Abstract
Many data suggest that alpha synuclein (α-syn) aggregation is involved in Parkinson's disease (PD) neurotoxicity and is accelerated by the pathogenetic point mutation A30P. The triplication of α-syn gene has been linked to early-onset familial PD, suggesting that the cellular dosage of α-syn is an important modulator of its toxicity. To verify this point, we developed an inducible model of α-syn expression (both wild type [WT] and mutated A30P) in rat PC12/TetOn cells. At low expression level, both α-syn(WT) and (A30P) did not aggregate, were not toxic, and displayed a protective action against oxidative stress triggered by hydrogen peroxide (H2O2). By increasing α-syn expression, its antioxidant function was no longer detectable as for the A30P form, but again no aggregation and cell death were present both for the WT and the mutated protein. To clarify why α-syn did not accumulate at high expression level, we inhibited macroautophagy by 3-methyladenine (3-MA) and the proteasome by MG132. In presence of 3-MA, α-syn(WT) accumulated, A11 anti-oligomer antibody-positive aggregates were detectable, and cell toxicity was evident, while proteasome inhibition did not increase α-syn(WT) accumulation. Macroautophagy or proteasome inhibition slightly increased α-syn(A30P) toxicity, with no detectable aggregation. This model can provide useful details about α-syn function, aggregation, and degradation pathways.
Key words: alpha-synuclein, protein aggregation, macroautophagy, proteasome, oxidative stress, Parkinson's disease
Abbreviations: CMA, chaperone-mediated autophagy; DOXY, doxycycline; FCS, fetal calf serum; HS, horse serum; LB, Lewy bodies; LDH, lactate dehydrogenase release to lactate dehydrogenase; LN, Lewy neurites; PD, Parkinson's disease; UPS, ubiquitin-proteasome system
Graphical abstract
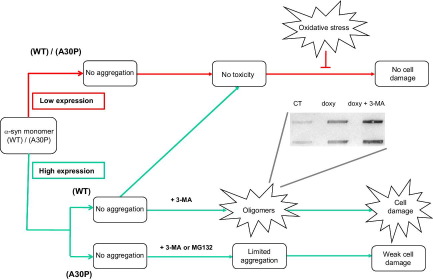
Graphical abstract summarizing the main findings of the article. The red flow chart describes the system when α-syn expression was induced at low level, while the green part splits the different scenario for α-syn(WT) and α-syn(A30P) at high expression level. The slot blot shows the appearance of A11 immunoreactivity. 3-MA, 3-methyladenine; doxy, doxycycline. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Highlights
▶We developed a model of alpha-synuclein (wild type or A30P) expression in PC12 cells. ▶At low alpha-synuclein expression, we found no aggregation but neuroprotection. ▶At high alpha-synuclein expression macroautophagy was activated without aggregation. ▶Macroautophagy inhibition lead to alpha-synuclein wild type aggregation and toxicity. ▶The A30P form was not toxic and its removal involved also the proteasome.
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the degeneration of the dopaminergic neurons of the substantia nigra pars compacta. This event correlates with a deficit of dopamine in the striatum that is at the basis of PD clinical features, including bradykinesia, resting tremor, rigidity, and postural instability (Fahn, 2003). From the neuropathologic point of view, PD is characterized in almost all forms by proteinaceous intracytoplasmic inclusion bodies called Lewy bodies (LB) and by the presence of abnormally shaped neurites named Lewy neurites (LN) (Dauer and Przedborski, 2003). The etiopathogenesis of PD is probably multifactorial, including both environmental and genetic factors (Di Monte, 2003; Gasser, 2009; Warner and Schapira, 2003). Several genes (α-syn, parkin, UCH-L1, DJ-1, LRRK2, PINK-1, and NR4A2) have been linked to genetic cases of PD (Bekris et al., 2010; Hashimoto et al., 2003; Giasson and Lee, 2003; Cookson, 2003). Alpha-syn is the principal component of LB and LN (Fahn, 2003), and three autosomal dominant missense mutations (an Ala to Pro substitution at codon 30 [A30P], an Ala to Thr substitution at codon 53 [A53T] and a Glu to Lys [E46K] at codon 46) have been linked to familial PD (Polymeropoulos et al., 1997; Krüger et al., 1998; Zarranz et al., 2004). The available data about α-syn physiological functions deal with presynaptic plasticity, dopamine trafficking and homeostasis, exocytotic vesicle interaction, regulation of monoamine transporters, and chaperone-like activity (Burré et al., 2010; Scott et al., 2010; Garcia-Reitböck et al., 2010; Lykkebo and Jensen, 2002; Wersinger et al., 2003; Oaks and Sidhu, 2011; Osterova et al., 1999). A30P and A53T mutations affect the ability of α-syn to modulate dopamine vesicle trafficking, modify its interaction with cellular membranes (Saha et al., 2004; Jensen et al., 1998), and increase its natural propensity to aggregate (Anderson et al., 2010; Volles and Lansbury, 2003; el-Agnaf and Irvine, 2002; Conway et al., 2000).
The description of a family with early-onset PD that showed a triplication of a chromosomic region containing α-syn gene suggested that the cellular dosage of the protein is critical for PD etiopathogenesis (Singleton et al., 2003; Uversky and Eliezer, 2009). Moreover, many putative triggers of idiopathic PD (mitochondrial complex I inhibitors, environmental toxins, oxidative stress, or proteasome impairment) cause α-syn modifications that are sufficient to alter its intracellular concentration, leading to aggregation and related toxicity (Riedel et al., 2011; Esteves et al., 2011; Xie et al., 2010; Sherer et al., 2003; Norris et al., 2003; Ischiropoulos and Beckman, 2003). From the other side, the existence of a neuroprotective pathway mediated by native α-syn is supported by some evidence (Jin et al., 2011; Lee et al., 2010; Jensen et al., 2003; Manning-Bog et al., 2003; Seo et al., 2002; Hashimoto et al., 2002).
To investigate whether α-syn level modulates its function, aggregation, and toxicity we have developed an inducible model of α-syn expression in rat PC12/TetOn cells, comparing the WT form to the mutated A30P, too.
Experimental procedures
PC12/TetOn(α-syn) cell lines
A commercial PC12/TetOn cell line was purchased from Clontech (Palo Alto, CA, USA) (Rossi and Blau, 1998). To express α-syn(WT) the corresponding cDNA was amplified by mean of rapid amplification of cDNA ends - polymerase chain reaction (RACE-PCR) from a human brain cDNA bank and then cloned into an expression vector (pRSET, Invitrogen, Carlsbad, CA, USA) and fully sequenced. This plasmid served as template for introducing the A30P mutation by a site-directed mutagenesis commercial kit (Invitrogen). To produce the TetOn/pBI-G vectors, α-syn cDNA sequences were excised from pRSET plasmids using the unique restriction enzymes NotI and SalI (Roche, Basel, Switzerland) and inserted into different pBI-G vectors, cut with the same restriction enzymes.
PC12/TetOn cells were cultured on disposable plasticware coated with poly-d-lysine at 37 °C, 5% CO2 in RPMI 1640 medium (Invitrogen) supplemented with 10% tetracycline-free horse serum (HS), 5% fetal calf serum (FCS) (Sigma Aldrich, St. Louis, MO, USA), 2 mM l-glutamine, 100UI/ml penicillin, and 100 mg/ml streptomycin (Invitrogen) in humidified atmosphere. Alpha-synuclein expression was induced by doxycycline addition (range: 0.1–1 μg/ml) directly into culture medium.
Cell viability and oxidative stress challenge
Cell viability was assessed by MTT colorimetric assay (conversion of a soluble tetrazolium salt (3–[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) into an insoluble formazan precipitate) and confirmed by lactate dehydrogenase release (LDH) assay performed according to the manufacturer's instructions (BioRad Laboratories, Hercules, CA, USA).
To challenge PC12/TetOn cells with oxidative stress, 100×103 undifferentiated cells were seeded in a 96-well plate and incubated overnight. Next day, a freshly prepared H2O2 dilution was added (final concentration: 150 μM), and cells were incubated for 72 h. At the end of the experiment, the number of viable cells was estimated as previously described.
Thioflavin-T binding assay for amyloid aggregate detection
For thioflavin-T staining, cells (about 50×103) were cultured on poly-d-lysine-coated glass coverslips and treated with H2O2, as previously reported. Then, cells were fixed for 20 min with a 4% paraformaldehyde/PBS solution, incubated 8 min with a 0.05% thioflavin-T solution and washed twice with PBS. Thioflavin-T reactivity was analyzed using a fluorescence microscope coupled to a digital camera (Olympus Corporation, Tokyo, Japan).
Immunoblotting
Cellular total protein extracts were lysed in Laemmli SDS-PAGE 1× sample buffer, subjected to SDS-PAGE electrophoresis, and transferred to a nitrocellulose membrane (BioRad Laboratories). The membrane was then incubated overnight with a primary antibody diluted in 5% nonfat dry milk in PBS-T (PBS+0.1% Tween-20). After washing, the membrane was incubated for 1 h with a secondary antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) diluted 1:5000 in 5% nonfat dry milk in PBS-T. The membrane was finally subjected to ECL detection technique (GE Healthcare, Chalfont St. Giles, UK).
Immunocytochemistry
Cells (50×103) were cultured on poly-d-lysine-coated chamber slides and fixed for 20 min with 4% paraformaldehyde/PBS solution. The fixed cells were permeabilized using 0.5% Triton-X 100, 0.2% FCS in PBS for 5 min and incubated overnight with a primary antibody diluted 1:100 in PBS with 1% HS. After washing, an FITC-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted 1:200 in PBS with 1% HS was added for 30 min, and cells were analyzed by a fluorescence microscope coupled to a digital camera (Olympus Corporation, Tokyo, Japan).
Macroautophagy and proteasome inhibition
Alpha-synuclein expression was induced for 48 h by doxycycline addition (0.1 μg/ml or 1 μg/ml), and then the autophagy inhibitor 3-methyladenine (3-MA) 10 mM (Sigma Aldrich Co.) or the proteasome inhibitor MG132 10 μM (Sigma Aldrich Co.) was added to the medium for further 18 h. Cell viability was then assessed by MTT assay and cell protein lysates were collected for Western blotting.
Antibodies
The following primary antibodies were used to run immunoblotting or immunocytochemistry: anti α-syn monoclonal antibody (dilution 1:2500) (Transduction Laboratories, Lexington, KY, USA); anti α-tubulin monoclonal antibody (dilution 1:5000) (Abcam, Cambridge, UK); anti LC3 polyclonal antibody (dilution 1:200) (Abcam, Cambridge, UK); anti-oligomer (A11) polyclonal antibody (dilution 1:250) (Invitrogen).
Results
Modulation of α-synuclein expression in PC12/TetOn cells
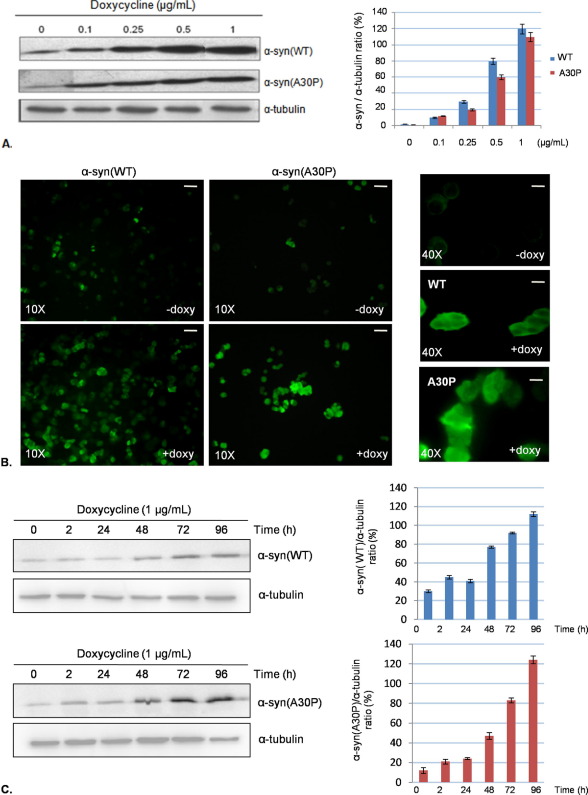
Fig. 1A shows a Western blotting analysis assessing α-syn expression in PC12/TetOn cells. Cells were exposed for 96 h to increasing doxycycline concentration. We found a positive correlation between doxycycline amount and protein expression level. In PC12/TetOn cells transfected by an empty pBI-G vector, the basal level of α-syn was negligible and was not affected by doxycycline addition (data not shown). We further confirmed α-syn-inducible expression by immunocytochemistry (Fig. 1B). PC12/TetOn cells were exposed to 1 μg/ml doxycycline for 48 h, and then α-syn immunoreactivity was assessed. We were able to detect a cytoplasmic increase of the protein (both WT and A30P).
Fig. 1.
Development of an inducible α-syn expression system in PC12 cells. (A) Representative Western blot showing human α-syn-inducible expression. PC12 cells were transfected with a tetracycline responsive plasmid (pBI-G) carrying human α-syn(WT or A30P) full-length cDNAs as described in methods. The dynamic range of α-syn expression was tested by addition of the antibiotic doxycycline (doxy.) for 96 h to culture medium in a dose ranging between 0.1 and 1 μg/ml. The bar graph (right) is the densitometric analysis of three independent Western blot experiments, using α-tubulin immunoreactivity as normalization standard. (B) Immunocytochemistry of human α-syn expression (WT or A30P) in PC12/TetOn cells. The upper picture for each cell line is the basal expression in absence of doxycycline (−doxy), while the lower picture shows α-syn immunoreactivity after 48-h induction by 1 μg/ml doxycycline (+doxy) (magnification 10×; bar=10 μm). Alpha-syn expression at higher magnification (40×) before and after doxycycline induction (1 μg/ml for 48 h) is also presented (right-Bar=5 μm). (C) Western blot showing α-syn(WT) and (A30P) cellular bioavailability over time. The bar graphs (right) represent the densitometric analysis of three independent Western blot assays.
Finally, we verified the cellular presence of α-syn over time (Fig. 1C). Alpha-syn expression was induced with 1 μg/ml doxycycline for increasing time intervals (0–96 h). The protein level doubled 48 h after doxycycline addition, and after 96 h, it reached a strong level of expression in comparison with the basal condition both for the WT and the A30P form.
Effect of α-syn expression level on oxidative stress response and cellular viability
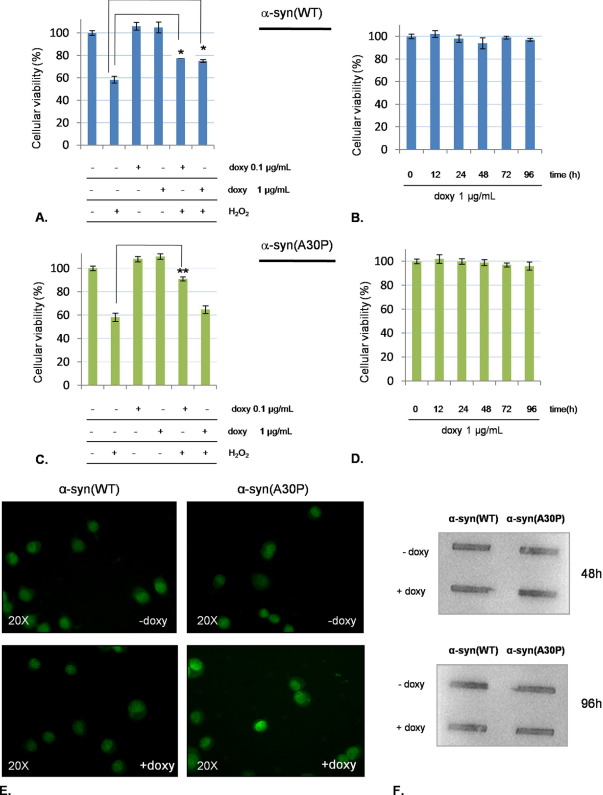
We have previously shown that low amounts of α-syn are protective against oxidative stress in vitro (Albani et al., 2004). In order to confirm this point with an independent model, we decided to challenge PC12/TetOn cells with hydrogen peroxide (H2O2) for 72 h while we were inducing α-syn expression with low amount of doxycycline (0.1 μg/ml). Our results are shown in Fig. 2A, C. The oxidative treatment was toxic to uninduced cells, with a reduction of viability of about 50% in comparison with control. On the contrary, when we exposed PC12/TetOn to doxycycline 0.1 μg/ml before oxidative challenge, a significant increase of cell viability was assessed. In this situation, no difference was detectable between α-syn(WT) and (A30P). When we treated mock-transfected PC12/TetOn cells with hydrogen peroxide (H2O2), no protection from H2O2 toxicity was detectable (data not shown).
Fig. 2.
Neuroprotective effect of human α-syn against oxidative stress without cellular toxicity. PC12/TetOn cells were seeded in 96-well plates at 100×103 cells/well and grown overnight. Next day, cells were exposed to doxycycline 0.1 μg/ml or 1 μg/ml for 48 h. Afterward, H2O2 150 μM was added for further 72 h and then cell viability was assessed by MTT or cells were fixed with 4% PFA and stained with 0.05% thioflavin-T as described in methods. (A) Viability of PC12/TetOn cells expressing different levels of human α-syn(WT) challenged by H2O2; (B) Viability assay of PC12/TetOn expressing α-syn(WT) after addition of doxycycline 1 μg/ml for increasing time intervals; (C) Evaluation of viability of PC12/TetOn expressing different levels of human α-syn(A30P) after oxidative challenge by H2O2. (D) Viability of PC12/TetOn stimulated with doxycycline 1 μg/ml to express human α-syn(A30P) for increasing time intervals. *P<0.05; **P<0.01, one-way ANOVA followed by Tukey's post-hoc test. (E) Absence of α-syn aggregate formation assessed by thioflavin-T staining in PC12/TetOn cells after 48 h stimulation with doxycycline 1 μg/ml. The upper pictures show the basal condition (−doxy), while the +doxy conditions are the stimulated cells. (F) Representative dot blot assessing A11 reactivity in uninduced (−doxy) and induced (+doxy) condition for both cell lines at two different time-points (48 and 96 h) from doxycycline addition. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
We have also verified cell viability after α-syn overexpression induced by doxycycline 0.1 μg/ml in absence of H2O2 by MTT and LDH release assays. At the same time, α-syn aggregation was checked by α-syn immunostaining or A11 anti-oligomer antibody reactivity. We did not find any toxic effect or evidence of α-syn aggregation, both for the WT and the A30P form (data not shown).
The further step was to evaluate our model when we strongly induced α-syn expression level. In this situation, we expected to find α-syn toxicity and aggregation, with loss of neuroprotection against oxidative stress. At first, we added to PC12/TetOn growth medium 1 μg/ml doxycycline for increasing time intervals (0–96 h) and then we quantified cellular viability by MTT assay. As shown in Fig. 2B, D, no toxicity was recorded even at 96 h, and this situation was the same for the WT and the mutated form. We have also increased the induction time with doxycycline 1 μg/ml up to 7 days, without any toxicity (data not shown). When exposed to doxycycline at 1 μg/ml, cells expressing α-syn(WT) were still able to counteract oxidative stress, while cells carrying the mutated form were not different from control (Fig. 2A, C). To investigate the formation of α-syn aggregates in this situation of high α-syn expression level, we stained cells with thioflavin-T, but no difference between control and induced cells came to light (Fig. 2E). Finally, to assess whether at least α-syn oligomers were accumulating, we performed an A11 antibody assay at increasing time points (48–96 h). We did not find any positivity (Fig. 2F).
Macroautophagy and proteasome inhibition in PC12/TetOn cells
As in our model we did not find evidence of α-syn aggregation, we hypothesized that α-syn was efficiently removed from the cytosol. To test this, we considered the involvement of the macroautophagy system and the ubiquitin-proteasome system (UPS), two pathways responsible for proteins, protein aggregates, or organelles degradation and recycling. We have verified that macroautophagy and the proteasome were functions of our cell model and were inhibited by 3-MA and MG132, respectively (data not shown).
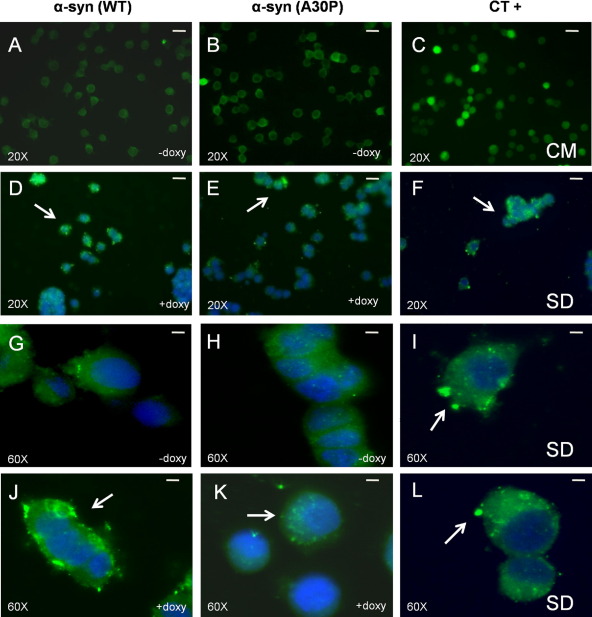
Alpha-synuclein expression was induced by doxycycline (1 μg/ml) for 48 h in both WT and A30P transfected cells, and then we evaluated macroautophagy activation by LC3-II immunocytochemistry (Fig. 3). As positive control (CT+), we cultured mock-transfected PC12/TetOn cells for 18 h in absence of serum to induce starvation. We noticed macroautophagy activation, with the appearance of LC3-II-positive spots in serum-starved and doxycycline-induced cells, with a stronger reactivity for the α-syn(WT) form in comparison with the mutated A30P. We have also checked whether macroautophagy was activated by the addition of doxycycline 0.1 μg/ml for 48 h, but no positive LC3-II reactivity was found (data not shown).
Fig. 3.
Macroautophagy activation after doxycycline addition and α-syn expression in PC12/TetOn cells. Cells were seeded at 50×103 cell/well in a multiwell chamber slide and grown overnight. The next day, doxycycline was added (1 μg/ml) for 48 h and then macroautophagic organelle formation was monitored by LC3-II immunoreactivity (highlighted by arrows), while nuclei were stained by Hoechst 33342. As positive control (CT+), PC12/TetOn mock-transfected with empty pBI-G vector were seeded as previously described but then were kept for 18 h in a starving condition (serum deprivation[SD]). (A–C) Control condition showing LC3-II immunoreactivity in uninduced PC12/TetOn cells (−doxy) or in PC12/TetOn mock transfected cells cultured in presence of complete medium (CM). Bar=10 μm (magnification 20). (D–F) LC3-II immunoreactivity as above but after 48 h induction by doxycycline (+doxy) or 18 h SD. Bar=10 μm (magnification 20×). (G–I) Control condition as in (A–C) but at higher magnification (60×; bar=2.5 μm). (J–L) Same situation as in (D–F) (60× magnification; bar=2.5 μm).
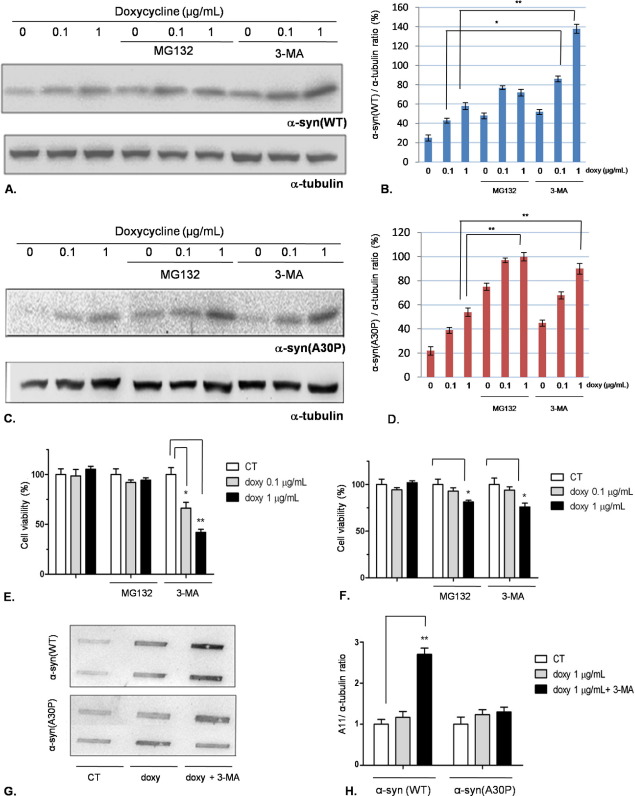
Then, we decided to block macroautophagy (3-MA 10 mM for 18 h) or the UPS (MG132 10 μM for 18 h) and assess α-syn accumulation and cellular viability in presence of doxycycline (0.1 or 1 μg/ml). The results are shown in (Fig. 4). In PC12/TetOn cells expressing α-syn(WT), the inhibition of macroautophagy, but not the proteasome, led to an accumulation of the protein (Fig. 4A, B). As for cell viability, we detected an increase of cell toxicity in cells incubated with 3-MA only, and the effect was stronger at doxycycline 1 μg/ml (Fig. 4E). On the other hand, in PC12/TetOn cells expressing the mutated A30P, both 3-MA and MG132 induced protein accumulation but to a smaller extent than in the WT line (Fig. 4C, D) with a weak decrease of cell viability at doxycycline 1 μg/ml only.
Fig. 4.
Inhibition of macroautophagy by 3-MA leads to α-syn(WT) accumulation, toxicity, and oligomer formation in PC12/TetOn. (A) PC12/TetOn cells expressing α-syn(WT) were exposed to 0.1 or 1 μg/ml of doxycycline for 48 h, and then the UPS inhibitor MG132 or the macroautophagy inhibitor 3-MA was added for further 18 h. Alpha-syn(WT) expression level was then quantified by Western blotting, using α-tubulin as internal standard. The blot quantification (three replicates for each condition) is shown in (B). **: P<0.01, one-way ANOVA followed by Tukey's post-hoc test. (C) The same experiment was carried out using PC12/TetOn overexpressing α-syn(A30P). The graph shown in (D) reports the quantification by densitometric analysis (three replicates for each condition). **: P<0.01, one-way ANOVA followed by Tukey's post-hoc test. (E) Cell viability assessment for the PC12/TetOn cells expressing α-syn(WT). The experimental scheme was the same described in (A). *: P<0.05; **P<0.01, one-way ANOVA followed by Tukey's post-hoc test. (F) Cell viability assay for PC12/TetOn cells expressiong α-syn(A30P) after doxycycline stimulation. The experiment was carried out as detailed in (A); *: P<0.05, one-way ANOVA followed by Tukey's post-hoc test. (G) Representative dot blot showing the effect of 3-MA treatment on α-syn oligomer formation detected by A11 antibody. PC12/TetOn cells were incubated with doxycycline (1 μg/ml) for 48 h, and then 3-MA 10 mM was added for further 18 h. Oligomeric species formation was assessed by A11 reactivity. The bar graph in (H) shows the densitometric quantification of three independent replicates, performed by normalizing the A11 signal to α-tubulin immunoreactivity in the same blot (not shown). For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Provided that with the 3-MA treatment α-syn(WT) showed a marked toxic phenotype, we investigated in this scenario the appearance of α-syn aggregates or oligomeric species. After the treatment with doxycycline 1 μg/ml and 3-MA, thioflavin-T staining did not show evidence of insoluble α-syn(WT) fibrils (data not shown), whereas we noticed an increase of oligomers detected by A11 antibody. In α-syn(A30P) expressing cells, we did not find A11 reactivity following the previously described experimental scheme (Fig. 4G, H).
Discussion
Even if α-syn aggregation is considered of relevance for PD pathogenic process, the molecular mechanism triggering PD starting from α-syn homeostasis alteration is still a matter of debate.
Here, we describe a tightly controllable cellular model of α-syn expression that allowed us to finely tune α-syn intracellular level and find out how the protein concentration affects its function and/or toxicity. Moreover, we compared the familial PD-linked mutation A30P with the wild type form of the protein. When α-syn was expressed at low level, we confirmed a neuroprotective role against oxidative stress, as we have already demonstrated in a different cellular model (Albani et al., 2004). This α-syn-mediated protective function is to be underlined, as oxidative stress is a main feature of PD and neurodegeneration in general, so a loss of this α-syn positive action, for instance as a consequence of its sequestration into LB, might contribute to PD onset. Alpha-syn neuroprotective action was independent from the presence of the mutation A30P, suggesting that this amino acidic substitution does not map within the protein domain required for neuroprotection, probably the C-terminal part that is also involved in other trophic functions of α-syn, like the proliferation of dopaminergic cells (Albani et al., 2004; Kim et al., 2000, 2002; Park et al., 2002; Yin et al., 2011). In this condition of low α-syn expression, we did not notice α-syn aggregation (oligomers or fibrils) or toxicity, likely as a critical concentration threshold value was not reached. Coherently, with doxycycline 0.1 μg/ml no macroautophagy activation was found.
When we tried to induce in PC12/TetOn α-syn aggregation by an enhanced level of α-syn expression obtained by the addition of doxycycline 1 μg/ml, we did not find any aggregate or A11 reactivity both for α-syn(WT) and (A30P), but in this case the autophagic machinery of the cells was activated, suggesting that they were starting protective pathways to cope with the excess of α-syn and prevent aggregate formation. It is of note that, under increased α-syn expression, while α-syn(WT) was still able to counteract oxidative stress, the mutated form lost this function, raising the possibility that in this situation the presence of the A30P mutation had some dose-dependent deleterious effect that nullified the neuroprotective phenotype. On the contrary, the WT form did not aggregate as it was removed, but the amount of soluble α-syn molecules was probably still sufficient to keep the antioxidant property.
As in our model we had evidence of macroautophagy activation after the addition of doxycycline 1 μg/ml, we tried to accumulate α-syn by macroautophagy inhibition. In this situation, α-syn(WT) accumulated inside PC12/TetOn cells, leading to cell death and the formation of oligomers. This result is in agreement with the literature, where macroautophagy was reported to be crucial for α-syn(WT) degradation in neurons (Vogiatzi et al., 2008; Xilouri et al., 2008). Moreover, it was also reported that α-syn was able to inhibit macroautophagy, and probably this strongly contributes to its accumulation with a kind of positive loop, considering that macroautophagy is activated for α-syn removal (Winslow et al., 2010). Proteasome inhibition in PC12/TetOn had no effect on α-syn(WT) accumulation and toxicity, suggesting a minor role for the proteasome in degrading α-syn(WT) in our model. In our experimental setting, α-syn(A30P) was removed by the cells even in presence of macroautophagy inhibition, and in fact the α-syn(A30P) form did not show toxicity and oligomer formation after 3-MA treatment, even if its concentration was slightly increased, but probably to an extent not sufficient to produce a detectable amount of aggregated forms. Clearly, in the case of α-syn(A30P) other cellular degrading pathways are involved, as we demonstrated by proteasome inhibition that increased α-syn(A30P) accumulation, even if also in this case the fraction of α-syn(A30P) that was not degraded was limited and unable to have strong toxic effects. We can speculate that in PC12/TetOn cells α-syn(A30P) expression at high level started multiple degrading pathways, including autophagy and the proteasome, maybe for a more toxic phenotype of the molecule in comparison with the WT, a point in agreement with the loss of viability protection against oxidative stress in the case of α-syn(A30P) only. Consequently, to find aggregation and toxicity of α-syn(A30P) in our model, it is likely that both macroautophagy and the proteasome should be inhibited at the same time. We have attempted to perform this protocol, but unfortunately the double treatment was too toxic for cells. Of course, we do not rule out that in our PC12/TetOn α-syn(A30P) may be partly degraded also by other autophagic pathways like the chaperone-mediated autophagy (CMA), as already reported (Xilouri et al., 2009; Yang et al., 2009).
In conclusion, our results confirmed a neuroprotective role of α-syn at low level of expression against oxidative stress independently from the presence of a pathogenetic mutation and explain why our PC12/TetOn cells overexpressing α-syn(WT) or (A30P) did not show any reduction in cell viability, as they are able to efficiently cope with α-syn overproduction by activating degrading mechanisms, including macroautophagy and the proteasome. However, α-syn(WT) and (A30P) follow different pathways of removal with a stronger involvement of macroautophagy for the WT form, while the mutant A30P elicited multiple degrading pathways (Fig. 5).
Acknowledgments
We are grateful to Alessandro Negro for α-synp RSET plasmids preparation. This work was supported by Italian Telethon Foundation grant n°E.0959 awarded to G.F.
References
- Albani D., Peverelli E., Rametta R., Batelli S., Veschini L., Negro A., Forloni G. Protective effect of TAT-delivered alpha-synuclein: relevance of the C-terminal domain and involvement of HSP70. FASEB J. 2004;18:1713–1715. doi: 10.1096/fj.04-1621fje. [DOI] [PubMed] [Google Scholar]
- Anderson V.L., Ramlall T.F., Rospigliosi C.C., Webb W.W., Eliezer D. Identification of a helical intermediate in trifluoroethanol-induced alpha-synuclein aggregation. Proc Natl Acad Sci U S A. 2010;107:18850–18855. doi: 10.1073/pnas.1012336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris L.M., Mata I.F., Zabetian C.P. The genetics of Parkinson disease. J Geriatr Psychiatry Neurol. 2010;23:228–242. doi: 10.1177/0891988710383572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M.R., Südhof T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway K.A., Lee S.J., Rochet J.C., Ding T.T., Williamson R.E., Lansbury P.T., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson M.R. Parkin's substrates and the pathways leading to neuronal damage. Neuromol Med. 2003;3:1–13. doi: 10.1385/NMM:3:1:1. [DOI] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Di Monte D.A. The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- el-Agnaf O.M., Irvine G.B. Aggregation and neurotoxicity of alpha-synuclein and related peptides. Biochem Soc Trans. 2002;30:559–565. doi: 10.1042/bst0300559. [DOI] [PubMed] [Google Scholar]
- Esteves A.R., Arduíno D.M., Silva D.F., Oliveira C.R., Cardoso S.M. Mitochondrial dysfunction: the road to alpha-synuclein oligomerization in PD. Parkinsons Dis. 2011;2011:693–761. doi: 10.4061/2011/693761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Reitböck P., Anichtchik O., Bellucci A., Iovino M., Ballini C., Fineberg E., Ghetti B., Della Corte L., Spano P., Tofaris G.K., Goedert M., Spillantini M.G. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson's disease. Brain. 2010;133:2032–2044. doi: 10.1093/brain/awq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T. Mendelian forms of Parkinson's disease. Biochim Biophys Acta. 2009;1792:587–596. doi: 10.1016/j.bbadis.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Giasson B.I., Lee V.M. Are ubiquitination pathways central to Parkinson's disease? Cell. 2003;114:1–8. doi: 10.1016/s0092-8674(03)00509-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Hsu L.J., Rockenstein E., Takenouchi T., Mallory M., Masliah E. Alpha-synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J Biol Chem. 2002;277:11465–11472. doi: 10.1074/jbc.M111428200. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Rockenstein E., Masliah E. Transgenic models of alpha-synuclein pathology: past, present, and future. Ann N Y Acad Sci. 2003;991:171–188. [PubMed] [Google Scholar]
- Ischiropoulos H., Beckman J.S. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P.H., Nielsen M.S., Jakes R., Dotti C.G., Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson's disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- Jensen P.J., Alter B.J., O'Malley K.L. Alpha-synuclein protects naive but not dbcAMP-treated dopaminergic cell types from 1-methyl-4-phenylpyridinium toxicity. J Neurochem. 2003;86:196–209. doi: 10.1046/j.1471-4159.2003.01835.x. [DOI] [PubMed] [Google Scholar]
- Jin H., Kanthasamy A., Ghosh A., Yang Y., Anantharam V., Kanthasamy A.G. Alpha-synuclein negatively regulates protein kinase Cdelta expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J Neurosci. 2011;31:2035–2051. doi: 10.1523/JNEUROSCI.5634-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.D., Paik S.R., Yang C.H. Structural and functional implications of C-terminal regions of alpha-synuclein. Biochemistry. 2002;41:13782–13790. doi: 10.1021/bi026284c. [DOI] [PubMed] [Google Scholar]
- Kim T.D., Paik S.R., Yang C.H., Kim J. Structural changes in alpha-synuclein affect its chaperone-like activity in vitro. Protein Sci. 2000;9:2489–2496. doi: 10.1110/ps.9.12.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J.T., Schöls L., Riess O. Ala30-to-pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Kim C., Lee S.J. Alpha-synuclein stimulation of astrocytes: potential role for neuroinflammation and neuroprotection. Oxid Med Cell Longev. 2010;3:283–287. doi: 10.4161/oxim.3.4.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkebo S., Jensen P.H. Alpha-synuclein and presynaptic function: implications for Parkinson's disease. Neuromol Med. 2002;2:115–129. doi: 10.1385/NMM:2:2:115. [DOI] [PubMed] [Google Scholar]
- Manning-Bog A.B., McCormack A.L., Purisai M.G., Bolin L.M., Di Monte D.A. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris E.H., Giasson B.I., Ischiropoulos H., Lee V.M. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003;278:27230–27240. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- Oaks A.W., Sidhu A. Synuclein modulation of monoamine transporters. FEBS Lett. 2011;585:1001–1006. doi: 10.1016/j.febslet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterova N., Petrucelli L., Farrer M., Mehta N., Choi P., Hardy J., Wolozin B. Alpha-synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.M., Jung H.Y., Kim T.D., Park J.H., Yang C.H., Kim J. Distinct roles of the N-terminal-binding domain and the C-terminal-solubilizing domain of alpha-synuclein, a molecular chaperone. J Biol Chem. 2002;277:28512–28520. doi: 10.1074/jbc.M111971200. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E.S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W.G., Lazzarini A.M., Duvoisin R.C., Di Iorio G., Golbe L.I., Nussbaum R.L. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Riedel M., Goldbaum O., Wille M., Richter-Landsberg C. Membrane lipid modification by docosahexaenoic acid (DHA) promotes the formation of α-synuclein inclusion bodies immunopositive for SUMO-1 in oligodendroglial cells after oxidative stress. J Mol Neurosci. 2011;43:290–302. doi: 10.1007/s12031-010-9439-5. [DOI] [PubMed] [Google Scholar]
- Rossi F.M., Blau H.M. Recent advances in inducible gene expression systems. Curr Opin Biotechnol. 1998;9:451–456. doi: 10.1016/s0958-1669(98)80028-1. [DOI] [PubMed] [Google Scholar]
- Saha A.R., Hill J., Utton M.A., Asuni A.A., Ackerley S., Grierson A.J., Miller C.C., Davies A.M., Buchman V.L., Anderton B.H., Hanger D.P. Parkinson's disease alpha-synuclein mutations exhibit defective axonal transport in cultured neurons. J Cell Sci. 2004;117:1017–1724. doi: 10.1242/jcs.00967. [DOI] [PubMed] [Google Scholar]
- Scott D.A., Tabarean I., Tang Y., Cartier A., Masliah E., Roy S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.H., Rah J.C., Choi S.H., Shin J.K., Min K., Kim H.S., Park C.H., Kim S., Kim E.M., Lee S.H., Lee S., Suh S.W., Suh Y.H. Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. FASEB J. 2002;16:1826–1828. doi: 10.1096/fj.02-0041fje. [DOI] [PubMed] [Google Scholar]
- Sherer T.B., Kim J.H., Betarbet R., Greenamyre J.T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M.R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Uversky V.N., Eliezer D. Biophysics of Parkinson's disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci. 2009;10:483–499. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzi T., Xilouri M., Vekrellis K., Stefanis L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem. 2008;283:23542–23556. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volles M.J., Lansbury P.T., Jr Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson's disease. Biochemistry. 2003;42:7871–7878. doi: 10.1021/bi030086j. [DOI] [PubMed] [Google Scholar]
- Warner T.T., Schapira A.H. Genetic and environmental factors in the cause of Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S16–S23. doi: 10.1002/ana.10487. [DOI] [PubMed] [Google Scholar]
- Wersinger C., Prou D., Vernier P., Niznik H.B., Sidhu A. Mutations in the lipid-binding domain of alpha-synuclein confer overlapping, yet distinct, functional properties in the regulation of dopamine transporter activity. Mol Cell Neurosci. 2003;24:91–105. doi: 10.1016/s1044-7431(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Winslow A.R., Chen C.W., Corrochano S., Acevedo-Arozena A., Gordon D.E., Peden A.A., Lichtenberg M., Menzies F.M., Ravikumar B., Imarisio S., Brown S., O'Kane C.J., Rubinsztein D.C. α-Synuclein impairs macroautophagy: implications for Parkinson's disease. J Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Li X., Li C., Zhu W., Jankovic J., Le W. Proteasome inhibition modeling nigral neuron degeneration in Parkinson's disease. J Neurochem. 2010;115:188–199. doi: 10.1111/j.1471-4159.2010.06914.x. [DOI] [PubMed] [Google Scholar]
- Xilouri M., Vogiatzi T., Vekrellis K., Park D., Stefanis L. Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One. 2009;4(5):e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M., Vogiatzi T., Vekrellis K., Stefanis L. Alpha-synuclein degradation by autophagic pathways: a potential key to Parkinson's disease pathogenesis. Autophagy. 2008;4:917–919. doi: 10.4161/auto.6685. [DOI] [PubMed] [Google Scholar]
- Yang Q., She H., Gearing M., Colla E., Lee M., Shacka J.J., Mao Z. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science. 2009;323:124–127. doi: 10.1126/science.1166088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Han J., Zhang C., Ma Q.L., Li X., Cheng F., Liu G., Li Y., Uéda K., Chan P., Yu S. C-terminal part of α-synuclein mediates its activity in promoting proliferation of dopaminergic cells. J Neural Transm. 2011;118:1155–1164. doi: 10.1007/s00702-011-0592-y. [DOI] [PubMed] [Google Scholar]
- Zarranz J.J., Alegre J., Gómez-Esteban J.C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D.G., de Yebenes J.G. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]