Highlights
► The surface of endosomes provides a stage to assemble signaling complexes, to transport signaling molecules and to modify and terminate signal transduction. ► Several important signaling molecules, including RhoGTPases, Src, and MAPK were shown to utilize signaling endosomes to regulate cell migration.
Abstract
Cell migration is a complex biological process that is under the tight control of diverse signaling events. While many of the involved signaling molecules diffuse rapidly within cells, it now seems that certain key regulators of cell migration prefer to travel on endosomes. In this review we will discuss the multiple roles of signaling endosomes in regulation of local migration stimuli, dynamics of focal adhesions, cell contractility and locomotion.
Introduction
Cellular movement on the extracellular matrix requires the correct processing of a multitude of in-going and out-going signals. These signals control different events that, once coordinated and fine-tuned, enable a cell to migrate into a certain direction [1–3]. At first, cells polarize and spread out lamellipodia where multiple focal complexes (FC) are formed. Then, FC either rapidly turnover or grow and mature into focal adhesions (FA) [4–7]. Mature FA form the anchoring plaques that connect the cells with extracellular matrix during cell migration. Once mature, FA will stop to grow, remain stationary and in the end gradually disassemble at the trailing edge as the cell proceeds locomotion [8]. Sophisticated mechanisms coordinate these events in space and time. The role of the endosomal membrane system has recently attracted much attention, since these intracellular vesicles unexpectedly appear to manage critical aspects of cell migration [9–12]. In some ways, endosomes could be considered as containers, that utilize microtubule or/and actin filaments, to be directed to the right place at the right time [13]. There, the cytoplasmic surface of endosomes provides a platform for the assembly of specific signaling complexes, which will affect key processes during cell migration. Thus, the chief object of this review is to delineate the intimate relationship of endosomal signaling and directed cell movement. The role of endosomal integrin trafficking, recycling, and degradation in cell migration will not be discussed here but has been subject to excellent reviews elsewhere [14–16]. We will focus on specific sub-populations of endosomes carrying signaling molecules that regulate cell migration. What makes these endosomes different from other endosomes is a key question that for now must remain unanswered.
While the role of endosomes as a principal force for forward movement has been postulated already some years back by Mark Bretscher [17,18], it is now becoming evident that early as well as late endosomes play a rather surprising role in the local regulation of each step of cell migration [19•,20••,21••,22•] (see Figure 1).
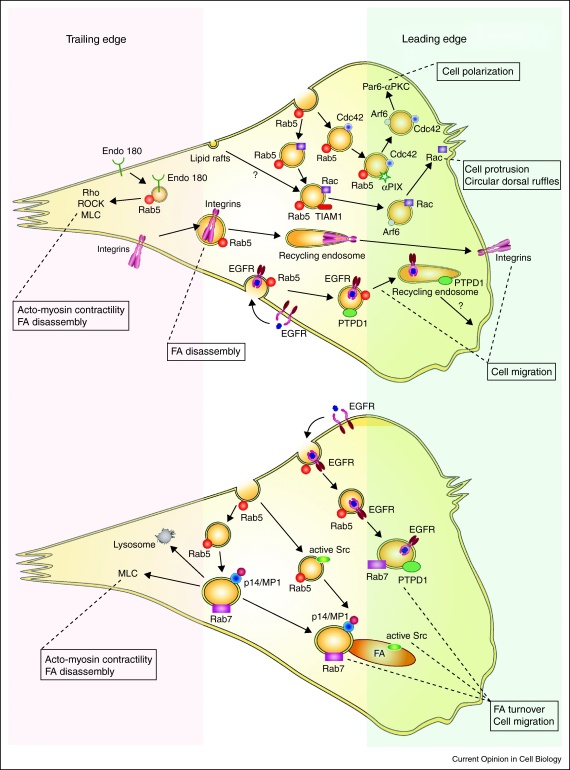
Figure 1.
Regulation of cell migration by signaling endosomes. Leading edge: Cellular polarization, formation of the cell protrusion, lamellipodia and circular ruffles are controlled by the small GTPases Cdc42 and Rac. They are transported toward the plasma membrane on early endosomes in an Arf6-dependent manner. Activation of Cdc42 and Rac involves endosomal association of their GEFs, αPIX and Tiam1, respectively. Src moves on early endosomes toward focal adhesions. PTPD1 localizes to early, late and recycling endosomes upon EGF receptor endocytosis. Late endosomes carrying p14/MP1 MAPK scaffold complex can target focal adhesion and serve as a signaling platform for MAPK pathway (lower panel). Trailing edge: Focal adhesion disassembly requires integrin endocytosis and acto-myosin contractility. Integrins are endocytosed into early endosomes and are further recycled back to the leading edge to form new adhesions. Acto-myosin contractility is locally regulated by Endo180-carrying early endosomes through Rho-ROCK-MLC pathway and potentially through p14/MP1 late endosomes (lower panel).
Cell polarity and formation of the leading edge
Formation and protrusion of the leading edge involves the activation of the small Ras-like GTPases Rho, Rac and Cdc42 [23–25]. Downstream effectors of Rac coordinate actin assembly, formation of lamellipodia and membrane ruffles [26]. Cdc42 regulates cell polarity and the formation of filopodia through a wide variety of downstream effectors [27]. A precise spatial and temporal control of Rac/Cdc42 activation at the plasma membrane is therefore required to coordinate cell migration. Interestingly, both Rac and Cdc42 utilize early Rab5 positive endosomes to traffic to specific regions at the plasma membrane [20••,21••,28] (Figure 1, upper panel).
Rac is recruited to Tiam1 (RacGEF)-containing early endosomes that subsequently transport Rac towards the plasma membrane during the formation of circular dorsal ruffles [20••]. Interestingly, the targeting of these ‘Rac-vesicles’ to the plasma membrane requires the activity of yet another GTPase, Arf6 (ADP-ribosylation factor 6). Arf6 functions as a regulator of endocytosis, endosomal recycling (e.g. beta1 integrin) as well as actin and membrane remodeling [29–31]. At the plasma membrane, integrin-mediated adhesions provide high-affinity binding sites for Rac in the form of lipid rafts [32]. Thus it seems plausible that Rac may be delivered on early endosomes to integrin containing microdomains at the plasma membrane (Figure 1).
Like Rac, Cdc42 was detected on early endosomes that target the leading edge of migrating cells. While the recruitment of Cdc42 to endosomes is independent of Arf6, the re-localization of Cdc42 and its GEF betaPIX from endosomes to the leading edge requires Arf6 activity [21••] (see Figure 1, upper panel). This Arf6-dependent recruitment of Cdc42 to the leading edge was important for the activation of Cdc42-mediated polarity, the subsequent activation of the Par6-aPKC pathway and the polarized reorganization of the microtubule network (APC accumulation at the microtubule tips) [21••]. By controlling the re-localization of the early endosomal pool of Cdc42 and Rac to the leading edge, Arf6 could be considered as a master regulator of endosomal signaling during cell migration [33].
However, unanswered remains the question, whether Rac and Cdc42 are recruited to the same specialized subpopulation of early endosomes or to distinct subpopulations to act together? Recent work suggests a common regulatory mechanism for Rac and Cdc42 endosomal trafficking after PDGF stimulation through RhoB [28], the latter one known to localize to endosomes and to regulate endosomal transport events. While it is evident that the endocytic transport of integrins from and back to the cell surface plays a key role during cell migration [34,35], experimental evidence for the direct targeting of FC/FA by early Rac/Cdc42-carrying endosomes is still missing. The use of fluorescent protein biosensors in combination with fast high resolution life cell microscopy could help to better define the function of Rac/Cdc42-carrying endosomes.
Regulation of FA turnover
The assembly and disassembly of FA are controlled by many different signaling molecules [2,23,25,36,37]. For example, the cytosolic non-receptor protein-tyrosine phosphatase PTPD1 localizes to actin as well as to FA, where it recruits Src. Once recruited, Src modulates Src-FAK (focal adhesion kinase) signaling [38] and thereby FA dynamics. Interestingly both PTPD1 and Src utilize, to some extent, endosomes to regulate cell migration [22•,39] (Figure 1, upper panel). PTPD1 is rapidly recruited in a kinesin (KIF16B) dependent manner to EGF-receptor (EGF-R) positive endosomes, upon growth factor stimulation (EGF) [40•]. At first, PTPD1 is present in early (EEA1) and late endosomes (Rab7) but finally accumulates in recycling endosomes (Rab11) (Figure 1, upper and lower panels). Interestingly PTPD1 appears to function during cell migration and endosomal traffic, as RNAi mediated PTPD1 depletion slows down cell migration and enhances lysosomal degradation of the EGF-R. However, it remains an open question how PTPD1 exerts these different functions. If it either dephosphorylates endosomal targets and/or rather acts as a scaffold protein for signaling molecules remains unclear. Thus, a direct role of endosomal PTPD1 in FA signaling still requires further investigation.
Src localizes to endosomes and to FA [41–43] and appears to dynamically shuttle between these different locations [39,44]. Yet, the role of endosomal Src remains largely unclear. On the one hand, it appears that the late endosomal fraction of Src can be activated and that this could be an essential step in the recruitment to FA, without the need to transit Rab11 positive recycling endosomes. Instead the Endosomal Sorting Complexes Required for Transport (tsg101, an ESCRT-I subunit) were required to transport activated Src along late endosomes to FA [22•] (Figure 1, lower panel). On the other hand, it seems that inactive c-Src localizes to endosomes, whereas the active Src fraction resides at FA [41–44,45], which requires the endocytic recycling compartment [42]. While much evidence argues in favor of endosomal Src signaling, the underlying molecular mechanisms remain to be defined.
Acto-myosin contractility
The assembly and disassembly of FA requires contractile forces. The Rho-ROCK cascade regulates acto-myosin contractility through myosin light chain 2 (MLC2). Its signaling appears to be, at least in part, regulated, by the directional endosomal transport of Endo180 (also named CD280, uPARAP, and MRC2), a pro-migratory collagen receptor involved in Rho-ROCK signaling [19•,46–48]. While Endo180 traffics on early and recycling endosomes, it co-localizes with MLC2 in places with increased contractile signals [19•] (Figure 1, upper panel). Interestingly, Endo180 mediates phosphorylation of MLC2 and thereby regulates tail-retraction [19•]. Moreover, Endo180 also seems to be required for the activation of Rho-ROCK signaling, specifically the subsequent phosphorylation of myosin phosphatase 1 (MYPT1) and Lim Kinase 1/2 (LIMK). When Endo180 is trapped on recycling endosomes, the phosphorylation of MLC2 and in consequence the contractile response increases. Thus, Endo180 containing endosomes are localized directly at sites of adhesion turnover to transmit their contractile signals through Rho-ROCK-MLC2 directly at sites of adhesion turnover [19•]. However, it is not yet clear, how Endo180 connects to Rho-ROCK-MLC2 signaling and if the role of Endo180 in cell contractility is a direct consequence of Endo180–collagen interaction.
Summary and outlook
Extracellular signals, that originate at the plasma membrane can be effectively extended and modified by endosomal signaling. Different subpopulations of specialized endosomes could contribute to the modulation of compartmentalized signal transduction in distinct ways. In a ‘passive performance’ model, different signaling pathways are associated with distinct endosomal populations and thus are separated from each other. Such ‘passive performance’ could occur on the surface of early, late and recycling endosomes. Upon ligand binding, activated receptors are removed from the cell surface and transported into the endosomal membrane system. During their endosomal passage the activated receptors will continue to activate cytoplasmic signaling cascades, mainly by the help of endosomal scaffold proteins. How these specialized signaling endosomes receive their guiding cue to home into their destinations is not clear. One possible mechanism could potentially involve signaling from internalized receptors to either recruit or control motor proteins to carry these signaling endosomes toward specific locations. Once their tasks are completed and receptor signaling is no longer required, these receptors become finally ubiquitinated and will be degraded in lysosomes via the multivesicular body pathway. During cell migration, such a mechanism could assure execution of the contractile signaling in the trailing edge, where increasing numbers of signaling endosomes will guarantee compartmentalized signaling fidelity. This might be the case for Endo180-carrying endosomes [19•], p14/MP1 late endosomes (unpublished data) as well as for the Src trafficking toward FA [22•].
Yet, the rapid endosomal transport of Rac and Cdc42 on early endosomes also suggests the existence of an ‘active performance’ model, where (most frequently) early endosomes deliver signaling components very quickly and with high accuracy. This model most would involve the rapid recycling of activated receptors back to the plasma membrane thereby enabling rapid reconstitution of the pool membrane receptors to receive and process ongoing migration/chemotactic stimuli. How this scenario functions in cell migration, initiates signaling and the recruitment of motor proteins ‘on demand’ requires further investigations.
Probably the most likely scenario is a mixed version, in which some of the signaling molecules use endosomes for both modes as ‘passive’ and ‘active’ signaling entities. This appears plausible for both, PTPD1 and Src [40•], which emerge to have broader functions in cell migration. Maybe recruitment of PTPD1 to late endosomes could assist Src trafficking and function, whereas recycling of PTPD1 through Rab11 endosomes favors EGF-R recycling [40•].
Focal adhesions contain a complex and multilayered molecular architecture [49•]. At least three spatial and functional FA compartments are described: an integrin signaling layer, which also includes paxillin and FAK; a force transduction layer and an actin regulatory layer, consisting of different FA and actin-binding proteins. How are these different layers affected and regulated by endosomal trafficking? How is this astonishing specificity reached? One could assume that the integrin signaling layer is regulated mainly through internalization and endocytosis of the β-integrin subunits. Force transduction and actin regulatory layers might be regulated, at least in part, by signaling endosomes that deliver small GTPases, MAPKs, etc.
The advent of high resolution microscopy techniques as well as quantitative proteomics in combination with clever genetic manipulation may help to address how endosomes can target the ‘deepest’ integrin signaling layer to manipulate phosphorylation events; and how these endosomes differ from the ones, which regulate the actin regulatory and force transduction layers.
Signaling endosomes are about to become key players in cells. Yet the molecular mechanisms that send these players to their ‘home base’ are unclear. Which are the signals that target them to specific locations and which motor protein takes them to their site of action? In the future it will be important to understand the positioning of signaling endosomes and their targeting to specific subcellular locations.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We would like to apologize to our colleagues whose work could not be cited here because of space restrictions. Work in the Huber and Teis laboratories is supported by the SFB021 ‘Cell Proliferation and Cell Death in Tumors’ of the FWF and the COMET Center ONCOTYROL, by the Federal Ministry for Transport Innovation and Technology (BMVIT), the Federal Ministry of Economics and Labour the Federal Ministry of Economy, Family and Youth (BMWA/BMWFJ) and the Tiroler Standortagentur and HFSP.
References
- 1.Lauffenburger D.A., Horwitz A.F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 2.Huang C., Jacobson K., Schaller M.D. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 3.Wehrle-Haller B., Imhof B. The inner lives of focal adhesions. Trends Cell Biol. 2002;12:382–389. doi: 10.1016/s0962-8924(02)02321-8. [DOI] [PubMed] [Google Scholar]
- 4.Bershadsky A.D., Tint I.S., Neyfakh A.A., Jr., Vasiliev J.M. Focal contacts of normal and RSV-transformed quail cells. Hypothesis of the transformation-induced deficient maturation of focal contacts. Exp Cell Res. 1985;158:433–444. doi: 10.1016/0014-4827(85)90467-7. [DOI] [PubMed] [Google Scholar]
- 5.Clark E.A., King W.G., Brugge J.S., Symons M., Hynes R.O. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rottner K., Hall A., Small J.V. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 7.Riveline D., Zamir E., Balaban N.Q., Schwarz U.S., Ishizaki T., Narumiya S., Kam Z., Geiger B., Bershadsky A.D. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfenson H., Lubelski A., Regev T., Klafter J., Henis Y.I., Geiger B. A role for the juxtamembrane cytoplasm in the molecular dynamics of focal adhesions. PLoS One. 2009;4:e4304. doi: 10.1371/journal.pone.0004304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baass P.C., Di Guglielmo G.M., Authier F., Posner B.I., Bergeron J.J. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 1995;5:465–470. doi: 10.1016/s0962-8924(00)89116-3. [DOI] [PubMed] [Google Scholar]
- 10.Grimes M.L., Zhou J., Beattie E.C., Yuen E.C., Hall D.E., Valletta J.S., Topp K.S., LaVail J.H., Bunnett N.W., Mobley W.C. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadowski L., Pilecka I., Miaczynska M. Signaling from endosomes: location makes a difference. Exp Cell Res. 2009;315:1601–1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Scita G., Di Fiore P.P. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 13.Goode B.L., Drubin D.G., Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 14.Wickstrom S.A., Fassler R. Regulation of membrane traffic by integrin signaling. Trends Cell Biol. 2011 doi: 10.1016/j.tcb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Caswell P., Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008 doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Lobert V.H., Brech A., Pedersen N.M., Wesche J., Oppelt A., Malerod L., Stenmark H. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin–integrin complexes. Dev Cell. 2010;19:148–159. doi: 10.1016/j.devcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Bretscher M.S. Directed lipid flow in cell membranes. Nature. 1976;260:21–23. doi: 10.1038/260021a0. [DOI] [PubMed] [Google Scholar]
- 18.Bretscher M.S. Endocytosis: relation to capping and cell locomotion. Science. 1984;224:681–686. doi: 10.1126/science.6719108. [DOI] [PubMed] [Google Scholar]
- 19•.Sturge J., Wienke D., Isacke C.M. Endosomes generate localized Rho-ROCK-MLC2-based contractile signals via Endo180 to promote adhesion disassembly. J Cell Biol. 2006;175:337–347. doi: 10.1083/jcb.200602125. [DOI] [PMC free article] [PubMed] [Google Scholar]; Endo180 located to early endosomes was shown to locally regulate Rho-ROCK-MLC2 contractility.
- 20••.Palamidessi A., Frittoli E., Garre M., Faretta M., Mione M., Testa I., Diaspro A., Lanzetti L., Scita G., Di Fiore P.P. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]; Early endosomal trafficking of Rac was demonstrated to regulate lammelipodia formation and cell migration in Arf6-dependent manner.
- 21••.Osmani N., Peglion F., Chavrier P., Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol. 2010;191:1261–1269. doi: 10.1083/jcb.201003091. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cdc42 localization and transport on early endosomes toward the leading edge was shown to regulate cell polarity.
- 22•.Tu C., Ortega-Cava C.F., Winograd P., Stanton M.J., Reddi A.L., Dodge I., Arya R., Dimri M., Clubb R.J., Naramura M. Endosomal-sorting complexes required for transport (ESCRT) pathway-dependent endosomal traffic regulates the localization of active Src at focal adhesions. Proc Natl Acad Sci U S A. 2010;107:16107–16112. doi: 10.1073/pnas.1009471107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Src was found to be active when transported on late Rab7 endosomes. Endosomal trafficking was shown to be crucial for the Src function in cell migration and focal adhesion regulation.
- 23.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 24.Ridley A.J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Parri M., Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 27.Etienne-Manneville S. Cdc42--the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 28.Huang M., Satchell L., Duhadaway J.B., Prendergast G.C., Laury-Kleintop L.D. RHOB links pdgf signaling to cell migration by coordinating activation and localization of CDC42 and Rac. J Cell Biochem. 2011 doi: 10.1002/jcb.23069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown F.D., Rozelle A.L., Yin H.L., Balla T., Donaldson J.G. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Souza-Schorey C., Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 31.Powelka A.M., Sun J., Li J., Gao M., Shaw L.M., Sonnenberg A., Hsu V.W. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 32.del Pozo M.A., Alderson N.B., Kiosses W.B., Chiang H.H., Anderson R.G., Schwartz M.A. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 33.Schweitzer J.K., Sedgwick A.E., D'Souza-Schorey C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin Cell Dev Biol. 2011;22:39–47. doi: 10.1016/j.semcdb.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caswell P.T., Vadrevu S., Norman J.C. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 35.Subramani D., Alahari S.K. Integrin-mediated function of Rab GTPases in cancer progression. Mol Cancer. 2010;9:312. doi: 10.1186/1476-4598-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiger B., Spatz J.P., Bershadsky A.D. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 37.Parsons J.T., Martin K.H., Slack J.K., Taylor J.M., Weed S.A. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 38.Carlucci A., Gedressi C., Lignitto L., Nezi L., Villa-Moruzzi E., Avvedimento E.V., Gottesman M., Garbi C., Feliciello A. Protein-tyrosine phosphatase PTPD1 regulates focal adhesion kinase autophosphorylation and cell migration. J Biol Chem. 2008;283:10919–10929. doi: 10.1074/jbc.M707248200. [DOI] [PubMed] [Google Scholar]
- 39.Fincham V.J., Brunton V.G., Frame M.C. The SH3 domain directs acto-myosin-dependent targeting of v-Src to focal adhesions via phosphatidylinositol 3-kinase. Mol Cell Biol. 2000;20:6518–6536. doi: 10.1128/mcb.20.17.6518-6536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Carlucci A., Porpora M., Garbi C., Galgani M., Santoriello M., Mascolo M., di Lorenzo D., Altieri V., Quarto M., Terracciano L. PTPD1 supports receptor stability and mitogenic signaling in bladder cancer cells. J Biol Chem. 2010;285:39260–39270. doi: 10.1074/jbc.M110.174706. [DOI] [PMC free article] [PubMed] [Google Scholar]; PTPD1 was found to localize to endosomes and to utilize both recycling and degradation endocytic pathways to support cell migration.
- 41.Sandilands E., Brunton V.G., Frame M.C. The membrane targeting and spatial activation of Src, Yes and Fyn is influenced by palmitoylation and distinct RhoB/RhoD endosome requirements. J Cell Sci. 2007;120:2555–2564. doi: 10.1242/jcs.003657. [DOI] [PubMed] [Google Scholar]
- 42.Sandilands E., Cans C., Fincham V.J., Brunton V.G., Mellor H., Prendergast G.C., Norman J.C., Superti-Furga G., Frame M.C. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell. 2004;7:855–869. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Kasahara K., Nakayama Y., Kihara A., Matsuda D., Ikeda K., Kuga T., Fukumoto Y., Igarashi Y., Yamaguchi N. Rapid trafficking of c-Src, a non-palmitoylated Src-family kinase, between the plasma membrane and late endosomes/lysosomes. Exp Cell Res. 2007;313:2651–2666. doi: 10.1016/j.yexcr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Fincham V.J., Frame M.C. The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J. 1998;17:81–92. doi: 10.1093/emboj/17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fincham V.J., James M., Frame M.C., Winder S.J. Active ERK/MAP kinase is targeted to newly forming cell–matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.East L., McCarthy A., Wienke D., Sturge J., Ashworth A., Isacke C.M. A targeted deletion in the endocytic receptor gene Endo180 results in a defect in collagen uptake. EMBO Rep. 2003;4:710–716. doi: 10.1038/sj.embor.embor882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelholm L.H., List K., Netzel-Arnett S., Cukierman E., Mitola D.J., Aaronson H., Kjoller L., Larsen J.K., Yamada K.M., Strickland D.K. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J Cell Biol. 2003;160:1009–1015. doi: 10.1083/jcb.200211091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sturge J., Wienke D., East L., Jones G.E., Isacke C.M. GPI-anchored uPAR requires Endo180 for rapid directional sensing during chemotaxis. J Cell Biol. 2003;162:789–794. doi: 10.1083/jcb.200302124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Kanchanawong P., Shtengel G., Pasapera A.M., Ramko E.B., Davidson M.W., Hess H.F., Waterman C.M. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors elegantly showed multilayer structure of focal adhesion on the protein level, combining previously obtained knowledge with advanced microscopy techniques.