Abstract
The paucity of costimulation at the tumor site compromises the ability of tumor-specific T cells to eliminate the tumor. Here, we show that bi-specific oligonucleotide aptamer conjugates can deliver costimulatory ligands to tumor cells in situ and enhance antitumor immunity. In poorly immunogenic subcutaneously implanted tumor and lung metastasis models, systemic delivery of an agonistic 4-1BB aptamer ligand conjugated to a prostate specific membrane antigen (PSMA)-binding tumor-targeting aptamer led to inhibition of tumor growth, was more effective than, and synergized with, vaccination, and exhibited a superior therapeutic index compared to costimulation with 4-1BB antibodies. Tumor inhibition was dependent on homing to PSMA-expressing tumor cells and 4-1BB costimulation. Aptamer targeted costimulation is a broadly applicable and clinically feasible approach to enhance the costimulatory environment of disseminated tumor lesions. This study suggests that potentiating naturally occurring antitumor immunity via tumor-targeted costimulation could be an effective approach to elicit protective immunity to control tumor progression in cancer patients.
Introduction
Costimulation is critical for the survival and expansion of antigen-activated T cells.1,2 Deficiency of costimulation at the tumor site is a major reason why tumors are not responsive to vaccine-induced tumor immunity. By and large, tumors do not express costimulatory ligands such as B7-1, B7-2, 4-1BBL, CD70, or LIGHT, which are required to cross-link and activate the cognate receptors on tumor-infiltrating lymphocytes. The importance of costimulation at the tumor site was demonstrated by studies showing that intratumoral administration of costimulatory ligands such as B7-1/2,3 4-1BBL,4 OX40L,5 CD40,6 or LIGHT7 enhance tumor immunogenicity and inhibit tumor growth. Nonetheless, since metastatic disease is the primary cause of death among cancer patients, the challenge to promote costimulation within the disseminated tumor lesions of the patient remains unresolved. Although systemic administration of costimulatory ligands in the form of soluble ligand-Fc fusions or agonistic antibodies can promote tumor immunity,1,2 it also runs the risk of enhancing sub-threshold autoreactive immune responses. For example, administration of superagonistic CD28 antibodies to human volunteers is associated with severe toxicity,8 and administration of agonistic 4-1BB antibodies in mice results in nonspecific immune stimulation and other immune-related anomalies.9,10
Targeting costimulatory ligands to disseminated tumor lesions could reduce drug associated toxicities. To date, tumor-targeted immune stimulatory ligands have been described whereby the therapeutic ligand was conjugated to a tumor-targeting single-chain antibody.11,12,13,14 Notably, bi-specific CD3-CD19 antibodies that target T cells to CD19 expressing B-lymphoma cells exhibited remarkable therapeutic effects in non-Hodgkin's lymphoma patients.15 Yet the complexity and high cost of generating clinical grade protein-based therapeutic reagents, especially bi-specific monoclonal antibodies or antibody-ligand conjugates, have hindered their use as therapeutic agents.16 In addition, human protein reagents, including fully humanized monoclonal antibodies, are not totally devoid of immunogenicity, which can become a limiting factor upon repeated administrations.17 Viral vectors such as poxvirus- or adenovirus-based vector could be potentially adapted for tumor delivery of costimulatory ligands by reducing their cytolytic functions and exploiting their tumor-specific replication properties. Nonetheless, the notoriously poor penetration of viral vectors into solid tumors, the immunogenicity of the viral backbone, and the complexities associated with manufacturing clinical grade viral vectors, all but preclude their consideration for this application.18,19,20
Here, we describe the development of bi-specific ligands composed of oligonucleotide aptamers21,22 to target costimulatory ligands to tumor cells in vivo.
Results
Functional characterization of a bi-specific PSMA-4-1BB aptamer conjugate
4-1BB is a major costimulatory receptor promoting the survival and expansion of activated CD8+ T cells.23,24 We have previously shown that a bivalent 4-1BB binding aptamer formed by conjugation of two monomeric aptamers costimulates CD8+ T cells and promotes tumor immunity in mice.25 To mitigate the risk of autoimmunity,9,10 the bivalent 4-1BB aptamer was targeted to tumor cells by conjugation to a prostate specific membrane antigen (PSMA) binding aptamer (Figure 1a and Supplementary Figure S1). PSMA is a prostate tissue specific product which is upregulated on human prostate tumor cells. Since the human PSMA binding aptamer does not cross-react with murine PSMA (data not shown), in order to analyze the immunological and antitumor effects of PSMA-targeted costimulation in immune competent mice, murine CT26 colorectal carcinoma (H-2d) and B16/F10 melanoma (H-2b) tumor cells were stably transfected with a human PSMA expression plasmid.
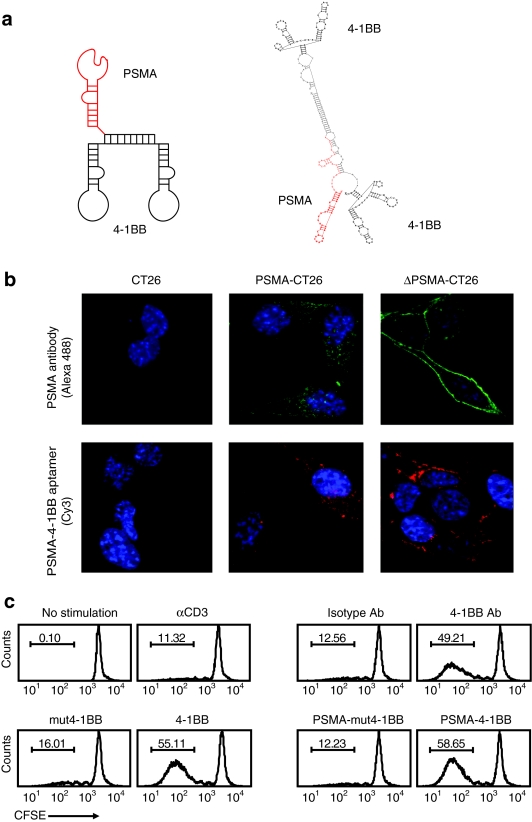
Figure 1.
Functional characterization of a bi-specific PSMA-4-1BB aptamer conjugate. (a) Sequence and computer generated secondary structure of the PSMA-4-1BB aptamer conjugate. See Methods section for full sequence. (b) Binding to PSMA-expressing CT26 tumor cells. Parental CT26 cells, CT26 cells expressing a wild-type PSMA (PSMA-CT26) or CT26 cells expressing an internalization-deficient mutant (ΔPSMA-CT26) were incubated with anti-PSMA antibody (green) or Cy3-conjugated PSMA-4-1BB aptamer conjugate (red) and analyzed by confocal microscopy (×60 magnification). Nuclei were stained with DAPI (blue) (N = 3). (c) 4-1BB costimulation. CD8+ T cells were labeled with CFSE, activated with suboptimal concentrations of anti-CD3 antibody, and incubated with anti-4-1BB antibody/isotype control IgG, unconjugated agonistic 4-1BB/costimulation-deficient mut4-1BB aptamers, or with PSMA-4-1BB /PSMA-mut4-1BB aptamer dimer conjugates. Two days later cells were analyzed by flow cytometry (N = 2). CFSE, carboxyfluorescein succinimidyl ester; PSMA, prostate specific membrane antigen.
Tumor-targeted 4-1BB costimulatory ligands, like cytotoxic antibodies such as Rituximab or Trastuzumab that kill their tumor target via antibody dependent cell cytotoxicity (ADCC) or fix complement,26,27 need to engage receptors that either do not internalize or rapidly recycle without dissociating their cargo.28,29 Binding of Rituximab to CD2030 or m816C antibody to Tenascin31 are examples of the former, while binding of Trastuzumab to Erb2 may be an example of the latter,32 although another study suggested that Erb2 does not internalize efficiently upon antibody binding.33 PSMA, like many receptors, upon binding its ligand is internalized via a chlatrin-dependent endocytic mechanism.34 Thus, in order to simulate a non internalizing receptor, we introduced a deletion into the cytoplasmic domain of PSMA (ΔPSMA) that reduces its internalization upon ligand binding.34
Both PSMA and ΔPSMA, were stably transfected into CT26 colon carcinoma and B16/F10 melanoma tumor cells. Flow cytometry confirmed that both PSMA proteins were expressed at comparable levels on the surface of transfected tumor cells (data not shown), and that these levels were three to fivefold lower compared to human prostate tumor LNCaP cells (Supplementary Figure S2). The binding and subcellular localization of the PSMA-4-1BB aptamer conjugate in CT26 tumor cells was determined by confocal microscopy. The PSMA-4-1BB aptamer conjugates or anti-PSMA antibody bound to PSMA-CT26 cells and was internalized, whereas they bound to ΔPSMA-CT26 cells but remained on the cell surface (Figure 1b).
We next tested whether the 4-1BB aptamer, when conjugated to the PSMA aptamer, retained its capacity to costimulate CD8 T cells. Costimulation was determined by measuring the proliferation of suboptimally activated, carboxyfluorescein succinimidyl ester (CFSE)-labeled CD8+ T cells as described.25 4-1BB antibody, unconjugated 4-1BB aptamer, and the PSMA-conjugated 4-1BB aptamer, but not PSMA-mut4-1BB which contains a nonfunctional 4-1BB aptamer, induced a comparable level of T cell proliferation (Figure 1c). These experiments show that conjugation of the PSMA and 4-1BB aptamers did not adversely affect binding to PSMA-expressing cells or costimulation, respectively.
Inhibition of tumor growth in mice treated with PSMA-4-1BB aptamer conjugates
We next determined whether systemic administration of PSMA-4-1BB aptamer conjugates affects tumor growth in tumor-bearing mice using the subcutaneously implanted CT26 colon carcinoma and the B16 clone F10 (B16/F10) lung metastasis models. Dose titration experiments showed no significant differences in the growth potential of parental and ΔPSMA-expressing CT26 or B16/F10 tumor cells, suggesting that expression of ΔPSMA did not significantly alter the poor immunogenicity of the tumor cells (data not shown). Treatment of day 3 subcutaneously implanted ΔPSMA-CT26 tumor-bearing mice with PSMA-4-1BB aptamer conjugate had a significant inhibitory effect on tumor growth (Figure 2a), with four out of eight mice surviving long-term (Figure 2b). Consistent with the conclusion that aptamer treatment elicited a tumor-specific immune response, CD8+ T cells isolated from the treated mice proliferated in response to ΔPSMA-CT26 tumor cells (Supplementary Figure S3). The mice that rejected the implanted tumor, but not age-matched control mice, were resistant to a rechallenge with CT26 tumor cells (Supplementary Figure S4), showing that tumor-targeted 4-1BB costimulation can engender long-term protective immunological memory against the tumors that do not present 4-1BB ligands. Little to no inhibition of tumor growth was seen in mice implanted with the wild-type PSMA-expressing tumor cells which supports ligand internalization, suggesting that 4-1BB costimulation of the tumor-infiltrating T cells is preceded by the binding of the aptamer conjugates to the ΔPSMA-expressing tumor cells (Supplementary Figure S5).
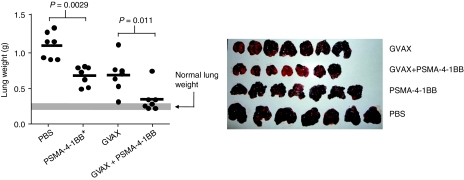
Figure 2.
Inhibition of tumor growth in mice treated with PSMA-4-1BB aptamer conjugate. (a) Subcutaneous tumor model. Mice bearing ΔPSMA-CT26 tumors, at days 3, 4, 5, and 7 mice were treated with 50 pmol of PSMA-4-1BB aptamer conjugate, 4-1BB aptamer, PSMA aptamer, mixture of 4-1BB and PSMA aptamer, or with phosphate buffered-saline (PBS), and monitored for tumor growth. Statistical analysis of average tumor size at day 20. PBS versus PSMA, 4-1BB, 4-1BB & PSMA and PSMA-4-1BB conjugate, P = 0.1567, 0.0347, 0.075, <0.0001, respectively. PSMA-4-1BB conjugate versus PSMA & 4-1BB, P = 0.0008. (N = 1). (b) Survival curves of the aptamer treated mice. Statistical analysis. PBS versus 4-1BB & PSMA or PSMA-4-1BB conjugate, P = 0.0003 and <0.0001, respectively. 4-1BB & PSMA versus PSMA-4-1BB conjugate, P < 0.0001. (N = 1). (c) Lung metastasis model. Mice were implanted with ΔPSMA-B16/F10 cells and injected with 50 pmol of PSMA-4-1BB or PSMA-mut4-1BB aptamer conjugates at days 5, 8, 11, 14. When about half of the mice in the control groups have shown signs of morbidity (circa days 25–28), mice were killed and lungs were weighed (N = 2). PSMA, prostate specific membrane antigen.
We have repeatedly noted that injection of PSMA aptamer conjugated to nonfunctional cargo such as non functional siRNAs35 or mutant 4-1BB (Figure 4b) had a small inhibitory effect on tumor growth. This can be attributed to either nonspecific immune stimulation by the oligonucleotide backbone of the aptamer conjugate36 or to direct binding of the aptamer conjugate to the PSMA-expressing tumor cells. Since unconjugated 4-1BB aptamer at 50 pmol/injection used in this experiment also leads to a small inhibition of tumor growth (see also Figure 4b), we tested the possibility that the antitumor effect of the PSMA-4-1BB aptamer conjugate was due to the separate additive effects of the PSMA and 4-1BB aptamers on tumor growth. Using a PSMA aptamer that was not capable of annealing to the 4-1BB aptamer (see Methods section), we found no significant enhancement of antitumor immunity when mice were treated with a mixture of the PSMA and 4-1BB aptamers (Figure 2a).
We also evaluated the ability of the PSMA-4-1BB aptamers to inhibit lung metastasis. To this end, C57BL/6 mice were injected intravenously with ΔPSMA-B16/F10 tumor cells and treated with PSMA-4-1BB or PSMA-mut4-1BB aptamer conjugates starting at day 5 after tumor inoculation. PSMA-4-1BB, but not PSMA-mut4-1BB treatment inhibited the development of lung metastasis (Figure 2c). By visual inspection at the time of sacrifice, six out of eight mice that had been treated with the PSMA-4-1BB aptamer conjugate were free of metastasis, whereas the lungs of all mice from the control and PSMA-mut4-1BB treated groups were covered with many metastatic nodules (Supplementary Figure S6).
We next tested whether tumor-targeted costimulation can potentiate a vaccine-induced antitumor response. To this end, day 5 B16/F10 tumor-bearing mice were vaccinated with GM-CSF expressing irradiated B16/F10 tumor cells (GVAX) and/or treated with PSMA-4-1BB aptamer conjugates. Consistent with previous observations,37,38,39 GVAX vaccination of tumor-bearing mice resulted in a partial inhibition of metastasis (Figure 3), thereby simulating a “weak” vaccination protocol. To measure the effect of both vaccination and costimulation, the PSMA-4-1BB aptamer conjugates were injected at half the concentration used in Figure 2c to avoid the almost complete inhibition of metastasis by this treatment alone. GVAX vaccination combined with PSMA-4-1BB aptamer costimulation was significantly more effective compared to each treatment alone (Figure 3). Interestingly, three out of seven mice in this group developed coat discoloration reminiscent of vitiligo, an antimelanocyte autoimmune response (Supplementary Figure S7).
Figure 3.
PSMA-targeted 4-1BB costimulation potentiates vaccine-induced tumor immunity. Mice bearing B16.F10 tumors were treated with 25 pmol per injection of PSMA-4-1BB aptamer conjugates and/or vaccinated with GM-CSF expressing irradiated tumor cells38 at day 5 post-tumor inoculation. Lung metastasis was determined by measuring lung weight (left) and visual inspection (right) (N = 1). PSMA, prostate specific membrane antigen.
Mechanism of tumor inhibition-PSMA targeting and 4-1BB costimulation
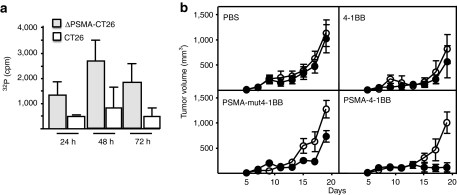
To determine whether the observed tumor inhibition shown in Figure 2 is dependent on PSMA targeting, mice were coimplanted in opposite flanks with parental CT26 and ΔPSMA-CT26 tumor cells and injected via the tail vein with either PSMA-4-1BB aptamer conjugate, unconjugated 4-1BB aptamer, or the costimulatory-deficient PSMA-mut4-1BB aptamer conjugate. Systemically injected 32P-labeled PSMA-4-1BB aptamer conjugates accumulated preferentially in the ΔPSMA-expressing as compared to parental CT26 tumor cells (Figure 4a). Treatment with the PSMA-4-1BB aptamer conjugate inhibited the growth of PSMA-expressing, but not the contralaterally implanted parental CT26 tumor cells (Figure 4b). Treatment with unconjugated 4-1BB had a small inhibitory effect on both ΔPSMA-expressing and parental tumor cells reflecting the effect of limited 4-1BB costimulation at the dose of 50 pmol/injection used (see also Figure 6a). Treatment with the costimulatory-deficient PSMA-mut4-1BB aptamer conjugate had a small inhibitory effect on the ΔPSMA-expressing, but not the contralaterally implanted parental tumor cells. This implies that the observed inhibition reflects binding of aptamer conjugates to the (PSMA expressing) tumor cells rather than a nonspecific immune stimulatory effect of nucleic acids. At day 19, when mice were killed because the parental CT26 tumors reached maximum allowable size, only PSMA-expressing, but not parental, CT26 tumors in mice treated with PSMA-4-1BB aptamer conjugate exhibited significant inhibition of growth; in three mice small tumors were palpable whereas in two mice tumors initially grew, became palpable, but fully regressed at the time of sacrifice (Supplementary Figure S8).
Figure 4.
PSMA-binding-4-1BB aptamer conjugate mediated inhibition of tumor growth is dependent on PSMA expression on the tumor cells. Balb/c were coimplanted subcutaneously with ΔPSMA-expressing (left flank) and parental (right flank) CT26 tumor cells and treated with PSMA-binding-4-1BB aptamer conjugate. (a) 15 days post-tumor inoculation 32P-labeled aptamer conjugate was injected, and 6, 24, and 48 hours later tumors were excised and 32P content determined (three mice per group). (N = 3). (b) 3 days post-tumor inoculation mice were treated with 50 pmol per injection of PSMA-4-1BB, PSMA-mut4-1BB aptamer conjugates, or unconjugated 4-1BB aptamer (five mice per group) and tumor growth monitored.(open circle) Parental CT26, (closed circle) ΔPSMA-CT26. Statistical analysis of average tumor size at day 19: ΔPSMA-CT26 versus CT26 tumor size in the PSMA-mut4-1BB treated mice, P = 0.0051, and in the PSMA-4-1BB treated mice, P = 0.0013. ΔPSMA-CT26 tumor size in the PSMA-mut4-1BB versus PSMA-4-1BB treated mice, P = 0.0007. ΔPSMA-CT26 versus CT26 tumor size in the PSMA-4-1BB treated mice at day 15 P = 0.0096, and day 17 P = 0.0076. (N = 2). PSMA, prostate specific membrane antigen.
This experiment, therefore, shows that the inhibition of tumor growth is mediated via PSMA aptamer targeting to tumor cells expressing the cognate receptor, it depends on a costimulation-competent 4-1BB aptamer, and that inhibition is, at least initially, local. This, however, appears to conflict with the repeated observations that mice which rejected the CT26 tumors, as shown in Figures 2b or 6a below, were resistant to a subsequent tumor challenge (Supplementary Figure S4), suggesting that aptamer treatment induced systemic, and not local, antitumor immunity. A plausible explanation that reconciles both observations is that the tumor-targeted 4-1BB costimulation does potentiate a systemic immune response but its dissemination is delayed, becoming effective if the tumor challenge occurs subsequent to PSMA-4-1BB treatment (Supplementary Figure S4), but not if tumor challenge is concurrent with PSMA-4-1BB therapy (Figure 4b).
To obtain direct evidence that the PSMA-binding-4-1BB aptamer conjugate is capable of costimulating tumor-infiltrating CD8+ T cells in a 4-1BB-dependent manner, we determined whether intratumoral accumulation of tumor-specific CD8+ T cells is dependent on 4-1BB/4-1BBL interactions. To this end, we tested whether the intratumoral accumulation of adoptively transferred Pmel-1 CD8+ T cells which recognize an epitope of gp100, a tumor antigen expressed in B16/F10 tumor cells,40 can be inhibited by intratumoral injection of a 4-1BB-Fc fusion protein. Mice implanted with a ΔPSMA-B16 tumor and treated with PSMA-binding-4-1BB aptamer conjugate resulted in significant intratumoral accumulation of Pmel-1 cells (Figure 5). Importantly, treatment with a 4-1BB-Fc fusion, which blocks 4-1BB/4-1BBL interactions, but not with an isotype control, inhibited the intratumoral accumulation of the Pmel-1 cells. Collectively, the experiments shown in Figures 4 and 5 provide complementary evidence that systemic administration of PSMA-4-1BB aptamer conjugates inhibits tumor growth in mice via tumor-targeted (PSMA aptamer-dependent) 4-1BB costimulation.
Figure 5.
PSMA- and 4-1BB-dependent intratumoral infiltration of transgenic Pmel-1 CD8 T cells in mice treated with PSMA-4-1BB aptamer conjugate. At days 11, 12, 13, and 17 B16/F10 (B16) or ΔPSMA-expressing B16/F10 (ΔPSMA-B16) tumor-bearing mice implanted with Pmel-1 CD8+ T cells were treated with PSMA-4-1BB or PSMA-binding-mut4-1BB aptamer conjugate (50 pmol per injection) or with PBS. At day 21 mice were killed, tumor isolated, and the number of tumor-infiltrating Pmel-1 cell was determined by flow cytometry. Where indicated, anti-4-1BB or isotype antibody was injected intratumorally immediately after aptamer injection. N = 2. PSMA, prostate specific membrane antigen.
Tumor targeting improves the safety profile of 4-1BB costimulation
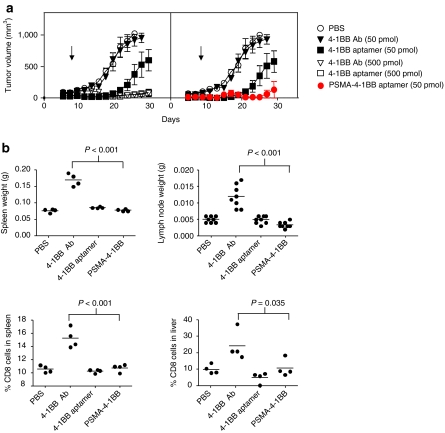
Since the systemic administration of agonistic 4-1BB antibodies results in nonspecific immune stimulation,9,10 we tested whether tumor-targeted delivery of the 4-1BB aptamer ligands would reduce the severity of adverse effects associated with 4-1BB costimulation. Using the subcutaneous CT26 tumor model we first compared the antitumor activity of an agonistic anti-4-1BB antibody, unconjugated 4-1BB aptamer, and the PSMA-4-1BB aptamer conjugate. Systemic administration of high levels of unconjugated 4-1BB aptamer or 4-1BB antibody (500 pmol/injection) inhibited tumor growth to near completion, whereas concentrations tenfold lower exerted only a partial inhibitory effect (4-1BB aptamer) or failed to inhibit tumor growth (4-1BB antibody). This is consistent with our previous observation that immune modulatory CTLA-4, 4-1BB, or OX40 aptamers were equally or slightly more potent than their corresponding antibodies.25,41,42 Similarly, low levels of PSMA-targeted 4-1BB aptamer conjugate was nearly as effective as tenfold increases in 4-1BB aptamer or antibody levels in inhibiting tumor growth. These data show that targeting 4-1BB ligands to tumor cells achieve a therapeutic benefit at lower doses, and that the PSMA-4-1BB aptamer conjugate was more effective than 4-1BB antibody.
We measured adverse effects in mice treated with therapeutic doses of 4-1BB antibody (500 pmol/injection), unconjugated 4-1BB aptamer (500 pmol/injection) and PSMA-conjugated 4-1BB aptamer (50 pmol/injection). Treatment of mice with 4-1BB Ab recapitulated the effects described in previous studies, which include enlarged spleen and lymph nodes and elevated levels of CD8+ T cells in the spleen and liver.9,10 In contrast, treatment of mice with unconjugated 4-1BB aptamer or with PSMA-4-1BB aptamer conjugate did not result in enlarged spleen or lymph nodes nor in the accumulation of CD8+ T cells in the spleen or liver. These results show that the unconjugated and PSMA-targeted 4-1BB aptamers exhibit a superior safety profile compared to antibodies, and that treatment with the tumor-targeted 4-1BB aptamers requires less reagent to achieve a therapeutic effect.
Discussion
In this study we describe a clinically feasible method to promote costimulation at the site of disseminated tumors using bi-specific oligonucleotide aptamers to target costimulatory ligands to tumor cells in situ. Systemic administration of PSMA-4-1BB aptamer conjugates to tumor-bearing mice led to significant inhibition of tumor growth and long-term tumor rejection (Figures 2–4 and 6). Moreover, targeted costimulation with bi-specific aptamers can synergize with and potentiate vaccine-induced immunity (Figure 3). The magnitude of the protective antitumor response engendered by the PSMA-4-1BB bi-specific aptamers in this study (using a first-generation reagent and non-optimized treatment schedule) has been rarely observed with other immune potentiating single-agent monotherapies and appears to be superior to that of vaccination with GVAX, a potent vaccination protocol in mice (Figures 2 and 3 and references).37,38,39 The tumor inhibitory effect of administering PSMA-4-1BB aptamer conjugates to the tumor-bearing mice reflected the potentiation of a naturally occurring, though weak and nonproductive, immune response elicited by the poorly immunogenic CT26 or B16/F10 tumors. Since tumor progression in cancer patients often elicits, albeit ineffective, antitumor immune responses,43 these observations suggest that tumor-targeted costimulation may be capable of potentiating the naturally occurring antitumor immune responses in cancer patients to control tumor progression to a level comparable if not exceed that of vaccination (Figure 3).
Figure 6.
Therapeutic index of costimulatory 4-1BB ligands. (a) Comparative analysis of the tumor inhibitory capacity of PSMA-4-1BB aptamer conjugate, free 4-1BB aptamer and 4-1BB antibody. ΔPSMA-CT26 tumor-bearing mice were treated with PBS (open circle), 50 (closed triangle) or 500 (open triangle) pmol per injection of anti-4-1BB antibody, 50 (closed square) or 500 (open square) pmol per injection of unconjugated 4-1BB aptamer, or 50 pmol per injection of PSMA-binding-4-1BB aptamer conjugates (closed circle), starting at day 3 post-tumor implantation (10 mice per group). Data were separated into two panels for clarity purposes. Left panel compares the 4-1BB antibody to the unconjugated 4-1BB aptamer and the right panel compares the unconjugated to the PSMA-conjugated 4-1BB aptamer. (N = 1). (b) Evaluation of nonspecific immune stimulatory effects in mice treated with therapeutic doses of 4-1BB ligands. Balb/c mice were injected with 4-1BB antibody (500 pmol per injection), unconjugated 4-1BB aptamer (500 pmol per injection), PSMA-4-1-BB aptamer conjugate (50 pmol per injection), or PBS at days 1, 2, 3, 5. Two weeks after the last injection mice were killed, the spleen and the two inguinal lymph nodes were weighed and percentage of CD8 T cells in spleen and liver was determined by flow cytometry. (N = 2). PSMA, prostate specific membrane antigen.
Drug toxicity is a major impediment in developing effective treatments for cancer. For example, in human volunteers, administration of superagonistic CD28 antibodies was associated with severe toxicity,8 and in mice administration of agonistic 4-1BB antibodies resulted in nonspecific immune stimulation and other immune-related anomalies.9,10 Targeting poorly specific drugs to tumor cells should, therefore, mitigate their undesirable effects on normal cells. Here we show that using bi-specific aptamers to target 4-1BB ligands to tumor cells can reduce the therapeutic dose compared to untargeted ligand (Figure 6a), and is not associated with adverse effects as compared to using a 4-1BB antibody (Figure 6b).
Several studies have shown that optimal activation of T cells by costimulation through 4-1BB,44,45 OX40,46 or GITR47,48 can promote their resistance to the immune suppressive effects of foxp3+ regulatory T cells (Treg), and conceivably other immune attenuating mediators. Effective costimulation targeted to the tumor site with bi-specific aptamers could, therefore, confer increased resistance to the local immune suppressive effects of Treg without affecting the physiological functions of Treg elsewhere in the body. It is, therefore, tempting to speculate that effective tumor-targeted costimulation may reduce, though not eliminate, the need to develop strategies to counter tumor-induced suppression mechanisms.
In summary, aptamer-based bi-specific ligands represent a new platform technology to endow costimulatory capacity to disseminated tumors which will synergize with vaccination protocols to enhance the susceptibility of disseminated tumors to naturally occurring or vaccine-induced antitumor immune responses. The PSMA-4-1BB aptamer conjugate described in this study is a first-generation prototype aptamer conjugate that can be used to deliver other, and perhaps more effective, costimulatory ligands to tumor cells such as CD70, CD40L, or LIGHT. Targeted delivery of aptamer-based costimulatory ligands to tumor cells in situ could, therefore, serve as a powerful approach to achieve protective antitumor immunity.
Methods
PSMA-4-1BB aptamer conjugates. The PSMA aptamer, 5′GGGAGGACGAUGCGGAUCAGCC AUGUUUACGUCACUCCUUGUCAAUCCUCAUCGGCAGACGACUCGCCCGA 3′49 was cloned into pUC57 between KpnI and BamHI restriction sites and PCR amplified using forward primer 5′TAATACGACTCACTATAGGGAGGACGATGCGG3′ and reverse primer 5′GCTATAAGTGTGCATGAGAACTCGGGCGAGTCGTCTG3′. The reverse primer encodes a sequence which is complementary to the linker sequence of the dimeric form of 4-1BB aptamer.25 A reverse primer that doesn't encode a complementary sequence to the 4-1BB linker, 5-TCGGGCGAGTCGTCTG-3, was used to amplify a PSMA aptamer sequence that cannot anneal to the 4-1BB dimer. Using overlapping oligonucleotides the 4-1BB dimer was cloned in pGem-t-easy plasmid (Promega, Madison, WI) and PCR amplified using the forward primer 5′CAGGCGGCCGCGAATT3′ and reverse primer 5′CGTCGCATGCTCCCGGC3′. To generate a mutated form of the 4-1BB dimer, (mut4-1BB), PCR amplification was carried out in the presence of 8-oxo-2'-deoxyguanosine-5'-Triphosphate and 2'-deoxy-pyrene-5'-triphosphate (Trilink, Millersvile, MD). The PCR products were cloned into pGem-t-easy plasmid and sequenced. A clone that had no predicted impact in the secondary structure of the PSMA aptamer was chosen for further studies. The sequence of the 4-1BB dimer:
5′GGGAGAGAGGAAGAGGGAUGGGCG ACCGAACGUGCCCUUCAAAGCCGUUCACUAACCAGUGGCAUAACCCAGAGGUCGAUAGU ACUGGAUCCCCCCCCCGCUAUAAGUGUGCAUGAGAACCCCGGGGGGAGAGAGGAAGAGGG AUGGGCGACCGAACGUGCCCUUCAAAGCCGUUCACUAACC AGUGGCAUAACCCAGAGGUCGAUAGUACUGGAUCCCCCC3′
The sequence of mut4-1BB dimer (differences are shown in bold):
5′GGGAGAGAGGAAGGGGGAUGGGCGACCGAGCG UGCCCUCCAGAGCCGUUCACCAGCCAGUGGCA UAGCCCAGAGGUCGAUAAUACUGGACCCCCCCCCC GCUAUAAGCGGGCAUGAGAACCCCGGGGGGAGAG AGGAAGGGGGAUGGGCGACCGAACGUGCCCCUCA AAGCCGUCCACUAACCAGCGGCACAGCCCAGAGG CCGAUAGUACUGGACCCCCCC3′
The PSMA and 4-1BB aptamer PCR products were purified using the QIAprep Spin columns (Qiagen, Valencia, CA). RNA was transcribed using the T7(Y639F) polymerase as previously described50 and annealed to form the PSMA-4-1BB aptamer conjugates. The products were separated on a polyacrylamide gel. The conjugate was purified by polyacrylamide gel electrophoresis and concentrated on a 30 Kda Amicon Ultra-4 column (Millipore, Billerica, MA)
Confocal microscopy. The 4-1BB aptamer dimer was labeled with Cy3 before hybridization to the PSMA aptamer using the Silencer RNA labeling kit (Ambion, Austin, TX). Tumor cells were washed with phosphate-buffered saline and incubated with 40 nmol/l of Cy3-labeled aptamer conjugate or with 10 mg/ml anti-PSMA Ab (MBL, Woburn, MA) and Alexa Fluor 488 goat anti-mouse IgG (Molecular Pobes, Eugene, OR). Coverslips were mounted with Prolong Gold-DAPI (Molecular Pobes, Eugene, OR)
Derivation of PSMA-expressing CT26 tumor cell lines. The PSMA cDNA, kindly provided by Dr. Ponmarev, was PCR amplified using forward primer 5́GATCAGCGGCCGCGCCACCATGTGGAATCTCCTTCACG3′ and reverse primer 5′GTTAAGTCGACGAGGATCCTCGAGAATCCTCTTAGGCTACTTCACTC3′. ΔPSMA was generated by deleting the N-terminal 13 amino acids WNLLHETDSAVAT using forward primers 5′GATCAGCGGCCGCGCCACCATGGCGCGCCGCCCGCGCTGGCTG3′ and reverse primer 5′GTTAAGTCGACGAGGATCCTCGAGAATCCTCTTAGGCTACTTCACTC3′. The PCR products were cloned into the SalI and Not1 restriction sites of the retroviral vector pBMN (Addgene, Cambridge, MA) and transiently transfected into the Phoenix-AMPHO 293 packaging cell lines. Viral supernatant was used to transduce CT26 colon carcinoma (H-2d) and B16/F10 melanoma (H-2b) tumor cell lines and PSMA-expressing cells were isolated by sorting using PE-labeled anti-PSMA antibody from MBL, Woburn, MA.
CFSE proliferation. CD8 cell were isolated from spleen and lymph nodes, using the stem Sep negative selection kit (StemCell Technologies, Vancouver, CA). Purified CD8 cells were stained with 2 µmol/l of CFSE. CFSE labeled cells were incubated with 1 µg/ml of CD3 (BD Bioscience, San Jose, CA) and either 5 µg/ml of anti-murine 4-1BB (3H3) Ab kindly provided by Dr Robert Mittler, 5 µg/ml isotype control Ab, 100 nmol/l of 4-1BB aptamer dimer or 100 nmol/l PSMA-4-1BB aptamer conjugates and analyzed by flow cytometry.
Intratumoral infiltration of Pmel-1 CD8+ T cells. C57BL/6 mice (Thy 1.2) were implanted subcutaneously in one flank with 3 × 104 B16/F10 tumor cells and in the contralateral flank with 105 ΔPSMA-B16/F10 tumor cells. At day 6, 5 × 106 gp100-specific Pmel-1 (Thy 1.1) CD8+ T cells were injected via the tail vein and 2 days later injected with 5 mg of gp100 peptide (KVPRNEDWL) plus 5 mg LPS. At days 11, 12, 13, and 17 mice were injected with 50 pmol of PSMA-4-1BB aptamer conjugate. In some mice 100 pmol of 4-1BB-Ig fusion protein or isotype IgG (R&D, Minneapolis, MN) were injected intratumorally coincident with injection of aptamer conjugates. Mice were killed at day 21, tumors were removed, treated with collagenase, stained with APC-labeled anti-CD8 Ab (BD Bioscience) and anti PE-labeled anti-Thy1.1 Ab (BD Bioscience) and analyzed by flow cytometry.
Tumor immunotherapy studies. 3 × 105 parental CT26 or ΔPSMA-CT26 tumor cells were implanted subcutaneously in Balb/c mice. At days 3, 4, 5, and 7 the tumor-bearing mice were injected via the tail vein with 50 pmol of PSMA-4-1BB, 50 or 500 pmol of 4-1BB aptamer dimer, or with 50 or 500 pmol of anti-4-1BB 3H3 antibody (kindly provided by Dr Robert Mittler).
To monitor for metastasis, C57BL/6 mice were implanted with 105 ΔPSMA-B16/F10 cells via the tail vein and injected with 50 pmol PSMA-4-1BB aptamer conjugates at days 5, 8, 11, 14. When about half of the mice in the control groups have shown signs of morbidity (circa days 25–28), mice were killed and lungs were weighed. GM-CSF expressing B16/F10 tumor cells, kindly provided by Dr G. Dranoff, were irradiated (5,000 rad) and 5 × 105 cells were injected subcutaneously at days 5, 8, and 11.38
Statistical analysis. For statistical analysis of tumor growth P values were calculated using Student's t-test. For survival, P values were determined using the Log-rank (Mantel–Cox) test.
Tumor homing or 32P-labeled aptamer conjugates. The 4-1BB aptamer dimer was transcribed in vitro in the present of 1/1,000 parts of α32P-ATP (3,000 Ci/mmol) (PerkinElmer, Boston, MA) and annealed to the PSMA aptamer as described above. Balb/c mice were coimplanted with CT26 and ΔPSMA-CT26 tumor cells in the opposite flanks and 15 days later injected via the tail vein with 5 × 105 cpm 32P-labeled aptamer conjugate. At indicated times tumors were surgically removed, cells dispersed by incubation with 400 U/ml of collagenase, washed three times with phosphate-buffered saline, and cell associated 32P was measured in a scintillation counter
SUPPLEMENTARY MATERIAL Figure S1. Heteroduplex formation between PSMA and 4-1BBB aptamers. Figure S2. Expression of human PSMA on the surface of tumor cells. Figure S3. Induction of T cell responses in tumor-bearing mice treated with PSMA-binding-4-1BB aptamer conjugate. Figure S4. Mice that rejected the ΔPSMA-CT-26 tumor are resistant to a rechallenge with parental CT-26 tumor cells. Figure S5. Inhibition of tumor growth in mice bearing internalizing and non internalizing PSMA receptors treated with PSMA-binding-4-1BB aptamers. Figure S6. Lungs of mice treated with PSMA-binding-4-1BB aptamer conjugates at day of sacrifice. Figure S7. Coat discoloration in mice vaccinated with GVAX and treated with PSMA-binding-4-1BB aptamer conjugates. Figure S8. Tumor size at day of sacrifice in mice coimplanted with PSMA-CT26 and parental CT26 tumor cells.
Acknowledgments
We thank Jian Zhang (Medical School, University of Miami) for assistance in the murine studies, Paloma Giangrande (University of Iowa) for advice in generating aptamer conjugates, and Gabriel Gaidosh (Medical School, University of Miami) for helping with the confocal microscopy. This work was supported by the Dodson foundation and the Sylvester Comprehensive Cancer Center (Medical School, University of Miami). The authors declared no conflict of interest.
Supplementary Material
Heteroduplex formation between PSMA and 4-1BBB aptamers.
Expression of human PSMA on the surface of tumor cells.
Induction of T cell responses in tumor-bearing mice treated with PSMA-binding-4-1BB aptamer conjugate.
Mice that rejected the ΔPSMA-CT-26 tumor are resistant to a rechallenge with parental CT-26 tumor cells.
Inhibition of tumor growth in mice bearing internalizing and non internalizing PSMA receptors treated with PSMA-binding-4-1BB aptamers.
Lungs of mice treated with PSMA-binding-4-1BB aptamer conjugates at day of sacrifice.
Coat discoloration in mice vaccinated with GVAX and treated with PSMA-binding-4-1BB aptamer conjugates.
Tumor size at day of sacrifice in mice coimplanted with PSMA-CT26 and parental CT26 tumor cells.
REFERENCES
- Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Deraffele G, Mitcham J, Moroziewicz D, Cohen SM, Hurst-Wicker KS.et al. (2005Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma J Clin Invest 1151903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Breiter DR, Zheng G., and, Chen A. Enhanced antitumor responses elicited by combinatorial protein transfer of chemotactic and costimulatory molecules. J Immunol. 2007;178:3301–3306. doi: 10.4049/jimmunol.178.5.3301. [DOI] [PubMed] [Google Scholar]
- Piconese S, Valzasina B., and, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Guardino A, Chinsangaram L, Goldstein MJ, Panicali D., and, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of recombinant fowlpox virus encoding CD40 ligand. Cancer Res. 2007;67:7037–7044. doi: 10.1158/0008-5472.CAN-07-0224. [DOI] [PubMed] [Google Scholar]
- Tamada K, Shimozaki K, Chapoval AI, Zhu G, Sica G, Flies D.et al. (2000Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway Nat Med 6283–289. [DOI] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD.et al. (2006Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412 N Engl J Med 3551018–1028. [DOI] [PubMed] [Google Scholar]
- Lee SW, Salek-Ardakani S, Mittler RS., and, Croft M. Hypercostimulation through 4-1BB distorts homeostasis of immune cells. J Immunol. 2009;182:6753–6762. doi: 10.4049/jimmunol.0803241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D.et al. (2007Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice J Immunol 1784194–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Sadun RE, Arias RS, Flanagan ML, Sachsman SM, Nien YC.et al. (2007Targeted and untargeted CD137L fusion proteins for the immunotherapy of experimental solid tumors Clin Cancer Res 132758–2767. [DOI] [PubMed] [Google Scholar]
- Sharma S, Dominguez AL, Manrique SZ, Cavallo F, Sakaguchi S., and, Lustgarten J. Systemic targeting of CpG-ODN to the tumor microenvironment with anti-neu-CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB-neuT tolerant mice. Cancer Res. 2008;68:7530–7540. doi: 10.1158/0008-5472.CAN-08-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Wang H, Lu B, Li B, Hou S, Qian W.et al. (2008Cancer immunotherapy using in vitro genetically modified targeted dendritic cells Cancer Res 683854–3862. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA., and, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S.et al. (2008Tumor regression in cancer patients by very low doses of a T cell-engaging antibody Science 321974–977. [DOI] [PubMed] [Google Scholar]
- Pardoll D., and, Allison J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat Med. 2004;10:887–892. doi: 10.1038/nm0904-887. [DOI] [PubMed] [Google Scholar]
- De Groot AS., and, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Kirn DH., and, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Liu TC, Hwang TH, Bell JC., and, Kirn DH. Development of targeted oncolytic virotherapeutics through translational research. Expert Opin Biol Ther. 2008;8:1381–1391. doi: 10.1517/14712598.8.9.1381. [DOI] [PubMed] [Google Scholar]
- Parato KA, Senger D, Forsyth PA., and, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Gold L. Oligonucleotides as research, diagnostic, and therapeutic agents. J Biol Chem. 1995;270:13581–13584. doi: 10.1074/jbc.270.23.13581. [DOI] [PubMed] [Google Scholar]
- Nimjee SM, Rusconi CP., and, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- Vinay DS., and, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- Wang C, Lin GH, McPherson AJ., and, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH.et al. (2008Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice J Clin Invest 118376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Pop LM., and, Vitetta ES. Engineering therapeutic monoclonal antibodies. Immunol Rev. 2008;222:9–27. doi: 10.1111/j.1600-065X.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., and, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS., and, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Smythe E., and, Warren G. The mechanism of receptor-mediated endocytosis. Eur J Biochem. 1991;202:689–699. doi: 10.1111/j.1432-1033.1991.tb16424.x. [DOI] [PubMed] [Google Scholar]
- Pagel JM, Hedin N, Subbiah K, Meyer D, Mallet R, Axworthy D.et al. (2003Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas Blood 1012340–2348. [DOI] [PubMed] [Google Scholar]
- Brown MT, Coleman RE, Friedman AH, Friedman HS, McLendon RE, Reiman R.et al. (1996Intrathecal 131I-labeled antitenascin monoclonal antibody 81C6 treatment of patients with leptomeningeal neoplasms or primary brain tumor resection cavities with subarachnoid communication: phase I trial results Clin Cancer Res 2963–972. [PubMed] [Google Scholar]
- Austin CD, De Mazière AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX.et al. (2004Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin Mol Biol Cell 155268–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulida J, Kraus MH, Alimandi M, Di Fiore PP., and, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- Rajasekaran SA, Anilkumar G, Oshima E, Bowie JU, Liu H, Heston W.et al. (2003A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen Mol Biol Cell 144835–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor F, Kolonias D, Giangrande PH., and, Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465:227–230. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K., and, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K.et al. (1993Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity Proc Natl Acad Sci USA 903539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Peggs KS, Curran MA., and, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas A, Hurwitz AA., and, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA.et al. (2003Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells J Exp Med 198569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollins CM, Nair S, Boczkowski D, Lee J, Layzer JM, Gilboa E.et al. (2008Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer Chem Biol 15675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli-Marotto S, Nair SK, Rusconi C, Sullenger B., and, Gilboa E. Multivalent RNA aptamers that inhibit CTLA-4 and enhance tumor immunity. Cancer Res. 2003;63:7483–7489. [PubMed] [Google Scholar]
- Hodi FS., and, Dranoff G. Combinatorial cancer immunotherapy. Adv Immunol. 2006;90:341–368. doi: 10.1016/S0065-2776(06)90009-1. [DOI] [PubMed] [Google Scholar]
- Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS.et al. (20044-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells J Leukoc Biol 75785–791. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Messer RJ, Carmody AB, Mittler RS, Burlak C., and, Hasenkrug KJ. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J Immunol. 2008;180:5267–5274. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A.et al. (2007OX40 costimulation turns off Foxp3+ Tregs Blood 1102501–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji HB, Liao G, Faubion WA, Abadía-Molina AC, Cozzo C, Laroux FS.et al. (2004Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression J Immunol 1725823–5827. [DOI] [PubMed] [Google Scholar]
- Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM.et al. (2004Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells J Immunol 1735008–5020. [DOI] [PubMed] [Google Scholar]
- Lupold SE, Hicke BJ, Lin Y., and, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002;62:4029–4033. [PubMed] [Google Scholar]
- McNamara JO, 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E.et al. (2006Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras Nat Biotechnol 241005–1015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heteroduplex formation between PSMA and 4-1BBB aptamers.
Expression of human PSMA on the surface of tumor cells.
Induction of T cell responses in tumor-bearing mice treated with PSMA-binding-4-1BB aptamer conjugate.
Mice that rejected the ΔPSMA-CT-26 tumor are resistant to a rechallenge with parental CT-26 tumor cells.
Inhibition of tumor growth in mice bearing internalizing and non internalizing PSMA receptors treated with PSMA-binding-4-1BB aptamers.
Lungs of mice treated with PSMA-binding-4-1BB aptamer conjugates at day of sacrifice.
Coat discoloration in mice vaccinated with GVAX and treated with PSMA-binding-4-1BB aptamer conjugates.
Tumor size at day of sacrifice in mice coimplanted with PSMA-CT26 and parental CT26 tumor cells.