Abstract
Clinical trials have demonstrated the potential of ex vivo hematopoietic stem cell gene therapy to treat X-linked severe combined immunodeficiency (SCID-X1) using γ-retroviral vectors, leading to immune system functionality in the majority of treated patients without pretransplant conditioning. The success was tempered by insertional oncogenesis in a proportion of the patients. To reduce the genotoxicity risk, a self-inactivating (SIN) lentiviral vector (LV) with improved expression of a codon optimized human interleukin-2 receptor γ gene (IL2RG) cDNA (coγc), regulated by its 1.1 kb promoter region (γcPr), was compared in efficacy to the viral spleen focus forming virus (SF) and the cellular phosphoglycerate kinase (PGK) promoters. Pretransplant conditioning of Il2rg−/− mice resulted in long-term reconstitution of T and B lymphocytes, normalized natural antibody titers, humoral immune responses, ConA/IL-2 stimulated spleen cell proliferation, and polyclonal T-cell receptor gene rearrangements with a clear integration preference of the SF vector for proto-oncogenes, contrary to the PGK and γcPr vectors. We conclude that SIN lentiviral gene therapy using coγc driven by the γcPr or PGK promoter corrects the SCID phenotype, potentially with an improved safety profile, and that low-dose conditioning proved essential for immune competence, allowing for a reduced threshold of cell numbers required.
Introduction
X-linked severe combined immunodeficiency disease (SCID-X1) is a rare disorder caused by mutations in the interleukin-2 receptor γ gene (IL2RG) leading to a nonfunctional common γ-chain protein (γc). The γc protein is a shared subunit of IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 signaling, critical for growth and maturation of lymphocyte populations.1 Patients suffer from a complete absence of mature T and natural killer (NK) cells. B cells are present but dysfunctional in SCID-X1 humans and absent in Il2rg−/− mice. Without treatment, patients succumb to severe, recurrent infections within the first year of life. Bone marrow (BM) transplantation from an HLA-identical donor is life-saving with a high success rate.2 However, HLA-identical donors are only available to one out of every three patients. Non-HLA-identical BM transplantation has been attempted but carries a vastly increased risk of complications and mortality, making SCID-X1 an excellent candidate for clinical gene therapy trials for those patients who lack such a donor.
The efficacy of γ-retroviral (RV) gene therapy to successfully treat SCID-X1 was demonstrated in seminal clinical trials.3,4 However, some patients required immunoglobulin-replacement therapy. Additionally, 5 out of 20 patients developed leukemia several years post-therapy due to RV-induced insertional mutagenesis, which in most cases was successfully treated without reducing the effectiveness of the gene therapy.5,6,7
To increase safety of future gene therapy treatment, the use of self-inactivating (SIN) RV and lentiviral vectors (LVs) incorporating internal promoters has been proposed, which should reduce the risk of undesirable activation of genes adjacent to the integration site8,9 and the bias toward integrating near transcription start sites.10,11,12 LV are less vulnerable to silencing than RV13 and do not require ex vivo growth factor stimulation, thereby preventing loss of stem cell repopulating capacity.
We generated a series of third generation SIN LVs with a codon-optimized human IL2RG open reading frame (hereafter designated coγc) that markedly improved mRNA transcription and translation.14,15 Transgene expression was driven by the strong spleen focus forming virus (SF) promoter to determine the potential genotoxicity of the procedure. For clinical application, the human phosphoglycerate kinase16 (PGK) promoter and a ∼1.1 kb section of the native IL2RG promoter sequence17 (γcPr) were tested in Il2rg−/− mice, since cellular/physiological promoters have been suggested to further improve vector safety.18 We found that the clinical LVs provided safe and functional correction of the phenotype of SCID-X1 mice, and that the level of pretransplant conditioning was a critical determinant for successful B cell reconstitution and immunoglobulin responses.
Results
LV construction for SCID-X1 gene therapy
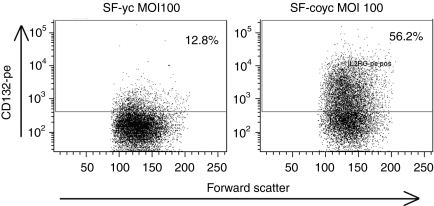
A SIN LV19,20 incorporating part of the native human IL2RG complementary DNA (cDNA) driven by the viral SF promoter21 was improved for efficacy by replacement of the native IL2RG by the coγc sequence, resulting in an average 8.4-fold increase in viral titer, a 3.6-fold increase in transgene mRNA expression per integration, and a 33-fold increase in IL2RG protein expression corresponding to a threefold increase in IL2RG protein membrane expression (Tables 1 and 2 and Figure 1). In addition, the cellular PGK or γcPr promoter was used to control coγc expression, the latter shown capable of driving transgene expression in lymphoid cell lines.17
Table 1. Titer comparison of SF-γc and SF-coγc LV vectors.
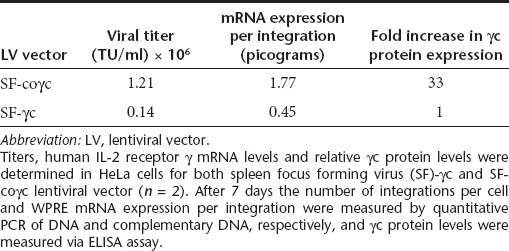
Table 2. Comparison of surface γc expression between SF-γc and SF-coγc LV.
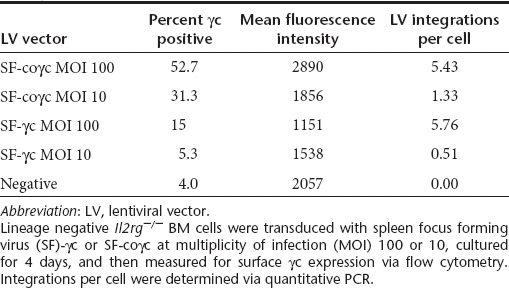
Figure 1.
Comparison of codon-optimized γc vector versus non-optimized in vitro. Flow cytometry images of lin- cells transduced with either LV-SF-γc or LV-SF-coγc at a HeLa MOI of 100. Surface expression of CD132 is much higher in the cells transduced with the codon-optimized vector, with a similar number of integrations per cell found in both vectors (5.4 for coγc and 5.8 for γc). LV, lentiviral vector; MOI, multiplicity of infection; SF, spleen focus forming virus.
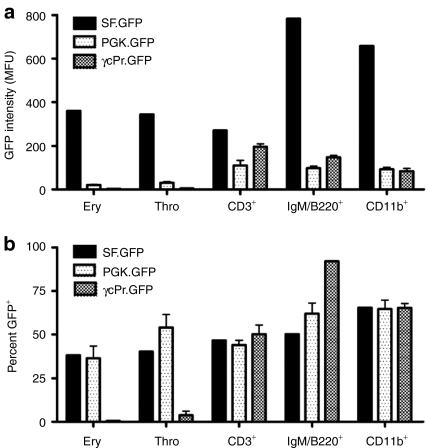
Wild-type lineage depletion (Lin−) BM cells were transduced with a non-saturating but fixed multiplicity of infection (MOI) of γcPROM-GFP (BM copy number per cell: 4.9), PGK-GFP (BM copy number per cell: not determined) or SF-GFP LV vector (BM copy number per cell: 3.2) and transplanted in 6 Gy sublethal TBI conditioned mice to compare promoter specificity and activity (Figure 2). In the γcPr-GFP mice, green fluorescent protein (GFP) expression in peripheral blood 3 months post-transplantation was present on average in 50% of CD3+ cells, 92% of IgM/B220+ cells and 65% of CD11b+ cells, whereas expression in erythrocytes and platelets was negligible. Similar percentages of GFP expression were observed in the constitutively active SF-GFP group, with detectable GFP levels in erythrocytes and thrombocytes (36–42%), similar to the constitutively active PGK promoter. Fluorescence intensity in CD3+ cells was similar across all three promoters, but GFP intensity was for the SF-GFP group fivefold higher in B cells and fourfold higher in CD11b+ cells compared to the physiological promoters (Figure 2b). GFP expression in γcPr-GFP-transduced cells was stable for 8 months. We concluded that the γc promoter element displayed improved stable selectivity with a lower expression level compared to the SF promoter.
Figure 2.
Comparison of SF, PGK, and γcPr promoter expression in vivo. (a) Represents the fluorescence intensity (mean fluorescent units or MFU) of GFP expression in erythrocytes (ery), thrombocytes (thro), T lymphocytes (CD3+), B lymphocytes (IgM/B220+) and myeloid cells (CD11b+) in Il2rg−/− mice 3 months after transplantation of wild-type Lin− cells transduced with SF-GFP (dark gray bars, n = 2), PGK-GFP (light gray bars, n = 3) or γcPr-GFP (black bars, n = 3). (b) Shows the proportion of GFP positive cells. GFP, green fluorescent protein; PGK, phosphoglycerate kinase; SF, spleen focus forming virus.
T- and B-cell reconstitution following lentiviral gene therapy for murine SCID-X1
4 × 105 SF-coγc, γcPr-coγc (both MOI 9–10) or PGK-coγc (MOI 2, due to low vector titer) transduced male Il2rg−/− Lin− bone marrow cells were transplanted into 6 Gy irradiated female Il2rg−/− recipients (n = 5 for PGK and n = 4 for SF and γcPr). As controls, Il2rg−/− and wild-type Lin− BM cells were transduced with γcPr-GFP and transplanted into Il2rg−/− mice (n = 4 and n = 3, respectively). The number of integrations per BM cell corrected for donor/recipient chimerism was proportional to the MOI, reaching 2.1, 0.9 and 2.0 for the groups SF-coγc, PGK-coγc and γcPr-coγc respectively (Supplementary Table S1). The control groups containing the γcPr-GFP vector on average had 4.9 integrations for wild-type transplanted cells and 4.0 integrations for Il2rg−/− cells. Polyclonal integration patterns were confirmed for all vectors by linear amplification–mediated PCR (LAM-PCR) of BM DNA at 8 months post-transplant (Supplementary Figure S1). An initial, limited analysis revealed the LV characteristic preferential integration in active transcription units, with no obvious preference for transcription start sites. Of note, potential oncogenes, listed in the mouse retrovirus tagged cancer gene database22 were over-represented among integrations of vectors containing the SF promoter. Contrary to the vectors with cellular promoters, almost 15% of SF integrations were found within 100 kb of a gene listed in the retrovirus tagged cancer gene database (Supplementary Table S3). Even in this limited data set, this difference is statistically significant (P = 0.02).
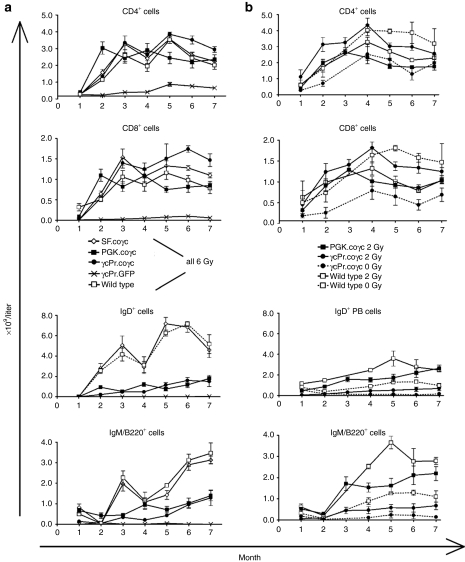
Peripheral white blood cell counts increased in all gene therapy treated mice and were stable for 7 months post-therapy. T- and B-lymphocyte reconstitution is shown in Figure 3a. Animals transplanted with Il2rg−/− cells treated with γcPr-GFP had 7–11% peripheral blood CD3+CD4+ cells, depending on the time interval after transplantation, and were negative for CD8+, B220+IgM+, IgD+ and CD19+ cells. Animals transplanted with Il2rg−/− cells treated with SF-coγc, PGK-coγc or γcPr-coγc began to recover T- and B-cell populations 2 months post-transplant, as did mice given wild-type cells. Reconstitution of CD4+ and CD8+ cells were similar for the coγc-treated groups and for the wild-type group, and T-cell populations remained stable. Two-sided analysis of variance comparing to the GFP group returned P values of <0.01 for both T and B lymphocytes for wild-type and all coγc-treated groups at months 5–7 after transplantation. B220+, IgM+ and IgD+ cell counts were similar for the wild-type and SF-coγc groups, whereas the PGK-coγc and γcPr-coγc mice had B-cell counts two to fourfold lower, but prominently increased relative to the GFP control (P < 0.001 for wild-type and coγc groups).
Figure 3.
T- and B-lymphocyte reconstitution in peripheral blood. Il2rg−/− mice were transplanted with lentiviral vector transduced wild-type or Il2rg−/− Lin− cells. Absolute lymphocyte cell numbers in peripheral blood of gene therapy treated mice were determined monthly by flow cytometry. (a) A comparison of all the lentiviral vectors tested is shown: Il2rg−/− Lin− cells with γcPr-GFP, SF-coγc, γcPr-coγc (n = 4 per group) or PGK-coγc (n = 5), and wild-type Lin− cells with γcPr-GFP (n = 3). Mice were subjected to 6 Gy irradiation and received 4 × 105 cells. T and B lymphocytes are presented as CD4+, CD8+ or IgD+, IgM/B220+ cells respectively. (b) Represents a comparison of a titration in irradiation conditioning: Il2rg−/− mice were subjected to 2Gy irradiation and transplanted with 5 × 105 cells, either wild-type Lin− cells (n = 3) or Il2rg−/− Lin− cells transduced with γcPr-coγc (n = 9) or PGK-coγc (n = 7). A further five Il2rg−/− mice were transplanted with Il2rg−/− Lin− cells transduced with γcPr-coγc (n = 3) or wild-type Lin− cells (n = 2), without pretransplant irradiation. GFP, green fluorescent protein; PGK, phosphoglycerate kinase; SF, spleen focus forming virus.
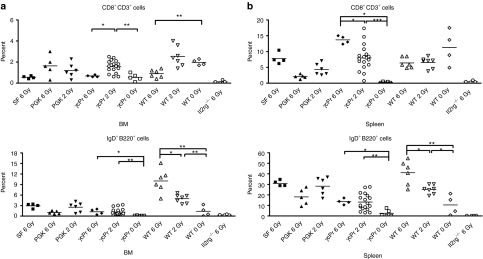
To further study the influence of conditioning intensity and cell number on engraftment, 30 Il2rg−/− mice were treated with LV-γcPr-coγc or LV-PGK-coγc transduced Il2rg−/− cells or wild-type cells with the pretransplant conditioning reduced to 2 Gy (n = 13 for γcPr-coγc, n = 7 for PGK-coγc and n = 3 for wild type) or no conditioning radiation (“0 Gy”) (γcPr-coγc n = 5 and wild type n = 2). Four γcPr-coγc treated mice in the 2 Gy group received 105 transduced cells compared to the 5 × 105 cells given to the other mice. Reconstitution of T and B cells is shown in Figure 3b. Reduction of pretransplant conditioning from 6 Gy to 2 Gy had little or no effect on the reconstitution of T and B cells in either coγc treated mice or the wild-type controls. Eliminating pretransplant conditioning altogether resulted in reduced T- and B-cell counts for γcPr-coγc treated mice (P < 0.01 for B cells compared to the 2 Gy group), whereas increased T-cell counts and reduced B cells seen in wild-type 0 Gy controls were lower, but not statistically significant relative to the 2 Gy wild-type group. Mice injected with 105 γcPr-coγc transduced cells had ∼25% lower T-cell counts relative to mice given 5 × 105 cells, however, this difference was not statistically significant either (data not shown). Mice were killed at 9 months post-therapy and T- and B-lymphocyte markers were determined in BM and spleen (Figure 4). Spleen T-cell populations were similar for mice treated with coγc vector transduced cells and recipients of wild-type cells. Mice treated with γcPr-GFP lacked CD8+ cells and had reduced levels of CD4+ cells. BM of conditioned mice treated with coγc vectors or wild-type cells had similar levels of B lymphocytes, whereas GFP-treated Il2rg−/− mice had virtually no B lymphocytes (Figure 4a). Similar results were found in the spleens (Figure 4b).
Figure 4.
Percentages of CD8+ and IgD+ cells in spleen and bone marrow (BM). Percentages of CD3/CD8+ and IgD/B220+ cells in (a) BM and (b) spleens of experimental mice. Mice were subject to 6 Gy, 2 Gy or 0 Gy irradiation and transplanted with 5 × 105 wild-type Lin− cells (n = 17) or 5 × 105 Il2rg−/− Lin− cells transduced with SF-coγc (n = 4), PGK-coγc (n = 11), γcPr-coγc (n = 27) or γcPr-GFP (n = 4). All groups consist of mice from two separate experiments, excepting the PGK and SF groups. Significant differences are seen in the percentages of IgD/B220+ cells between mice given pretransplant conditioning and those who did not (*P < 0.05, **P < 0.01). GFP, green fluorescent protein; PGK, phosphoglycerate kinase; SF, spleen focus forming virus.
Mice given wild-type or γcPr-coγc-treated cells with no pretransplant irradiation conditioning had significantly lower percentages of IgD/B220+ cells in their spleens and BM than mice given similar cells with conditioning. The average numbers of integrations per BM cell corrected for donor/recipient chimerism were 2.2 for the PGK-coγc mice and 3.5, 3.2 and 2.4 for the γcPr-coγc mice given 0 Gy TBI/5 × 105 cells, 2 Gy TBI/5 × 105 cells and 2 Gy TBI/105 cells, respectively (Supplementary Table S1).
To confirm that the γcPr-coγc vector could effectively restore T- and B-cell populations even at a low MOI, another 19 Il2rg−/− mice were administered 2 Gy irradiation and treated with 5 × 105 Il2rg−/− LV-γcPr-coγc transduced cells (n = 15) or wild-type cells (n = 4). Cells transduced with LV-γcPr-coγc using a HeLa transducing unit (HTU) MOI of 10, 3 or 1 were transplanted into Il2rg−/− mice (n = 5 per group). Reducing the MOI of the γcPr-coγc from 10 to 3 had no effect on the efficacy of leukocyte reconstitution, whereas reducing the MOI to 1 resulted in a more protracted course of T-cell reconstitution to reach levels of T and B cells similar to the MOI 10 and 3 γcPr-coγc groups at 5 months post-therapy (Supplementary Figure S2). Mice were killed at 8 months post-therapy and the average vector copy per BM cell, corrected for donor/recipient chimerism, was 1.9, 1.0, and 0.2 for the mice given cells transduced with a HTU MOI of 10, 3, and 1, respectively (Supplementary Table S1).
Humoral immune responses
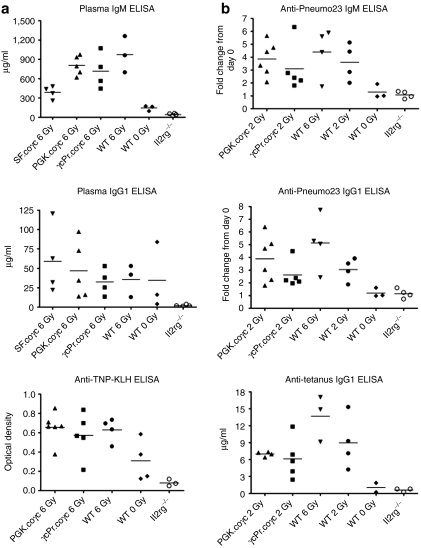
At 15 weeks post-transplant, peripheral blood plasma was tested for basal immunoglobulin levels. Plasma concentrations of IgM and IgG1 were similar among the wild-type group and the two coγc treated groups (Figure 5a) and clearly distinct from the absent antibodies in GFP-treated Il2rg−/− mice (P < 0.05). Natural antibodies specific to trinitrophenol keyhole limpet hemocyanin23 were absent in GFP-treated Il2rg−/− mice but present in PGK-coγc mice as well as wild-type controls. We conclude that natural antibodies normalized as well in successfully treated mice.
Figure 5.
Response of plasma immunoglobulin levels to immunization. (a) Plasma immunoglobulin levels quantified for IgM, IgG1 or trinitrophenol keyhole limpet hemocyanin. Il2rg−/− mice were transplanted with wild-type Lin− cells (n = 3) or Il2rg−/− Lin− cells transduced with γcPr-coγc (n = 4), SF-coγc (n = 4), PGK-coγc (n = 5) or γcPr-GFP (n = 4, group designated Il2rg−/−). Plasma was collected at 15 weeks after transplantation. All coγc-treated groups are significantly different than the GFP control group for levels of plasma IgM, IgG1 and anti-trinitrophenol keyhole limpet hemocyanin antibodies (P < 0.05). (b) Quantification of Pneumo23 and tetanus-specific immune responses. Il2rg−/− mice transplanted with 5 × 105 Il2rg−/− Lin− cells or wild-type (WT) cells were subjected to a Pneumo23 immunization scheme 6 months after transplantation, with plasma collected 10 days later. Shown are fold changes in Pneumo23-specific IgM and IgG1 antibody levels before and after immunization. Mice had been transduced with γcPr-coγc (n = 5) or PGK-coγc (n = 6) after 2Gy irradiation, or given Il2rg−/− Lin− cells with no vector (n = 4), or given wild-type cells after 6 Gy, 2 Gy or 0 Gy irradiation (n = 4 for 6 and 2 Gy and n = 3 for 0 Gy group). All groups except for the WT 0 Gy group are significantly different than the Il2rg−/− control group (P < 0.05). One month later, the surviving mice were subjected to a tetanus toxoid immunization scheme. Tetanus injections were repeated 2 times at 2-week intervals. Shown are plasma IgG1 antibody levels 2 weeks after the last boost. No antibodies to either Pneumo23 or tetanus were observed prior to immunization. GFP, green fluorescent protein; PGK, phosphoglycerate kinase; SF, spleen focus forming virus.
To confirm that a specific T-cell dependent antibody response was restored in γcPr-coγc and PGK-coγc mice, a tetanus toxoid immunization scheme was started at 4 months after transplantation in wild-type and γcPr-coγc recipient mice in the 2 Gy radiation groups (Figure 5b). Mice were injected three times with tetanus toxoid at 2-week intervals. Tetanus-specific IgG antibody levels increased over time in all groups with similar antibody responses between wild-type and coγc treated mice. The antibody titers were also similar among γcPr-coγc treated mice receiving 5 × 105 or 1 × 105 Lin− cells. The specific antibody response was absent in plasma of Il2rg−/− mice.
A T-cell independent antibody test was performed in mice that received wild type, PGK-coγc or γcPr-coγc-treated cells in the 2 and 0 Gy radiation groups (Figure 5b). Mice were injected with Pneumo23 and plasma was collected after 10 days had passed. IgM and IgG antibody levels in coγc and wild-type cell treated mice were clearly normalized relative to the nonresponding GFP-treated mice. Of note, in the 0 Gy TBI recipients of γcPr-coγc transduced cells, three out of four mice immunized failed to produce antibodies, significantly different from all other treated groups (P = 0.001) and consistent with the severely reduced B-cell reconstitution in these mice.
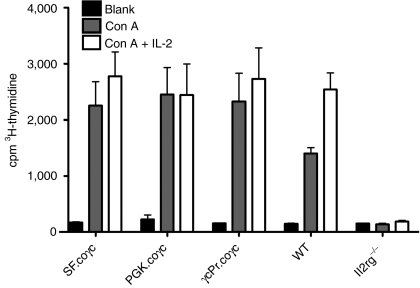
To assess T-lymphocyte response to cytokine signaling, the proliferative potential of spleen cells was determined in response to ConA and IL-2. As shown in Figure 6, cells of mice treated with SF, PGK or γcPr containing coγc vectors showed similar levels of cellular expansion in response to ConA and IL-2. A response was not obtained in spleen cells of Il2rg−/− mice treated with GFP (P < 0.01).
Figure 6.
Spleen cell responses to stimulation with ConA/IL-2. Spleen cells were collected from wild-type mice, Il2rg−/− mice, and Il2rg−/− mice transplanted with Il2rg−/− Lin− cells transduced with γcPr-coγc, SF-coγc or PGK-coγc (N = 3 for all groups). Measurements of coγc groups were adjusted for levels of spleen cell chimerism (Supplementary Table S1). Wild-type and coγc-treated groups were significantly different compared to Il2rg−/− group (**P < 0.01) for both Con A and Con A + IL-2 stimulation, whereas the gene therapy treated mice did not significantly differ from the wild-type mice. IL-2, interleukin-2; PGK, phosphoglycerate kinase; SF, spleen focus forming virus.
TCR repertoire
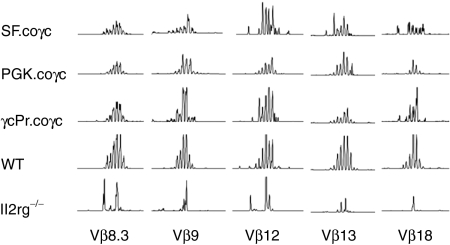
Functional T-cell receptor (TCR) rearrangement was determined from spleen cDNA with primers specific to the Constant (C) part of the T-cell antigen receptor β chain and one of the Variable (V) gene segment-specific primers.24,25 A total of 21 V gene segment primers were used to create PCR products, which were then diluted and analyzed on an ABI 3130xl sequencer. Peak patterns for mice treated with coγc vectors or wild-type cells were diverse, indicating the existence of a polyclonal T-cell population that is able to create a diverse, functional repertoire of antigen receptors (Figure 7). Peak patterns for Il2rg−/− mice treated with the GFP control vector tended to have only a few peaks, indicating that the antigen receptor repertoires in T cells in these mice had no or limited functionality.
Figure 7.
Genescan analysis of murine T cell receptor β (TCRβ) repertoire. PCR was performed on cDNA synthesized from total spleen RNA, using a specific primer for the Constant part of the TCRβ chain in combination with 21 Variable (V) gene segment-specific primers. Of the 21 V gene segments tested, the most representative samples are shown here. Diverse peak patterns indicate the rearranged TCRβ genes are derived from a polyclonal cell population, whereas samples with only a few peaks reflect inability to form a broad T-cell receptor repertoire.
Secondary transplants
All mice in these studies were killed 8–9 months after transplantation and BM cells were retransplanted into one or two Il2rg−/− mice following 6 Gy irradiation (2 × 106 total BM cells per mouse). The secondary transplants were monitored monthly by blood counts and flow cytometry as described for up to 8 months. Reconstitution was universally inferior to the primary transplants, with reduced circulating B-cell counts (for the γcPr-coγc secondary recipients group 0.1 ± 0.0 × 109 versus 1.2 ± 0.2 × 109 IgM+B220+ B cells/l and for wild-type secondary recipients, 1.9 ± 0.2 × 109 versus 4.7 ± 0.5 × 109 IgM+B220+ B cells/l) and poor antibody responses to Pneumo23 and tetanus seen in mice given coγc-treated or wild-type cells (data not shown). Some secondary transplants had undetectable levels of B cells, with this effect most often seen when the primary transplants were treated with physiological promoters or received no pretransplant conditioning.
NK cell reconstitution
A pilot experiment to obtain insight into NK cell reconstitution following transplantation of cells transduced with the therapeutic γc gene was performed in Rag2−/−/Il2rg−/− C57BL/6 mice, also using the opportunity to compare the LV-γcPr-coγc vector to the LV-UCOE-IL2RG construct published earlier.26 Il2rg−/− lin- cells were transduced using an MOI of 15 and of 10 for the γcPr and UCOE vectors, respectively, and transplanted into Rag2−/−/Il2rg−/− recipients. Wild-type C57BL/6 mice and untreated Rag2−/−/Il2rg−/− C57BL/6 mice were used as controls. Percentages of CD3+, B220+, and NK1.1+ cells were similar for the two vectors, and also similar to those obtained with the SF and PGK vectors in recipients at a BALB/c background, although the mice treated with the γcPr vector had a higher number of vector copies per cell (Supplementary Table S2), again stressing that vector copy number/cell has no major impact above a certain threshold level.
Discussion
To address the risk of γ-retroviral vector-mediated insertional mutagenesis in gene therapy of SCID-X1, we generated SIN HIV-1 derived LVs12 containing the bPRE4* element described previously.20 These vectors integrate with high efficiency in hematopoietic stem cells by an overnight transduction without the requirement of prestimulation with growth factors and have a reduced likelihood of integrating near transcription start sites, as well as improved resistance to silencing.10,13 Vectors for transfer of the therapeutic transgene were driven by the SF, PGK or γcPr promoters. Lineage negative Il2rg−/− cells transduced with these vectors and transplanted in clinically feasible numbers into Il2rg−/− recipient mice proved effective in restoring immune competence, as measured by T-cell numbers, polyclonal TCRβ gene rearrangements, ConA/IL-2 stimulated T-cell proliferation, T-cell dependent and independent B-cell antibody responses, and by restored levels of natural antibodies. All three vectors proved effective at restoring T-cell populations and immune system functionality to wild-type levels, provided a minimum level of pretransplant conditioning was applied. A series of GFP vectors served as controls, and additionally demonstrated improved selectivity of the γcPr promoter for expression in white blood cells.
We compared the efficacy of the SF-coγc, γcPr-coγc and PGK-coγc vectors at reconstituting T- and B-cell populations in Il2rg−/− mice. The levels of T- and B-cell reconstitution in SF-coγc vectors, which approach recovery rates of wild-type cells, are similar to the initial results of retroviral gene therapy for murine SCID-X1.27 Likewise, the lymphocyte numbers and the results of a spleen cell proliferation assay compare favorably with a more recent approach to murine SCID-X1 gene therapy with LVs using a similar MOI.26 The γcPr-coγc and PGK-coγc vectors fully restored T-lymphocyte populations in the periphery, BM and spleen, but were less effective in restoring B-cell populations. Mice treated with coγc vectors regained immune function as shown by spleen cell reactivity to proliferation signals and displayed a repertoire of TCRβ gene rearrangements. Although the B-cell populations were reduced, PGK and γcPr-coγc vector treated mice had plasma levels of IgM and IgG1 similar to mice given wild-type cells with a comparable pretransplant conditioning regimen, as well as fully normalized antibody responses to Pneumo23 and tetanus toxoid. The reduced number of circulating B cells in γcPr-coγc treated animals apparently does not negatively influence specific antibody production resulting from T-cell dependent or T-cell independent responses.
Due to a low vector titer, the MOI of the PGK-coγc vector was fivefold lower than the other vectors with a corresponding reduction in the number of integrations per cells. The integrations per cell were also dissimilar for the comparison study between the UCOE and γcPr vectors, which aimed at measuring NK cell levels. However, we demonstrated that reducing the γcPr MOI to 0.2 integrations per cell had no significant effect on reconstitution, thus allowing comparisons at different numbers of integrations per cell.
Although full functional restoration was accomplished, the reduced number of circulating B lymphocytes in γcPr-coγc and PGK-coγc treated mice compared to those from SF-coγc and wild-type groups is noteworthy. This might be the result of species-specific differences in lymphocyte development: humans with SCID-X1 are able to produce high levels of B cells through non-γc-dependent pathways,1 whereas Il2rg−/− mice do not have this ability. Another possibility is that the lower levels of γc protein expression driven by γcPr and PGK relative to that by the SF promoter reduces the selective advantage of B precursor cells to repopulate bone marrow, leading to lower circulating levels and/or proliferation. This may also underline the importance of pretransplant conditioning.
Reduction of the pretransplant conditioning of recipient mice from 6 Gy to 2 Gy had no significant effect on the efficacy of the gene therapy treatment, consistent with the principles of conditioning for autologous stem cell engraftment earlier established in thalassemic mice.28 However, also in line with the latter observations, eliminating the pretransplant conditioning altogether severely reduced the efficacy of the treatment in mice transplanted with either γcPr-coγc treated or wild-type cells, with a corresponding drop in donor cell BM chimerism, percentage of lymphocytes present in spleen and BM, and failures to produce antibodies upon immunization. Il2rg−/− recipients of wild-type cells but not preconditioned displayed low to undetectable B cells levels in blood, BM and spleen, and many of these mice had poor plasma antibody levels and specific immune responses. This is similar to conditions applied in the first γ-retroviral vector SCID-X1 trials,4 resulting in some of the patients requiring immunoglobulin-replacement therapy. To our knowledge, this is the first paper underlining the importance of pretransplant conditioning for murine SCID-X1. Extrapolation of this observation to human SCID-X1 gene therapy predicts improved immune reconstitution by applying mild conditioning and a reduced threshold of cell numbers required, thereby improving both efficacy and safety.
Titration of the LV-γcPr-coγc vector demonstrated convincingly that lowering the vector dose to reach the preferred one copy per cell for clinical application had no significant effect on the reconstitution of T-and B-cell lineages, in contrast to results previously described by Zhou et al.29 Using an HTU MOI of 1 resulted in on average 0.2 vector copies per BM cell in vivo, compared to 1.9 copies/BM cell in a mouse given an MOI of 10, with no significant difference in the T- and B-cell counts seen in these animals at 6 months post-therapy. Likewise, reduction of the Lin− cell number transplanted to 105/mouse (4 × 106/kg, equivalent to 4 × 107/kg unfractionated BM cells or roughly 106/kg CD34+ cells in humans) did not noticeably influence immune reconstitution.
In this manuscript, we also provide evidence that the codon optimization proved essential for improved viral vector titers, transgene transcript levels and protein production, as has been shown before for other vectors,15 allowing immune reconstitution at a level of one transgene copy per cell. This is in contrast to a recent report in which low copy numbers per cell of a vector with a 233-bp human EF1α promoter resulted in significantly reduced B and NK cell reconstitution and IgG plasma levels.29
The use of strong retroviral enhancer/promoter sequences is not desirable for clinical application since the leukemia cases that occurred in the initial gene therapy trials were caused by integrations in vulnerable regions near proto-oncogenes resulting in subsequent upregulation and constitutive expression.6,7 Furthermore, viral promoters are at risk of being silenced by methylation in mammalian cells.30,31,32,33 The SF promoter in particular has been shown to modify gene expression in regions greater than 100 kb,34 reducing the potential of activating neighboring genes by cellular promoters18 may be beneficial for clinical application. In a recent study, Zhou et al.29 tried to address whether a 233-bp human EF1α promoter was able to upregulate LMO2 with a LMO2 activation assay in human leukemic Jurkat T cells when inserted in the LMO2 locus, and compared to a γC genomic sequence inserted in the reverse orientation. However, LVs integrate throughout genes, not at fixed locations or orientations, and have shown no preference to integrate near oncogenes or near transcription start sites. In the present study, we encountered a significant number of integrations of the SF vector in the immediate vicinity of retrovirus tagged cancer gene database listed proto-oncogenes, which was not observed for the PGK or γcPr vectors (Supplementary Table S3).
Constitutive IL2RG expression has been postulated to pose an increased risk of oncogenesis.35 However, this implication of IL2RG acting as an oncogene under certain circumstances is controversial,36,37 since aberrant constitutive expression of LMO2 results in a differentiation block of developing T-cell precursors under proliferative pressure,38 which by itself would be sufficient to explain the onset of leukemia after a secondary oncogenic event. In this scenario, IL2RG expression is permissive, not causative. More recent evidence supports the notion that IL2RG and LMO2 require additional cooperating mutations.39 In addition, a novel model of SCID-X1 reconstitution suggests that the SCID-X1 phenotype itself causes cells to be more susceptible to insertional leukemogenesis by murine leukemia viruses or γ-retroviral vectors.40 Of note, no insertions were detected in the Lmo2 locus after thorough integration analysis of the leukemic clones obtained in the course of this study.
The study involved 70 primary recipients, of which bone marrow cells have been retransplanted 8 months after transplantation into secondary recipients to increase the proliferative pressure on the transduced cell population. Secondary recipients had poor lymphocyte reconstitution, particularly in the B-cell compartments. Current observation times of primary and secondary recipients are at least 15 months after transplantation. While we have detected leukemia and T-cell malignancies in some mice, none of these adverse events were shown to be vector-derived after quantitative PCR (qPCR) and LAM-PCR analysis and as such were concluded to be background malignancies inherent to the radiation exposure, mouse strain or other factors in the procedure.41 The mice and their retransplants will be monitored life-long in the context of a larger study directed at potential adverse effects and at a full integration analysis, to be reported separately. Some mice treated with similar vectors in experiments not included in this manuscript have developed leukemia that are likely vector derived, which will be reported on separately.
We conclude that the γcPr-coγc vector, as well as the PGK-coγc vector, provides an attractive, required alternative to replace constitutive viral promoters, allowing the development of efficacious therapeutic LV mediated gene transfer for the correction of SCID-X1 with a potentially improved safety profile both by the use of the SIN lentiviral backbone42,43 and as judged from an initial integration analysis. Additionally, our study underscores the importance of pretransplant conditioning for consistent successful correction, which should have an impact on current clinical protocol development.
Materials and Methods
Mice. BALB/c and Il2rg−/− mice were bred in the Experimental Animal Center of Erasmus MC. Il2rg−/− single KO mice with a targeted γc deletion were derived from a 10th generation backcross of BALB/c Rag2−/−/γc−/− mice, kindly provided by Dr Spits,44 with syngeneic BALB/c wild-type mice. All mice were used at 6–10 weeks of age and were maintained in specified pathogen free conditions. Experiments were approved by the institutional Animal Ethical Committee of Erasmus MC in accordance with legislation in the Netherlands. Comparison studies between the LV-γcPr-coγc vector described here and a LV-UCOE vector were performed using Il2rg−/− donor mice and Rag2−/−/Il2rg−/− recipients, plus wild-type controls (C57BL/6 background). These experiments were conducted at University College, London, UK according to their protocols,26 with approval in accordance with legislation in the UK.
LV plasmids. Third generation SIN LV incorporating the γc cDNA were constructed using the HIV-1 vector backbone42,43 with SF promoter and a modified woodchuck post-translational regulatory element (bPRE4*) as described previously, 20 but now incorporating the γc cDNA. The native human IL2RG cDNA was cloned from a LZRS-IL2RG-IRES-EGFP retroviral plasmid,44 kindly provided by Dr Pike-Overzet, Dept of Immunology, Erasmus MC, Rotterdam, the Netherlands, and cloned into pRRL.PPT.SF.EGFP.WPRE4*.SIN (LV-SF-GFP) to create LV-SF-γc. Codon-optimized human IL2RG sequence (coγc) predicted by GeneOptimizer software (GeneArt AG, Regensburg, Germany) was cloned into LV-SF-γc to generate the LV-SF-coγc vector.
The human IL2RG promoter fragment (γcPr) was obtained by PCR amplification of a ∼1.1 kb human DNA region17 using the following primers: 5′-CTCGAGAGGATGTCTTGTTGGTCT-3′, and 5′-GGATCCCGCTTGCTCTTCATTCCC-3′ with restriction sites underlined (Eurogentec, Maastricht, The Netherlands). Post-PCR, the fragment was ligated into pCR-TOPO-TA vector (Invitrogen, Carlsbad, CA) and verified by restriction digest and sequencing. The γcPr was subsequently ligated into a GFP LV in place of the PGK promoter by digestion with XhoI and BamHI to create LV-γcPr-GFP. Similarly, γcPr and PGK were cloned into SF-coγc to produce LV-γcPr-coγc and LV-PGK-coγc.
Production of lentiviral particles and titration. LVs were produced by standard calcium phosphate transfection of HEK 293T cells19 with the packaging plasmids pMDL-g/pRRE, pMD2-VSVg and pRSV-Rev. LV were concentrated by ultracentrifugation at 20,000 r.p.m. for 2 hours at 4 °C and stored at −80 °C. Viral vector titration was performed on HeLa cells by serial dilutions to determine transducing units (HTU) per ml. The transduced cells were harvested on day 11, DNA and total RNA were purified and the copy number per cell was determined by real-time qPCR.
Real-time qPCR. qPCR to quantify the integrated proviral copy number45 was performed on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) with 100 ng genomic DNA template and SYBR Green PCR Master Mix (Applied Biosystems) with HIV primer, 5′- CTGGAAGGGCTAATTCACTC-3′, and 5′-GGTTTC CCTTTCGCTTTCAG-3′ resulting in a 274 bp fragment of the proviral DNA. The PCR conditions were 95 °C for 10 minutes and 45 cycles as follows: 95 °C for 15 s and 62 °C for 1 minute. A standard calibration curve was determined from flow sorted mouse 3T3 cells transduced with LV-SF-GFP at a MOI of 0.06 containing one integration per genome. All PCR reactions were performed in triplicate and analyzed with SDS 2.2.2 software.
Determination of WPRE mRNA levels. Total RNA of transduced HeLa cells was converted to cDNA via the QuantiTect Reverse Transcription kit (Qiagen, Venlo, The Netherlands). qPCR reactions were performed using 5 ng cDNA and primer 5′-ATTGCCACCACCTGTCAACT-3′ and 5′-CCGACAACACCACGGAATTA-3′ to amplify a fragment of the WPRE. qPCR reactions were performed on the ABI Prism 7900HT using a serial dilution of SF-IL2RG plasmid as the standard curve and a 55 °C annealing temperature.
Chimerism assay. qPCR reactions were performed on spleen and BM DNA from Il2rg−/− mice transplanted with male donor cells transduced with LV vectors to amplify a region of the Y chromosome as described by Pujal et al.46 using 100ng of genomic DNA and primers 5′-TCATCGG AGGGCTAAAGTGTCAC-3′ and 5′-TGGCATGTGGGTTCCTGTCC-3′. A standard curve was generated using spleen and BM DNA from male BALB/c mice.
Ligation-mediated PCR and LAM-PCR. High-resolution insertion-site analysis by LAM-PCR and ligation-mediated PCR was performed on BM, peripheral blood or spleen DNA from Il2rg−/− mice transduced with LV vectors. For LAM-PCR,47 restriction enzymes Tsp509I or MseI were used with the lentiviral (HIV) primer set. PCR products were run on high-resolution polyacrylamide (Spreadex) gel. To the nested primers of the ligation-mediated PCR48,49 protocol, the following primers were added to create the sequencing tags: 3′-GCCTCCCTCGCGCCATCAG-5′ + MID (multiplex identifier) to the primer annealing to the viral LTR, and 3′-GCCTTGCCAGCCCGCTCAG-5′ + MID to the primer annealing to the linker.
Transduction and transplantation of lineage negative BM cells. BM cells from male Il2rg−/− and congenic wild-type mice were purified by Lin− (BD Biosciences, Santa Clara, CA). Lin− BM cells were transduced overnight at 106 cells/ml in serum free modified Dulbecco's medium with supplements50 in the presence of murine stem cell factor 100 ng/ml, human FMS-like tyrosine kinase 3-ligand 50 ng/ml and murine thrombopoietin 10 ng/ml at HTU MOI 9, except for the PGK-coγc vector (MOI 2) and a vector dose titration of γcPr-coγc from MOI 10 to 3 to 1. Subsequently, 5 × 105, 4 × 105 or 105 cells were injected into the tail vein of 6 Gy or 2 Gy irradiated female Il2rg−/− recipients or without conditioning.
Immunophenotyping by flow cytometry. Flow cytometric analyses were performed on cells obtained from blood, bone marrow and spleen. Peripheral blood was collected monthly in EDTA tubes by retro-orbital puncture under isoflurane anesthesia. Complete blood cell counts were measured using a Vet ABC hematology analyzer (Scil animal care, Viernheim, Germany). Blood was lysed and leukocytes were washed three times with Hank's balanced salt solution (Invitrogen) containing 0.5% (wt/vol) bovine serum albumin and 0.05% (wt/vol) sodium azide (HBN). Cells were incubated for 30 minutes at 4 °C in HBN containing 2% heat-inactivated normal mouse serum and antibodies against CD3, CD4, CD8, B220, CD19, IgM, IgD, CD11b Sca-1, C-kit and TCRβ directly conjugated to R-phycoerythrin, peridinin chlorophyll protein or allophycocyanin (all antibodies BD Biosciences). Subsequently, cells were washed and measured on a FACSCalibur (Becton Dickinson, San Jose, CA). BM and spleen cells were evaluated similarly. Additionally, GFP expression was measured in mice treated with the LV-SF-GFP or LV-ycPr-GFP vectors.
Determination of surface γc expression. Lineage negative Il2rg−/− BM cells were purified as described above and transduced with LV-SF-γc or LV-SF-coγc vectors at a HeLa MOI of 100, 10 or 1. Transduced cells were cultured for 4 days, washed and then surface γc expression was determined via flow cytometry. A CD132-pe antibody (BD Biosciences) was used to determine γc expression.
Immunizations and ELISAs. HeLa cells were transduced with a fixed MOI for either LV-SF-γc or LV-SF-coγc and kept in culture for 7 days. Copy number per cell was confirmed by qPCR. An enzyme-linked immunosorbent assay (ELISA) was performed on extracts from these transduced cells coated on the plates, which were normalized for protein content. Biotinylated goat anti-human common γ-chain (BAF284; R&D Systems, Minneapolis, MN) was applied to these plates, and signal was detected with streptavidine-peroxidase. The relative fold increase in γc expression was determined, adjusted for copy number per cell.
An ELISA was done on peripheral blood plasma collected 15 weeks after transplantation to determine basal IgM and IgG1 plasma levels. Briefly, Covalink 96-well plates (Nunc A/S, Roskilde, Denmark) were coated first with dithiobis (succinimidyl propionate) (Pierce Biotechnology, Rockford, IL) in methanol and then with unlabeled (capture) anti-mouse IgM (1B4B1) or IgG1 (H143.225.8) antibodies (SouthernBiotech, Birmingham, AL) for 2 hours. The wells were blocked overnight at 4 °C with 1% bovine serum albumin in phosphate-buffered saline. The next day the plates were washed six times with phosphate-buffered saline/0.05% Tween 20 and incubated with serially diluted serum for 1 hour at room temperature. Plates were washed and incubated with a goat anti-mouse horseradish peroxidase–conjugated antibody at room temperature for 1 hour. After subsequent washing the wells were stained with TMB substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) and the reaction was stopped after 10 minutes by adding 1 mol/l phosphoric acid. The absorbance of each plate was then read at 405 nm using a FLUOstar Optima plate reader. Antibody concentrations were calculated by using purified IgM (M5909) and IgG (I5381) antibody standards (Sigma, St Louis, MO).
An ELISA for natural antibodies to trinitrophenol keyhole limpet hemocyanin was done on peripheral blood taken from PGK-coγc-treated mice as well as mice given WT and Il2rg−/− cells. The ELISA was performed as described, using TNP(17)-KLH (T-5060-5, Biosearch Technologies, Novato, CA) and anti-KLH (12B4.G3.A8) antibody (AbCam, Cambridge, UK). Optical density was used to measure the antibodies.
T-cell dependent specific antibody responses were determined by intraperitoneal immunization with 16 IU of tetanus toxoid. This process was repeated twice at 2-week intervals, and plasma was collected 2 weeks after the last immunization. An ELISA was performed on plates coated with tetanus toxoid, and horseradish peroxidase goat-anti-mouse IgG1 (Invitrogen) was used to measure signal. Anti-tetanus antibody TetE3 was used to make a standard line (AbCam).
T-cell independent responses were obtained by subcutaneous immunization with 0.5 µg of each 23 purified pneumococcal capsular antigens (PNEUMO 23; Sanofi Pasteur MSD, Brussels, Belgium), and plasma was collected 10 days later. ELISA plates were coated with Pneumo23 and goat-anti-mouse IgM or goat-anti-mouse IgG1 were used to measure signal (Invitrogen). Optical density was measured of preimmunization and postimmunization plasma to determine the antibody response.
In vitro spleen cell proliferation assay. Spleen cells were cultured at 1 × 106 cells/ml in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and antibiotics (pen/strep). Concanavalin A (Con A, 2.5 µg/ml, Sigma) was added with or without human IL-2 (500 units/ml; Biogen Idec, Badhoevedorp, The Netherlands). On day 3, 0.5 µCi 3H-thymidine was added to each well. On day 4, cells were washed and 3H-thymidine incorporation was measured on a TopCount microplate scintillation counter (GMI, Ramsey, MN).
Genescan. Genescan analysis of murine TCRβ repertoire was performed on cDNA derived from 2 µg total RNA purified from fresh spleen cells using the Allprep DNA/RNA kit (Qiagen). cDNA was synthesized using the QuantiTect Reverse Transcription kit (Qiagen). PCR was performed on the cDNA using a specific primer for the Constant (C) part of the T cell antigen receptor β chain (5′-FAM-CTTGGGTGGAGTCACATTTCTC-3′) in combination with 21 Variable (V) gene segment-specific primers to form PCR products between 140 and 240 bp as described.25 All PCR products were analyzed on an ABI 3100 or 3130xl sequencer.
Statistics. Statistical analysis was performed using SPSS 11 (SPSS, Chicago, IL) and Graphpad Prism (GraphPad Software, San Diego, CA). Significance of difference was determined by a Mann–Whitney test, for categorical data by Fisher's Exact test. Data are presented ± SEM where applicable.
Acknowledgments
The authors thank Luigi Naldini for the third generation self-inactivating lentiviral vector, and recognize the assistance of Karin Pike-Overzet, Mark Rodijk and Frank J. T. Staal under the guidance of Jacques J.M. van Dongen in implementing the Gene Scan analysis as described by Pannetier et al. and in providing the LZRS-IL2RG-IRES-EGFP retroviral plasmid, and are grateful to Roya Sarwari for technical assistance and Elnaz Farahbakhshian for providing the Y chromosome primers. Funding was provided by the European Commission (5th, 6th and 7th Framework Programs, Contracts QLK3-CT-2001-00427-INHERINET, LSHB-CT-2004-005242-CONSERT, and LSHB-CT-2006-19038-Magselectofection, Grant Agreement 222878-PERSIST Grant agreement 261387 CELL-PID), The Netherlands Health Research and Development Organization ZonMw (Translational Gene Therapy Research program, project 43100016), the Wellcome Trust (A.J.T., M.P.B.) and the UK Biotechnological and Biological Sciences Research Council (F.Z.). Work was performed primarily in Rotterdam, the Netherlands. The authors declare no competing financial interests.
Supplementary Material
LAM-PCR of bone marrow DNA in gene therapy mice.
LV-γcPr-coγc titration FACS data.
Averages of chimerism and proviral copies per BM.
T, B and NK cell percentages in spleen.
Viral integrations near RTCGD genes, by promoter.
REFERENCES
- Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS.et al. (1993Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans Cell 73147–157. [DOI] [PubMed] [Google Scholar]
- Fischer A, Griscelli C, Friedrich W, Kubanek B, Levinsky R, Morgan G.et al. (1986Bone-marrow transplantation for immunodeficiencies and osteopetrosis: European survey, 1968-1985 Lancet 21080–1084. [DOI] [PubMed] [Google Scholar]
- Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J.et al. (2004Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector Lancet 3642181–2187. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC.et al. (2010Efficacy of gene therapy for X-linked severe combined immunodeficiency N Engl J Med 363355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman FD. Retroviral integration and human gene therapy. J Clin Invest. 2007;117:2083–2086. doi: 10.1172/JCI32949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E.et al. (2008Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1 J Clin Invest 1183132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H.et al. (2008Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients J Clin Invest 1183143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew C., and, Ihle JN. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol Cell Biol. 1991;11:1820–1828. doi: 10.1128/mcb.11.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlich U, Bohne J, Schmidt M, von Kalle C, Knöss S, Schambach A.et al. (2006Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity Blood 1082545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C.et al. (2006Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration Nat Biotechnol 24687–696. [DOI] [PubMed] [Google Scholar]
- Schambach A, Bohne J, Chandra S, Will E, Margison GP, Williams DA.et al. (2006Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells Mol Ther 13391–400. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L.et al. (1998Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery J Virol 729873–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer A, Ikawa M, Dayn Y., and, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci USA. 2002;99:2140–2145. doi: 10.1073/pnas.251682798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deml L, Bojak A, Steck S, Graf M, Wild J, Schirmbeck R.et al. (2001Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein J Virol 7510991–11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Carranza B, Gentsch M, Stein S, Schambach A, Santilli G, Rudolf E.et al. (2009Transgene optimization significantly improves SIN vector titers, gp91phox expression and reconstitution of superoxide production in X-CGD cells Gene Ther 16111–118. [DOI] [PubMed] [Google Scholar]
- Hannan GN, Lehnert SA, MacAvoy ES, Jennings PA., and, Molloy PL. An engineered PGK promoter and lac operator-repressor system for the regulation of gene expression in mammalian cells. Gene. 1993;130:233–239. doi: 10.1016/0378-1119(93)90424-2. [DOI] [PubMed] [Google Scholar]
- Markiewicz S, Bosselut R, Le Deist F, de Villartay JP, Hivroz C, Ghysdael J.et al. (1996Tissue-specific activity of the gammac chain gene promoter depends upon an Ets binding site and is regulated by GA-binding protein J Biol Chem 27114849–14855. [DOI] [PubMed] [Google Scholar]
- Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E.et al. (2008Physiological promoters reduce the genotoxic risk of integrating gene vectors Mol Ther 16718–725. [DOI] [PubMed] [Google Scholar]
- Follenzi A., and, Naldini L. HIV-based vectors. Preparation and use. Methods Mol Med. 2002;69:259–274. [PubMed] [Google Scholar]
- van Til NP, Stok M, Aerts Kaya FS, de Waard MC, Farahbakhshian E, Visser TP.et al. (2010Lentiviral gene therapy of murine hematopoietic stem cells ameliorates the Pompe disease phenotype Blood 1155329–5337. [DOI] [PubMed] [Google Scholar]
- Baum C, Hegewisch-Becker S, Eckert HG, Stocking C., and, Ostertag W. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells. J Virol. 1995;69:7541–7547. doi: 10.1128/jvi.69.12.7541-7547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi K, Suzuki T, Stephens RM, Jenkins NA., and, Copeland NG. RTCGD: retroviral tagged cancer gene database. Nucleic Acids Res. 2004;32 Database issue:D523–D527. doi: 10.1093/nar/gkh013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver K, Zeijlemaker WP, Schellekens PT., and, Vossen JM. Measurement of primary in vivo IgM- and IgG-antibody response to KLH in humans: implications of pre-immune IgM binding in antigen-specific ELISA. J Immunol Methods. 1984;74:241–251. doi: 10.1016/0022-1759(84)90291-6. [DOI] [PubMed] [Google Scholar]
- Pannetier C, Cochet M, Darche S., and, Kourilsky P.inventors (1998. Process for determining the quantity of a DNA fragment of interest by a method of enzymatic amplification of DNA. US patent 5,635,354.
- Pannetier C, Cochet M, Darche S, Casrouge A, Zöller M., and, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Schambach A, Sinclair J.et al. (2007Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells Blood 1101448–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M, Bloom ML, Imada K, Berg M, Bollenbacher JM, Bloom ET.et al. (1999Restoration of lymphoid populations in a murine model of X-linked severe combined immunodeficiency by a gene-therapy approach Blood 943027–3036. [PubMed] [Google Scholar]
- Wagemaker G, Visser TP., and, van Bekkum DW. Cure of murine thalassemia by bone marrow transplantation without eradication of endogenous stem cells. Transplantation. 1986;42:248–251. doi: 10.1097/00007890-198609000-00004. [DOI] [PubMed] [Google Scholar]
- Zhou S, Mody D, DeRavin SS, Hauer J, Lu T, Ma Z.et al. (2010A self-inactivating lentiviral vector for SCID-X1 gene therapy that does not activate LMO2 expression in human T cells Blood 116900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challita PM., and, Kohn DB. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc Natl Acad Sci USA. 1994;91:2567–2571. doi: 10.1073/pnas.91.7.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben RC, Migchielsen AA, van der Jagt RC, van Ormondt H., and, van der Eb AJ. Inactivation of the Moloney murine leukemia virus long terminal repeat in murine fibroblast cell lines is associated with methylation and dependent on its chromosomal position. J Virol. 1991;65:904–912. doi: 10.1128/jvi.65.2.904-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug CA, Cheshier S., and, Weissman IL. Inactivation of a GFP retrovirus occurs at multiple levels in long-term repopulating stem cells and their differentiated progeny. Blood. 2000;96:894–901. [PubMed] [Google Scholar]
- Pikaart MJ, Recillas-Targa F., and, Felsenfeld G. Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev. 1998;12:2852–2862. doi: 10.1101/gad.12.18.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustikova O, Fehse B, Modlich U, Yang M, Düllmann J, Kamino K.et al. (2005Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking Science 3081171–1174. [DOI] [PubMed] [Google Scholar]
- Woods NB, Bottero V, Schmidt M, von Kalle C., and, Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
- Pike-Overzet K, de Ridder D, Weerkamp F, Baert MR, Verstegen MM, Brugman MH.et al. (2006Gene therapy: is IL2RG oncogenic in T-cell development Nature 443E5; discussion E6–7. [DOI] [PubMed] [Google Scholar]
- Thrasher AJ, Gaspar HB, Baum C, Modlich U, Schambach A, Candotti F.et al.2006Gene therapy: X-SCID transgene leukaemogenicity Nature 443E5–6; discussion E6–7. [DOI] [PubMed] [Google Scholar]
- Pike-Overzet K, de Ridder D, Weerkamp F, Baert MR, Verstegen MM, Brugman MH.et al. (2007Ectopic retroviral expression of LMO2, but not IL2Rgamma, blocks human T-cell development from CD34+ cells: implications for leukemogenesis in gene therapy Leukemia 21754–763. [DOI] [PubMed] [Google Scholar]
- Davé UP, Akagi K, Tripathi R, Cleveland SM, Thompson MA, Yi M.et al. (2009Murine leukemias with retroviral insertions at Lmo2 are predictive of the leukemias induced in SCID-X1 patients following retroviral gene therapy PLoS Genet 5e1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie L, Hector RD, Grant L, Bell M, Nielsen AA, Meikle S.et al. (2009A novel model of SCID-X1 reconstitution reveals predisposition to retrovirus-induced lymphoma but no evidence of gammaC gene oncogenicity Mol Ther 171031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn SL, Liao SH, Dane AP, Hu M, Hyman J, Finnie JW.et al. (2010Lymphomagenesis in SCID-X1 mice following lentivirus-mediated phenotype correction independent of insertional mutagenesis and gammac overexpression Mol Ther 18965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailles LE., and, Naldini L. HIV-1-derived lentiviral vectors. Curr Top Microbiol Immunol. 2002;261:31–52. doi: 10.1007/978-3-642-56114-6_2. [DOI] [PubMed] [Google Scholar]
- Follenzi A., and, Naldini L. Generation of HIV-1 derived lentiviral vectors. Meth Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL.et al. (2004Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2-/- gammac-/- mice: functional inactivation of p53 in developing T cells Blood 1043886–3893. [DOI] [PubMed] [Google Scholar]
- van Til NP, Markusic DM, van der Rijt R, Kunne C, Hiralall JK, Vreeling H.et al. (2005Kupffer cells and not liver sinusoidal endothelial cells prevent lentiviral transduction of hepatocytes Mol Ther 1126–34. [DOI] [PubMed] [Google Scholar]
- Pujal JM., and, Gallardo D. PCR-based methodology for molecular microchimerism detection and quantification. Exp Biol Med (Maywood) 2008;233:1161–1170. doi: 10.3181/0802-RM-35. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Schwarzwaelder K, Bartholomae C, Zaoui K, Ball C, Pilz I.et al. (2007High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR) Nat Methods 41051–1057. [DOI] [PubMed] [Google Scholar]
- Kustikova OS, Modlich U., and, Fehse B. Retroviral insertion site analysis in dominant haematopoietic clones. Methods Mol Biol. 2009;506:373–390. doi: 10.1007/978-1-59745-409-4_25. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Steigerwald SD, Mueller PR, Wold B., and, Riggs AD. Genomic sequencing and methylation analysis by ligation mediated PCR. Science. 1989;246:810–813. doi: 10.1126/science.2814502. [DOI] [PubMed] [Google Scholar]
- Wognum AW, Visser TP, Peters K, Bierhuizen MF., and, Wagemaker G. Stimulation of mouse bone marrow cells with kit ligand, FLT3 ligand, and thrombopoietin leads to efficient retrovirus-mediated gene transfer to stem cells, whereas interleukin 3 and interleukin 11 reduce transduction of short- and long-term repopulating cells. Hum Gene Ther. 2000;11:2129–2141. doi: 10.1089/104303400750001435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LAM-PCR of bone marrow DNA in gene therapy mice.
LV-γcPr-coγc titration FACS data.
Averages of chimerism and proviral copies per BM.
T, B and NK cell percentages in spleen.
Viral integrations near RTCGD genes, by promoter.