support with granulocyte colony-stimulating factor (G-CSF) 5 μg/kg/day beginning on day 5 or 6 of each treatment cycle and continuing until an absolute neutrophil count ≥2 × 109/l past the nadir. All appropriate supportive care measures were permitted throughout treatment including tumour lysis syndrome prophylaxis, transfusion support and antibiotics. Dose modifications for toxicity were specified in the protocol. Protocol therapy was discontinued in the event of unacceptable toxicity, disease progression, or patient and/or physician discretion. Toxicities were reported in accordance with the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.(National Cancer Institute 2006)
Consolidation therapy with rituximab was administered as 4 consecutive weekly doses of rituximab 375 mg/m2 IV beginning within 4 weeks after completion of induction chemotherapy. Rituximab maintenance therapy began 12 weeks after the start of consolidation therapy, with administration of a single dose of rituximab 375 mg/m2 IV every 12 weeks for a total of 5 years (total of 20 maintenance doses).
Criteria for response
Restaging computerized tomography scans were performed after cycles 2, 4, and 6. Patients were considered evaluable for response if they completed at least 2 cycles of therapy and had undergone an initial response evaluation. Patients were considered evaluable for toxicity if they had received at least 1 dose of therapy. Responses were defined by the 1999 International Working Group criteria.(Cheson, et al 1999)
Statistical considerations
The primary endpoint of this study was the observed overall response (OR) and CR to the VcR-CVAD regimen at the completion of induction therapy. A balanced two-stage design was undertaken to test the null hypothesis that a true probability of OR is at most 0.7 versus the alternative hypothesis that it is at least 0.9.(Ye and Shyr 2007) The significance level of this test was 0.10 with a power of 0.9. Initially, 15 evaluable patients were to be assessed for response, with plans to terminate enrollment early if there were 11 or fewer objective responses observed. An additional 15 evaluable patients were to be enrolled if there were 12 or more objective responses observed in the first stage of the study. The critical value for rejecting the null hypothesis and declaring the proposed treatment as promising at the end of the study was 25 objective responses. Ninety-five percent confidence intervals (95% CI) of the overall and complete response rates were computed using the stage-wise ordering method, which takes into account the multiple testing procedure of the two-stage design.(Koyama and Chen 2008)
Secondary endpoints included determination of PFS and OS, and assessment of tolerability and toxicity associated with the treatment. PFS was calculated from the date of first VcR-CVAD administration until progression of disease or death from any cause. OS was calculated from the date of first VcR-CVAD administration until death from any cause.
The survival distribution for PFS and OS was estimated using the Kaplan-Meier method. Cox proportional hazard regression models were used to assess whether baseline characteristics, such as MCL International Prognostic Index (MIPI), score were predictive of PFS and OS.
Results
Patients
Thirty patients from four institutions were entered into this study between July 27, 2005, and May 5, 2008. The baseline patient characteristics are summarized in Table 1. The median age for enrolled patients was 61 years, and the majority of patients were men (80%). All patients had advanced stage disease, and six patients had blastic morphology of lymphoma. The MIPI risk score indicated medium or high risk disease in 60% of patients.(Hoster, et al 2008)
Table 1.
Baseline characteristics of study participants.
| Characteristics | No. |
|---|---|
| Total patients | 30 |
|
| |
| Evaluable for response | 30 (100%) |
|
| |
| Sex | |
| Male (%) | 24 (80%) |
| Female (%) | 6 (20%) |
|
| |
| Age, years (at on-study) | |
| Median | 61 |
| Range | 48–74 |
|
| |
| ECOG performance status (%) | |
| 0 | 6 (20%) |
| 1 | 19 (63%) |
| 2 | 5 (17%) |
|
| |
| MIPI risk factors (%) | |
| Low | 12 (40%) |
| Medium | 8 (27%) |
| High | 10 (33%) |
|
| |
| Stage (%) | |
| III | 4 (13%) |
| IV | 26 (87%) |
|
| |
| Elevated LDH (%) | 20 (67%) |
|
| |
| Elevated β2m (%) | 20 (67%) |
|
| |
| Blastic morphology | 6 (20%) |
|
| |
| Ki-67 score (%)† | |
| <10% | 11 (37%) |
| 10–30% | 12 (40%) |
| >30% | 5 (17%) |
Ki-67 analysis not available in 2 patients.
ECOG, Eastern Cooperative Oncology Group; MIPI, Mantle Cell Lymphoma International Prognostic Index; LDH, lactate dehydrogenase.
Responses
Twenty-seven patients experienced objective response during induction chemotherapy, with an OR rate of 90% (95% CI 76–95%) (Table 2). A CR or unconfirmed CR (CRu) was achieved in 23 patients (77%), and four patients (13%) achieved a partial response (PR). Three patients experienced progressive disease (PD) during induction chemotherapy, with two patients experiencing progression after the first cycle of chemotherapy, and another patient experiencing PD following cycle 2. All three patients with PD had leukaemic presentations with white blood cell counts of 70 × 109/l, 171 × 109/l, and 398 × 109/l. Three of four patients with a PR after induction chemotherapy improved to a CR during maintenance rituximab.
Table 2.
Response rates to VcR-CVAD induction chemotherapy.
| Clinical response | (95% CI) | |
|---|---|---|
| Overall response rate | 27 | 90% (76–95%) |
| CR/CRu | 23 | 77% (63–90%) |
| PR | 4 | 13% (5–30%) |
| PD | 3† | 10% (3–26%) |
All three patients with PD had leukaemic presentations of MCL.
CR/CRu, complete response/unconfirmed CR; PR, partial response; PD, progressive disease
Progression-free and overall survival
With a median follow-up of 42 months in surviving patients, 12 patients have experienced PD, and five deaths have been observed. Four deaths were due to progressive MCL, and one death was related to treatment complications following allogeneic stem cell transplant. All patients were included in the survival measures according to intention-to-treat analysis.
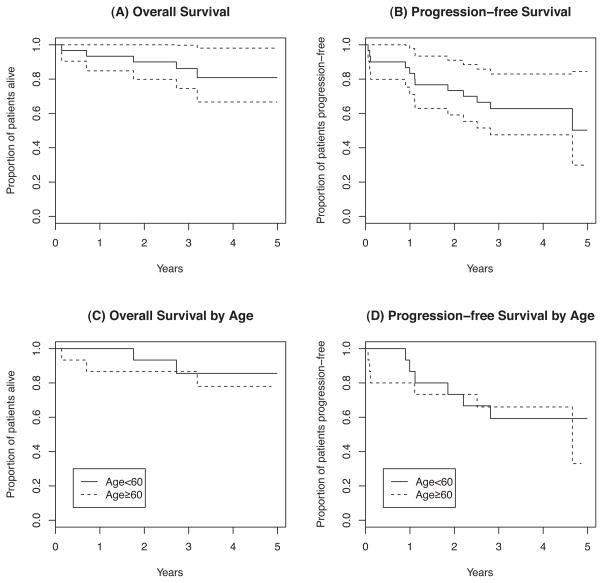
The 3-year PFS and OS were 63% (95% CI, 48–83%) and 86% (95% CI, 75%–100%), respectively. There were no significant differences noted in PFS or OS between patients less than age 60 and patients ≥ 60 years of age (Figure 2). There were no significant PFS or OS differences observed when analysed by MIPI risk category or by Ki-67 score (data not shown).(Determann, et al 2008, Hoster, et al 2008) However, a higher MIPI score appears to be associated with shorter PFS and OS according to Cox proportional hazards regression analysis. An increase in MIPI score by 1 was associated with a relative risk of 2.57 (p=0.019) and 3.15 (p=0.034) for PFS and OS, respectively.
Figure 2.
Kaplan-Meier curves for overall and progression-free survival. (A) Overall survival (95% confidence interval); (B) Progression-free survival (95% confidence interval); (C) Overall survival stratified by age (< 60 years vs. ≥ 60 years); (D) Progression-free survival stratified by age (< 60 years vs. ≥ 60 years).
Ten patients experienced progression after completing induction chemotherapy. Eight of these patients went on to non-myeloablative allogeneic transplant. One patient experienced progression and death while undergoing additional salvage chemotherapy, and another patient received therapy with a novel agent as part of a clinical trial.
Toxicity
Toxicity during induction chemotherapy was primarily expected myelosuppression (Table 3). Grade 3 and 4 neutropenia and thrombocytopenia were the most frequently experienced haematological events. Thirteen of 167 (8%) treatment cycles were complicated by neutropenic fever/infection. Grade 3 and 4 non-haematological toxicities were relatively uncommon during induction chemotherapy (Table 4).
Table 3.
Haematological toxicities and infection during VcR-CVAD induction chemotherapy (worst grade experienced per patient).
| Grade 3 | Grade 4 | |
|---|---|---|
| Thrombocytopenia | 8 | 15 |
| Neutropenia | 3 | 17 |
| Haemoglobin | 8 | 0 |
| Febrile neutropenia | 5 | 0 |
| Infection with neutropenia | 2 | 0 |
| Infection without neutropenia | 3 | 1 |
Table 4.
Non-haematological toxicities during VcR-CVAD induction chemotherapy (worst grade experienced per patient).
| Grade 3 | Grade 4 | |
|---|---|---|
| Hyperglycaemia | 4 | 0 |
| Hyponatraemia | 4 | 0 |
| Hypophosphataemia | 1 | 0 |
| Dehydration | 2 | 0 |
| Pulmonary embolism | 0 | 1 |
| Oesophagitis | 1 | 0 |
| Mood alteration | 1 | 0 |
| Pain | 2 | 0 |
| Sensory neuropathy | 8 | 1 |
| Fatigue | 2 | 0 |
Grade 3 PPN was observed in five of the first seven patients (Table 5). The bortezomib dose was reduced from 1.5 mg/m2 to 1.3 mg/m2. After four of the next seven patients experienced grade 3–4 PPN, the protocol was further modified to include a 50% dose reduction of vincristine to 1 mg. Of the final 16 patients enrolled, only one experienced severe (grade 3) PPN. Among the nine patients experiencing grade 3–4 PPN, six patients had improvement to grade 1, and three remain with grade 2 PPN. However, six patients require chronic medications for symptom control.
Table 5.
Sensory neuropathy during VcR-CVAD induction chemotherapy (worst grade experienced per patient).
| Patients | Bortezomib (mg/m2) | Vincristine (mg/m2) | Grade | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
|
| ||||||
| 1–7 | 1.5 | 2 | 2 | 0 | 5 | 0 |
|
| ||||||
| 8–14 | 1.3 | 2 | 0 | 3 | 2 | 1 |
|
| ||||||
| 15–30 | 1.3 | 1 | 7 | 0 | 1 | 0 |
Six events of delayed neutropenia were observed in five patients during maintenance rituximab. All events were asymptomatic and resolved with administration of pegylated G-CSF. To date, the cohort has completed a median of 9.5 doses of MR (range 0–19). Fourteen patients remain on MR and 13 patients have discontinued rituximab maintenance, six for disease progression, six for hypogammaglobulinaemia associated with recurrent infections, and one due to angina felt to be unrelated to treatment.
Serum immunoglobulin levels (quantitative levels of IgA, IgG, IgM) were evaluated in 22 patients during the course of protocol therapy. At least one quantitative immunoglobulin level was found to be below the lower limits of normal in 18 of 22 (82%) patients. Twelve patients experienced quantitative immunoglobulin ≤ 50% of the lower limit of normal (IgA ≤ 0.2 g/l, IgG ≤ 3 g/l, IgM ≤ 0.2 g/l) at least once and 7 of these patients experienced recurrent grade 1–2 respiratory infections (n=7). One patient developed pneumococcal sepsis and another developed recurrent Clostridium difficile colitis. Six patients required discontinuation of MR, after a median of eight doses, secondary to symptomatic hypogammaglobulinaema; four patients have received gamma globulin replacement therapy.
Discussion
The VcR-CVAD induction regimen produced a high CR rate (77%) and high OR rate (90%) in a representative MCL patient population (median age 61 years, 60% intermediate/high risk MIPI). When compared to our previous trial (Kahl et al 2006) using a similar induction, without bortezomib, we observed a higher CR rate (77% vs. 64%) and higher OR rate (90% vs. 77%). If these differences are “real”, we cannot be certain bortezomib is solely responsible, as the VcR-CVAD induction was administered on slightly more intensive schedule than the modified hyper-RCVAD regimen (21 vs. 28 days). Despite the uncertainties, the data are not inconsistent with the hypothesis that bortezomib potentiates cytotoxic chemotherapy in MCL.
The major toxicities from VcR-CVAD were myelosuppression and PPN. The severity of myelosuppression did not appear to be altered by the addition of bortezomib. However, there is clearly an increased risk for PPN when combining bortezomib and vincristine, and our data suggest the risk can be ameliorated with alternative dosing. The approved dose of bortezomib is 1.3 mg/m2 on days 1, 4, 8, and 11, repeated in 21 day cycles. In an effort to make the treatment schedule more patient-friendly and to maximize overlap with cytotoxic chemotherapy, we opted for two bortezomib doses (days 1 and 4) with each chemotherapy cycle. We initially selected a dose of 1.5 mg/m2, as existing data suggested superiority for this dose in MCL.(Goy, et al 2005, O’Connor, et al 2005) Once apparent that severe PPN was occurring at an unacceptable frequency, the protocol was modified (twice) resulting in our final dosing of bortezomib 1.3 mg/m2 days 1 and 4 and vincristine 1 mg each cycle. Using this strategy, only one of the last 16 patients enrolled experienced significant PPN, which developed after cycle 6. One of the interesting observations from the study was the onset of the PPN. For all patients affected by severe PPN, it developed quite suddenly after cycle 4, 5, or 6 and there was little opportunity for pre-emptive dose modifications.
We are aware of two other published studies that have combined bortezomib with vincristine (as part of the R-CHOP regimen) in B cell lymphomas. The French adult lymphoma study group (Groupe d’ Etude des Lymphomes de l’ Adulte) has published results from a randomized Phase 2 trial where bortezomib was added to R-CHOP using escalating doses and bi-weekly vs. weekly schedules.(Ribrag, et al 2009) These investigators also advise caution when combining both neurotoxic agents. The PPN was clearly increased when using higher doses (1.6 mg/m2) and when using the bi-weekly schedule. However, a report from a multicentre trial conducted in the U.S. did not find excessive neurotoxicity when combining bortezomib 1.3 mg/m2 days 1 and 4 with R-CHOP 21, using full-dose vincristine (2 mg).(Furman, et al, 2010)
With a median follow up of 42 months, the 3-year PFS and OS were 63% and 86%, respectively. These results compare favourably to the parent study (no bortezomib) in which the 3-year PFS and OS were 50% and 75%, respectively (Kahl et al 2006). Again, if these differences are real, we cannot say whether they are due to the incorporation of bortezomib, intensification of the induction regimen from 28 to 21 days, or prolongation of the MR from 2 to 5 years.
The inclusion of MR for MCL has been controversial. Maintenance rituximab for 2 years was proven to be of no clinical benefit in a curable lymphoma (DLBCL).(Habermann, et al 2006) Maintenance rituximab for 2 years has prolonged PFS in incurable lymphomas, including follicular and other indolent histologies.(Hochster, et al 2004, Salles, et al 2011, van Oers, et al 2006). Similar to other incurable B cell lymphomas, we hypothesized MR would have a beneficial effect in MCL. Two small, randomized controlled trials have generated conflicting results. The German Low Grade Lymphoma Study Group enrolled patients with relapsed MCL, re-established remission with chemotherapy, with or without rituximab and then randomized to MR for 9 months or observation.(Forstpointner, et al 2006) Only 47 patients were analysed in the MCL cohort, but a statistically significant improvement in response duration was observed in favour of MR (remission beyond 2 years 45% vs. 9%, p = 0.049). The Swiss Group for Clinical Cancer Research (Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung) enrolled 104 patients with a mixture of untreated and relapsed MCL and randomized them to a single 4-week rituximab treatment or a prolonged rituximab schedule of a 4-week treatment followed by a single dose every 8 weeks × 4 doses.(Ghielmini, et al 2005) The extended schedule did not improve response rates, response duration, or event-free survival. The reasons for these discrepant results are unclear, but we speculate that the quality of the response to induction therapy may affect the likelihood of MR benefit. The question may soon be put to rest in MCL, as an independent Data and Safety Monitoring Board recently recommended early closure of a large randomized trial that demonstrates a significant benefit for patients receiving MR after R-CHOP chemotherapy (Martin Dreyling, University of Munich-Grosshadern, Germany, personal communication).
The duration of MR is another question in need of further study in B cell lymphomas. Most published studies in FL and MCL have utilized a 1 or 2 year maintenance strategy.(Ghielmini, et al 2005, Hochster, et al 2004, Salles, et al 2011, van Oers, et al 2006) In this study, we sought to determine the safety and efficacy of a longer duration of MR. Infections, seemingly related to hypogammaglobulinaemia, caused premature discontinuation of MR in six patients. Two infections were severe (sepsis and intractable clostridium difficile colitis), and four patients have gone on to gamma globulin replacement therapy. Although the majority of patients have tolerated the prolonged MR without difficulty, the intolerance rate of 22% leads us to recommend that 2 years of MR remain the standard until randomized controlled trials comparing 2 years against more prolonged schedules are completed.
The 3-year PFS of 63% and OS of 86% observed in this trial are comparable to other studies that used more intensive regimens. For example, with nearly identical follow up time, Romaguera et al (2005) reported a 3-year PFS of 64% and 3-year OS of 82% for the conventional R-hyperCVAD regimen. The Cancer and Leukemia Group B has published the results of an intensive immunochemotherapy induction regimen followed by autologous stem cell transplantation (ASCT), producing a 3-year PFS of 63% and 3-year OS of 83%.(Damon, et al 2009) Two multicentre European studies, utilizing high dose cytarabine in the induction and consolidative ASCT have generated slightly superior PFS results, but both were limited to patients aged 65 years and younger, making comparison with the present trial difficult.(Delarue, et al 2008, Geisler, et al 2008)
To determine if these promising results could be replicated in a cooperative setting, ECOG protocol E1405 was initiated and utilized the same VcR-CVAD induction therapy followed by MR for 2 years. Enrollment completed in November 2008 and the OR (96%) and CR (75%) rates were similar to those observed in the present study.(Kahl, et al 2009) More follow up time is needed before we can report on the 3-year PFS and OS from E1405.
Further progress in the treatment of MCL will require larger trials capable of generating robust, comparative data. The U.S. cooperative groups have agreed in principle to an intergroup trial for the older MCL patients, which will test the contribution of bortezomib in a randomized fashion. The chemotherapy backbone for this trial will be bendamustine plus rituximab (BR), a logical choice given the promising activity and toxicity profile of the BR regimen.(Robinson, et al 2008, Rummel, et al 2005) The role of MR will also be evaluated in this trial. In a separate intergroup trial, younger patients will be randomized to receive either BR or conventional R-hyperCVAD, with all patients receiving ASCT as part of an intensive therapy strategy. These intergroup efforts represent a genuine opportunity to move the MCL field forward by establishing standard regimens upon which novel agents can be systematically evaluated.
Acknowledgments
University of Wisconsin Carbone Cancer Center, the Cancer Center Support Grant P30 CA14520, Millennium Pharmaceuticals, Inc., and the University of Wisconsin Forward Lymphoma Research Fund.
Funding
This work was supported by the National Institutes of Health [P30 CA14520], Millennium Pharmaceuticals, Inc., and the University of Wisconsin Forward Lymphoma Research Fund.
Footnotes
Author contributions:
Julie Chang: Collected and analysed data, prepared manuscript.
Sangbum Choi: Analysed data.
KyungMann Kim: Analysed data, assisted with manuscript preparation.
Jens Eickhoff: Assisted with research design, approved manuscript.
David Yang: Analysed data, assisted with manuscript preparation.
Leslie Gilbert: Trial implementation and data management.
Eric Rogers: Trial implementation and data management.
Jae Werndli: Trial design and implementation, data management.
Michael Huie: Enrolled research subjects, approved manuscript.
Thomas McFarland: Enrolled research subject, approved manuscript.
Michael Volk: Enrolled research subject, approved manuscript.
Jules Blank: Enrolled research subject, approved manuscript.
Natalie Callander: Enrolled research subject, approved manuscript.
Walter Longo: Enrolled research subject, approved manuscript.
Christopher Peterson: Enrolled research subject, approved manuscript.
Brad Kahl: Designed clinical trial, analysed data, prepared manuscript.
Conflicts of interest:
Julie Chang none
Christopher Peterson none
Sangbum Choi none
Jens Eickhoff none
KyungMann Kim none
Leslie Gilbert none
Eric Rogers none
Jae Werndli none
Michael Huie none
Thomas McFarland none
Michael Volk none
Jules Blank none
Natalie Callander research funding - Millennium
Walter Longo none
Brad Kahl research funding and consulting –Millennium, research funding and consulting – Genentech
References
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, Lacasce AS, Blum KA, Byrd JC, Kelly M, Stock W, Linker CA, Canellos GP. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue R, Haioun C, Ribrag V, Brice P, Delmer A, Tilly H, Salles G, Van Hoof A, Casasnovas O, Brousse N, Lefrere F, Hermine O. RCHOP and RDHAP followed by autologous stem cell transplantation (ASCT) in mantle cell lymphoma (MCL): final results of a phase II study from the GELA. Blood (ASH Annual Meeting Abstracts) 2008;112:581. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- Determann O, Hoster E, Ott G, Wolfram Bernd H, Loddenkemper C, Leo Hansmann M, Barth TE, Unterhalt M, Hiddemann W, Dreyling M, Klapper W. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood. 2008;111:2385–2387. doi: 10.1182/blood-2007-10-117010. [DOI] [PubMed] [Google Scholar]
- Dreyling M, Hoster E, van Hoof A, Metzner B, Gisselbrecht C, Reiser M, Pfreundschuh M, Trumper L, Steinhauer H, Boiron J, Boogaerts M, Aldaoud A, Silingardi V, Kluin-Nelemans H, Unterhalt M, Hiddemann W. Early consolidation with myeloablative radiochemotherapy followed by autologous stem cell transplantion in first remission of mantle cell lymphoma. Blood (ASH Annual Meeting Abstracts) 2008;112:285. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- Fayad L, Thomas DA, Romaguera J. Update of the M.D. Anderson Cancer Center Experience with HyperCVAD and Rituximab for the Treatment of Mantle Cell and Burkitt-Type Lymphomas. Clin Lymphoma Myeloma. 2007;8:S57–62. doi: 10.3816/clm.2007.s.034. [DOI] [PubMed] [Google Scholar]
- Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O’Connor OA, Shi H, Boral AL, Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- Forstpointner R, Unterhalt M, Dreyling M, Bock HP, Repp R, Wandt H, Pott C, Seymour JF, Metzner B, Hanel A, Lehmann T, Hartmann F, Einsele H, Hiddemann W. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG) Blood. 2006;108:4003–4008. doi: 10.1182/blood-2006-04-016725. [DOI] [PubMed] [Google Scholar]
- Furman RR, Martin P, Ruan J, Cheung YK, Vose JM, Lacasce AS, Elstrom R, Coleman M, Leonard JP. Phase 1 trial of bortezomib plus R-CHOP in previously untreated patients with aggressive non-Hodgkin lymphoma. Cancer. 2010;116:5432–5439. doi: 10.1002/cncr.25509. [DOI] [PubMed] [Google Scholar]
- Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, Eriksson M, Nordstrom M, Kimby E, Boesen AM, Kuittinen O, Lauritzsen GF, Nilsson-Ehle H, Ralfkiaer E, Akerman M, Ehinger M, Sundstrom C, Langholm R, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghielmini M, Schmitz SF, Cogliatti S, Bertoni F, Waltzer U, Fey MF, Betticher DC, Schefer H, Pichert G, Stahel R, Ketterer N, Bargetzi M, Cerny T. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK) J Clin Oncol. 2005;23:705–711. doi: 10.1200/JCO.2005.04.164. [DOI] [PubMed] [Google Scholar]
- Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, Fayad L, Dang NH, Samaniego F, Wang M, Broglio K, Samuels B, Gilles F, Sarris AH, Hart S, Trehu E, Schenkein D, Cabanillas F, Rodriguez AM. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, Reiser M, Forstpointner R, Metzner B, Peter N, Wormann B, Trumper L, Pfreundschuh M, Einsele H, Hiddemann W, Unterhalt M, Dreyling M. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- Hochster HS, Weller E, Ryan T, et al. Results of E1496: A phase III trial of CVP with or without maintenance rituximab in advanced indolent lymphoma (NHL) [abstract 6502] J Clin Oncol. 2004;22:556. [Google Scholar]
- Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wormann B, Ludwig WD, Duhrsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- Kahl B, Longo W, Eickhoff J, Zehnder J, Jones C, Blank J, McFarland T, Bottner W, Rezazedeh H, Werndli J, Bailey H. Maintenance rituximab following induction chemoimmunotherapy may prolong progression-free survival in mantle cell lymphoma: a pilot study from the Wisconsin Oncology Network. Ann Oncol. 2006;17:1418–1423. doi: 10.1093/annonc/mdl127. [DOI] [PubMed] [Google Scholar]
- Kahl BS, Li H, Smith MR, Gascoyne RD, Paietta EP, Advani R, Horning SJ. The VcR-CVAD regimen produces a high complete response rate in untreated Mantle Cell Lymphoma (MCL): First analysis of E1405 - A phase II study of VcR-CVAD with maintenance rituximab for MCL. Blood. 2009;114:663. doi: 10.1182/blood-2013-08-523845. abstract 1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med. 2008;27:3145–3154. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Chadburn A, Christos P, Furman R, Ruan J, Joyce MA, Fusco E, Glynn P, Elstrom R, Niesvizky R, Feldman EJ, Shore TB, Schuster MW, Ely S, Knowles DM, Chen-Kiang S, Coleman M, Leonard JP. Intensive treatment strategies may not provide superior outcomes in mantle cell lymphoma: overall survival exceeding 7 years with standard therapies. Ann Oncol. 2008;19:1327–1330. doi: 10.1093/annonc/mdn045. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Schlossman R, Munshi NC, Hideshima T, Anderson KC. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0. 2006 Available at: http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-Hodgkin’s Lymphomas. 2008;3 doi: 10.6004/jnccn.2010.0021. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- O’Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, Straus D, Portlock C, Hamlin P, Choi E, Dumetrescu O, Esseltine D, Trehu E, Adams J, Schenkein D, Zelenetz AD. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Paoluzzi L, O’Connor OA. Mechanistic rationale and clinical evidence for the efficacy of proteasome inhibitors against indolent and mantle cell lymphomas. Bio Drugs. 2006;20:13–23. doi: 10.2165/00063030-200620010-00002. [DOI] [PubMed] [Google Scholar]
- Ribrag V, Gisselbrecht C, Haioun C, Salles G, Golfier JB, Ertault M, Ferme C, Briere J, Brice P, Mounier N. Efficacy and toxicity of 2 schedules of frontline rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone plus bortezomib in patients with B-cell lymphoma: a randomized phase 2 trial from the French Adult Lymphoma Study Group (GELA) Cancer. 2009;115:4540–4546. doi: 10.1002/cncr.24518. [DOI] [PubMed] [Google Scholar]
- Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, Tulpule A, Schwartzberg LS, Lemieux B, Cheson BD. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4473–4479. doi: 10.1200/JCO.2008.17.0001. [DOI] [PubMed] [Google Scholar]
- Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, McLaughlin P, Younes A, Samaniego F, Goy A, Sarris AH, Dang NH, Wang M, Beasley V, Medeiros LJ, Katz RL, Gagneja H, Samuels BI, Smith TL, Cabanillas FF. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, Josten KM, Durk H, Rost A, Neise M, von Grunhagen U, Chow KU, Hansmann ML, Hoelzer D, Mitrou PS. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, Caballero D, Haioun C, Pedersen LM, Delmer A, Simpson D, Leppa S, Soubeyran P, Hagenbeek A, Casasnovas O, Intragumtornchai T, Ferme C, da Silva MG, Sebban C, Lister A, Estell JA, Milone G, Sonet A, Mendila M, Coiffier B, Tilly H. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- The Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, Jack A, Van’t Veer M, Vranovsky A, Holte H, van Glabbeke M, Teodorovic I, Rozewicz C, Hagenbeek A. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- Ye F, Shyr Y. Balanced two-stage designs for phase II clinical trials. Clin Trials. 2007;4:514–524. doi: 10.1177/1740774507084102. [DOI] [PubMed] [Google Scholar]