Summary
Immune reconstitution appears to be delayed following myeloablative conditioning (MAC) and umbilical cord blood transplantation (UCBT) in paediatric recipients. Although reduced toxicity conditioning (RTC) vs. MAC prior to allogeneic stem cell transplantation is associated with decreased transplant-related mortality, the effects of RTC vs. MAC prior to UCBT on immune reconstitution and risk of graft-versus-host disease (GVHD) are unknown. In 88 consecutive paediatric recipients of UCBT, we assessed immune cell recovery and immunoglobulin reconstitution at days +100, 180 and 365 and analysed risk factors associated with acute and chronic GVHD. Immune cell subset recovery, immunoglobulin reconstitution, and the incidence of opportunistic infections did not differ significantly between MAC vs. RTC groups. In a Cox model, MAC vs. RTC recipients had significantly higher risk of grade II-IV acute GVHD (Hazard Ratio [HR] 6.1, p=0.002) as did recipients of 4/6 vs. 5-6/6 HLA-matched UCBT (HR 3.1, p=0.03), who also had significantly increased risk of chronic GVHD (HR 18.5, p=0.04). In multivariate analyses, MAC vs. RTC was furthermore associated with significantly increased transplant-related (Odds Ratio 26.8, p=0.008) and overall mortality (HR=4.1, p=0.0001). The use of adoptive cellular immunotherapy to accelerate immune reconstitution and prevent and treat opportunistic infections and malignant relapse following UCBT warrants further investigation.
Keywords: cord blood transplantation, GVHD, paediatrics, immune, immunology
Introduction
Allogeneic haematopoietic stem cell transplantation (AlloSCT) offers potentially curative therapy for a subset of patients with numerous malignant and non-malignant diseases. Umbilical cord blood transplantation (UCBT) from an unrelated donor offers an alterative stem cell source for those patients lacking a suitably histocompatible related donor. We and others have demonstrated that myeloablative conditioning (MAC) followed by UCBT in paediatric patients with malignant and non-malignant diseases results in high rates of engraftment, sustained donor chimerism, risk of grade II-IV acute GVHD (aGVHD) of 25-50%, and transplant-related mortality (TRM) of 15-50% within the first 100 days following UCBT (Eapen, et al 2006, Eapen, et al 2007, Kurtzberg, et al 2008, Rubinstein, et al 1998, Styczynski, et al 2004). Compared to adult unrelated donor transplantation using bone marrow or peripheral blood stem cells, UCBT has the advantages of faster procurement and enhanced ability to cross human leucocyte antigen (HLA) disparities with lower risk of severe aGVHD. The disadvantages of UCBT include delayed haematopoietic recovery and increased risk of transplant-related mortality (TRM), particularly in the early post-transplant period (Liao, et al 2011, Szabolcs & Cairo 2010).
Reduced toxicity conditioning (RTC) has emerged as an alternative to traditional myeloablative conditioning (MAC). We and others have defined RTC as a regimen associated with myeloablation, but with decreased toxicity secondary to conditioning compared with traditional MAC (Alatrash, et al 2011, Styczynski, et al 2011). The purpose of RTC is to decrease TRM while establishing a platform of host-donor tolerance through immunosuppression prior to and following transplantation. RTC prior to UCBT has been successfully employed in adults (Ballen, et al 2007, Brunstein, et al 2007, Majhail, et al 2006). Additionally, we and others have reported that RTC prior to UCBT results in high rates of engraftment and low risk of TRM in small groups of paediatric patients (Bradley, et al 2007, Pulsipher, et al 2009).
Long-term survival following AlloSCT depends in large part on successful immune reconstitution to reduce the risk of opportunistic infection and malignant relapse. However, T-cells in UCB are nearly exclusively naïve, and few mature lymphocytes are transplanted following UCBT. The high rates of opportunistic infection commonly observed in the first 3-6 months following UCBT have been attributed to deficits in immune reconstitution, particularly via the thymic-independent pathway (Szabolcs & Cairo 2010). Moreover, those paediatric UCBT recipients who have successful antigen-specific T-cell proliferation have been found to have a lower risk of leukaemic relapse than those who do not (Parkman, et al 2006). Severe GVHD and the immunosuppressive therapies used to treat it may also inhibit thymopoiesis. While some reports have described faster recovery of total and CD4+ T-cells subsets and T-cell receptor excision circle (TREC) levels in the early post-transplant period following RTC vs. MAC in adult AlloSCT recipients (Chao, et al 2002, Jimenez, et al 2005, Schulenburg, et al 2005), other reports have not observed faster immune recovery following RTC AlloSCT (Maris, et al 2003). Such comparisons are further complicated by the diversity of immunosuppressive therapies employed in modern preparative regimens. Moreover, the effects of RTC prior to UCBT on immune reconstitution and risk of GVHD have not been compared to those of MAC in paediatric recipients. This study of 88 consecutive paediatric and adolescent UCBT recipients compared MAC and RTC recipients with respect to immune cell recovery and immunoglobulin reconstitution, incidence of post-transplant infections, probability of acute and chronic GVHD and survival outcomes.
Materials and methods
Eligibility
The sample consisted of 88 consecutive paediatric patients undergoing UCBT at Columbia University Medical Center (CUMC) between March 2000 and October 2008, with a cutoff date of January 2009 for analysis. Patients in this report were treated on one of several institutional protocols, based largely on their primary disease, with similar eligibility criteria for UCBT, UCB unit selection and handling procedures, GVHD prophylaxis, and supportive care. All research protocols incorporated assessments of GVHD and immune reconstitution as required observations, and a cohort of 88 consecutive patients undergoing MAC or RTC prior to UCBT was defined and analyzed retrospectively. All research protocols were approved by the CUMC Institutional Review Board (IRB) and were in compliance with the Declaration of Helsinki. All patients and parents were required to sign the CUMC IRB approved statements of informed consent and assent when applicable.
Patients ≤22 years of age with malignant and non-malignant diseases were eligible for an UCBT if they had no matched family donor or matched unrelated adult donor (9-10/10 HLA match), or if their disease status precluded waiting 2-3 months for a matched unrelated adult haematopoietic stem cell donor. Patients were eligible if they had a fully matched or a one or two antigen-mismatched unrelated cord blood unit with a minimum cryopreserved cell dose of ≥2 × 107 total nucleated cells/kg available. Patients were required to have a Lansky (≤16 years) or Karnofsky (>16 years) performance status score >50% prior to study entry.
Cord blood donor selection
HLA-A and HLA-B typing was performed by intermediate resolution molecular testing, and HLA-DRB1 typing was determined by hybridization of polymerase chain reaction (PCR)-amplified DNA with sequence specific oligonucleotide probes (Petersdorf, et al 1991). Confirmatory typing was performed at CUMC. Priority was given to select the most closely matched donor unit-recipient pair, and subsequently the unit with the largest nucleated cell dose was selected. Transplants were classified as HLA-mismatched with one or two differences if disparities were detected in HLA-A or HLA-B by low resolution typing, or in HLA-DRB1 by high resolution typing.
Conditioning regimens
The conditioning regimens were largely protocol-driven and disease-specific and consisted of both myeloablative conditioning (MAC) (n=49, 56%) and reduced toxicity conditioning (RTC) (n=39, 44%). Patients with co-morbid pre-transplant features pre-AlloSCT received only RTC regimens. Patients without co-morbid features received either a MAC or RTC regimen. Most MAC regimens consisted of total body irradiation (TBI, 1200 cGy) or busulfan (12.8 mg/kg in patients >4 years of age, 16 mg/kg in patients ≤4 years of age) in combination with melphalan (135 mg/m2) or cyclophosphamide as follows: TBI/melphalan (n=14), TBI/cyclophosphamide (n=9), busulfan/cyclophosphamide (n=19), busulfan/melphalan (n=2); other agents (n=5). Lung shielding was not used for TBI-containing regimens. Busulfan-containing conditioning regimens included busulfan pharmacokinetic studies, and were targeted to achieve 600-900 ng/ml steady state concentration. All RTC regimens were fludarabine-based (150-180 mg/m2) as follows: fludarabine/busulfan (12.8 mg/kg in patients >4 years of age, 16 mg/kg in patients ≤4 years of age) (n=30) and fludarabine/cyclophosphamide (n=9). Nearly all patients (n=80) received either anti-thymocyte globulin (ATG) or alemtuzumab as part of conditioning.
Cord blood thawing, nucleated cell count and CD34 enumeration
Following the method of Rubinstein et al. (1995) and the Cord Blood Transplantation Study (COBLT; (Cairo, et al 2005, Fraser, et al 1998, Kurtzberg, et al 2005), the cryopreserved UCB unit was placed in a sterile bag and then thawed in a 38°C waterbath with gentle agitation. After thawing, an equal volume of dextran/albumin solution was added over 10 min, centrifuged at 400g for 15 min at 10°C and the supernatant removed. The cell pellet was re-suspended in dextran/albumin and immediately infused into the patient over 30-60 min.
GVHD prophylaxis and grading
aGVHD prophylaxis consisted of tacrolimus and mycophenolate mofetil (MMF). Tacrolimus was administered starting at 0.03 mg/kg/day as continuous IV infusion or 0.12 mg/kg orally (PO) twice a day with dosage adjustment to maintain blood levels between 5 and 20 ng/ml starting on the first day of conditioning regimen or one day prior to transplant (day −1), as we have previously reported (Bhatia, et al 2010, Osunkwo, et al 2004). MMF was administered at 15-30 mg/kg q 6-12 hours either PO or IV starting the day following transplant (day +1), as we have previously described (Bhatia, et al 2010, Osunkwo, et al 2004). Tacrolimus and/or MMF were tapered if patients had ≤grade II aGVHD on day +30 for malignant diseases and day +180 for non-malignant diseases (Bhatia, et al 2010, Osunkwo, et al 2004). aGVHD and chronic GVHD (cGVHD) were graded according to Seattle consensus criteria (Glucksberg, et al 1974). All patients who achieved any level of donor chimerism were considered at risk for developing aGVHD. Only patients with sustained engraftment of donor haematopoiesis and surviving for more than 100 days after transplant were evaluated for the development of cGVHD.
Supportive care and infection prophylaxis
All patients received sargramostim (250 mcg/m2/day) IV daily from day 0 until the white blood cell count reached ≥0.3 × 109/l for two days and then were switched to filgrastim (10 μg/kg/day) either IV or subcutaneously until an absolute neutrophil count (ANC) ≥2.5 ×109/l was achieved for three days, as previously described (9). Herpes simplex virus (HSV) prophylaxis consisted of acyclovir (250 mg/m2) IV q 8 h from day −5 until engraftment and ≤grade II mucositis. Pneumocystis pneumonia (PCP) prophylaxis consisted of trimethoprim/sulfamethoxazole until day −2 and then resumed three times weekly after myeloid engraftment. Patients unable to tolerate trimethoprim/sulfamethoxazole received IV pentamidine prophylaxis every two weeks. Fungal prophylaxis consisted of liposomal amphotericin B (3 mg/kg/day) IV from day 0 to day +100 as previously described (Roman, et al 2008). Briefly, patients at risk of acquiring cytomegalovirus (CMV) infection (CMV-positive donor and/or recipient), after achieving an ANC >0.75 ×109/l following AlloSCT, received prophylaxis with foscarnet (90 mg/kg/dose) every other day alternating with gancyclovir (5 mg/kg/dose) every other day until day +100, as we have previously described (Shereck, et al 2007). Intravenous immune globulin (IVIG) 200 mg/kg was administered starting on day −1 and continued every three weeks until day +100. IVIG was discontinued on day +100 for patients with <grade II aGVHD. For patients with ≥grade II aGVHD on day +100, treatment was continued until the severity of aGVHD was <grade II. Patients with IgA deficiency were given IVIG products low in IgA. Patients that developed cGVHD or relapse of ≥grade II aGVHD resumed IVIG prophylaxis until severity of aGVHD was <grade II.
Engraftment and donor chimerism
Neutrophil recovery was defined as the first day the ANC was ≥0.5 ×109/l for three consecutive days following the neutrophil nadir. Platelet recovery was defined as the first day the platelet count was ≥20 ×109/l independent of platelet transfusions for at least seven consecutive days. Primary myeloid graft failure was defined as failure to achieve a donor-derived ANC ≥0.500 ×109/l by day +42 and/or ≤50% whole blood donor chimerism by day +60 in all except immune deficiency patients. In patients with T-cell or combined immune deficiency, primary graft failure was defined as ≤50% T-cell (CD3) donor chimerism by day +180. Donor myeloid and/or lymphoid chimerism was measured on days +30, 60, 100, 180 and 365 after transplant. The percentage of donor chimerism was determined by quantifying fluorescent-labelled PCR products from donor and recipient alleles at short tandem repeat loci, as recently described (Styczynski, et al 2011). Donor chimerism was determined for whole blood and cell subsets as required by the individual disease protocol. Cell subsets were isolated using Miltenyi magnetic particles. The purity of each subset was determined by flow cytometry.
Toxicity and definitions
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria version 2.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf) prior to 2003 and Common Terminology Criteria for Adverse Events version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf) after 2003. Patients with refractory malignant disease and/or in third complete response (CR3) or beyond were classified as poor risk. All other patients with malignant disease and all patients with non-malignant disease were considered to have average risk disease. Relapse was defined by morphological and/or radiological evidence of malignant disease in any site; time to relapse was measured as the time interval between UCBT and relapse, with censoring at death. Overall survival (OS) was the time between transplantation and death due to any cause, and was censored if patients were alive at the day of last follow-up.
Immune reconstitution
Immune cell recovery
Mononuclear cells (MNC) from patient samples were washed in phosphate-buffered saline supplemented with 1% heat activated fetal bovine serum (Sigma-Aldrich, St Louis, MO, USA) and 1% azide (Sigma-Aldrich). Cells were analysed as previously described (Ayello, et al 2006). Briefly, fluorescent-conjugated monoclonal antibodies (CD3, CD16, CD56, CD4, CD8, CD19, BD Biosciences, San Jose, CA, USA) were added to MNCs, incubated in the dark at 4° C for 20-30 min, washed in azide buffer and fixed with 0.5% paraformaldehyde (Sigma-Aldrich). Samples were analysed on a FACS Calibur cytometer (BD Biosciences) and then analysed using CellQuest software (BD Biosciences). The lymphocyte subpopulation was gated and used as a reference for the determination of CD3−CD16+CD56+ (natural killer [NK] cell), CD3+ (total T-cell), CD3+CD4+ (helper T-cell), CD3+CD8+ (cytolytic T-cell) and CD19+ (B-cell) subsets at days +100, +180 and +365 (±10 days). Each subset was then analysed for both its light scatter and forward side scatter characteristics and expression of the surface antigens recognized by the monoclonal antibodies mentioned above. Appropriate isotype controls were included, and ≥10,000 events were collected for each evaluation. Absolute immune cell subset counts for each patient were subsequently categorized as normal or low according to age-specific reference ranges provided by our institution’s laboratory.
Immunoglobulin level (IgG, IgA, IgM) reconstitution
Immunoglobulin levels (IgG, IgA and IgM) were measured using enzyme-linked immunosorbent assay (ELISA) at days +100, +180 and +365 (±10 days). Plasma or serum from patient samples was used and protein levels measured by quantitative ELISA according to the manufacturer’s kit instructions (R&D systems, Milwaukee, MN, USA; Raybiotec, Norcross, GA, USA). Samples were run in triplicate and data presented as mean ± standard deviation (SD). Immunoglobulin levels were assessed as being normal or low according to age-specific reference ranges as defined by Lockitch et al ( 1988).
Opportunistic systemic infections
Systemic viral infections (SVIs)
CMV Infection and CMV Disease
CMV infection was defined as isolation of the CMV virus and/or detection of viral nucleic acid by PCR in any body fluid or tissue specimen. PCR positivity for CMV was defined as ≥600 copies/ml. CMV systemic disease was defined as isolation of the CMV virus or detection of nucleic acid in any body fluid or tissue specimen and evidence of end-organ disease. Patients were stratified according to risk for CMV infection as low risk (donor and recipient CMV IgG negative), intermediate risk (donor CMV IgG positive and recipient CMV IgG negative), and high risk (recipient CMV IgG positive and donor CMV IgG positive or negative).
Adenovirus Infection
Patients were considered to have ADV infection at a specific site if they were culture-positive by tissue biopsy and/or PCR positive (≥100 copies/mL) for ADV with corresponding clinical signs and symptoms were considered to have ADV infection at that site. Disseminated ADV disease was defined as clinical evidence of ADV infection involving two or more organ sites. Laboratory investigations for adenovirus were pursued only in patients with corresponding clinical signs and symptoms.
Other Viral Infections
BK virus infection was defined as haemorrhagic cystitis with positive urine PCR. Human herpes virus-6 (HHV-6) infection was defined as a positive PCR (≥100 copies/ml) from blood and/or cerebrospinal fluid (CSF). BK virus and HHV-6 PCR studies were performed only on patients with corresponding clinical signs and symptoms. Upper respiratory illness was defined as the acute onset of rhinorrhea, sinusitis, pharyngitis or cough without clinical or radiological evidence of lower respiratory tract involvement and/or hypoxia, combined with detection of the virus in upper respiratory secretions. Lower respiratory illness was defined as clinical signs and symptoms of lower respiratory involvement with radiological evidence of new pulmonary infiltrates, with or without hypoxia, associated with the detection of the virus in nasal washes or bronchoalveolar lavage specimens (Ljungman, et al 2001).
Invasive fungal infections (IFIs)
Invasive fungal infections (IFI) were characterized as candidaemia, invasive aspergillosis, or other fungal infections. Invasive aspergillosis was defined as possible (clinical signs and symptoms plus a compatible thoracic computerized tomography scan or X-Ray), probable (clinical signs and symptoms, compatible X-ray findings, and a positive respiratory tract culture for Aspergillus spp. or positive galactomannan assay) and definite (positive histology for an invasive mould infection by Aspergillus) infections. Candida infection was defined as a positive fungal blood culture, urine culture or any organ system on radiographic evaluation or proven by biopsy.
Bacterial infection
Bacterial infection was defined as a blood, bronchoalveolar lavage, urine, or CSF culture positive for one or more bacteria in the presence of corresponding clinical signs and symptoms.
Statistical methods
Demographic and clinical characteristics of patients who received MAC vs. RTC were compared; the statistical significance of the differences was evaluated by means of chi-square or Fisher’s exact tests. Probabilities of neutrophil recovery, platelet recovery, ≥grade II aGVHD, cGVHD, day 100 TRM, and OS were estimated using the Kaplan-Meier product limit method (Kaplan & Meier 1958). Kaplan-Meier curves were compared between groups using log rank tests.
Continuous variables were summarized as mean ± standard deviation (SD) and categorical variables as percentages. Risk factors for abnormally low post-transplant absolute immune cell subset counts and immunoglobulin levels, day 100 TRM, and malignant relapse were assessed by univariate and multivariate logistic regression models. Multivariate Cox proportional hazards regression modelling was used to examine risk factors for ≥grade II aGVHD, cGVHD, and OS. Variables with p<0.2 in the univariate analyses for overall mortality were included in the multivariate analyses. A p-value less than 0.05 was considered significant.
Results
Demographics and clinical characteristics
The study sample consisted of 88 UCBT recipients (Table 1). Patients undergoing MAC differed significantly from those who received RTC with respect to their underlying disease (p<0.001), disease status (p<0.001), performance status prior to UCBT (p=0.04), and previous history of AutoSCT (p<0.001) but not HLA matching, pre-thaw total nucleated cells (TNC)/kg, or pre-thaw CD34 cell dose/kg. Disease status at the time of UCBT is summarized in Supplementary Table 1 for patients with malignancies.
Table 1.
Patient characteristics by conditioning regimen
| Myeloablative | Reduced Toxicity | Total | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | P-value | |
| N= | 49 | 55.7 | 39 | 44.3 | 88 | |
| Gender | 0.07 | |||||
| Male | 25 | 51.0 | 27 | 69.2 | 52 | |
| Female | 24 | 49.0 | 12 | 30.8 | 36 | |
| Mean age, years (SD) | 7.4 | (6.3) | 9.3 | (7.0) | 8.2 | 0.20 |
| Disease | <0.001 | |||||
| Acute lymphoblastic leukaemia | 18 | 36.7 | 0 | 0.0 | 18 | |
| Acute myeloid leukaemia | 13 | 26.5 | 2 | 5.1 | 15 | |
| Neuroblastoma | 1 | 2.0 | 10 | 25.6 | 11 | |
| Other malignancies | 2 | 4.1 | 12 | 30.8 | 14 | |
| Marrow failure | 6 | 12.2 | 3 | 7.7 | 9 | |
| Haemoglobinopathies | 3 | 6.1 | 5 | 12.8 | 8 | |
| Immunodeficiency diseases | 1 | 2.0 | 3 | 7.7 | 4 | |
| Other nonmalignant diseases | 5 | 10.2 | 4 | 10.3 | 9 | |
| Malignant disease | 0.29 | |||||
| No | 15 | 30.6 | 15 | 38.5 | 30 | |
| Yes | 34 | 69.4 | 24 | 61.5 | 58 | |
| Disease status | <0.001 | |||||
| Complete response | 29 | 59.2 | 8 | 20.5 | 37 | |
| Partial response | 0 | 0.0 | 7 | 17.9 | 7 | |
| Stable disease | 15 | 30.6 | 20 | 51.3 | 35 | |
| Progressive disease or relapse | 5 | 10.2 | 3 | 7.7 | 8 | |
| Other | 0 | 0.0 | 1 | 2.6 | 1 | |
| Performance status prior to UCBT | 0.04 | |||||
| 50-70 | 1 | 2.0 | 3 | 7.7 | 4 | |
| 80-90 | 11 | 22.4 | 9 | 23.1 | 20 | |
| 100 | 11 | 22.4 | 17 | 43.6 | 28 | |
| Not available | 26 | 53.1 | 10 | 25.6 | 36 | |
| Previous AutoSCT | <0.001 | |||||
| No | 49 | 100.0 | 26 | 66.7 | 75 | |
| Yes | 0 | 0.0 | 13 | 33.3 | 13 | |
| CMV donor/recipient | 0.33 | |||||
| −/− | 11 | 22.4 | 13 | 33.3 | 24 | |
| All other | 38 | 77.6 | 26 | 66.7 | 64 | |
| HLA match | 0.30 | |||||
| 4/6 | 22 | 44.9 | 24 | 61.5 | 46 | |
| 5/6 | 16 | 32.7 | 9 | 23.1 | 25 | |
| 6/6 | 11 | 22.4 | 6 | 15.4 | 17 | |
| N= | 49 | 55.7 | 39 | 44.3 | 88 | |
| Related donor | 0.40 | |||||
| No | 47 | 95.9 | 35 | 89.7 | 82 | |
| Yes | 2 | 4.1 | 4 | 10.3 | 6 | |
| Risk | 0.48 | |||||
| Average | 34 | 69.4 | 30 | 76.9 | 64 | |
| Poor | 15 | 30.6 | 9 | 23.1 | 24 | |
| ATG/Alemtuzumab | 0.08 | |||||
| ATG | 38 | 77.6 | 31 | 79.5 | 69 | |
| Alemtuzumab | 4 | 8.2 | 7 | 17.9 | 11 | |
| Neither | 7 | 14.3 | 1 | 2.6 | 8 | |
| CD34 weight-adjusted dose | ||||||
| Median (range) | 2.1 | (0.4-9.3) | 2.1 | (0.3-9.6) | 2.1 | 1.00 |
| TNC weight-adjusted dose | ||||||
| Median (range) | 4.0 | (0.9-17.3) | 3.6 | (0.9-22.6) | 3.8 | 0.39 |
| Time period | 1.00 | |||||
| Before 1/1/05 | 28 | 57.1 | 22 | 56.4 | 50 | |
| After 12/31/04 | 21 | 42.9 | 17 | 43.6 | 38 | |
| Conditioning | <0.001 | |||||
| Total body irradiation | 23 | 46.9 | 0 | 0.0 | 23 | |
| Busulfan/fludarabine | 1 | 2.0 | 30 | 76.9 | 31 | |
| Other | 25 | 51.0 | 9 | 23.1 | 34 | |
SD, standard deviation, UCBT, umbilical cord blood transplantation; AutoSCT, autologous stem cell transplantation; CMV, cytomegalovirus; HLA, human leucocyte antigen; ATG, antithymocyte globulin; TNC, total nucleated cells
Engraftment and donor chimerism
Nine patients (4 MAC and 5 RTC) experienced primary graft failure and were excluded from evaluation of engraftment, whole blood chimerism, and probability of aGVHD and cGVHD. In the patients who engrafted, the median time intervals until neutrophil and platelet engraftment were 29 and 53 days, respectively, in the RTC group and 24 and 118 days, respectively in the MAC group (p=NS). The MAC and RTC groups did not differ significantly with respect to mean ± SD whole blood donor chimerism at days 30, 60, 100 and 365 post-transplant: RTC, 68.0 ± 37.6, 79.4 ± 30.7, 88.8 ± 21.7, 83;1 ± 27.9, respectively; MAC, 72.3 ± 39.2, 83.4 ± 31.0, 89.9 ± 17.0, 85.0 ± 26.8, respectively (p=NS). The MAC and. RTC groups also did not differ significantly with respect to engraftment and percentage of donor chimerism.
Immune reconstitution
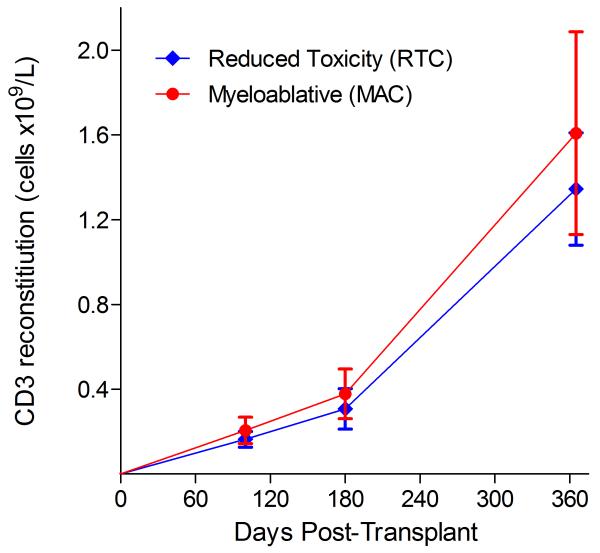
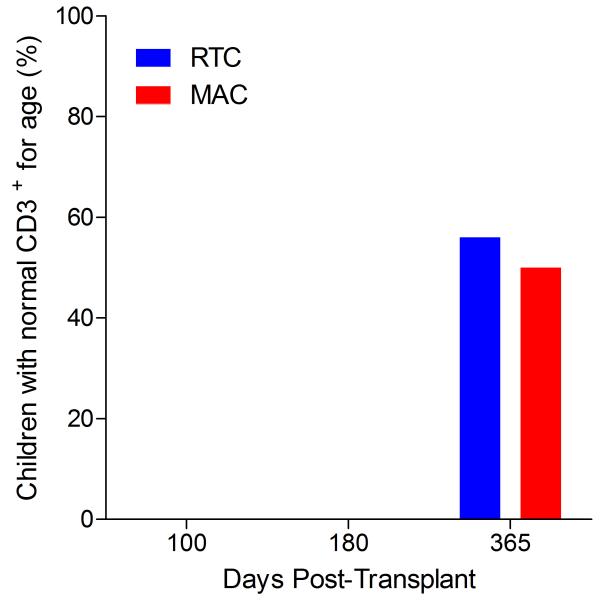
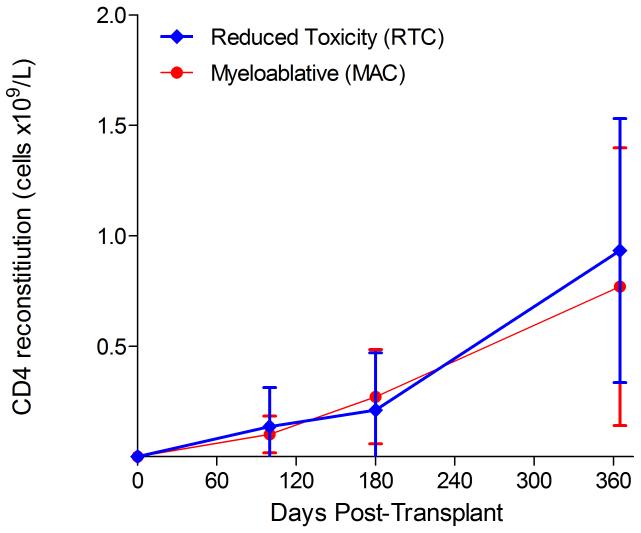
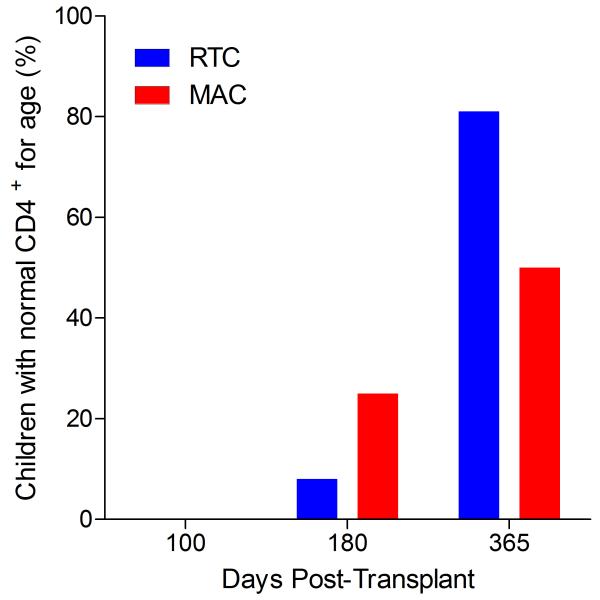
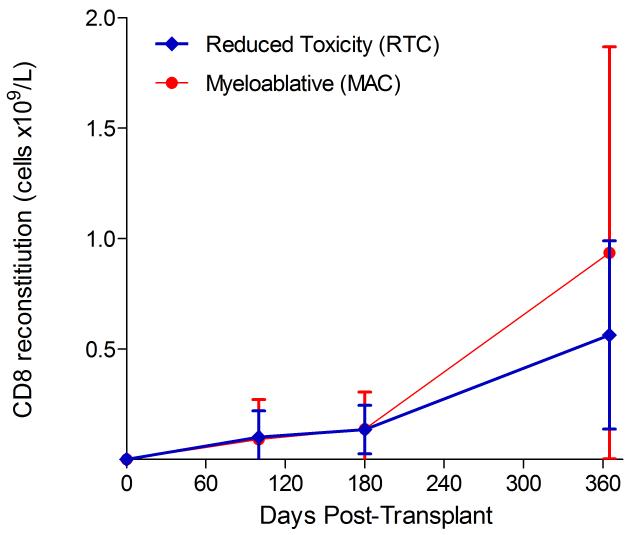
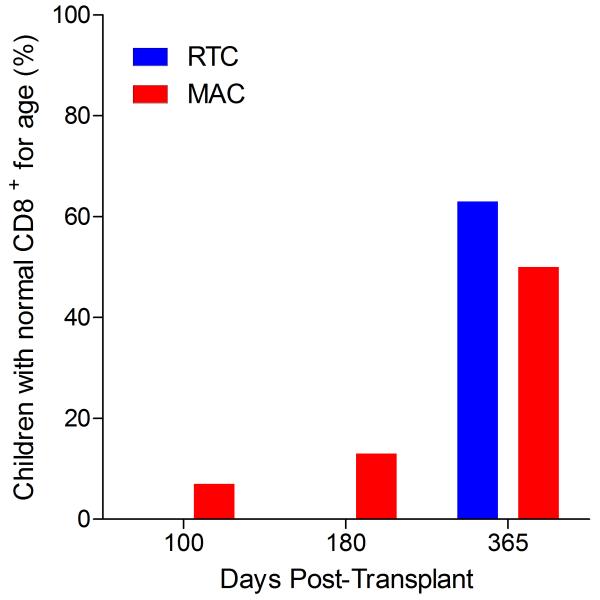
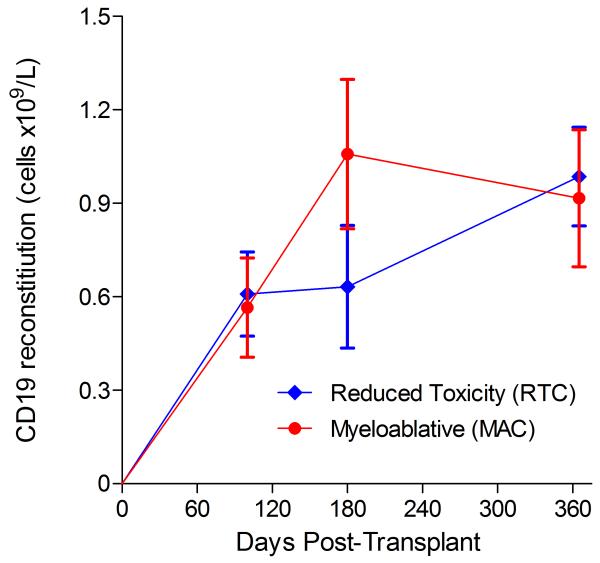
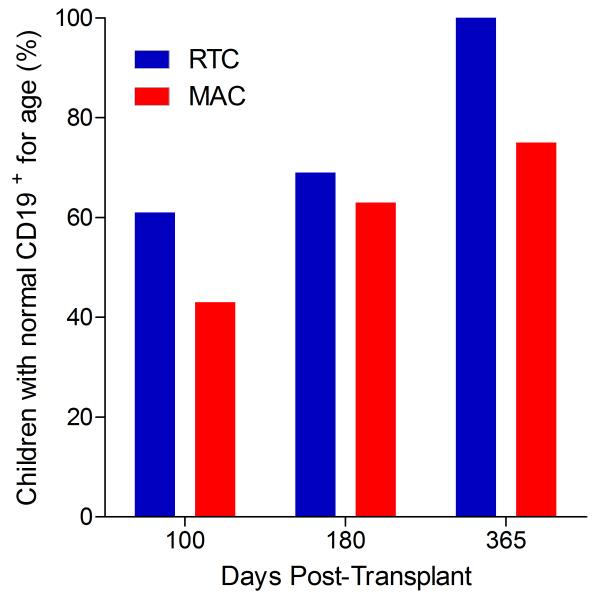
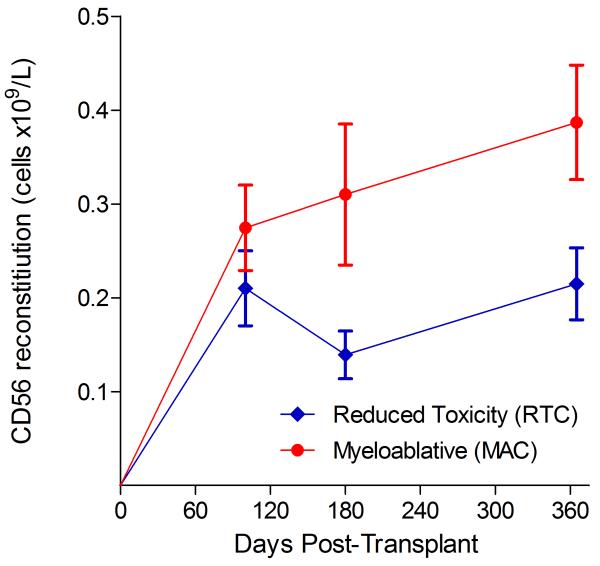
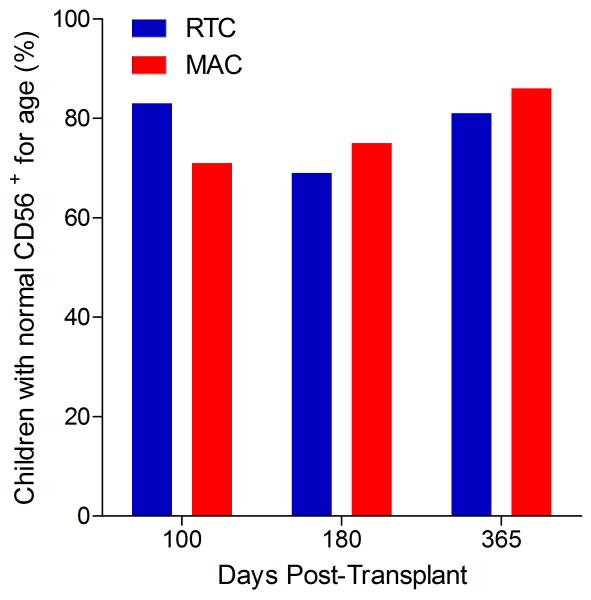
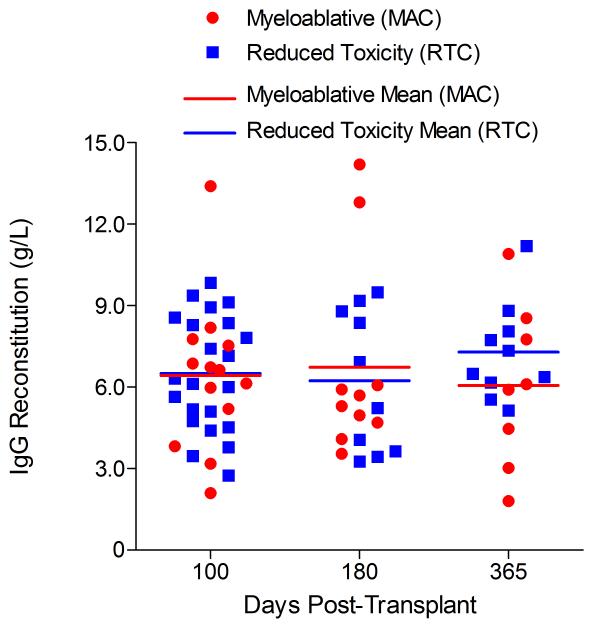
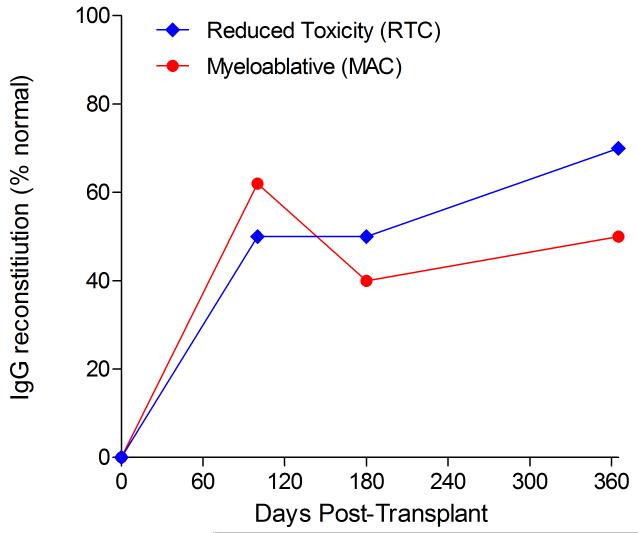
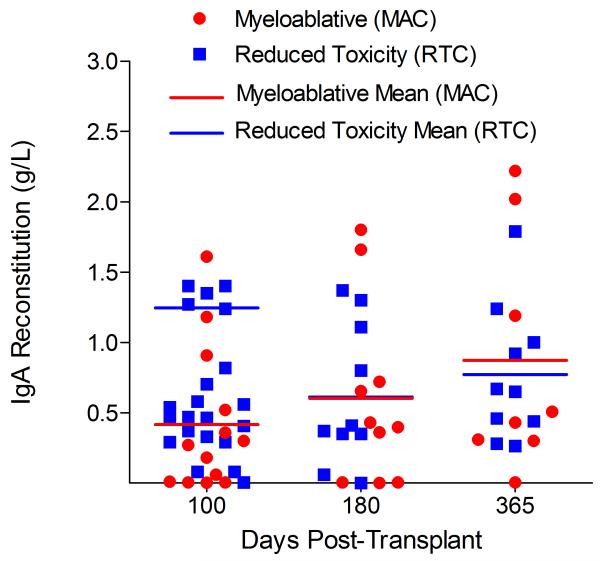
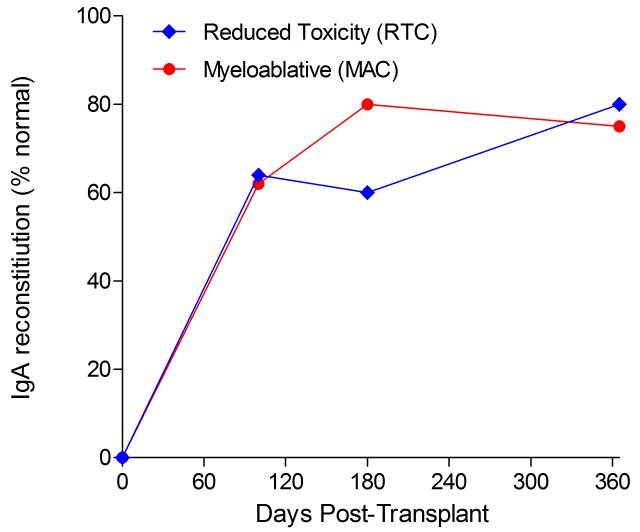
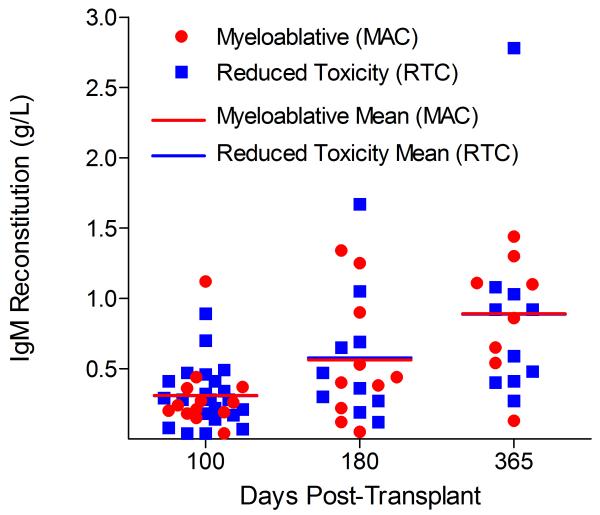
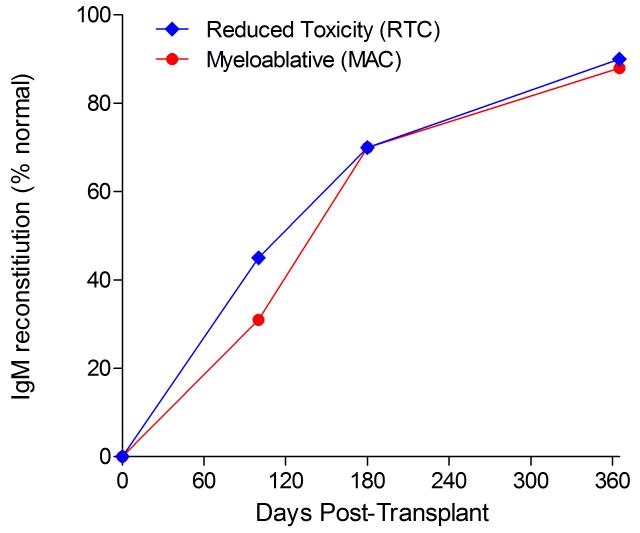
Immunophenotypic analysis of T-lymphocyte (CD3+, CD3+CD4+, CD3+CD8+), B-lymphocyte (CD19+) and NK-cell (CD3−CD56+) reconstitution at days +100, +180 and +365 is presented as mean immune cell subset counts (× 109/l) and percentages of children achieving normal immune cell subset counts (Figs. 1 and 2). Recovery of immunoglobulin levels (g/l) at days +100, +180 and +365 is depicted similarly (Fig. 3). At day 180, NK-cell levels were slightly higher in the MAC vs. RTC group (0.31±0.212 × 109 vs. 0.171±0.083 × 109 cells/l, p=0.05). Otherwise, absolute immune cell subset recovery, absolute immunoglobulin reconstitution, and percentages of children with normal immune cell subset counts and immunoglobulin levels, did not differ significantly with respect to conditioning regimen. Variables not significantly associated with absolute immune cell susbset counts, absolute immunoglobulin reconstitution, or achievement of age-specific normal immune cell subset recovery or immunoglobulin reconstitution included grade II-IV aGVHD status, ATG/alemtuzumab in the conditioning regimen, SVIs, IFIs, TNC dose/kg, and CD34 dose/kg.
Figure 1.
T-cell immune reconstitution by mean (±SD) absolute cell counts (× 109/l) and percentages of children achieving normal lymphocyte counts at days 100, 180 and 365 post-transplant for CD3+ (A&B), CD3+CD4+ (C&D) and CD3+CD8+ cells (E&F).
Figure 2.
B-lymphocyte and NK-cell reconstitution by mean (±SD) absolute cell counts (× 109/l) and percentages of children achieving normal counts at days 100, 180 and 365 post-transplant for CD19+ (A&B) and CD3−CD56+ cells (C&D).
Figure 3.
Immunoglobulin reconstitution by absolute immunoglobulin subset levels (g/l) and percentages of children achieving normal immunoglobulin subset levels at days 100, 180 and 365 post-transplant for IgG (A&B), IgA (C&D) and IgM (E&F).
Opportunistic infections
Bacterial infections, SVIs, and IFIs among patients receiving MAC vs. RTC are summarized in Table 2. The MAC and RTC groups did not differ significantly in incidence of opportunistic infections.
Table 2.
Infections by conditioning regimen
| Myeloablative | Reduced Toxicity | Total | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | P-value | |
| N= | 49 | 55.7 | 39 | 44.3 | 88 | |
| Viral infection | 0.16 | |||||
| None | 31 | 63.3 | 24 | 61.5 | 55 | |
| Respiratory syncytial virus (RSV) | 2 | 4.1 | 6 | 15.4 | 8 | |
| Influenza | 2 | 4.1 | 5 | 12.8 | 7 | |
| Adenovirus | 2 | 4.1 | 1 | 2.6 | 3 | |
| Human herpesvirus 6 (HHV6) | 1 | 2.0 | 0 | 0.0 | 1 | |
| Cytomegalovirus (CMV) | 6 | 12.2 | 1 | 2.6 | 7 | |
| >1 viral infection* | 5 | 10.2 | 2 | 5.1 | 7 | |
| Bacterial infection | 0.42 | |||||
| None | 38 | 77.6 | 34 | 87.2 | 72 | |
| Mycobacterium avium complex | 1 | 2.0 | 0 | 0.0 | 1 | |
| Escherichia coli | 1 | 2.0 | 0 | 0.0 | 1 | |
| Klebsiella pneumoniae | 2 | 4.1 | 2 | 5.1 | 4 | |
| Acinetobacter baumannii | 1 | 2.0 | 0 | 0.0 | 1 | |
| Enterobacter cloacae | 3 | 6.1 | 0 | 0.0 | 3 | |
| Staphylococcus epidermidis | 3 | 6.1 | 1 | 2.6 | 4 | |
| Staphylococcus aureus (MSSA) | 0 | 0.0 | 1 | 2.6 | 1 | |
| Acinetobacter lwoffi | 0 | 0.0 | 1 | 2.6 | 1 | |
| Fungal infection | 0.48 | |||||
| None | 43 | 87.8 | 36 | 92.3 | 79 | |
| Aspergillus flavus | 1 | 2.0 | 0 | 0.0 | 1 | |
| Aspergillus fumigatus | 1 | 2.0 | 0 | 0.0 | 1 | |
| Aspergillus (spp. unknown) | 1 | 2.0 | 0 | 0.0 | 1 | |
| Candida kefyr and Candida parapsilosis | 1 | 2.0 | 0 | 0.0 | 1 | |
| Candida lusitaniae | 1 | 2.0 | 0 | 0.0 | 1 | |
| Candida tropicalis | 1 | 2.0 | 0 | 0.0 | 1 | |
| Candida krusei | 0 | 0.0 | 1 | 2.6 | 1 | |
| Candida glabrata | 0 | 0.0 | 1 | 2.6 | 1 | |
| Candida guilliermondii | 0 | 0.0 | 1 | 2.6 | 1 | |
In the myeloablative group: 1 RSV+adenovirus, 1 influenza+adenovirus, 1 influenza+HHV6, 1 influenza+Epstein Barr virus, 1 adenovirus+HHV6; in the reduced toxicity group: 1 RSV+CMV, 1 CMV+adenovirus+influenza.
Acute and chronic GVHD
After excluding the nine patients who experienced primary graft failure, the probability of grade II-IV aGVHD in the entire cohort was 32.2% (95% confidence interval [CI95]: 17.7-47.7). There was a significant difference in the incidence of grade II-IV aGVHD in paediatric recipients following RTC vs. MAC and UCBT (RTC: 16.9% [CI95: 1.4-47.9], MAC: 44.4% [CI95: 26.4-61.0]), (p=0.017). The probability of cGVHD in the entire cohort was 16.6% (CI95: 2.1-41.4) and did not differ significantly with respect to MAC vs. RTC prior to UCBT.
In a Cox proportional hazards regression model (excluding patients with primary graft failure) that included conditioning regimen, risk (average vs. poor), HLA-match (4/6 vs. 5-6/6), CMV status (donor/recipient −/− vs. other), time period (before 2005 vs. after 2004), and TNC dose × 107/kg, MAC recipients had a significantly higher risk of grade II-IV aGVHD (Hazard Ratio [HR] 6.1 [CI95: 2.0-18.8]) (p=0.002) than RTC recipients. Those receiving a 4/6 HLA-matched cord blood unit also had a significantly higher risk of grade II-IV aGVHD than those who received a 5/6 or 6/6 HLA-matched graft (HR 3.0 [CI95: 1.1-8.6]) (p=0.03) (Table 3).
Table 3.
Cox proportional hazards regression models of factors associated with ≥grade II acute GVHD*
| Unadjusted | Multivariable** | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Conditioning regimen | 0.02 | 0.002 | ||||
| Reduced Toxicity | 1.0 | Referent | 1.0 | Referent | ||
| Myeloablative | 3.3 | 1.2-9.0 | 6.1 | 2.0-18.8 | ||
| Risk | 0.59 | 0.43 | ||||
| Average | 1.0 | Referent | 1.0 | Referent | ||
| Poor | 0.7 | 0.3-2.2 | 0.4 | 0.2-2.0 | ||
| HLA matching | 0.06 | 0.03 | ||||
| 5/6+6/6 | 1.0 | Referent | 1.0 | Referent | ||
| 4/6 | 2.3 | 1.0-5.5 | 3.1 | 1.1-8.6 | ||
| CMV status | 0.55 | 0.76 | ||||
| −/− | 1.0 | Referent | 1.0 | Referent | ||
| All other | 0.8 | 0.3-1.9 | 0.9 | 0.3-2.4 | ||
| Time period | 0.22 | 0.21 | ||||
| Before 1/1/05 | 1.0 | Referent | 1.0 | Referent | ||
| After 12/31/04 | 0.6 | 0.2-1.4 | 0.5 | 0.2-1.4 | ||
| TNC (continuous) | 0.9 | 0.8-1.1 | 0.34 | 1.0 | 0.9-1.2 | 0.78 |
Patients with primary graft failure excluded.
Hazard ratios for each variable are adjusted for all other variables shown.
CMV, cytomegalovirus; HLA, human leucocyte antigen; TNC, total nucleated cells.
In a similar Cox proportional hazards regression model analysing predictors of chronic GVHD in those paediatric UCBT recipients who survived to day 100 post-transplant and did not suffer graft failure, patients receiving a 4/6 HLA-matched cord blood unit had a markedly higher risk of cGVHD (HR 18.5 [CI95: 1.3-274.8]) (p=0.04) vs. those with 5/6 or 6/6 HLA matches (Table 4).
Table 4.
Cox proportional hazards regression models of factors associated with chronic GVHD*
| Unadjusted | Multivariable** | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Conditioning regimen | 0.32 | 0.10 | ||||
| Reduced Toxicity | 1.0 | Referent | 1.0 | Referent | ||
| Myeloablative | 2.1 | 0.5-8.6 | 3.6 | 0.8-17.1 | ||
| Risk | 0.41 | 0.24 | ||||
| Average | 1.0 | Referent | 1.0 | Referent | ||
| Poor | 2.0 | 0.4-10.2 | 3.6 | 0.4-31.2 | ||
| HLA matching | 0.04 | 0.04 | ||||
| 5/6+6/6 | 1.0 | Referent | 1.0 | Referent | ||
| 4/6 | 8.9 | 1.1-72.8 | 18.5 | 1.3-274.8 | ||
| CMV status | 0.29 | 0.93 | ||||
| −/− | 1.0 | Referent | 1.0 | Referent | ||
| All other | 0.5 | 0.1-1.9 | 0.9 | 0.2-5.5 | ||
| Time period | 0.72 | 0.06 | ||||
| Before 1/1/05 | 1.0 | Referent | 1.0 | Referent | ||
| After 12/31/04 | 1.3 | 0.3-5.2 | 5.4 | 0.9-30.7 | ||
| TNC (continuous) | 0.8 | 0.6-1.1 | 0.18 | 0.8 | 0.5-1.3 | 0.4 |
Patients with primary graft failure or surviving <100 days excluded.
Hazard ratios for each variable are adjusted for all other variables shown.
CMV, cytomegalovirus; HLA, human leucocyte antigen; TNC, total nucleated cells.
Malignant relapse
Among the 47 patients in the cohort with haematological malignancies, the probability of malignant relapse was 38.3% (95% CI: 24.5-53.6). The RTC group (42.9%) and MAC group (36.3%) did not differ significantly in incidence of malignant relapse (p=0.75 [NS]). Other variables not significantly predictive of relapse in this sample included gender, HLA matching, conditioning regimen composition, and primary graft failure. Failure to achieve normal T-cell, B-cell, or NK-cell counts at days +100, +180, and +365 was not predictive of relapse in patients with haematological malignancies.
Toxicities
In univariate analysis, the MAC group had a higher risk of grade III and IV pulmonary toxicity definitely, probably, or possibly related to conditioning than the RTC group (27% vs. 0%) (p=0.0004). All such pulmonary toxicities occurred in the MAC group and were grade IV, representing the need for intubation/mechanical ventilation in the setting of respiratory distress/dyspnea (n=13), with most of these patients having signs of pneumonitis/pulmonary infiltrates (n=12) and eventual acute respiratory distress syndrome (n=10). Marginal elevation in the risk of grade III-IV renal toxicity definitely, probably, or possibly related to conditioning was also observed in patients receiving MAC vs. RTC (14% vs. 3%) (p=0.07). All such renal toxicities were grade III elevations in the serum creatinine. Similarly, the proportion of patients with grade III-IV hepatic toxicity definitely, probably, or possibly related to conditioning was marginally higher among patients receiving MAC vs. RTC (37% vs. 18%) (p=0.06). Several patients experienced multiple hepatic toxicities; in the MAC group, hepatic toxicities included grade III aspartate transaminase (AST) elevations (n=6), grade IV AST elevations (n=2), grade III ALT elevations (n=9), grade IV ALT elevations (n=3), grade III hyperbilirubinaemia (n=6), and grade IV hyperbilirubinaemia (n=2); in the RTC group, hepatic toxicities included grade III AST elevations (n=1), grade IV AST elevations (n=2), grade III ALT elevations (n=5), and grade III hyperbilirubinaemia (n=3).
Transplant-related mortality
The probability of day 100 TRM in the entire cohort was 18.0% (CI95: 5.8-35.7). The probability of day 100 TRM following RTC was 2.6% (CI95: 0-59.9) vs. MAC: 29.3% (CI95: 12.4-48.5), (p=0.0026). In the univariate analysis, the only risk factor associated with a significant increase in day 100 TRM was MAC vs. RTC (Odds Ratio [OR]: 15.200 [CI95: 1.899-121.676]) (p=0.0103). Similarly, in the multivariate analysis the only risk factor associated with a significant increase in day 100 TRM was MAC vs. RTC (OR: 26.752 [CI95: 2.352-304.246]) (p=0.0081). When patients with acute lymphoblastic leukamia (ALL) and acute myeloid leukaemia (AML) were excluded, MAC vs. RTC was the strongest predictor of day 100 TRM in the univariate analysis (OR: 5.576 [CI95: 0.954-32.584]) (p=0.0564) and was even stronger in the multivariate analysis (OR: 9.985 [CI95: 1.059-94.165]) (p=0.045); older age was also associated with increased risk in the multivariate analysis (OR: 1.257 [CI95: 1.021-1.548]) (p=0.0312). As immune cell recovery and immunoglobulin reconstitution were not regularly measured prior to day +100, the impact of early immune reconstitution on day 100 TRM was not assessed.
Overall survival
The probability of OS in the entire cohort was 41.2% (CI95: 30.1-51.9) at 5 years of follow-up. The probability of 5-year OS following RTC was 58.3% (CI95: 40.0-72.8) vs. MAC: 26.7% (CI95: 14.4-40.7), (p=0.0037). In the univariate analysis, the risk factors associated with decreased OS included older age (HR: 1.052 [CI95: 1.009-1.097]) (p=0.0179), MAC vs. RTC (HR: 2.434 [CI95: 1.322-4.481]) (p=0.0043), poor vs. average risk disease (HR: 2.907 [CI95: 1.627-5.195]) (p=0.0003), CD34/kg × 105 <2.3 vs. ≥2.3 (HR: 1.962 [CI95: 1.079-3.568]) (p=0.0271), malignant vs. non-malignant disease (HR: 2.493 [CI95: 1.242-5.003]) (p=0.0102) and poor performance status pre-UCBT (HR: 0.963 [CI95: 0.931-0.996]) (p=0.0271). In the multivariate analysis the risk factors associated with a significant decrease in OS included older age (HR: 1.141 [CI95: 1.064-1.223]) (p=0.0002), MAC vs. RTC (HR: 4.134 [CI95: 2.016-8.477]) (p=0.0001) and CD34/kg × 105 <2.3 vs.≥ 2.3 (HR: 3.538 [CI95: 1.698-7.375]) (p=0.0007). When patients with ALL and AML were excluded, MAC vs. RTC remained a significant predictor of overall mortality in the multivariate analysis (HR: 3.116 [CI95: 1.029-9.433]) (p=0.0443), in addition to poor vs. average risk disease (HR: 4.240 [CI95: 1.636-10.986]) (p=0.0029). In the subset of 30 patients with non-malignant diseases, MAC vs. RTC was also strongly associated with overall mortality (HR: 4.116 [CI95: 1.179-14.38]) (p=0.0266). Failure to achieve normal T-cell, B-cell, or NK-cell recovery at days +100, +180, and +365 was not predictive of overall survival.
Causes of death
At the end of follow-up, 15 of 49 (30.6%) patients undergoing MAC prior to UCBT and 24 of 39 (61.5%) patients undergoing RTC were alive. Of the 34 deaths in the MAC group, 14 (41%) were due to multiple organ system failure, 12 (35%) to progressive disease, and 3 (9%) to aGVHD. Of the 15 deaths in the RTC group, 12 (80%) were attributable to progressive disease, 2 to aspergillosis (13%), and 1 (7%) to CMV pneumonitis.
Discussion
UCBT has been increasingly employed in paediatric patients and now accounts for approximately 40% of unrelated donor stem cell transplants in patients ≤20 years of age (Pasquini & Wang 2009). RTC regimens offer the potential to decrease TRM, but the effect of RTC vs. MAC followed by UCBT on the tempo of immune reconstitution and risk of aGVHD and cGVHD has not been reported. In the present series of 88 paediatric UCBT recipients, we found no significant difference in absolute T-, B-, and NK-cell counts or immunoglobulin reconstitution between recipients of RTC and recipients of MAC at days +100, +180, and +365, and no significant association between immune cell recovery subset at these time points and malignant relapse or overall mortality. Only a few patients achieved age-specific T-lymphocyte counts above the lower limit of normal within the first 180 days post-transplant. However, B- and NK-cell recovery was considerably more rapid. In Cox proportional hazards regression models, recipients of RTC had lower risk of grade II-IV aGVHD, as well as lower TRM and overall mortality than recipients of MAC. The incidence of opportunistic infections was not associated with intensity of conditioning.
Several studies have noted higher rates of infections and TRM, particularly in the first 100 days, following UCBT than following stem cell transplantation from other sources, suggesting that UCBT recipients may experience unique deficiencies in immunity (Eapen, et al 2006, Eapen, et al 2007, Kurtzberg, et al 1996, Kurtzberg, et al 2008, Prasad, et al 2008, Rocha, et al 2001, Rubinstein, et al 1998, Szabolcs & Cairo 2010, Szabolcs & Niedzwiecki 2007, Wagner, et al 2002, Wagner, et al 1996). Large registry series from the Center for International Blood and Marrow Transplant Research (CIBMTR) have also noted that paediatric recipients of UCBT have higher TRM than matched unrelated donor bone marrow transplant (MUD BMT) recipients, presumably due to slower myeloid recovery and higher rates of infection-related deaths (Eapen, et al 2006, Eapen, et al 2007). Similarly, Laughlin et al. (2004) noted a higher proportion of infection-related deaths within the first 100 days post-transplant in adults with leukaemia who received UCBT than among those who received matched or mismatched unrelated donor BMT. However, after 100 days the groups did not differ in risk of infection-related death (Laughlin, et al 2004). Hamza et al. (2004) noted that UCBT recipients had higher risks of bacterial infection than MUD BMT recipients during the first 50 days post-transplant but similar risks of bacterial, viral, and fungal infections at later time points. Other reports have found similar risk of infection among paediatric UCBT and T-cell-replete unrelated donor BMT recipients (Barker, et al 2005). Although some studies have suggested that UCBT recipients are comparable to BMT recipients in immune cell recovery (Moretta, et al 2001), others have suggested that UCBT recipients have delayed immune reconstitution, particularly in the CD3+CD8+ compartment (Thomson, et al 2000). NK- and B-cell recovery is relatively rapid following UCBT, with a median time of reconstitution of approximately 3 months and approximately 6 months post-transplant needed to achieve age-specific normal levels, respectively. However, the median time needed to achieve normal numbers of cytolytic T-cells (CTLs) among UCBT recipients is approximately 8-9 months, compared to 1-3 months following transplantation from other stem cell sources. The median time to achieve normal total and helper T-cell recovery is longer still, approaching 12 months in some series (Komanduri, et al 2007, Niehues, et al 2001, Szabolcs & Cairo 2010, Thomson, et al 2000). T-cell recovery following AlloSCT derives from antigen-driven peripheral expansion of mature, post-thymic donor cells (the thymic-independent pathway) and pre-thymic cells that must undergo differentiation in the host (the thymic-dependent pathway) (Crooks, et al 2006). As very few mature donor lymphocytes are transferred in UCBT, less robust thymic-independent T-cell proliferation may be expected compared to related or unrelated BMT (Cairo, et al 2005, Niehues, et al 2001). However, at 2 years follow-up, T-cell receptor (TCR) diversity is greater among UCBT recipients than among recipients of matched sibling donor BMT, as measured by TCR excision circles (Talvensaari, et al 2002). Parkman et al. (2006) noted an association between successful immune reconstitution following UCBT in children and decreased risk of leukaemic relapse and improved relapse-free survival. Specifically, robust antigen-specific T-cell proliferation in response to CMV, HSV or varicella zoster virus was a strong predictor of relapse-free survival. In addition, those without robust T-cell responses were more likely to die of infectious causes (Parkman, et al 2006). In the present series, the benefit of RTC vs. MAC with respect to TRM and overall mortality was not associated with increased velocity of cellular or humoral immune reconstitution as assessed by immune cell recovery and immunoglobulin reconstitution, respectively. Alemtuzumab was a component of several conditioning regimens employed and may persist at cytotoxic concentrations for months following AlloSCT. Previous studies of alemtuzumab-containing regimens suggest the incidence of infection may have been heightened and immune cell recovery further delayed in the subgroup of patients in our sample receiving alemtuzumab (Chakraverty, et al 2010, Morris, et al 2003). In addition, one third of RTC UCBT recipients had undergone prior MAC AutoSCT, which may also have contributed to a delay in immune reconstitution.
The shorter duration of profound neutropenia and less severe mucositis following RTC than following MAC AlloSCT suggest that RTC may carry lower risk of infection in the early post-transplant period (McSweeney, et al 2001). In addition, the persistence of host T-cells following non-myeloablative conditioning regimens provides theoretical protection against opportunistic infection prior to immune reconstitution via donor haematopoiesis. Several reports have noted that RTC recipients have lower risks of bacterial, viral, and fungal infection than MAC recipients in the first 30-100 days following AlloSCT (Junghanss, et al 2002, Maris, et al 2003, Schulenburg, et al 2005). Junghanss et al. (2002) observed significantly decreased incidence of CMV viraemia and disease following RTC vs. MAC during the first 100 days post-transplant. However, later CMV disease was more common among recipients of RTC, possibly because of greater immune suppression in these patients (Junghanss, et al 2002). Other reports have similarly demonstrated decreases in high-grade CMV infection rates among seropositive AlloSCT recipients within the first 100 days post-transplant following RTC vs. MAC, with similar or slightly higher risk of late CMV disease (Nakamae, et al 2009). We recently reported similar risk of systemic viral infection and higher risk of invasive fungal infection following RTC vs. MAC followed by AlloSCT in paediatric recipients, although prior MAC autologous transplantation in RTC AlloSCT recipients may have increased the underlying risk of infection in this patient subset (Satwani, et al 2009). Similarly, in the present series, RTC vs. MAC prior to UCBT was not associated with the incidence of bacterial, viral, or fungal infections post-UCBT.
Unrelated donor bone marrow and peripheral blood stem cell transplantation confers a high risk of aGVHD and cGVHD, which increases with greater HLA disparity (Anasetti & Hansen 1994). Although randomized trials comparing outcomes of unrelated bone marrow transplantation (UBMT) to UCBT are lacking, many large retrospective registry-based studies have observed significantly lower incidence of grade II-IV aGVHD following UCBT than following UBMT. Among adult patients, Laughlin et al. (2004) reported lower risk of grade II-IV aGVHD among mismatched UCBT recipients (41%) than among mismatched UBMT recipients (52%). Among adults with acute leukaemia, Rocha et al. (2004) noted lower cumulative incidence of grade II-IV aGVHD following mostly one or two antigen-mismatched UCBT (26%) than following fully matched UBMT (39%). Eapen et al. (2007) reported a reduced incidence of grade II-IV aGVHD among paediatric recipients of matched UCBT (24%) compared to matched UBMT (46%) or mismatched UBMT (60%). Most recently, Barker et al. (2010) presented a retrospective study of 1061 UCBT recipients, mostly children and adolescents, which noted increasing HLA disparity to be an independent predictor of greater risk of grade III-IV aGVHD and, when considering 3/6 HLA matched UCBT recipients, of cGVHD as well. The probability of grade II-IV aGVHD in our study was 32.2%, comparable to other paediatric UCBT series, which have reported the incidence of grade II-IV aGVHD to be approximately 25-45% (Kurtzberg, et al 2008, Martin, et al 2006, Michel, et al 2003, Prasad, et al 2008).
In the present study, MAC vs. RTC prior to UCBT was a significant independent predictor of grade II-IV aGVHD. Several reports have noted decreased risk of grade II-IV aGVHD following nonmyeloablative vs. myeloablative conditioning prior to AlloSCT using bone marrow or peripheral blood (Couriel, et al 2004, Mielcarek, et al 2003). Our group previously reported a low incidence of grade II-IV aGVHD among a small group of paediatric patients receiving RTC and UCBT (Bradley, et al 2007). The incidence of aGVHD was similarly low among a very small group of paediatric recipients of RTC and UCBT in a Paediatric Blood and Marrow Transplant Consortium study (Pulsipher, et al 2009). Data from adult UCBT recipients have not demonstrated a clear reduction in aGVHD risk associated with RTC, though the use of double UCBT, which is itself a risk factor for aGVHD, may be partially responsible for these findings (Ballen, et al 2007, Brunstein, et al 2007, MacMillan, et al 2009, Majhail, et al 2006, Yuji, et al 2005).
In patients with malignant disease, the use of less intensive preparative regimens prior to AlloSCT implies increased reliance on the donor’s immune cells (through allogeneic graft-versus-cancer effects) rather than on the conditioning regimen itself for disease eradication. Rodriguez et al. (2006) noted higher risk of relapse in adults with non-Hodgkin lymphoma following RTC vs. MAC prior to AlloSCT. Most recently, a report from the University of Minnesota noted significantly higher 3-year risk of relapse among adults with AML receiving RTC (44%) than among those receiving MAC (9%) prior to UCBT (Oran, et al 2011). The Paediatric Blood and Marrow Transplant Consortium reported 43% cumulative incidence of in 47 paediatric patients with haematological malignancies following RTC and AlloSCT, similar to the 43% risk of relapse observed in the present study (Pulsipher, et al 2009). Although we did not find an association between intensity of conditioning and the risk of relapse, our study lacked power to detect a moderate but clinically significant difference. In addition, we were unable to detect a significant association between malignant relapse and failure to normalize T-cell, B-cell, or NK-cell reconstitution at any of the study time points.
We observed robust early reconstitution of NK-cells and B-cells but delayed T-cell recovery among UCBT recipients, regardless of conditioning intensity. The high incidence of TRM observed following UCBT is attributable in part to increased risk of life-threatening viral infection, implicating deficiencies in T-cell mediated immunity (Eapen, et al 2006, Eapen, et al 2007, Kurtzberg, et al 1996, Kurtzberg, et al 2008, Prasad, et al 2008, Rubinstein, et al 1998, Szabolcs & Cairo 2010, Szabolcs & Niedzwiecki 2007, Wagner, et al 2002, Wagner, et al 1996). Donor lymphocyte infusions (DLI) may be used to prevent and treat viral infections and Epstein-Barr virus (EBV)-associated post-transplant lymphoproliferative disease prior to recovery of virus-specific T-cells, but carry substantial risk of GVHD (Fujita, et al 2008). However, cord blood does not afford a readily available source of donor lymphocytes. Significant ex vivo cord blood T-cell expansion has been demonstrated by several groups (Mazur, et al 2008, Parmar, et al 2006). Mazur et al. (2008) reported 76-fold expansion of cord blood T-cells after 12-14 days of CD3/28 stimulation and interleukin (IL)-2, with retention of a naïve phenotype, increased T-helper cell type 1 (Th1) cytokine production, and a lack of allogeneic cytotoxicity. Early reports from halpoidentical and matched unrelated donor AlloSCT recipients additionally suggest that infusion of donor-derived CTLs can prevent and treat EBV and adenovirus, without significant risk of GVHD or other toxicities (Leen, et al 2009). Hanley et al. (2009) have recently shown that multivirus-specific CTLs can be derived from naïve cord blood T-cells to target CMV, EBV and adenovirus; the expression of a chimeric antigen receptor targeting CD19 on these cord blood-derived CTL lines may allow for coupling of antiviral and antileukaemic activity in those UCBT recipients with Pre-B ALL (Hanley, et al 2009, Micklethwaite, et al 2010). In addition, expanded cord blood-derived regulatory T-cells (Tregs) have been shown to suppress cytokine production in mixed lymphocyte reactions and offer potential adoptive cellular immunotherapy for facilitating engraftment and reducing the risk of GVHD following UCBT (Godfrey, et al 2005, Hippen, et al 2008, Porter, et al 2006). A clinical trial to assess the safety and efficacy of ex vivo expanded and activated Tregs in non-myeloablative UCBT recipients is ongoing (Brunstein, et al 2009). Although we observed rapid NK reconstitution following UCBT in our cohort, an association has recently been observed between low NK-cell counts at 60 days following RTC AlloSCT and increased risk of relapse of haematological malignancies and overall mortality, similar to that reported in prior studies of halopidentical AlloSCT recipients (Dunbar, et al 2008). However, our group and others have demonstrated ex vivo expansion of NK-cells and NKT-cells from cryopreserved and rethawed cord blood after incubation with CD3 monoclonal antibodies, IL-2, IL-7, and IL-12, with demonstrated increases in NK-cell activating receptor expression and cytotoxicity against leukaemic cell lines and in NOD/SCID murine xenografts (Ayello, et al 2009, Ayello, et al 2006, Condiotti, et al 2001, Frias, et al 2008). Ongoing and future preclinical and clinical investigations may thus identify a role for cord-blood derived NK-cell and T-cell based therapies to enhance immune reconstitution following UCBT for the prevention and treatment of opportunistic infections and malignant relapse.
This study has several limitations, including small sample size, a heterogeneous group of primary diseases and conditioning regimens, a non-randomized design, differences between MAC and RTC groups with respect to underlying disease, prior treatment and performance status, and a lack of functional studies to complement the immune cell recovery counts and immunoglobulin reconstitution data presented. While all patients received granulocyte-macrophage colony-stimulating factor (GM-CSF) followed by G-CSF as growth factor support in the immediate post-transplant period, we cannot exclude the possibility that this approach, compared with administration of G-CSF alone, may have affected the speed of engraftment and early immune reconstitution or the incidence of grade II-IV aGVHD or opportunistic infection in the cohort as a whole. Experience with GM-CSF post-transplant to hasten engraftment is more limited than experience with G-CSF. However, a large meta-analysis identified no significant increase in the risk of acute or chronic GVHD following treatment with GM-CSF (Ho, et al 2003). Achievement of age-specific normal levels of lymphocytes was not significantly predictive of relapse or development of SVIs or IFIs. However, quantitative immune cell subsets alone may be poorly correlated with risk of relapse and/or opportunistic infection compared to antigen-specific T-cell proliferation or other measures of immunological function. As with any non-randomized study, we furthermore cannot exclude selection bias when explaining differences in outcomes between the MAC and RTC cohorts, particularly as a much greater proportion of patients with ALL and AML received MAC vs. RTC, despite the subgroup analyses performed for TRM and OS to exclude patients with ALL and AML and to exclude all patients with malignant diseases, respectively. In addition, despite the use of logistic regression and Cox proportional hazards models to account for several potential risk factors, we cannot exclude the possibility of uncontrolled confounding variables to explain the observed associations between grade II-IV aGVHD and MAC vs. RTC, between HLA disparity and grade II-IV aGVHD and cGVHD, or between TRM, OS, and variables including MAC vs. RTC.
In summary, this report demonstrated no significant differences in immune cell subset recovery and immunoglobulin reconstitution and no significant difference in the incidence of bacterial infection, SVI, or IFI between paediatric recipients of MAC vs. RTC prior to UCBT. Immune cell recovery was not significantly predictive of overall mortality, relapse in patients with haematological malignancies or with the development of systemic viral infections or invasive fungal infections. Thus, our data suggest that the therapeutic advantages of RTC vs. MAC UCBT are not explained by more rapid immune recovery. Future studies should also incorporate measures of functional immune reconstitution, such as antigen-specific T-cell proliferation and response to mitogenic stimulation. Adoptive cellular immunotherapy using cord-blood derived T-cell and NK-cell based therapies may play a role in promoting early immune reconstitution and the prevention and treatment of opportunistic infections and malignant relapse following UCBT and warrants further investigation (Szabolcs & Cairo 2010).
Supplementary Material
Acknowledgements
The authors would like to thank the patients and their families who participated in these clinical trials and the inpatient and outpatient bedside nurses who provided expert care on a daily basis to these patients at our institution.
M.B.G. performed the research, analysed the data and wrote the paper, J.S.J. analysed the data and wrote the paper, J.F., D.G., P.S., M.B., J.H.G., M.B.B., V.M., L.H. performed the research, C.V.V. analysed the data, E.M., P.D-L., and J.S. performed the research and wrote the paper, L.A.B-L. performed the research and wrote the paper, and M.S.C. designed the research study, performed the research, analysed the data and wrote the paper.
Supported in part by grants from the Pediatric Cancer Research Foundation, National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21 AR49330; MSC), Dreaming for Discovery and Cure Fund, Marisa Fund, Sonia Scaramella Fund, Paul Luisi Foundation, Brittany Barron Fund, Andrew J. Gargiso, Jr. Foundation, and the Doris Duke Charitable Foundation.
Footnotes
Presented in part at the American Society of Hematology Meeting, December 2009, New Orleans, Louisiana and the Blood and Marrow Transplant Tandem Meetings, February 2010, Orlando, Florida
Competing interests: M.B.B. is Director of Oncology Global Clinical Research, Research and Development, Bristol-Myers Squibb. All other authors declare no conflict of interest.
References
- Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, Xiao L, Kerbauy F, Chiattone A, Rondon G, Qazilbash MH, Giralt SA, de Padua Silva L, Hosing C, Kebriaei P, Zhang W, Nieto Y, Saliba RM, Champlin RE, Andersson BS. Myeloablative Reduced-Toxicity i.v. Busulfan-Fludarabine and Allogeneic Hematopoietic Stem Cell Transplant for Patients with Acute Myeloid Leukemia or Myelodysplastic Syndrome in the Sixth through Eighth Decades of Life. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.02.007. doi: 10.1016/j.bbmt.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anasetti C, Hansen JA. Effect of HLA incompatibility in marrow transplantation from unrelated and HLA-mismatched related donors. Transfus Sci. 1994;15:221–230. doi: 10.1016/0955-3886(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Ayello J, van de Ven C, Fortino W, Wade-Harris C, Satwani P, Baxi L, Simpson LL, Sanger W, Pickering D, Kurtzberg J, Cairo MS. Characterization of cord blood natural killer and lymphokine activated killer lymphocytes following ex vivo cellular engineering. Biol Blood Marrow Transplant. 2006;12:608–622. doi: 10.1016/j.bbmt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Ayello J, van de Ven C, Cairo E, Hochberg J, Baxi L, Satwani P, Cairo MS. Characterization of natural killer and natural killer-like T cells derived from ex vivo expanded and activated cord blood mononuclear cells: implications for adoptive cellular immunotherapy. Exp Hematol. 2009;37:1216–1229. doi: 10.1016/j.exphem.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, Haspel R, Kao G, Liney D, Alyea E, Lee S, Cutler C, Ho V, Soiffer R, Antin JH. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JN, Hough RE, van Burik JA, DeFor TE, MacMillan ML, O’Brien MR, Wagner JE. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005;11:362–370. doi: 10.1016/j.bbmt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115:1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Militano O, Jin Z, Figurski M, Shaw L, Moore V, Morris E, Tallamy B, van deVen C, Ayello J, Baxter-Lowe L, Satwani P, George D, Bradley MB, Garvin J, Cairo MS. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16:333–343. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Bradley MB, Satwani P, Baldinger L, Morris E, van de Ven C, Del Toro G, Garvin J, George D, Bhatia M, Roman E, Baxter-Lowe LA, Schwartz J, Qualter E, Hawks R, Wolownik K, Foley S, Militano O, Leclere J, Cheung YK, Cairo MS. Reduced intensity allogeneic umbilical cord blood transplantation in children and adolescent recipients with malignant and non-malignant diseases. Bone Marrow Transplant. 2007;40:621–631. doi: 10.1038/sj.bmt.1705785. [DOI] [PubMed] [Google Scholar]
- Brunstein C, Hippen KL, McKenna DH, Cao Q, Curtsinger J, Sumstad D, Levine BL, Riley JL, June CH, Miller JS, Blazar BR, Wagner JE. Adoptive Transfer of Umbilical Cord Blood (UCB)-Derived Regulatory T Cells (Tregs) to Recipients of Nonmyeloablative Unrelated Double UCB Transplantation. ASH Annual Meeting Abstracts. 2009;114:513. [Google Scholar]
- Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, McGlave PB, Wagner JE. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo MS, Wagner EL, Fraser J, Cohen G, van de Ven C, Carter SL, Kernan NA, Kurtzberg J. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: a Cord Blood Transplantation (COBLT) Study report. Transfusion. 2005;45:856–866. doi: 10.1111/j.1537-2995.2005.04429.x. [DOI] [PubMed] [Google Scholar]
- Chakraverty R, Orti G, Roughton M, Shen J, Fielding A, Kottaridis P, Milligan D, Collin M, Crawley C, Johnson P, Clark A, Parker A, Bloor A, Pettengell R, Snowden J, Pettitt A, Clark R, Hale G, Peggs K, Thomson K, Morris E, Mackinnon S. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116:3080–3088. doi: 10.1182/blood-2010-05-286856. [DOI] [PubMed] [Google Scholar]
- Chao NJ, Liu CX, Rooney B, Chen BJ, Long GD, Vredenburgh JJ, Morris A, Gasparetto C, Rizzieri DA. Nonmyeloablative regimen preserves “niches” allowing for peripheral expansion of donor T-cells. Biol Blood Marrow Transplant. 2002;8:249–256. doi: 10.1053/bbmt.2002.v8.pm12064361. [DOI] [PubMed] [Google Scholar]
- Condiotti R, Zakai YB, Barak V, Nagler A. Ex vivo expansion of CD56+ cytotoxic cells from human umbilical cord blood. Exp Hematol. 2001;29:104–113. doi: 10.1016/s0301-472x(00)00617-2. [DOI] [PubMed] [Google Scholar]
- Couriel D, Caldera H, Champlin R, Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101:1936–1946. doi: 10.1002/cncr.20613. [DOI] [PubMed] [Google Scholar]
- Crooks GM, Weinberg K, Mackall C. Immune reconstitution: from stem cells to lymphocytes. Biol Blood Marrow Transplant. 2006;12:42–46. doi: 10.1016/j.bbmt.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Dunbar EM, Buzzeo MP, Levine JB, Schold JD, Meier-Kriesche HU, Reddy V. The relationship between circulating natural killer cells after reduced intensity conditioning hematopoietic stem cell transplantation and relapse-free survival and graft-versus-host disease. Haematologica. 2008;93:1852–1858. doi: 10.3324/haematol.13033. [DOI] [PubMed] [Google Scholar]
- Eapen M, Rubinstein P, Zhang MJ, Camitta BM, Stevens C, Cairo MS, Davies SM, Doyle JJ, Kurtzberg J, Pulsipher MA, Ortega JJ, Scaradavou A, Horowitz MM, Wagner JE. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol. 2006;24:145–151. doi: 10.1200/JCO.2005.02.4612. [DOI] [PubMed] [Google Scholar]
- Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP, Horowitz MM, Wagner JE. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- Fraser JK, Cairo MS, Wagner EL, McCurdy PR, Baxter-Lowe LA, Carter SL, Kernan NA, Lill MC, Slone V, Wagner JE, Wallas CH, Kurtzberg J. Cord Blood Transplantation Study (COBLT): cord blood bank standard operating procedures. J Hematother. 1998;7:521–561. doi: 10.1089/scd.1.1998.7.521. [DOI] [PubMed] [Google Scholar]
- Frias AM, Porada CD, Crapnell KB, Cabral JM, Zanjani ED, Almeida-Porada G. Generation of functional natural killer and dendritic cells in a human stromal-based serum-free culture system designed for cord blood expansion. Exp Hematol. 2008;36:61–68. doi: 10.1016/j.exphem.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Rooney CM, Heslop HE. Adoptive cellular immunotherapy for viral diseases. Bone Marrow Transplant. 2008;41:193–198. doi: 10.1038/sj.bmt.1705906. [DOI] [PubMed] [Google Scholar]
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- Hamza NS, Lisgaris M, Yadavalli G, Nadeau L, Fox R, Fu P, Lazarus HM, Koc ON, Salata RA, Laughlin MJ. Kinetics of myeloid and lymphocyte recovery and infectious complications after unrelated umbilical cord blood versus HLA-matched unrelated donor allogeneic transplantation in adults. Br J Haematol. 2004;124:488–498. doi: 10.1046/j.1365-2141.2003.04792.x. [DOI] [PubMed] [Google Scholar]
- Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, Decker W, Molldrem JJ, Liu H, Gee AP, Rooney CM, Heslop HE, Dotti G, Brenner MK, Shpall EJ, Bollard CM. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, Bina M, Panoskaltsis-Mortari A, Rubinstein P, Van Rooijen N, Golovina TN, Suhoski MM, Miller JS, Wagner JE, June CH, Riley JL, Blazar BR. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VT, Mirza NQ, Junco Dd D, Okamura T, Przepiorka D. The effect of hematopoietic growth factors on the risk of graft-vs-host disease after allogeneic hematopoietic stem cell transplantation: a meta-analysis. Bone Marrow Transplant. 2003;32:771–775. doi: 10.1038/sj.bmt.1704228. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Martinez C, Ercilla G, Carreras E, Urbano-Ispizua A, Aymerich M, Villamor N, Amezaga N, Rovira M, Fernandez-Aviles F, Gaya A, Martino R, Sierra J, Montserrat E. Reduced-intensity conditioning regimen preserves thymic function in the early period after hematopoietic stem cell transplantation. Exp Hematol. 2005;33:1240–1248. doi: 10.1016/j.exphem.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Junghanss C, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, Chauncey T, McSweeney PA, Little MT, Corey L, Storb R. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99:1978–1985. doi: 10.1182/blood.v99.6.1978. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, Bryan SG, Kaur I, Martin S, Wieder ED, Worth L, Cooper LJ, Petropoulos D, Molldrem JJ, Champlin RE, Shpall EJ. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, Ciocci G, Carrier C, Stevens CE, Rubinstein P. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J, Cairo MS, Fraser JK, Baxter-Lowe L, Cohen G, Carter SL, Kernan NA. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion. 2005;45:842–855. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J, Prasad VK, Carter SL, Wagner JE, Baxter-Lowe LA, Wall D, Kapoor N, Guinan EC, Feig SA, Wagner EL, Kernan NA. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, Marks DI, van Rood JJ, Scaradavou A, Horowitz MM. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy-Nasser AA, Leung KS, Gee AP, Krance RA, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Geyer MB, Yang AJ, Cairo MS. Cord blood transplantation and stem cell regenerative potential. Exp Hematol. 2011;39:393–412. doi: 10.1016/j.exphem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Ljungman P, Ward KN, Crooks BN, Parker A, Martino R, Shaw PJ, Brinch L, Brune M, De La Camara R, Dekker A, Pauksen K, Russell N, Schwarer AP, Cordonnier C. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- Lockitch G, Halstead A, Quigley G, MacCallum C. Age- and sex-specific pediatric reference intervals: study design and methods illustrated by measurement of serum proteins with the Behring LN Nephelometer. Clin Chem. 1988;34:1618–1621. [PubMed] [Google Scholar]
- MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, Blazar BR, Wagner JE. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006;107:3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M, Sandmaier B, Maloney D, Storb R, Storek J. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol. 2003;31:941–952. doi: 10.1016/s0301-472x(03)00201-7. [DOI] [PubMed] [Google Scholar]
- Martin PL, Carter SL, Kernan NA, Sahdev I, Wall D, Pietryga D, Wagner JE, Kurtzberg J. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12:184–194. doi: 10.1016/j.bbmt.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biol Blood Marrow Transplant. 2008;14:1190–1196. doi: 10.1016/j.bbmt.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, Gooley TA, Hegenbart U, Nash RA, Radich J, Wagner JL, Minor S, Appelbaum FR, Bensinger WI, Bryant E, Flowers ME, Georges GE, Grumet FC, Kiem HP, Torok-Storb B, Yu C, Blume KG, Storb RF. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- Michel G, Rocha V, Chevret S, Arcese W, Chan KW, Filipovich A, Takahashi TA, Vowels M, Ortega J, Bordigoni P, Shaw PJ, Yaniv I, Machado A, Pimentel P, Fagioli F, Verdeguer A, Jouet JP, Diez B, Ferreira E, Pasquini R, Rosenthal J, Sievers E, Messina C, Iori AP, Garnier F, Ionescu I, Locatelli F, Gluckman E. Unrelated cord blood transplantation for childhood acute myeloid leukemia: a Eurocord Group analysis. Blood. 2003;102:4290–4297. doi: 10.1182/blood-2003-04-1288. [DOI] [PubMed] [Google Scholar]
- Micklethwaite KP, Savoldo B, Hanley PJ, Leen AM, Demmler-Harrison GJ, Cooper LJ, Liu H, Gee AP, Shpall EJ, Rooney CM, Heslop HE, Brenner MK, Bollard CM, Dotti G. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010;115:2695–2703. doi: 10.1182/blood-2009-09-242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek M, Martin PJ, Leisenring W, Flowers ME, Maloney DG, Sandmaier BM, Maris MB, Storb R. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- Moretta A, Maccario R, Fagioli F, Giraldi E, Busca A, Montagna D, Miniero R, Comoli P, Giorgiani G, Zecca M, Pagani S, Locatelli F. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol. 2001;29:371–379. doi: 10.1016/s0301-472x(00)00667-6. [DOI] [PubMed] [Google Scholar]
- Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH, Mackinnon S, Hale G. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102:404–406. doi: 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- Nakamae H, Kirby KA, Sandmaier BM, Norasetthada L, Maloney DG, Maris MB, Davis C, Corey L, Storb R, Boeckh M. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehues T, Rocha V, Filipovich AH, Chan KW, Porcher R, Michel G, Ortega JJ, Wernet P, Gobel U, Gluckman E, Locatelli F. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children -- a Eurocord analysis. Br J Haematol. 2001;114:42–48. doi: 10.1046/j.1365-2141.2001.02900.x. [DOI] [PubMed] [Google Scholar]
- Oran B, Wagner JE, DeFor TE, Weisdorf DJ, Brunstein CG. Effect of Conditioning Regimen Intensity on Acute Myeloid Leukemia Outcomes after Umbilical Cord Blood Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 doi: 10.1016/j.bbmt.2011.01.007. doi: 10.1016/j.bbmt.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Van de Ven C, del Toro G, Garvin J, George D, Bradley MB, Wolownik K, Wischhover C, Levy J, Skerrett D, Cairo MS. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2004;10:246–258. doi: 10.1016/j.bbmt.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Parkman R, Cohen G, Carter SL, Weinberg KI, Masinsin B, Guinan E, Kurtzberg J, Wagner JE, Kernan NA. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:919–927. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Parmar S, Robinson SN, Komanduri K, St John L, Decker W, Xing D, Yang H, McMannis J, Champlin R, de Lima M, Molldrem J, Rieber A, Bonyhadi M, Berenson R, Shpall EJ. Ex vivo expanded umbilical cord blood T cells maintain naive phenotype and TCR diversity. Cytotherapy. 2006;8:149–157. doi: 10.1080/14653240600620812. [DOI] [PubMed] [Google Scholar]
- Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation. Part I: CIBMTR summary slides. CIBMTR Newsletter. 2009;1:7–11. [Google Scholar]
- Petersdorf EW, Smith AG, Haase AM, Martin PJ, Hansen JA. Polymorphism of HLA-DRw52-associated DRB1 genes as defined by sequence-specific oligonucleotide probe hybridization and sequencing. Tissue Antigens. 1991;38:169–177. doi: 10.1111/j.1399-0039.1991.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Porter SB, Liu B, Rogosheske J, Levine BL, June CH, Kohl VK, Wagner JE, Miller JS, Blazar BR. Suppressor function of umbilical cord blood-derived CD4+CD25+ T-regulatory cells exposed to graft-versus-host disease drugs. Transplantation. 2006;82:23–29. doi: 10.1097/01.tp.0000225824.48931.af. [DOI] [PubMed] [Google Scholar]
- Prasad VK, Mendizabal A, Parikh SH, Szabolcs P, Driscoll TA, Page K, Lakshminarayanan S, Allison J, Wood S, Semmel D, Escolar ML, Martin PL, Carter S, Kurtzberg J. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–2989. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher MA, Boucher KM, Wall D, Frangoul H, Duval M, Goyal RK, Shaw PJ, Haight AE, Grimley M, Grupp SA, Kletzel M, Kadota R. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114:1429–1436. doi: 10.1182/blood-2009-01-196303. [DOI] [PubMed] [Google Scholar]
- Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S, Tiedemann K, Busca A, Chan KW, Kato S, Ortega J, Vowels M, Zander A, Souillet G, Oakill A, Woolfrey A, Pay AL, Green A, Garnier F, Ionescu I, Wernet P, Sirchia G, Rubinstein P, Chevret S, Gluckman E. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, Jacobsen N, Ruutu T, de Lima M, Finke J, Frassoni F, Gluckman E. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Nademanee A, Ruel N, Smith E, Krishnan A, Popplewell L, Zain J, Patane K, Kogut N, Nakamura R, Sarkodee-Adoo C, Forman SJ. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12:1326–1334. doi: 10.1016/j.bbmt.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Roman E, Osunkwo I, Militano O, Cooney E, van de Ven C, Cairo MS. Liposomal amphotericin B prophylaxis of invasive mold infections in children post allogeneic stem cell transplantation. Pediatr Blood Cancer. 2008;50:325–330. doi: 10.1002/pbc.21239. [DOI] [PubMed] [Google Scholar]
- Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, Taylor PE, Stevens CE. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE, Rosenfield RE, Stevens CE. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- Satwani P, Baldinger L, Freedman J, Jacobson JS, Guerra J, van de Ven C, Morris E, Garvin J, George D, Bradley MB, Bhatia M, Tallamy B, Schwartz J, Jin Z, Cairo MS. Incidence of Viral and fungal infections following busulfan-based reduced-intensity versus myeloablative conditioning in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2009;15:1587–1595. doi: 10.1016/j.bbmt.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Schulenburg A, Fischer M, Kalhs P, Mitterbauer M, Rabitsch W, Greinix HT, Leitner G. Immune recovery after conventional and non-myeloablative allogeneic stem cell transplantation. Leuk Lymphoma. 2005;46:1755–1760. doi: 10.1080/10428190500264496. [DOI] [PubMed] [Google Scholar]
- Shereck EB, Cooney E, van de Ven C, Della-Lotta P, Cairo MS. A pilot phase II study of alternate day ganciclovir and foscarnet in preventing cytomegalovirus (CMV) infections in at-risk pediatric and adolescent allogeneic stem cell transplant recipients. Pediatr Blood Cancer. 2007;49:306–312. doi: 10.1002/pbc.21043. [DOI] [PubMed] [Google Scholar]
- Styczynski J, Cheung YK, Garvin J, Savage DG, Billote GB, Harrison L, Skerrett D, Wolownik K, Wischhover C, Hawks R, Bradley MB, Del Toro G, George D, Yamashiro D, van de Ven C, Cairo MS. Outcomes of unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transplant. 2004;34:129–136. doi: 10.1038/sj.bmt.1704537. [DOI] [PubMed] [Google Scholar]
- Styczynski J, Tallamy B, Waxman I, van de Ven C, Milone MC, Shaw LM, Harrison L, Morris E, Satwani P, Bhatia M, George D, Bradley MB, Garvin JH, Schwartz J, Baxter-Lowe LA, Cairo MS. A pilot study of reduced toxicity conditioning with BU, fludarabine and alemtuzumab before the allogeneic hematopoietic SCT in children and adolescents. Bone Marrow Transplant. 2011;46:790–799. doi: 10.1038/bmt.2010.209. [DOI] [PubMed] [Google Scholar]
- Szabolcs P, Cairo MS. Unrelated umbilical cord blood transplantation and immune reconstitution. Semin Hematol. 2010;47:22–36. doi: 10.1053/j.seminhematol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M, Garnier F, Douek D, Gluckman E, Charron D, Toubert A. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002;99:1458–1464. doi: 10.1182/blood.v99.4.1458. [DOI] [PubMed] [Google Scholar]
- Thomson BG, Robertson KA, Gowan D, Heilman D, Broxmeyer HE, Emanuel D, Kotylo P, Brahmi Z, Smith FO. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–2711. [PubMed] [Google Scholar]
- Wagner JE, Rosenthal J, Sweetman R, Shu XO, Davies SM, Ramsay NK, McGlave PB, Sender L, Cairo MS. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- Waxman IM, Militano O, Baldinger L, Roman E, Qualter E, Morris E, Garvin J, Bradley MB, Bhatia M, Satwani P, George D, Del Toro G, Hawks R, Wolownik K, Foley S, Cheung YK, Schwartz J, van de Ven C, Baxter-Lowe LA, Cairo MS. Sequential administration of sargramostim and filgrastim in pediatric allogeneic stem cell transplantation recipients undergoing myeloablative conditioning. Pediatr Transplant. 2009;13:464–474. doi: 10.1111/j.1399-3046.2008.01000.x. [DOI] [PubMed] [Google Scholar]
- Yuji K, Miyakoshi S, Kato D, Miura Y, Myojo T, Murashige N, Kishi Y, Kobayashi K, Kusumi E, Narimatsu H, Hamaki T, Matsumura T, Kami M, Fukuda T, Masuo S, Masuoka K, Wake A, Ueyama J, Yoneyama A, Miyamoto K, Nagoshi H, Matsuzaki M, Morinaga S, Muto Y, Takeue Y, Taniguchi S. Reduced-intensity unrelated cord blood transplantation for patients with advanced malignant lymphoma. Biol Blood Marrow Transplant. 2005;11:314–318. doi: 10.1016/j.bbmt.2005.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.